Abstract
Background:
Chronic oxidative stress and inflammation severely affect the normal physiology of neurons and lead to neurodegenerative disorders such as Alzheimer's disease (AD). Polyphenols proved a boon in the prevention of dementia due to their antioxidant and neuroprotective potential. Caffeic acid phenethyl ester (CAPE) is a natural polyphenolic compound attributed with antioxidant, immunomodulatory, and neuroprotective properties.
Objective:
The present study investigates the effect of CAPE on experimental dementia in rats.
Methods:
Intracerebroventricle (ICV) injection of streptozotocin (STZ; 3 mg/kg) was given to Wistar rats (200 g, either sex) on days 1 and 3 to induce dementia of AD type. CAPE (3 and 6 mg/kg, i.p.) was administered to separate groups of rats for 28 successive days daily. Morris water maze and elevated plus maze served as exteroceptive behavioral models to measure the memory of the rats.
Results:
The present study illustrated that CAPE treatment for 28 consecutive days arrested the development of cognitive deficits in STZ-ICV-treated rats, that is, a significant (P < 0.05) reduction in the mean escape latency during acquisition trial and increased (P < 0.05) time spent in target quadrant during retrieval trial in Morris water maze test and reduction (P < 0.05) in transfer latency in elevated plus maze test. Furthermore, both the doses of CAPE when administered to rats that were previously treated with STZ-ICV prevented the rise of brain thiobarbituric acid reactive substance as well as TNF-α and simultaneously enhanced the GSH content.
Conclusion:
CAPE administration ameliorated STZ-ICV-induced dementia through the attenuation of oxidative stress and inflammation.
SUMMARY
Intracerebroventricular administration of streptozotocin (STZ-ICV) induced cognitive deficits, enhanced brain oxidative stress as well as inflammation in rats.
Treatment with Caffeic Acid Phenethyl Ester (CAPE; dose 3 and 6 mg/kg, i.p.) for 28 days once daily, enhanced the memory and prevented the development of STZ-ICV-induced dementia in rats.
The CAPE treated rats showed decrease in mean escape latency and increase in time spent in target quadrant in Morris Water Maze test. A decline in transfer latency of CAPE treated rats was observed in Elevated Plus Maze model.
Profound rise in brain GSH levels and diminution of TBARS as well as TNF-α content was observed in brains of CAPE treated rats. Hence, the memory enhancing activity of CAPE against STZ-ICV-induced dementia is attributed to its robust antioxidant and anti-inflammatory property.
Abbreviation used: AD: Alzheimer's disease, ANOVA: Analysis of Variance, aCSF: Artificial cerebrospinal fluid, CAPE: Caffeic acid phenethylester, EPM: Elevated plus maze, ELT: Escape latency time, GSH: Reduced glutathione, IL: Interleukin, ICV: Intracerebroventricular, MDA: Malondialdehyde, MEL: Mean escape latency, MWM: Morris water maze, NFTs: Neurofibrillary tangles, RNS: Reactive nitrogen species, ROS: Reactive oxygen species, SEM: Standard error of mean, STZ: Streptozotocin, TBARS: Thiobarbituric reactive substances, TSTQ: Time spent in target quadrant, TL: Transfer latency, TNF-α: Tumor necrosis factor alpha.
Keywords: Alzheimer's disease, caffeic acid phenethyl ester, inflammation, oxidative stress, streptozotocin
INTRODUCTION
Alzheimer's disease (AD) is the most prevalent type of dementia that affects elderly people.[1] Neurodegeneration initiates from entorhinal cortex and leads to gross cortical and hippocampal atrophy in the later stages. Glutamate excitotoxicity, loss of cholinergic inputs from nucleus basalis of Meynert, synaptic dysfunction, and glial activation are characteristic features of AD.[2] Extracellular senile plaques and intracellular neurofibrillary tangles (NFTs) are the foremost histopathological hallmarks.[1,2] Senile plaques are extracellular deposits of insoluble amyloid-β40-42 fibrils in brain, while NFTs are axonal aggregates of paired helical filaments that contain hyperphosphorylated tau protein. Advancing age, cardiovascular disorders, impaired glucose metabolism, dyslipidemia, and brain injury are the major risk factors of AD, which also accelerates free radical formation. Oxidative stress and inflammation precede the AD pathology and are associated with increased cholinergic deficit, β-amyloidosis, and taupathy.[3] Moreover, impaired glucose metabolism in synergy with oxidative stress impedes the clearance of amyloid-β40-42 and NFTs from the brain. A number of studies indicate that antioxidants such as resveratrol, catechins, and α-tocopherol confer neuroprotection, enhance cholinergic activity, and retard the progression of AD.[1,4] Natural antioxidants are reported to increase memory and, hence, are promising in therapeutic management of dementia.
Caffeic acid phenethyl ester (2-phenylethyl (2E)-3-(3, 4-dihydroxyphenyl) acrylate; CAPE) is a polyphenolic compound present in honey and propolis of honeybee hives.[5] The phenylpropanoid scaffold of caffeic acid (3, 4-dihydroxycinnamic acid) is commonly used as the template to develop caffeic acid derivatives (alkyl esters) and new chemical entities possessing robust antioxidant property. The phenethyl ester derivative of caffeic acid has better pharmacokinetic profile compared with caffeic acid, although in vivo CAPE is hydrolyzed to caffeic acid.[6] CAPE is known to possess various pharmacologic activities such as antioxidant, anti-inflammatory, immunomodulatory, and neuroprotective activities.[7,8] However, to the best of our knowledge, there is hardly any report in the literature regarding the effect of CAPE in dementia. Therefore, this study explores the effect of CAPE on Alzheimer's disease pathology and cognitive functions against streptozotocin (STZ)-induced dementia in rats.
MATERIALS AND METHODS
Animals
Adult Wistar rats (180-200 g, either sex) were procured from DFSAH, LUVAS, Hisar, and maintained at Central Animal Facility of the institute under standard laboratory conditions with controlled temperature (23 ± 2°C), humidity (40 ± 10 %), and natural light-dark cycle (12 h each). The animals were nourished with standard rodent pellet diet (Ashirwad Industries, Mohali) and water ad libitum. The experiment was carried out between 09:00 and 18:00 h. The care of laboratory animals was done following the guidelines of CPCSEA, Ministry of Forests and Environment, Government of India.
Drugs and reagents
CAPE procured from Sigma-Aldrich was dissolved in sterile dimethyl sulfoxide (DMSO) diluted with isotonic saline (1:5). STZ was procured from Sisco Research Laboratories Pvt. Ltd., Mumbai. Dihydrogen orthophosphate, 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB) (Himedia Laboratories, Mumbai); thiobarbituric acid, trichloroacetic acid, sodium dodecyl sulphate (Loba Chemie, Mumbai); and TNF-α ELISA kit (Krishgen, Mumbai) were used. Artificial cerebrospinal fluid (aCSF) was prepared as follows: (in mmol/L) 147 mM NaCl, 2.9 mM KCl, 1.6 mM MgCl2, 1.7 mM CaCl2, 2.2 mM dextrose were dissolved in 10 mL of water for injection.[9] All the drug solutions were freshly prepared before injections.
Intracerebroventricular injection of streptozotocin (STZ-ICV)
The rats were anaesthetized with chloral hydrate (300 mg/kg, i.p.). The head was positioned in the frame of stereotaxic apparatus (INCO, Ambala, India), middle sagittal incision was made in the scalp, and skull was exposed. Two holes were drilled through the skull for bilateral placement of microinjector into the lateral cerebral ventricles using the following coordinates: 0.8 mm posterior to bregma, 1.5 mm lateral to sagittal suture, and 3.6 mm beneath the surface of the brain.[10] STZ was dissolved in freshly prepared aCSF. STZ (3 mg/kg) was injected bilaterally in two divided doses on days 1 and 3. The concentration of STZ in aCSF was adjusted so as to deliver 10 µL (5 µL in each ventricle) of the solution.[11,12] Rate of injection was maintained at 1 µL/min. Surgical control animals were given same volume of aCSF on days 1 and 3. After days 1 and 3 injections, the skin was sutured and antiseptic powder (Cipladine®) was applied.
Experimental protocol
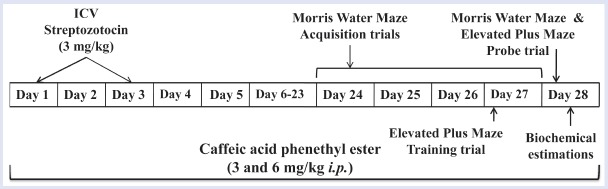
The animals were randomly divided into eight different groups having six to eight animals each: Group I: normal saline, sterile DMSO diluted with isotonic saline (1:5) for 28 consecutive days (i.p.); Group II: CAPE per se, CAPE (6 mg/kg, i.p.) for 28 consecutive days; Group III: donepezil per se, donepezil (1 mg/kg, p.o.) for 25 days daily; Group IV: sham control, aCSF 10 µL in both ventricles (5 µL each) on days 1 and 3; Group V: STZ-ICV, STZ (3 mg/kg/10 µL in both ventricles in two divided doses) on days 1 and 3; Group VI: STZ + CAPE (3), CAPE (3 mg/kg, i.p.) 60 min before STZ-ICV administration on day 1 and then consecutively up to day 28; Group VII: STZ + CAPE (6), CAPE (6 mg/kg, i.p.) 60 min before STZ-ICV administration on day 1 and then consecutively up to day 28; Group VIII: STZ + donepezil, donepezil (1 mg/kg, p.o.) for 25 consecutive days starting 60 min before STZ-ICV administration on day 1. Two doses of CAPE (3 and 6 mg/kg, i.p.)[13] were administered daily to separate groups of rats for 28 consecutive days. The memory of rats was evaluated from 24th day onwards upto 28th day and thereafter were sacrificed for biochemical studies [Figure 1]. The locomotor activity of animals was also measured before surgery and before behavioral studies. Donepezil (1 mg/kg, p.o.) served as the standard drug.[12] After behavioral studies, the animals were sacrificed by decapitation to estimate brain thiobarbituric acid reactive substances (TBARS), GSH, and TNF-α level.
Figure 1.
Experimental protocol and drugs treatment schedule
Morris water maze test
Morris water maze (MWM) is a swimming-based model used to assess spatial memory of rodents. In this task, the animal learns to escape on to a hidden platform. It consists of large, black, circular pool (200 cm in diameter, 60 cm in height, filled to a depth of 30 cm with water at temperature 25 ± 1°C). The tank was divided into four equal quadrants (Q1, Q2, Q3, and Q4) with help of two threads, fixed at right angle to each other on the rim of the pool, and numbered clock wise. A submerged platform (11 cm2) was placed inside the target quadrant (Q4) of this pool, 1 cm below surface of water. The position of platform was kept unaltered throughout the training session. Each animal was subjected to four consecutive training trials on each day with intertrial gap of 5 min. The rat was gently placed in the water between quadrants, facing the wall of pool with drop location changing from Q1 to Q4 on day 1, from Q2 to Q1 on day 2, from Q3 to Q2 on day 3, and from Q4 to Q3 on day 4 for each trial, and then allowed 120 s to locate submerged platform. Then, it was allowed to stay on the platform for 20 s. If it failed to find the platform within 120 s, it was guided gently onto platform and allowed to remain there for 20 s. Escape latency time (ELT) is the time taken by the rat to locate the hidden platform in water maze. Day 4 ELT vs. day 1 ELT was noted as index of acquisition or spatial learning. On the fifth day during the probe trial, platform was removed and each rat was allowed to explore the pool for 120 s. Mean time spent in all four quadrants was noted. The mean time spent by the animal in target quadrant (TSTQ) searching for the hidden platform was noted as index of retrieval or reference memory. The experimenter always stood at the same position. Care was taken that relative location of water maze with respect to other objects in the laboratory that served as prominent visual clues were not disturbed during the total duration of study.[9]
Elevated plus maze test
The elevated plus maze (EPM) apparatus for rats consisted of a central platform (10 cm × 10 cm) connected to two open arms (50 cm × 10 cm) and two covered (enclosed) arms (50 cm × 40 cm × 10 cm). The maze was elevated to a height of 50 cm from the floor. Transfer latency (TL) is defined as the time taken by the animal to move from the open arm into one of the covered arms with all of its four paws. In order to record TL, each rat was placed at the end of an open arm facing away from the central platform. TL was recorded on first day for each animal. The rat was allowed to explore the maze for 20 s and then returned to its home cage. The cut-off time to reach the closed arm is 90 s. In case the rat does not locate the closed arm in 90 s, it is gently guided to one of the closed arm. Retention of this learned task was examined 24 h after the first day trial.[4,9]
Locomotor activity
The locomotor activity was recorded using actophotometer (INCO, Ambala, India) for a period of 5 min. Animal was placed in the actophotometer for habituation (3 min). The animals were then observed for 5 min and expressed as counts per 5 min.[9]
Preparation of brain homogenate
After behavioral study, the animals were sacrificed by decapitation. The brains were removed and placed on ice followed by rinsing with ice-cold isotonic saline (0.9% NaCl). A (10% w/v) brain homogenate was prepared in 0.1 mM phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 g for 15 min and supernatant was separated for biochemical estimation.
Measurement of brain TBARS
TBARS assay measures the lipid peroxidation product malondialdehyde (MDA) in tissue sample.[14] The absorbance of supernatant was noted at 532 nm employing double-beam UV-visible spectrophotometer (Shimadzu).
Measurement of reduced glutathione
GSH was measured according to the method of Ellman.[15] Absorbance was noted at 412 nm using double beam UV-visible spectrophotometer (Shimadzu).
Determination of TNF-α
TNF-α value was measured as per the instructions given on the immunoassay kit. The absorbance was noted at 450 nm using ELISA reader (Biorad).
Statistical analysis
All the results are expressed as mean ± SEM. The data of all the groups were analyzed by one-way ANOVA followed by Tukey's test using software Graph Pad In Stat (Graph Pad Software Inc., USA). A P value less than 0.05 was considered to be significant.
RESULTS
CAPE and STZ treatments did not affect the locomotor activity of rats. The normal control and drug-treated groups have no significant statistical differences (P > 0.05) in locomotion as measured in digital actophotometer.
Effect of CAPE on MEL and TSTQ of rats in MWM task
Normal and sham control group animals showed reduction (P < 0.05) on day 4 MEL as compared with their day 1 MEL during the training trials, thereby exhibiting learning by virtue of training. Administration of CAPE (6 mg/kg, i.p.) for 28 consecutive days caused reduction (P < 0.05) of day 4 MEL as compared with normal control group, which indicates enhanced learning. However, intracerebroventricular administration of STZ prevented the reduction of day 4 MEL as compared with day 1 MEL. CAPE treatment in both doses (3 and 6 mg/kg, i.p.) caused significant (P < 0.05) reduction of day 4 MEL in STZ-treated rats. Donepezil-treated groups reflected considerably shortened day 4 MEL in the MWM test [Table 1].
Table 1.
Effect of caffeic acid phenethyl ester (CAPE) on mean escape latency (MEL) and time spent in target quadrant (TSTQ) of rats in Morris water maze paradigm.
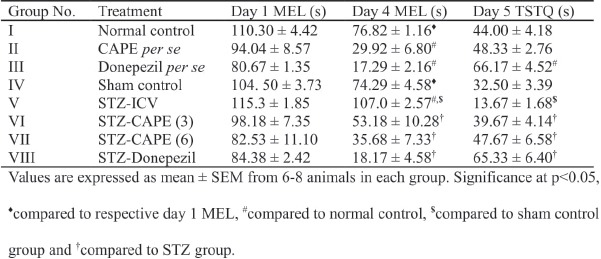
STZ-ICV injection resulted in lowering (P < 0.05) of TSTQ as compared with both normal control and sham control rats. However, rats that were treated for 28 days with CAPE (3 and 6 mg/kg) showed profound enhancement of TSTQ value vs STZ-ICV-administered rats. Furthermore, the standard drug donepezil was able to comprehensively increase the TSTQ value [Table 1].
Effect of CAPE on TL in EPM model
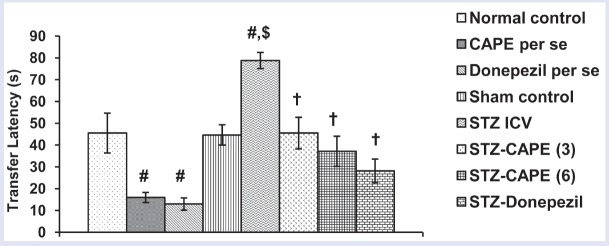
Administration of CAPE (6 mg/kg, i.p.) resulted in profound (P < 0.05) decrease in TL relative to normal control group, which testifies the memory-enhancing property of CAPE. STZ caused significant (P < 0.05) memory impairment in rats as reflected by higher day 2 TL value vs. sham group value. However, CAPE ameliorated the TL of STZ-treated rats, with the higher dose having more pronounced effect. Donepezil (standard drug) decreased TL value in normal as well as STZ-ICV-treated rats [Figure 2].
Figure 2.
Influence of CAPE treatment on mean TL of rats in EPM model. Values are expressed as mean ± SEM from six to eight animals in each group. Significance at P < 0.05: #compared with normal control, $compared with sham control group, and †compared with STZ group
Effect of CAPE on brain TBARS
STZ administration caused increase in brain TBARS level of rats compared with normal control and sham control. However, CAPE administration to STZ-ICV-treated rats prevented (P < 0.05) the rise of brain TBARS. Donepezil reduced the brain TBARS level in normal control as well as STZ-treated groups of rats [Table 2].
Table 2.
Repercussions of caffeic acid phenethyl ester (CAPE) treatment on brain TBARS and reduced GSH levels in rats
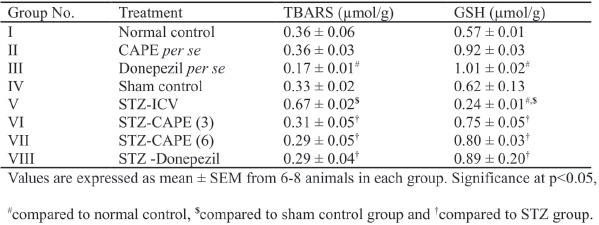
Effect of CAPE on reduced GSH in brain
STZ-ICV significantly (P < 0.05) decreased the GSH levels in brain of rats as compared with normal controls as well as sham controls. It was found that both doses of CAPE caused significant (P < 0.05) elevation of brain GSH levels in rats that were previously treated with STZ intracerebroventricularly. No statistical significant difference was found between the brain GSH levels of normal control and CAPE-treated rats. Donepezil, however, significantly (P < 0.05) reversed the STZ-induced reduction in GSH levels in brain of rats [Table 2].
Effect of CAPE on brain TNF-α
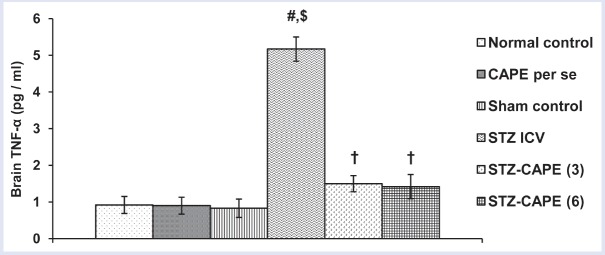
Brain TNF-α level was significantly (P < 0.05) enhanced by STZ treatment. But both doses of CAPE prevented (P < 0.05) this surge of TNF-α due to STZ. The higher dose (6 mg/kg, i.p.) was more effective in suppressing this pro-inflammatory cytokine. There was no significant difference between the TNF-α level of normal control and CAPE per se treated groups [Figure 3].
Figure 3.
Effect of administration of CAPE for 28 consecutive days on brain TNF-α level in separate groups of rats. Values are expressed as mean ± SEM from six to eight animals in each group. Significance at P < 0.05: #compared with normal control, $compared with sham control group, and †compared with STZ group
DISCUSSION
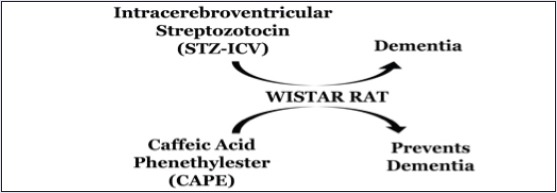
STZ-ICV injection causes the rise in free radicals in the brain, thereby enhancing oxidative stress.[11] The same has been observed in the present study in the form of higher brain TBARS levels and lower GSH levels in STZ-ICV-treated group. Measurement of MDA by TBARS assay is widely used to assess oxidative stress.[16] MDA is the most mutagenic and toxic product of lipid peroxidation, which causes magnitude-based cell senescence, autophagy, apoptosis, and necrosis. MDA also forms biomolecular adducts and MDA-acetaldehyde adducts, which are highly immunogenic.[17] The decline in GSH content has been well studied in AD-affected brain autopsy, which invariably leads to enhanced carbonylation of brain proteins and lipid peroxidation.[18] Although the GSH content of neurons is low, still GSH is the primary antioxidant defense of brain, which makes it a reliable oxidative stress biomarker. GSH is a tripeptide essential for detoxification of free radicals, maintenance of cellular redox status, and required as cofactor by numerous antioxidant enzymes.[19] In the present study, CAPE reinforced the antioxidant guard by enhancing the GSH levels in rat brains. Pretreatment of CAPE also prevented the rise of TBARS level in rat brains that were previously administered with STZ-ICV [Figure 4].
Figure 4.
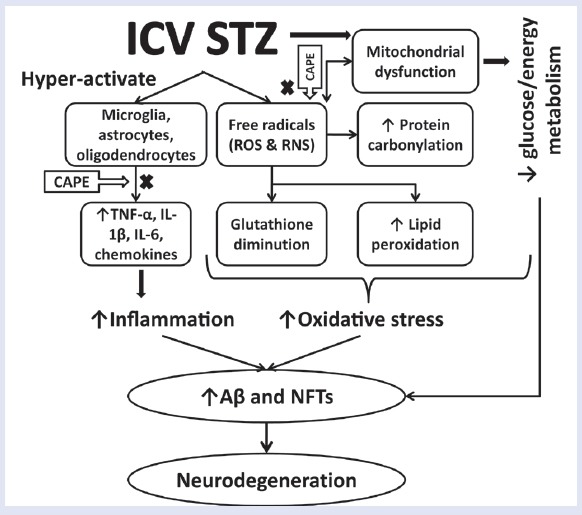
Proposed mechanism of amelioration of STZ-ICV-induced neurodegeneration through administration of CAPE in rats
Furthermore, the rats that were administered with STZ-ICV showed high brain TNF-α content as compared with their sham control counterparts. STZ-ICV treatment overactivates microglia and astrocytes, thereby enhancing the expression of pro-inflammatory cytokines like TNF-α, IL-1, and IL-6.[20] The literature reports of postmortem of AD-affected brains have revealed high TNF-α level.[21] TNF-α is vital for initiation and regulation of cytokine production during an inflammatory response. It can cause glutamate-mediated excitotoxic necrosis, chemokine-mediated cell apoptosis, and an increase in amyloid-β precursor protein (APP) transcription.[22] Administration of CAPE for 28 days prevented the surge in TNF-α level in rats that were previously treated with STZ-ICV [Figure 4].
MWM test employed in the present study is one of the most widely accepted model to evaluate spatial learning and memory of the rodents.[23] A lower value of day 4 MEL during acquisition trials denoted normal acquisition of memory and a higher value of TSTQ in search of missing platform during retrieval indicated retrieval of memory. In the present study, STZ-ICV impaired the spatial learning and memory of rats as evident by significantly higher day 4 MEL and lower TSTQ value vs. sham control. The oxidative stress and enhanced inflammation may have contributed to this observed memory impairment in rats. EPM is another animal model commonly used for evaluation of spatial memory of rodents.[4] Time taken by the animal to move from open arm to closed arm was taken as TL. STZ-ICV-treated rats took longer to enter into the closed arm of EPM and showed higher TL values. We observed that CAPE treatment for 28 days not only kept day 4 MEL at lower level but also rendered higher day 5 TSTQ in rats that were previously administered with STZ-ICV. Moreover, administration of CAPE to STZ-ICV-treated rats resulted in reduction of their TL values. These results are consistent with the reports from other laboratories that showed similar findings in response to STZ-ICV treatment.[4,9] CAPE per se group animals also showed significantly lower day 4 MEL, higher TSTQ, and reduced TL, which signify the memory-enhancing property of CAPE in addition to prevention of memory-impairing activity. These findings are dependable to the outcome of behavioral studies from other researchers that signify the memory-boosting property of CAPE.[7,8] The locomotor activity of normal control, STZ group, and CAPE per se rats showed no significant difference. This excludes the possibility that locomotor activity per se may have contributed to any changes during behavioral studies.
CONCLUSION
It can be concluded that CAPE showed memory improvement, prevention of memory impairment, and antioxidant properties. Therefore, it can be a utilized as a possible remedy for the management of dementia. However, further studies are very much needed to enlighten and consolidate the mechanism of action by employing suitable markers, agonists, or antagonists through various animal models.
Financial support and sponsorship
The authors are thankful to AICTE, New Delhi for providing financial support under research promotion scheme for this study.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors are thankful to AICTE, New Delhi, for providing financial support and are grateful to the management of ASBASJSM college of Pharmacy, Bela, affiliated to IKG PTU, Kapurthala, for providing necessary facilities.
REFERENCES
- 1.Feng Y, Wang X. Antioxidant therapies for Alzheimer's disease. Oxid Med Cell Longev 2012. :472932. doi: 10.1155/2012/472932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer's disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer's disease. Biochem Pharmacol. 2014;88:548–59. doi: 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiwari V, Kuhad A, Bishnoi M, Chopra K. Chronic treatment with tocotrienol, an isoform of vitamin E, prevents intracerebroventricular streptozotocin-induced cognitive impairment and oxidative-nitrosative stress in rats. Pharmacol Biochem Behav. 2009;93:183–9. doi: 10.1016/j.pbb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Magnani C, Isaac VLB, Correa MA, Salgado HRN. Caffeic acid: a review of its potential use in medications and cosmetics. Anal Methods. 2014;6:3203–10. [Google Scholar]
- 6.Wang X, Pang J, Maffucci JA, Pade DS, Newman RA, Kerwin SM, et al. Pharmacokinetics of caffeic acid phenethyl ester and its catechol-ring fluorinated derivative following intravenous administration to rats. Biopharm Drug Dispos. 2009;30:221–8. doi: 10.1002/bdd.657. [DOI] [PubMed] [Google Scholar]
- 7.Murtaza G, Karim S, Akram MR, Khan SA, Azhar S, Mumtaz A, et al. Caffeic acid phenethyl ester and therapeutic potentials. Biomed Res Int 2014. 2014 doi: 10.1155/2014/145342. 145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armutcu F, Akyol S, Ustunsoy S, Turan FF. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (review) Exp Ther Med. 2015;9:1582–88. doi: 10.3892/etm.2015.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misra S, Kuhad A, Chopra K. Neurobiological effect of 7-nitroindazole, a neuronal nitric oxide synthase inhibitor, in experimental paradigm of Alzheimer's disease. Indian J Exp Biol. 2013;51:1086–93. [PubMed] [Google Scholar]
- 10.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–49. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68:1021–9. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- 12.Sonkusare S, Srinivasan K, Kaul C, Ramarao P. Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci. 2005;77:1–14. doi: 10.1016/j.lfs.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Wang XF, Shi JJ, Li YP, Yang N, Zhai S, et al. Caffeic acid phenethyl ester inhibits liver fibrosis in rats. World J Gastroenterol. 2015;21:3893–903. doi: 10.3748/wjg.v21.i13.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 16.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Ayala A, Munoz FM, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014. 2014 doi: 10.1155/2014/360438. 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bizzozero OA, Ziegler JL, De Jesus G, Bolognani F. Acute depletion of reduced glutathione causes extensive carbonylation of rat brain proteins. J Neurosci Res. 2006;83:656–67. doi: 10.1002/jnr.20771. [DOI] [PubMed] [Google Scholar]
- 19.Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8:385–7. doi: 10.1016/s1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 20.Rai S, Kamat PK, Nath C, Shukla R. A study on neuroinflammation and NMDA receptor function in STZ (ICV) induced memory impaired rats. J Neuroimmunol. 2013;254:1–9. doi: 10.1016/j.jneuroim.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–8. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 22.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–21. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]