Abstract
Context:
Glycoside-based standardized fenugreek seed extract (SFSE-G) demonstrated promising efficacy in animal models of immune-inflammatory conditions.
Aim:
The present study was aimed at embryo-fetal development toxicity evaluation of SFSE-G in Wistar rats as per guideline No. 414 of the Organization for Economic Co-operation and Development (OECD).
Material and Methods:
Mated female rats were randomized into four groups of 30 each and received oral doses of either SFSE-G at 250, 500, and 1000 mg/kg or vehicle (water) during the period of gestation (postconception) from gestational day 5 (GD5, an implantation day) until 1 day before cesarean sections (GD19). Maternal food consumption, body weights, and clinical signs were monitored throughout gestation. Cesarean sections were performed on GD20 and fetal observations (gravid uterine weight, implantation sites, early and late resorptions, live and dead fetuses) were recorded. Live fetuses were weighed and examined for external, visceral, and skeletal variations and malformations.
Results:
None of the SFSE-G-treated groups showed maternal and embryo–fetal toxicity. Occasional and incidental skeletal and visceral malformations were observed and found to be spontaneous and unrelated to the treatment.
Conclusion:
Oral exposure of SFSE-G during the prenatal period did not show significant maternal and embryo-fetal toxicity up to a dose of 1000 mg/kg in rats. Therefore, the no-observed-adverse-effect level for SFSE-G for prenatal oral exposure was considered to be 1000 mg/kg.
SUMMARY
Prenatal toxicity of glycoside-based standardized fenugreek seed extract (SFSE-G) was evaluated.
SFSE-G was orally gavaged to rats on gestational days 5-19 with a limit dose of 1000 mg/kg.
SFSE-G did not show maternal or developmental toxicity.
SFSE-G showed NOAEL of 1000 mg/kg for prenatal exposure in female rats.
Abbreviations used: CPCSEA: Committee for the Purpose of Control and Supervision of Experiments on Animals; GD: Gestational day; GRAS: Generally recognized as safe; HED: Human equivalent dose; NOAEL: No-observed adverse effect levels; OECD: Organization for Economic Co-operation and Development; SFSE-G: glycoside-based standardized fenugreek seed extract.
Keywords: Prenatal development toxicity, glycosides, standardized fenugreek seed extract, OECD No. 414, no-observed-adverse-effect level, rats
INTRODUCTION
Inadequate nutrition is a prevalent condition that adversely affects global health. Dietary supplements and food fortification could help meet requirements for individuals at risk of deficiencies.[1] In the past decades, the interest and use of natural products and traditional medicine have increased globally among consumers, resulting in the growth of herbal medicine and dietary supplement industry.[2,3,4] At the same time, consumers, clinicians, and health educators have expressed concerns regarding the potential safety of herbal medicines.[3,5] It is broadly accepted that the fetus may be more sensitive to chemical exposures than the adult organism.[6] Furthermore, many diseases may have origins during the development period of pregnancy.[7] Therefore, the safety of dietary supplements needs to be ensured during prenatal exposure during pregnancy period to ensure safety.[8]
One of the potential natural sources of dietary supplement is fenugreek (Trigonella foenum-graecum L., Family: Fabaceae) seed. Fenugreek is an annual herb with medicinal properties and has been known as the oldest herbal medicine known for many physiological and health benefits.[9,10,11] The use of fenugreek in females dates back to the ancient Egypt when it was used to facilitate childbirth and increase mother's milk decreasing dysmenorrhea.[12] Fenugreek seeds have a generally recognized as safe (GRAS) status in the USA.
One of the important phytoconstituents of fenugreek seeds is the glycosides content. These include a variety of furostanol[13] and flavonol[14] glycosides. The ameliorating effects of fenugreek seed extract[15,16] and fenugreek glycosides[17,18] have been reported in an animal model of inflammation and rheumatoid arthritis. The benefits of glycoside-based standardized fenugreeks seed extract (SFSE-G) as a constituent of dietary supplement have been demonstrated for androgenic and anabolic potential in sedentary[19] and resistance trained male subjects.[20] SFSE-G has demonstrated good efficacy in an animal model of autoimmune inflammatory conditions such as interstitial pulmonary fibrosis[21] and glomerulonephritis.[22] A strong inflammatory component, with infiltration of tissue with macrophages and lymphocytes, is associated with many organ-specific autoimmune diseases.[23] Autoimmunity (a system of immune responses of an organism against its own healthy cells and tissue) is known to cause bone and joint health-related disorders. Therefore, SFSE-G can be explored as a dietary supplement for bone and joint health improvement. During the 90-day repeated dose toxicity study, SFSE-G showed no-observed-adverse-effect levels (NOAEL) of 1000 mg/kg/day in male and 500 mg/kg/day in female rats.[24] However, the safety of SFSE-G during prenatal exposure in female animals during the period of gestation has not yet being reported. Therefore, the present study was undertaken with the objective to evaluate effects of oral exposure of SFSE-G in pregnant female rats and their fetuses during the period of gestation (prenatal development period).
MATERIALS AND METHODS
Animals
Adult and young healthy female Wistar rats (of age 14-15 weeks) and proven fertile male rats bred in the animal house of Intox Private Limited, Pune, India, were used. Rats were housed in solid polypropylene cages with stainless steel grill tops and bedding of clean and sterilized paddy husk for at least 8 days before mating. The rats were provided with extruded rodent pelleted feed (“Nutrilab” brand, M/s Provimi Animal Nutrition India Pvt. Ltd., Bangalore) and filtered water ad libitum throughout the study. During the entire study, the rats were kept at the following controlled conditions: temperature of 22 ± 2°C, 50 ± 20% of relative humidity, and a light/dark cycle of 12 h. Two females cohabited overnight with a male and examined for the presence of sperm in their vagina and were defined as gestational day 0. After mating is confirmed, “0” day pregnant, the rats were randomly assigned into groups. The study was conducted at a GLP Certified laboratory and with prior approval for protocol from Institutional Animal Ethics Committee in accordance with guidelines set by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India.
The treatments
The test compound, SFSE-G, was supplied by Indus Biotech Private Limited (Pune, India) after preparation and characterization as per reported procedure.[24] The test compound, SFSE-G, is a standardized extract of fenugreek seeds containing 89.42 % of total glycosides. SFSE-G is commercially available as TestosurgeTM. The solution was freshly prepared as 1% w/v in the vehicle (Water, analytical grade) and orally gavaged to rats in a volume of 5 mL/kg body weight. The treatments were administrated once daily between 11.00 am to 1.00 pm. The same sequence and time of the day for the rats were followed throughout the treatment period.
The study design
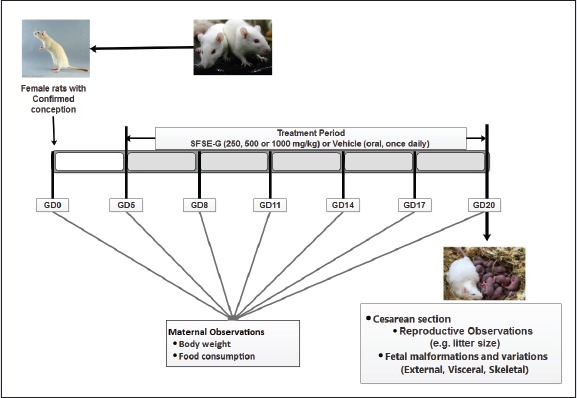
The study was conducted in accordance with the Organization for Economic Co-operation and Development (OECD) guidelines (Test No. 414: Prenatal Development Toxicity Study).[25] A dose range finding study was performed to determine doses for subsequent main study. Based on past toxicity data in rats,[24] the dose level of 1000 mg/kg was selected as highest dose as per as per OECD 414 guidelines.[25] The two- to fourfold intervals are frequently optimal for setting the descending dose levels.[25] Therefore, the doses of 250 mg/kg (one quarter of 1000 mg/kg), 500 mg/kg (one half of 1000 mg/kg), and 1000 mg/kg were selected for a dose range finding study.
Prenatal developmental toxicity: Dose-range finding study
Female rats were cohabited with male rats (1 male:2 females) overnight and examined for the presence of spermatozoa in vaginal smear next day morning. The day on which the sperms were found in the vagina was considered as gestational day 0 (GD0). The rats were randomly divided into four groups of seven female rats each (“0” day pregnant). They were administered with either vehicle or SFSE-G (250, 500, or 1000 mg/kg) from GD5 to GD19 and checked for systemic toxicity, body weight, and food consumption. All dams were sacrificed on GD20 and subjected to necropsy examination for ovaries and uterine contents. Fetal abnormalities were assessed by external examination. The highest dose level of 1000 mg/kg body weight did not result in any remarkable maternal or fetal toxicity in the exposed rats. Based on the findings, doses of 250, 500, and 1000 mg/kg body weight were selected for the main study.
Prenatal developmental toxicity: main study
One hundred and twenty female rats (“0” day pregnant) were selected and randomized into four groups (n = 30 in each group). The day on which the sperms were found in the vagina was considered as GD0. The rats were gavaged daily from GD5 to GD19. Each group of rats was treated either with vehicle (G1: VC) or SFSE-G (G2: 250 mg/kg, G3: 500 mg/kg, or G4: 1000 mg/kg). Cage-side observations of females were conducted at least once daily. Food consumption was measured at 3-day intervals starting on GD5. Maternal body weights were recorded on GD0, GD5, GD8, GD11, GD14, GD17, and before their terminal sacrifice on GD20. All animals were sacrificed on GD20 by carbon dioxide asphyxiation. The uterus from each female was examined for the number and placement of uterine implantation sites, number of live and dead fetuses, number of early and late resorptions, and any abnormalities of the uterus or embryonic sac. Dams were examined for the number of corpora lutea of ovaries. Sex and the body weight of each fetus were determined. Pre-implantation and postimplantation loss was calculated as follows: pre-implantations loss = [(no. of corpora lutea - no. of implantations)/no. of corpora lutea] × 100 and post-implantation loss = [(no. of dead implants)/no. of total implantations] ×100.[26] Uteri that had no visible implantation sites were stained with ammonium sulfide (10%) to detect very early resorptions.[27] All live fetuses were individually weighed, sexed, and examined for external anomalies in a uniform order (from head to tail) for external malformations. Live fetuses were euthanized by using diethyl ether vapors. One half of the fetuses from each litter was selected in a random manner and subjected to visceral examination by Wilson's Technique for assessment of soft tissue development.[28,29] The remaining half number of the fetuses were processed for skeletal examination using Alizarin Red S staining method[30] as follows: the fetuses were sacrificed, eviscerated, and prepared by the wet skinning method. The fetuses were then fixed in 70% isopropyl alcohol overnight. Next day, fetuses were transferred to Alizarin Red staining solution containing 1% KOH for 24 h. The KOH solutions were changed at 24 h interval to 1% and then to 0.5% concentration. The fetuses were then transferred to the clearing solution (glycerol and alcohol) and examined.
Statistical analysis
The data was presented as mean ± standard deviation (SD). The litter was used as the basis for the analysis of fetal variables. Gestational body weights, corrected body weight, body weight gain, and food consumption of pregnant rats, litter weight (total, male and female), average pup weight, uterus weight (absolute and relative), pre-implantation loss (%), postimplantation loss (%), dead and live fetuses (%) were analyzed by one-way analysis of variance, followed by Dunnett's test. Number of male and female pup, sex ratio, number of corpora lutea, number of implantation, number of live and dead fetuses, and number of early and late resorptions and incidences of fetal visceral and skeletal malformations were evaluated by using the Kruskal–Wallis test, followed by the Mann–Whitney test. Number of pregnant /nonpregnant females and number of live and dead females were analyzed using Fisher's test. The values of treatment groups were compared with VC and 5% level of difference (P < 0.05) was considered significant.
RESULTS
Maternal toxicity
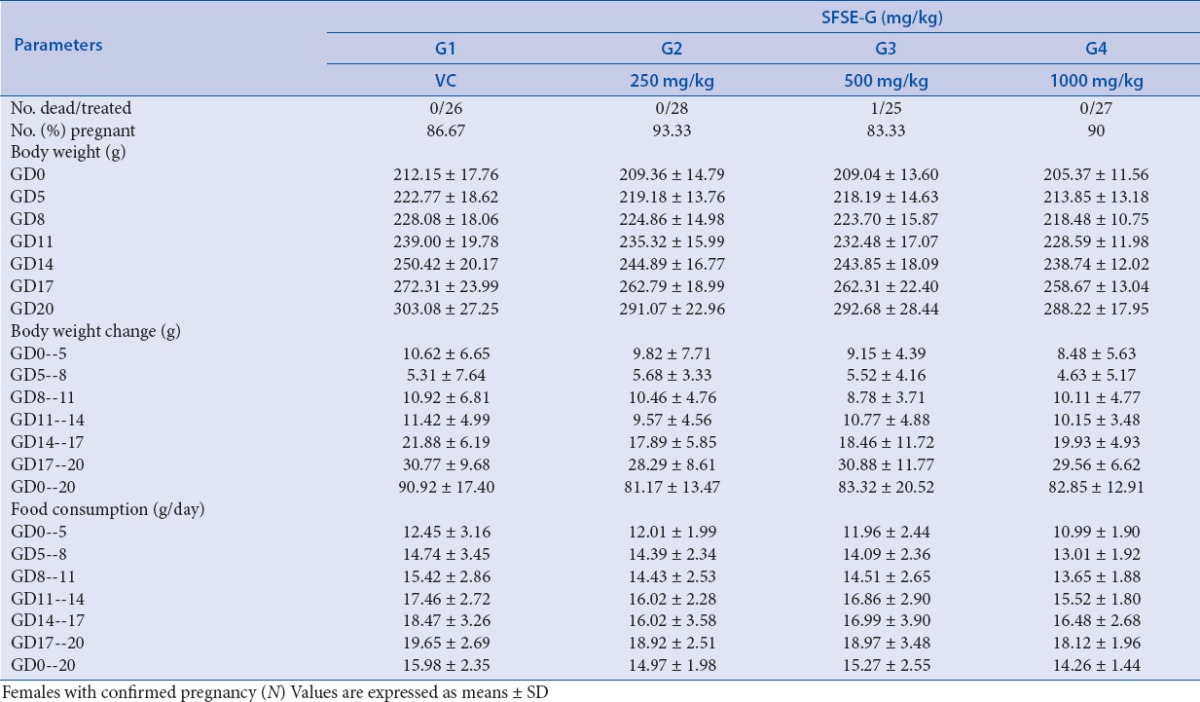
No treatment-related mortality was observed in any of treatment groups during the study period except the incidental death of one dam from G3 (on GD13 due to gavage error). No evidence of abortion was observed in any dam during the study period. One dam from G3 showed premature delivery (on GD18), which could be because of missed detection of sperms in the vagina on GD0. There were no significant body weight gains of pregnant rats from SFSE-G treated rats (G2, G3 or G4) as compared to VC group [Table 1]. No significant differences were found between the treatment groups and VC for the maternal weight and food consumption. No significant difference was found in the pregnancy rate of SFSE-G treated groups, i.e., G2, G3, and G4 with a pregnancy rate of 93.33, 83.33, and 90.0%, respectively, as compared with a pregnancy rate of VC group (86.67%).
Table 1.
SFSE-G: maternal observations
Reproductive findings related to embryo-fetal toxicity
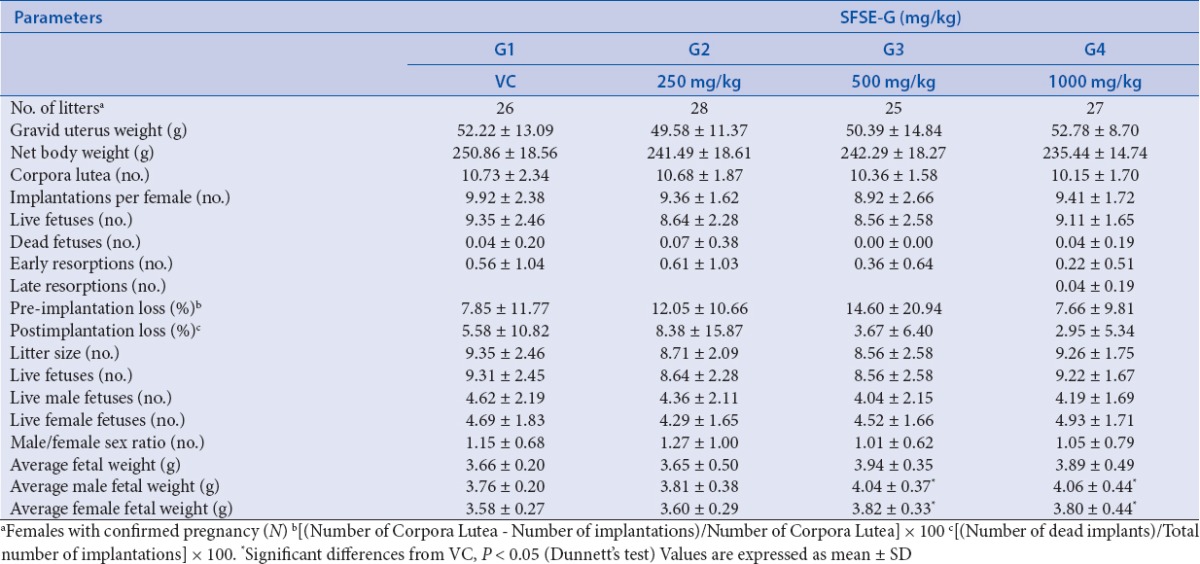
The findings related to reproductive data of embryo-fetal toxicity is presented in Table 2. The gravid uteri of females sacrificed on GD20 did not reveal any remarkable alterations indicative of adverse effects of SFSE-G. There were no significant differences in the corpora lutea, live fetuses, and implantation sites, early and late resorptions, pre- and postimplantation loss in SFSE-G treated groups as compared with VC group. The fetal weights of male and female fetuses in SFSE-G (500 and 1000 mg/kg) were significantly (P < 0.05) more than that of VC groups.
Table 2.
SFSE-G: reproductive observations
Fetal malformations and variations
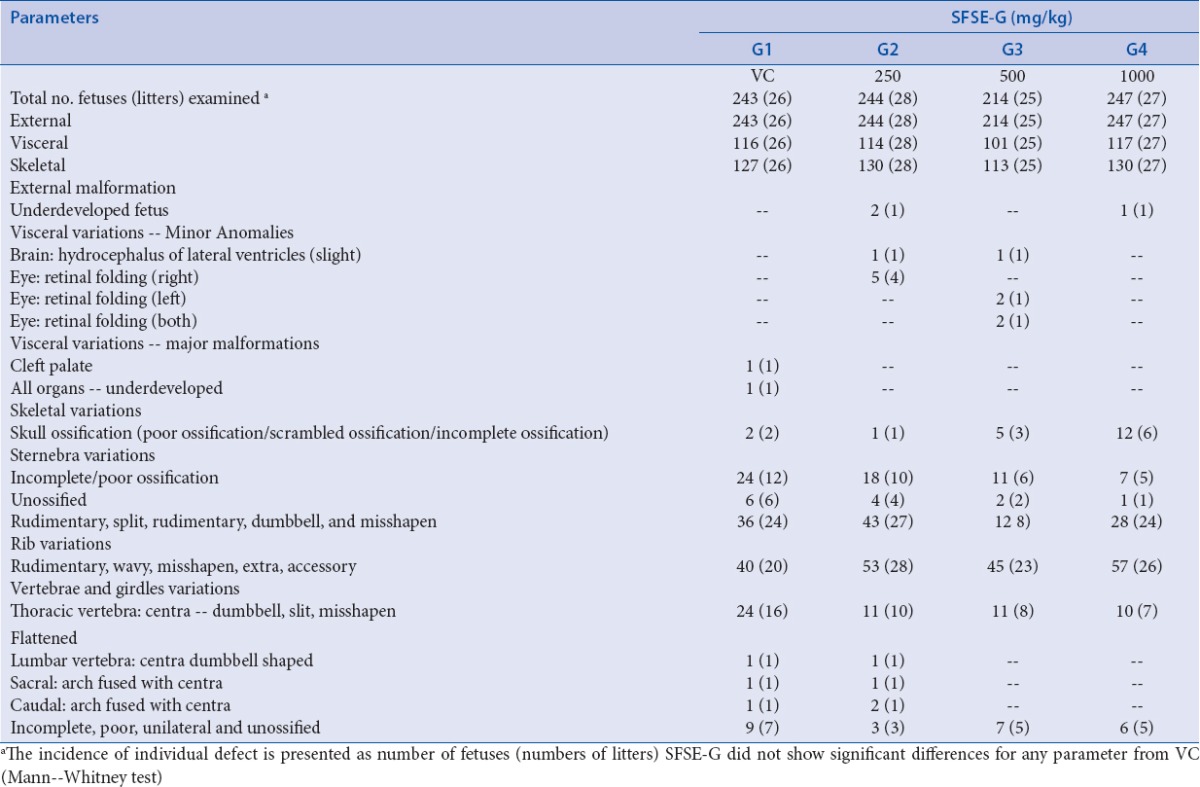
Each fetus was examined for any abnormalities (a permanent change that alters survival, development, or function) or the variations (a change that may not be permanent and does not affect survival or health). Each fetus was exposed to examine for any findings on length, cranium, eyes, palate, limbs, tail, genitals, sex, along with skeletal and visceral variations of exposed fetuses and data are presented in Table 3. No statistical difference was observed in the frequency of external abnormalities or variations in SFSE-G-treated groups (V/S. VC group).
Table 3.
SFSE-G: fetal observations (abnormalities and variations)
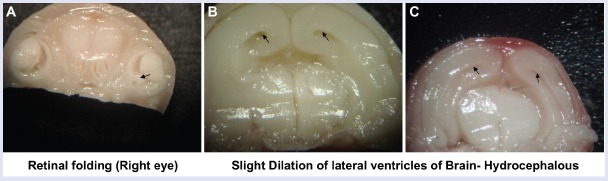
The representative images of variations seen in the visceral examination are presented in Figure 1. The retinal folding was observed in five fetuses from SFSE-G (250 mg/kg) and four fetuses from SFSE-G (500 mg/kg) group. The retinal folds are the artifacts produced at the time of fixation of the fetuses in 70% isopropyl alcohol and occurred with equal incidence in both sexes. Hydrocephalous of lateral ventricles of the brain was observed in one fetus from SFSE-G (250 mg/kg) and one fetus from SFSE-G (500 mg/kg) group. Major malformation like cleft palate was observed in one fetus from VC group. All organs were observed to be well developed except one fetus from VC group. These incidences of fetal soft tissue or skeletal variations are normal variants or minor anomalies and considered to be incidental and were not treatment related.
Figure 1.
The variations observed during fetal examination: (A) retinal folding in eye and (B and C) dilation of lateral ventricles of brain of representative fetus. The black arrow represents variations
The skeletal variations are in the form of ossification patterns that can be seen as unossified, scrambled, and poorly and incompletely ossified skulls. Such variations are commonly seen in 20-day old rat normal fetuses. Such variations were observed in two fetuses from the VC, one from SFSE-G (250 mg/kg), five from SFSE-G (500 mg/kg), and 12 from SFSE-G (1000 mg/kg) group. They were classified as normal variants. Poor and incomplete ossifications of sternebrae were also seen in 24 fetuses from VC, 18 from SFSE-G (250 mg/kg), 11 from SFSE-G (500 mg/kg), and seven from SFSE-G (1000 mg/kg) group. Unossified sternebrae were also encountered in all treatment groups. These are six in VC, four in SFSE-G (250 mg/kg), two in SFSE-G (500 mg/kg), and one in SFSE-G (1000 mg/kg) group. These were all classified as normal variants. Poor, incomplete, and not ossified caudal vertebrae were observed in nine fetuses in VC, three in SFSE-G (250 mg/kg), seven in SFSE-G (500 mg/kg), and six in SFSE-G (1000 mg/kg) group. These were all classified as normal variants. Minor anomalies like split, rudimentary, dumbbell shaped, asymmetrically dumbbell shaped, and misshapen sternebrae were seen in all treatment groups including VC group. These anomalies were observed in 36 fetuses from VC, 43 from SFSE-G (250 mg/kg), 12 from SFSE-G (500 mg/kg), and 28 from SFSE-G (1000 mg/kg) group. Minor anomalies in ribs like rudimentary, wavy, extra, accessory, and misshapen ribs were observed in 40 fetuses from VC, 53 from SFSE-G (250 mg/kg), 45 from SFSE-G (500 mg/kg), and 57 from SFSE-G (1000 mg/kg) dose group. Dumbbell/asymmetrically dumbbell shaped, split, misshapen, and flattened centras in thoracic vertebrae were observed in 24 fetuses from VC, 11 from SFSE-G (250 mg/kg), 11 from SFSE-G (500 mg/kg), and 10 from SFSE-G (1000 mg/kg) group. One fetus each from VC and SFSE-G (250 mg/kg) group was observed with dumbbell-shaped centra of lumbar vertebra and arch fused with centra sacral and caudal vertebra. None of these changes were found significant between SFSE-G-treated group as compared with VC group (Mann-Whitney test). Therefore, all the fetal skeletal variations encountered in the present study were considered to be incidental. They were of no teratological or toxicological significance, either because of their being normal variant or minor in nature, or their incidence being isolated and/or not significantly different from that of VC group.
DISCUSSION
The fenugreek seed extract especially SFSE-G are clinically proven efficacious and safe dietary supplementation for androgenic and anabolic effects.[19,20] However, the potential of SFSE-G as agent for bone and joint health or autoimmune inflammatory conditions in male or female subjects yet to be explored clinically. Due to hormonal differences, the higher prevalence of autoimmune diseases was found in females than in male population.[23,31,32] The prevalence of rheumatoid arthritis in women in low- and middle-income economic countries reached 0.75% (vs. 0.16% in male).[33] In addition, sex hormones related immunity with changes in the severity of autoimmune diseases is observed during pregnancy, when estrogens and progesterone reach the highest levels.[32] Therefore, SFSE-G needs to be evaluated for the safety in female population especially during pregnancy period before it is explored clinically for bone and joint health.
Prenatal developmental testing mainly concerns with the effects of prenatal exposure to the pregnant test animal and on the developing organism, including assessment of maternal effects as well as death, structural abnormalities, or altered growth in the fetus. Such abnormalities can be either irreversible or reversible. Teratological studies are the most commonly performed study type for the investigation of maternal, embryonic, and fetal toxicity. The present study, for the first time, evaluated the SFSE-G for prenatal developmental toxicity during the gestational period (prenatal exposure, GD5 through GD19) on maternal and fetus development in three dose levels (250, 500, and 1000 mg/kg, oral exposure).
During the present study, SFSE-G was found to be safe at the highest dose (1000 mg/kg) without adverse effects. Therefore, the NOAEL for SFSE-G for prenatal exposure was considered as 1000 mg/kg/day. Any treatment-related decrease in body weight gain and food consumption, clinical signs, organ toxicity, and female mortality[32,34] in developmental toxicity studies are indicators of maternal toxicity. In the past, there were few reports of increased number of resorptions with prenatal exposure to fenugreek seed powder in rats during the first 10 days of gestation,[35,36,37] reductions of fetal and placental weights, with less litter size.[38] These studies were not in compliance with recommendations or guidelines of reputed international agencies such as OECD for evaluation of embryo-fetal toxicity. Furthermore, these studies were not performed using standardized products with nonoptimal duration of treatment. The prenatal exposure for the whole period of organogenesis is recommended as exposure duration for embryo–fetal toxicity study.[25,39] Therefore, the reported information on embryo–fetal toxicity of fenugreek seed or powder is considered to be a limited value for any prenatal exposure toxicological or safety risk assessment. On the other hand, only reported study with recommended exposure (the whole gestation period) of up to 20% fenugreek seed powder in the diet of rats did not show any adverse effects,[40] supporting the safety results found in the present study.
During the present study, after prenatal exposure of SFSE-G did not cause any treatment-related observations. The pregnancy rate, total number of implantation, pre- and postimplantation loss, early and late resorptions, maternal body weights, and food consumptions were comparable in SFSE-G-treated group as that of VC groups. In the present study, fetal evaluations included body weight, sex ratio, number per dam (litter size), and external, visceral, and skeletal examinations. There were no significant differences observed in any of the fetal parameters during the fetal evaluations. In general, the skeletal examination did not show any major abnormalities, which could have caused any functional damage to these fetuses if allowed to grow in normal course. The variations noticed could be considered as the variations that occur during normal development as well but are repaired and normalized during the normal course of development. In general, the skeletal examination did not show any major variations that could have caused any functional damage to these fetuses if allowed to grow in the normal course. Therefore, SFSE-G was found devoid of toxic embryo/fetal effects such as resorptions, deaths or growth retardation, or structural malformations of the fetus on prenatal exposure of 1000 mg/kg from implantation throughout gestation (GD5-GD19).
It is well known that pregnancy can induce changes in the plasma concentrations of some drugs.[41,42] During pregnancy, an expansion of intravascular water content creates a larger space within which hydrophilic drugs may distribute.[42] Therefore, changes in maternal physiology during pregnancy impact on pharmacokinetics with a tendency to reduced plasma concentrations.[42] Drug absorption is usually decreased, which tends to decrease total plasma concentration of the drug.[42] Furthermore, subacute exposure (28 days) of SFSE-G to non-pregnant rats was reported to have good safety profile.[43] Therefore, absorption of glycosides in SFSE-G is expected to have significant reduction during pregnancy status and to not cause adverse effects on maternal physiological parameters.
There are no direct reports of placental concentration after SFSE-G exposure to pregnant or pregnant rats (or human). However, the existing evidence indicated a lack of placental penetration or accumulation of SFSE-G itself or its probable metabolites. First, the placental transfer occurs mainly via diffusion, thus it does not favor the movement of hydrophilic agents such as glycosides.[42] Second, agents with high protein binding and large molecular weight do not cross the placental barrier.[42] The maker glycosides of SFSE-G, i.e., Trigoneoside Ib and Vicenin 1, are having large molecular weights of 906 and 564 respectively.[44] In addition, the high residence time of SFSE-G as reported tissue distribution study in nonpregnant rats is indicative of high protein binding.[44] Third, the glycosides were not found to be present as their parent forms in the circulation after the oral administration to rats, rabbits, or humans.[45] The glycosides are reported to be metabolized by deglycosylation to aglycones in the intestine before absorption.[45] The phenolic aglycones of flavonol glycosides are metabolized as sulfated/glucuronidated form by gut and/or liver and then circulated in the blood as conjugates of their aglycones.[45] These conjugates have large molecular weight and often reside in the body for a prolonged time due to enteric recycling.[45] Similar properties (the slow distribution and high residence time) of major glycoside markers of SFSE-G[44,46] are reported. Taken together, the hydrophilic nature, high protein binding, and presence of large molecular weight molecules makes SFSE-G incapable to cross the placental barrier and to cause any adverse effects on embryo–fetal development.
The NOAEL is an important part of the nonclinical risk assessment. In the recent past, SFSE-G was reported to be safe for subchronic (90 days repeated dose) exposure in female rats with NOAEL of 500 mg/kg with no mutagenic potential.[47] The results of the present study confirmed the safety of oral gavage exposure of SFSE-G during pregnancy period with a higher level of NOAEL (1000 mg/kg). The more safety of SFSE-G during prenatal exposure might be due to less duration of exposure (15 vs. 90 days of repeated dosing) and/or the adaptive changes in immune system function that are reported during pregnancy period.[32] These results are also supported by epidemiologic reports that treatment of autoimmune diseases improved the prognosis for mother and child.[48]
Thus, the NOAEL of SFSE-G for maternal toxicity was found to be greater than or equal to 1000 mg/kg/day. The human equivalent dose (HED), considering the average human adult weight of 60 kg, as derived from NOAEL using guidance for industry issued by US Food and Drug Administration[49] as 9.7 g/day. In clinical practice, the maximum recommended starting dose (MRSD) for the clinical use is determined by dividing the HED derived from the animal NOAEL by the safety factor of 10 (the default safety factor).[49] Therefore, the prenatal exposure of 970 mg/day of SFSE-G or lower can be considered as safe in pregnant females during clinical use.
CONCLUSION
In conclusion, prenatal oral exposure of SFSE-G (dose up to 1000 mg/kg/day) to pregnant female rats did not show significant maternal and embryo–fetal toxicity during the gestational period. Therefore, oral dose of 1000 mg/kg/day and 970 mg/day can be considered as NOAEL and MRSD in pregnant rats and human, respectively.
Financial support and sponsorship
Indus Biotech Private Limited, Pune, India
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rautiainen S, Manson JE, Lichtenstein AH, Sesso HD. Dietary supplements and disease prevention-a global overview. Nat Rev Endocrinol. 2016;12:407–20. doi: 10.1038/nrendo.2016.54. [DOI] [PubMed] [Google Scholar]
- 2.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beg S, Swain S, Hasan H, Barkat M, Hussain MS. Systematic review of herbals as potential anti-inflammatory agents: recent advances, current clinical status and future perspectives. Pharmacogn Rev. 2011;5:120–37. doi: 10.4103/0973-7847.91102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthaman K, Mohamed Saleem T. Nutraceutical value of sesame oil. Pharmacogn Rev. 2009;3:264–9. [Google Scholar]
- 5.Owens C, Toone T, Steed-Ivie M. A survey of dietary supplement knowledge, attitudes, and use in a rural population. J Nutr Food Sci. 2014;4:304–8. [Google Scholar]
- 6.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease cause and prevention. Curr Opin Pediatr. 2015;27:248–53. doi: 10.1097/MOP.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picciano MF, McGuire MK. Use of dietary supplements by pregnant and lactating women in North America. Am J Clin Nutr. 2009;89:663S–7S. doi: 10.3945/ajcn.2008.26811B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haritha C, Reddy A, Reddy Y, Anjaneyulu Y, Rao T, Kumar B, et al. Evaluation of protective action of fenugreek, insulin and glimepiride and their combination in diabetic Sprague Dawley rats. J Nat Sci Biol Med. 2013;4:207–12. doi: 10.4103/0976-9668.107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perera P, Li Y. Functional herbal food ingredients used in type 2 diabetes mellitus. Pharmacogn Rev. 2012;6:37–45. doi: 10.4103/0973-7847.95863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav UC, Baquer NZ. Pharmacological effects of Trigonella foenum-graecum L in health and disease. Pharm Biol. 2014;52:243–54. doi: 10.3109/13880209.2013.826247. [DOI] [PubMed] [Google Scholar]
- 12.Younesy S, Amiraliakbari S, Esmaeili S, Alavimajd H, Nouraei S. Effects of fenugreek seed on the severity and systemic symptoms of dysmenorrhea. J Reprod Infertil. 2014;15:41–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Kang LP, Zhao Y, Pang X, Yu HS, Xiong CQ, Zhang J, et al. Characterization and identification of steroidal saponins from the seeds of Trigonella foenum-graecum by ultra high-performance liquid chromatography and hybrid time-of-flight mass spectrometry. J Pharm Biomed Anal. 2013;74:257–67. doi: 10.1016/j.jpba.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Pang X, Kang LP, Yu HS, Zhao Y, Xiong CQ, Zhang J, et al. New kaurene diterpenoid glycosides from fenugreek seeds. Nat Prod Res. 2013;27:1202–7. doi: 10.1080/14786419.2012.722087. [DOI] [PubMed] [Google Scholar]
- 15.Suresh P, Kavitha CN, Babu SM, Reddy VP, Latha AK. Effect of ethanol extract of Trigonella foenum graecum (Fenugreek) seeds on Freund's adjuvant-inducedl arthritis in albino rats. Inflammation. 2012;35:1314–21. doi: 10.1007/s10753-012-9444-7. [DOI] [PubMed] [Google Scholar]
- 16.Sindhu G, Ratheesh M, Shyni GL, Nambisan B, Helen A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int Immunopharmacol. 2012;12:205–11. doi: 10.1016/j.intimp.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata T, Cui MY, Hasegawa T, Takano F, Ohta T. Anti-inflammatory and anti-melanogenic steroidal saponin glycosides from Fenugreek (Trigonella foenum-graecum L.) seeds. Planta Med. 2011;77:705–10. doi: 10.1055/s-0030-1250477. [DOI] [PubMed] [Google Scholar]
- 18.Mandegary A, Pournamdari M, Sharififar F, Pournourmohammadi S, Fardiar R, Shooli S. Alkaloid and flavonoid rich fractions of fenugreek seeds (Trigonella foenum-graecum L.) with antinociceptive and anti-inflammatory effects. Food Chem Toxicol. 2012;50:2503–7. doi: 10.1016/j.fct.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Mokashi M, Singh-Mokashi R, Mohan V, Thakurdesai PA. Effects of glycosides based fenugreek seed extract on serum testosterone levels of healthy sedentary male subjects: a exploratory double blind, placebo controlled, crossover study. Asian Pharm Clin Res. 2014;7:177–81. [Google Scholar]
- 20.Wilborn C, Taylor L, Poole C, Foster C, Willoughby D, Kreider R. Effects of a purported aromatase and 5 α-reductase inhibitor on hormone profiles in college-age men. Int J Sport Nutr. 2010;20:457. doi: 10.1123/ijsnem.20.6.457. [DOI] [PubMed] [Google Scholar]
- 21.Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-kappa B, Muc5ac, TNF-alpha and IL-1beta. Chem Biol Interact. 2015;237:151–65. doi: 10.1016/j.cbi.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Thakurdesai PA, Vichare R, Bodhankar S, Aswar U, Mohan V. Proceedings of 2nd International Congress of Society for Ethnopharmacology (SFEC - 2015) Nagpur: Society for Ethnopharmacology; 2015. Glycosides based standardized extract of fenugreek seeds ameliorates anti-glomerular basement membrane antibodyinduced glomerulonephritis in rats. [Google Scholar]
- 23.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–69. doi: 10.1016/j.yfrne.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Thakurdesai PA, Mohan V, Bhaskaran S. Proceedings of 46th Annual Conference of Indian Pharmacological Society and International Conference on Translational Medicine; December 16–18. Bangalore, India: Indian Pharmacological Society; 2013. Toxicological evaluation of flavonoid glycosides based standardized fenugreek seed extract [TOX/PP-15] [Google Scholar]
- 25.OECD Test No 414: Prenatal Development Toxicity Study OECD Guidelines for the Testing of Chemicals Section 4. Health Effects. Paris: OECD Publishing; 2001. [Google Scholar]
- 26.Burdan F, Szumiło J, Dudka J, Klepacz R, Błaszczak M, Solecki M, et al. Morphological studies in modern teratological investigations. Folia Morphol (Praha) 2005;64:1–8. [PubMed] [Google Scholar]
- 27.Salewski E. Färbemethode zum makroskopischen Nachweis von Implantationsstellen am Uterus der Ratte. Naunyn Schmiedebergs Arch Pharmacol. 1964;247:367. [Google Scholar]
- 28.Wilson JG. Experimental studies on congenital malformations. J Chronic Dis. 1959;10:111–30. doi: 10.1016/0021-9681(59)90026-8. [DOI] [PubMed] [Google Scholar]
- 29.Seegmiller RE, Cook N, Goodwin K, Leishman T. Assessment of gross fetal malformations: the modernized Wilson technique and skeletal staining. Methods Mol Biol. 2012;889:451–63. doi: 10.1007/978-1-61779-867-2_28. [DOI] [PubMed] [Google Scholar]
- 30.Taylor P. Practical teratology. London: Academic Press; 1986. [Google Scholar]
- 31.Brunelleschi S. Immune response and auto-immune diseases: gender does matter and makes the difference. Italian J Gender Specific Med. 2016;2:5–14. [Google Scholar]
- 32.Ortona E, Pierdominici M, Maselli A, Veroni C, Aloisi F, Shoenfeld Y. Sex-based differences in autoimmune diseases. Ann Ist Super Sanita. 2016;52:205–12. doi: 10.4415/ANN_16_02_12. [DOI] [PubMed] [Google Scholar]
- 33.Rudan I, Sidhu S, Papana A, Meng SJ, Xin-Wei Y, Wang W, et al. Prevalence of rheumatoid arthritis in low- and middle-income countries: a systematic review and analysis. J Global Health. 2015;5:010409. doi: 10.7189/jogh.05.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khera K. Maternal toxicity in humans and animals: effects on fetal development and criteria for detection. Teratog Carcinog Mutagen. 1987;7:287–95. doi: 10.1002/tcm.1770070309. [DOI] [PubMed] [Google Scholar]
- 35.Khera K. Maternal toxicity: a possible etiological factor in embryo-fetal deaths and fetal malformations of rodent-rabbit species. Teratology. 1985;31:129–53. doi: 10.1002/tera.1420310115. [DOI] [PubMed] [Google Scholar]
- 36.Sethi N, Nath D, Singh R, Srivastava R. Antifertility and teratogenic activity of some indigenous medicinal plants in rats. Fitoterapia. 1990;61:64–7. [Google Scholar]
- 37.Elbetieha A, Al-Hamood M, Al-Kofahi A. Anti-implantation potential of some medicinal plants in female rats. Arch STD/HIV. 1996;10:181–7. [Google Scholar]
- 38.Kassem A, Al-Aghbari A, Al-Habori M, Al-Mamary M. Evaluation of the potential antifertility effect of fenugreek seeds in male and female rabbits. Contraception. 2006;73:301–6. doi: 10.1016/j.contraception.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 39.European Medicine Agency. Assessment report on Trigonella foenum-graecum L semen. London, UK: Committee on Herbal Medicinal Products (HMPC); 2010 May. Report No.: EMA/HMPC/146220/2010 [Google Scholar]
- 40.Mital N, Gopaldas T. Effect of fenugreek (Trigonella foenum graecum) seed based diets on the birth outcome in albino rats. Nutr Rep Int. 1986;33:363–9. [Google Scholar]
- 41.Anderson GD. Pregnancy-induced changes in pharmacokinetics. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 42.Dawes M, Chowienczyk PJ. Pharmacokinetics in pregnancy. Best Prac Res Clin Obstetr Gynaecol. 2001;15:819–26. doi: 10.1053/beog.2001.0231. [DOI] [PubMed] [Google Scholar]
- 43.Kandhare A, Bodhankar S, Mohan V, Thakurdesai P. Acute and repeated doses (28 days) oral toxicity study of glycosides based standardized fenugreek seed extract in laboratory mice. Regul Toxicol Pharmacol. 2015;72:323–34. doi: 10.1016/j.yrtph.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Kandhare A, Bodhankar SL, Mohan V, Thakurdesai PA. Pharmacokinetics, tissue distribution and excretion study of furostanol glycosides based standardized fenugreek seed extract in rats through solid-liquid extraction method. Ren Fail. 2015;37:1208–18. doi: 10.3109/0886022X.2015.1057472. [DOI] [PubMed] [Google Scholar]
- 45.Chao PDL, Hsiu SL, Hou YC. Bioavailability, metabolism, and pharmacokinetics of glycosides in Chinese herbs. Herbs: Challenges in Chemistry and Biology. American Chemical Society. 2006:212–23. [Google Scholar]
- 46.Kandhare A, Bodhankar SL, Mohan V, Thakurdesai PA. Development and validation of HPLC method for Vicenin-1 isolated from fenugreek seeds in rat plasma—application to pharmacokinetic, tissue distribution and excretion studies. Pharm Biol. 2016;14:1–9. doi: 10.3109/13880209.2016.1172245. [DOI] [PubMed] [Google Scholar]
- 47.Deshpande P, Mohan V, Thakurdesai P. Preclinical safety assessment of glycosides based standardized fenugreek seeds extract. J Appl Pharm Sci. 6:179–88. [Google Scholar]
- 48.Borchers AT, Naguwa SM, Keen CL, Gershwin ME. The implications of autoimmunity and pregnancy. J Autoimmun. 2010;34:J287–99. doi: 10.1016/j.jaut.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Center for Drug Evaluation and Research Guidance for Industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers Rockville, MD. USA: Center for Drug Evaluation and Research; 2005. [Google Scholar]