Abstract
Background:
Root of Panax ginseng C. A. Mey (Renseng in Chinese) is a famous Traditional Chinese Medicine. Ginsenosides are the major bioactive components. However, the shortage and high cost of some ginsenoside reference standards make it is difficult for quality control of P. ginseng.
Objective:
A method, single standard for determination of multicomponents (SSDMC), was developed for the simultaneous determination of nine ginsenosides in P. ginseng (ginsenoside Rg1, Re, Rf, Rg2, Rb1, Rc, Rb2, Rb3, Rd).
Materials and Methods:
The analytes were separated on Inertsil ODS-3 C18 (250 mm × 4.6 mm, 5 μm) with gradient elution of acetonitrile and water. The flow rate was 1 mL/min and detection wavelength was set at 203 nm. The feasibility and accuracy of SSDMC were checked by the external standard method, and various high-performance liquid chromatographic (HPLC) instruments and chromatographic conditions were investigated to verify its applicability. Using ginsenoside Rg1 as the internal reference substance, the contents of other eight ginsenosides were calculated according to conversion factors (F) by HPLC.
Results:
The method was validated with linearity (r2 ≥ 0.9990), precision (relative standard deviation [RSD] ≤2.9%), accuracy (97.5%–100.8%, RSD ≤ 1.6%), repeatability, and stability. There was no significant difference between the SSDMC method and the external standard method.
Conclusion:
New SSDMC method could be considered as an ideal mean to analyze the components for which reference standards are not readily available.
SUMMARY
A method, single standard for determination of multicomponents (SSDMC), was established by high-performance liquid chromatography for the simultaneous determination of nine ginsenosides in Panax ginseng (ginsenoside Rg1, Re, Rf, Rg2, Rb1, Rc, Rb2, Rb3, Rd)
Various chromatographic conditions were investigated to verify applicability of Fs
The feasibility and accuracy of SSDMC were checked by the external standard method.
Abbreviations used: DRT: Different value of retention time; F: Conversion factor; HPLC: High-performance Liquid Chromatography; LOD: Limit of detection; LOQ: Limit of quantitation; PD: Percent difference; PPD: 20(S)-protopanaxadiol; PPT: 20(S)-protopanaxatriol; RSD: Relative standard deviation; SSDMC: Single Standard for Determination of Multicomponents; TCM: Traditional Chinese Medicine.
Keywords: Chemometrics, conversion factor, ginsenosides, Panax ginseng, single standard for determination of multicomponents
INTRODUCTION
Traditional Chinese Medicine (TCM), a significant branch of the health-care system in China, exerts its therapeutic effect with multicomponent and multitarget. Hence, it is hard to control the quality of TCM for only determining the single component. Determination of multicomponents has been considered to be one of the key methods by Chinese Pharmacopoeia. Chinese Pharmacopoeia Commission directed that analytical method and testing items of monograph being revised should embody the idea of comprehensive quality control of TCMs, which multicomponents or fingerprint should be analyzed rather than single marker compound.[1] However, shortage and high cost of reference standards limit the application of the multi-index quality control.
Single standard to determine multicomponents (SSDMC) method was an economic and environmentally friendly method to the simultaneous assay of multicomponents, because only one reference standard would be needed to determine all the analytes in the sample when the method was officially accepted.[2] Therefore, this method can realize the multi-ingredient quality control and address the shortage of reference substance. Recently, triterpene acids in Ganoderma lucidum,[3] anthraquinone ingredients in rhubarb,[4] and diverse constituents in Euphorbia kansui[5] were also investigated successfully using this SSDMC method. In addition, the SSDMC standard of Echinacea angustifolia[6] and Salviae miltiorrhiza[7] has been adopted by USP monograph and Ch.P. 2015 edition individually. Therefore, analysis using SSDMC in Panax ginseng, one of the most frequently used and expensive botanical drugs, is an effective method for its quality control.
P. ginseng is derived from the root or rhizome of P. ginseng C.A. Mey, which reported antitumor, anti-inflammation, and antioxidant effect.[8,9,10,11,12,13] The major active components of P. ginseng are ginsenosides, polysaccharides, organic acids, volatile oils, amino acids, and enzymes. Particularly, ginsenosides are the most important component of P. ginseng and are critical for its therapeutic effect.[14] More than 40 ginsenosides found in P. ginseng plants have been reported, including two structural types such as the 20(S)-protopanaxadiol and 20(S)-protopanaxatriol.[15] However, ginsenoside reference standards of P. ginseng plants are expensive and in short supply, which has limited the application of analytical methods for the P. ginseng plants. For these purposes, it is necessary to determine the conversion factor (F) of the ginsenosides using one stable component which is easy to obtain. By establishing the F, it should be a practical way to determine multiple analogs and evaluate the quality of P. ginseng plants, their extracts, and related products.
In this study, a high-performance liquid chromatography (HPLC) method was developed and validated for the simultaneous determination of nine ginsenosides in P. ginseng using a single marker. Various HPLC instruments and chromatographic conditions were investigated to verify its applicability. Using ginsenoside Rg1 as the internal reference substance, the contents of other eight ginsenosides were calculated according to conversion factors (F) by HPLC. Finally, the accuracy of the method was assessed through comparing with the external standard method, aiming at realizing a better quality control to P. ginseng.
MATERIALS AND METHODS
Chemicals and materials
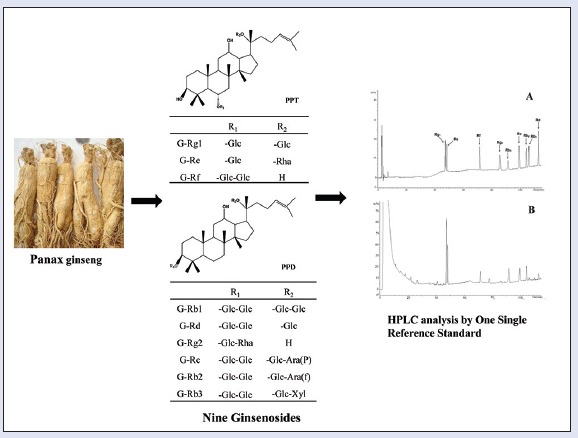
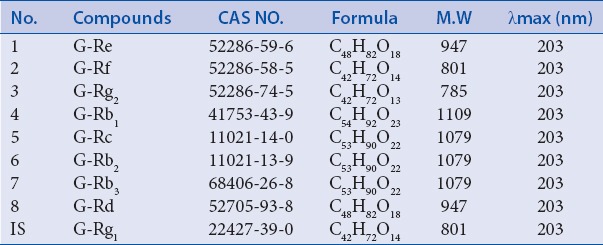
Sixteen P. ginseng samples were acquired from two provinces in China, including Nos. 1–5, 7–13, 15 (Jilin Province), 6, 14, 16 (Liaoning Province). All samples were identified by an expert in Guangdong Pharmaceutical University and are retained in the Department of TCM of Guangdong Pharmaceutical University. The reference substances, ginsenoside Rg1, Re, Rb1, Rb2, Rb3, Rd, were purchased from the National Institute for the Control of Pharmaceutical and Biological Products. Ginsenoside Rf, Rg2, and Rc were purchased from Chengdu Must Bio-Technology Co., Ltd. The purity of these reference substances reached over 98%. The structures and information of the nine ginsenosides are shown in Figure 1 and Table 1. HPLC-grade methanol and acetonitrile were purchased from Oceanpak Alexative Chemical, Ltd. Purified distilled was purchased from Watsons (Guangzhou Watson's Food and Beverage Co., Ltd.). Other reagents were of analytical grade.
Figure 1.
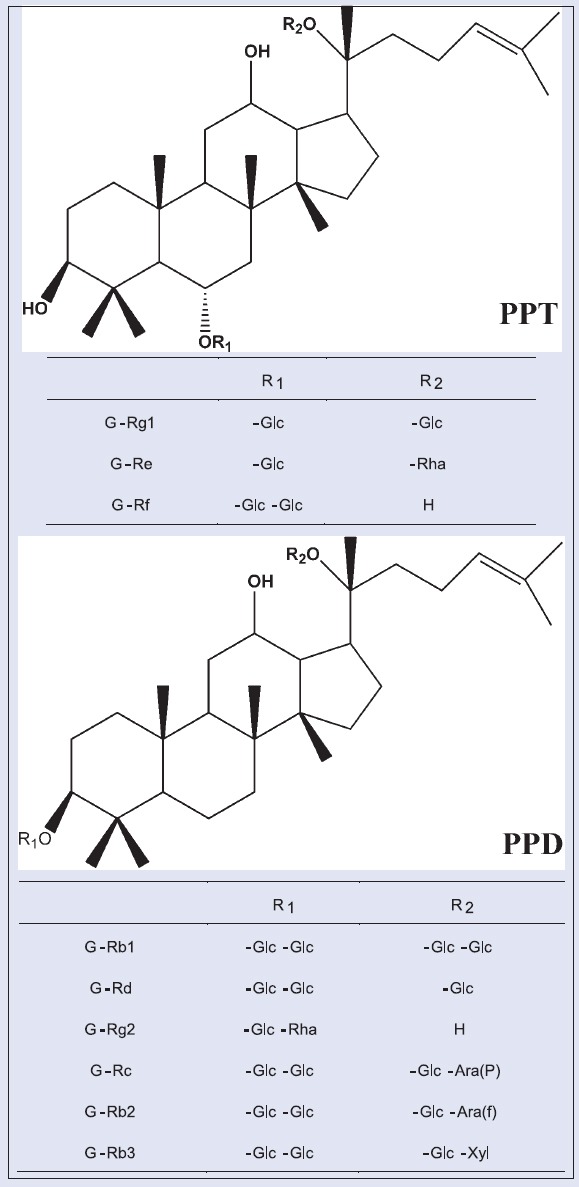
Chemical structures of ginsenoside Rg1, Re, Rf, Rg2, Rb1, Rc, Rb2, Rb3, Rd
Table 1.
Information of 9 ginsenosides
Apparatus and conditions
Analyses were performed using HPLC system, which were Shimadzu LC-20A including LC-20AT solvent delivery unit, SIL-20A autosampler, SPD-M20A diode array detector, and LC solution chromatography workstation (Shimadzu Corporation, Japan); Waters Alliance HPLC, including 2695 solvent management systems, 2996 diode array detector, Empower 3 chromatography workstation (Waters Corporation, USA). Columns used in the analysis were Inertsil ODS-3 C18 (250 mm × 4.6 mm, 5 μm, Shimadzu), Venusil XBP C18 (250 mm × 4.6 mm, 5 μm, Agela), and SunFire C18 (250 mm × 4. 6 mm, 5 μm, Waters). Mobile phase consisted of (a) acetonitrile and (b) water and separation was achieved using the following gradient: 0–28 min, 19% A; 28–58 min, 19%–29% A; 58–85 min, 29% A; 85–125 min, 29%–40% A. The flow rate was set at 1.0 mL/min and the column temperature was maintained at 30°C. The detection wavelength was at 203 nm, and the injection volume was 20 μL. Chromatograms are shown in Figure 2.
Figure 2.
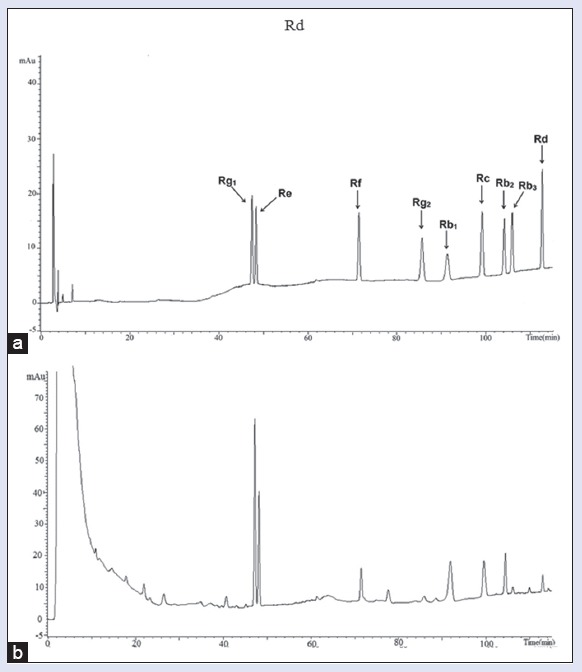
Chromatograms of nine mixed ginsenoside reference standards (a) and the samples of P. ginseng (b)
Preparation of standard solutions
The nine kinds of ginsenoside were accurately weighed, respectively (Rg1 4.99 mg, Re 5.01 mg, Rf 5.26 mg, Rg2 5.04 mg, Rb1 5.08 mg, Rc 5.04 mg, Rb2 5.35 mg, Rb3 5.42 mg, Rd 7.77 mg), in a 5 mL volumetric flask. They were dissolved with methanol and stored at 4°C. The mixed stock solutions were serially diluted (dilution factor = 5, 6, 8, 10, 12.5, 20, 40, 200) to produce calibration standard solutions.
Preparation of sample solution
Sample powder (over the 4th sieve) was accurately weighed (about 1 g) and transferred into 150 mL conical flask. They were extracted with 50 mL of water-saturated butanol in an ultrasonic bath for 30 min. 25 mL successive filtrate was evaporated in the evaporating dish. The residue was dissolved in methanol and transferred to a 5 mL volumetric flask. Before injection, the supernatant was filtered through 0.45 μm syringe filter.
Calculation of conversion factor and difference value of retention time
The conversion factor of the reference standard X (Fx) was calculated as the previous report based on the linearity data.[1] It was briefly described as following two equations:

The Asi and Axi are the peaks of ginsenoside Rg1, the internal standard, and the other reference standard (X), at the concentration level i. The Csi and Cxi are the concentrations of the ginsenoside Rg1 and the other reference standard (X), at concentration level i. N is the numbers of linearity data.
The difference value of retention time (DRT) was calculated as the DRT of the reference standard X (RTx) and the ginsenoside Rg1 (RTs).
DRTx =RTx - RTs (3)
Validation and assessment of the single standard for determination of multicomponents method
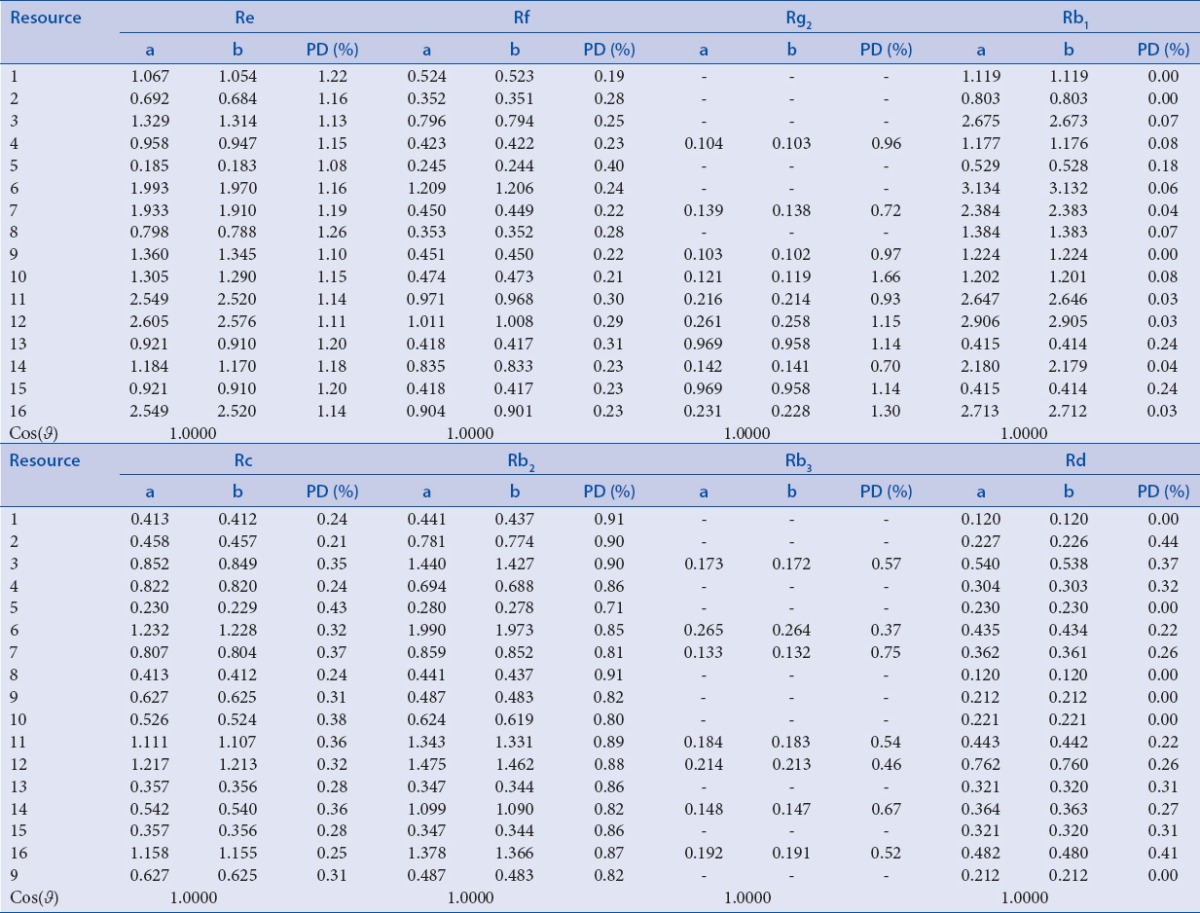
Sixteen batches of P. ginseng were collected, and content of the nine ginsenosides was calculated on SSDMC method and determined by the external standard method. Percent difference (PD) and Cos (θ), cosine similarity between two vectors, were employed.[16] The calculation of PD is: 100× (|x1 − x2|)/[(x1 + x2)/2]. The calculation of Cos (θ) is as the following equation:
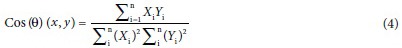
X and Y are the contents produced by SSDMC method and external standard method, and n is the number of data sets.
RESULTS AND DISCUSSION
Optimization of extraction conditions
According to the previous reports,[17,18] effects on extraction rate of nine ginsenosides in P. ginseng with water-saturated butanol, methanol, and 70% ethanol were investigated. The results indicated that the contents of ginsenosides in water-saturated butanol were higher and each target peak had a better separation in the liquid chromatograph. Furthermore, extraction method (reflux and ultrasonic) and time (30, 45, 60 min) were also investigated. The result showed that the most suitable method was the water-saturated butanol in an ultrasonic bath for 30 min.
Method validation
Specificity
The specificity was investigated by comparing the retention time (RT) between sample and the corresponding reference standard. Figure 1a and b showed that the nine peaks in the chromatogram could be identified by the corresponding standards.
Calibration curves, limit of quantification, and limit of detection
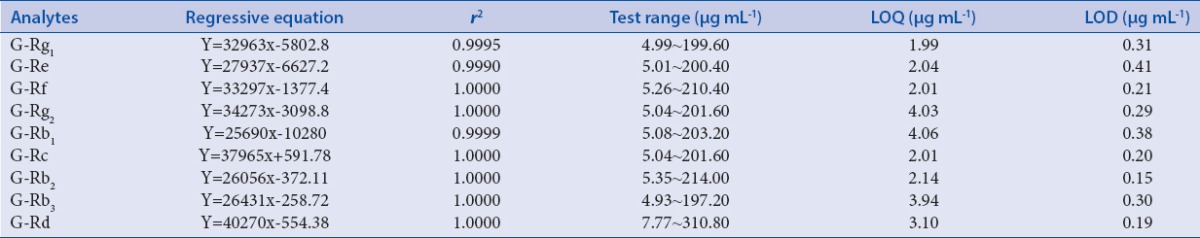
The mixed reference solution was diluted to appropriate concentrations with methyl alcohol for the establishment of calibration curves. The results exhibited a good linearity (r2 ≥ 0.9990) over the concentration range. The limits of quantification and detection for nine ginsenosides were measured based on a signal-to-noise (S/N) ratio at about 10 and 3. The data are listed in Table 2.
Table 2.
The regression data, LOQ and LDQ for nine ginsenosides
Precision and accuracy
Intra- and inter-day variations were chosen to determine the precision of the developed assay. For intraday variability test, the mixed reference solutions were analyzed for six replicates within 1 day. For interday variability test, the solutions were examined in six duplicates for consecutive 3 days. Variations were expressed by relative standard deviation (RSD). The recovery was used to evaluate the accuracy of the method. Known amounts of individual standards were added into a certain amount of P. ginseng samples, and then six duplicates of the mixed samples were extracted and analyzed using the method mentioned above. The recovery for each analyte was calculated as follows: recovery (%) =100 × (amount found − original amount)/amount spiked.
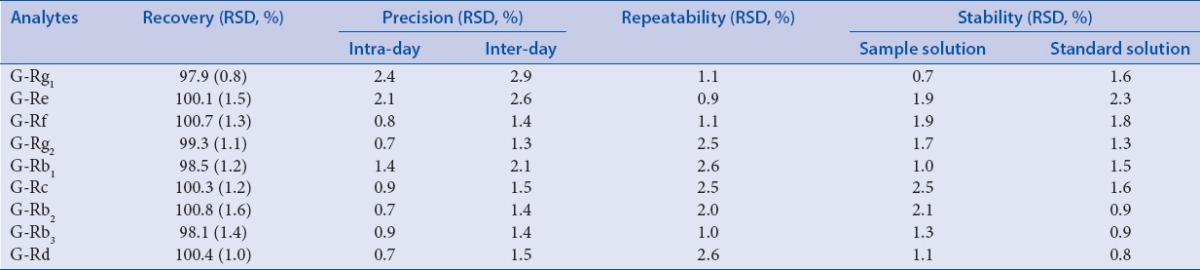
For overall intra- and inter-day variations, the RSDs of all of the analytes were less than 2.5% and 3.2%, respectively [Table 3]. For the recovery test, the recoveries of all of the analytes were in the range 97.9%–100.8% with the RSDs lower than 1.6%, showed that the instrument had a high precision and the accuracy of this method was good.
Table 3.
Accuracy, precision, repeatability and stability (n=6)
Stability and repeatability
The repeatability test was conducted by analyzing the same batch of P. ginseng for six replicates and represented as RSD. For measurement of stability, the sample solution and standards solution were stored at room temperature. The analyses were performed at 0, 2, 4, 8, 12, 16, and 24 h, respectively. RSD values of peak areas were calculated. For repeatability test, RSD of all analytes was less than 1.5% [Table 3], which indicated the method has good repeatability. The results of stability test showed sample solution and standards solution was stable for almost 24 h, with the RSDs of peak areas less than 2.6%.
Calculation and validation of the conversion factors (F)
In this study, the ginsenoside Rg1 was chosen as internal referring substance for its relatively high content, easier accessibility, better stability, and relatively lower cost. The maximum absorption wavelength of nine ginsenosides was about 203 nm. The Fs of other eight ginsenosides were calculated using above formulas. The result calculated from the eight gradient concentrations showed that Fs of Re, Rf, Rg2, Rb1, Rc, Rb2, Rb3, Rd were 0.846, 0.969, 1.036, 0.750, 1.160, 0.753, 0.753, 1.250, respectively.
The conversion factors (Fs) were investigated through following experiments: (a) different columns and instruments; (b) different wavelength (±1 nm), column temperature (±2°C), injection volume (15, 20 and 25 μL).
The results are as follows: (A) different instruments and columns were the most important factors to evaluate the robustness of Fs.[1] In this study, two instruments (Shimadzu LC-20A, Waters Alliance 2695 LC) and three columns (Inertsil ODS-3 C18, Venusil XBP C18, and SunFire C18) were used to explore. The result showed that the RSDs of Fs were all lower 3.6% for various instruments and different columns [Table 4], indicating that the different types of columns and instruments had no significant effects on Fs.
Table 4.
Variations of Fs on different conditions
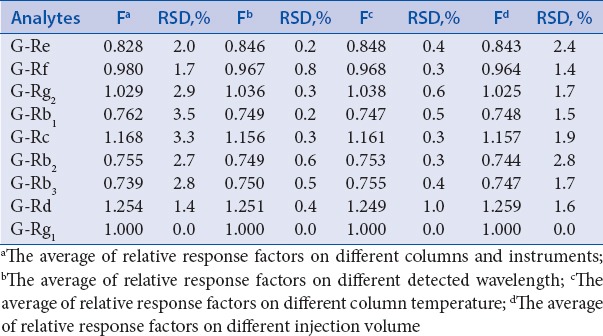
(B) The Fs were investigated on Shimadzu LC-20A system and Inertsil ODS-3 C18 (4.6 mm × 250 mm, 5 μm) column. The result showed that the RSDs of Fs were ranged from 0.2%–2.8% for different chromatographic conditions [Table 4], revealing that the Fs were steadily and can be adjusted in a narrow range.
Investigation of difference value of retention time
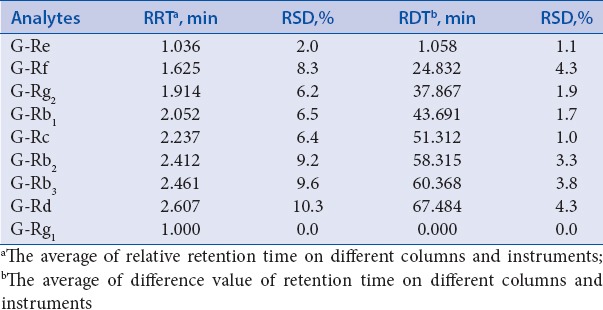
The calculation of DRT was necessary so that the peaks could be identified with only one single Reference Standard (IS). For ginsenoside Re, Rf, Rg2, Rb1, Rc, Rb2, Rb3, Rd, the DRTs were 1.05, 24.83, 37.86, 43.69, 51.31, 58.31, 60.36, 67.48 min, respectively. These values were stable on different days.
For a comprehensive and valid SSDMC method, it is necessary to position the peaks of analytes. First, two methods were used to positioning peaks including DRT and relative retention time (RRT). Second, different columns and instruments were used to evaluate the robustness of positioning peaks as described above.
The results are as follows: RRT was calculated as the ratio of RT of the reference standard X and the ginsenoside Rg1. The RT originally obtained at different situation was easily affected, which would lead to obvious fluctuate of RRT. In this study, the RRT was instability with RSDs ranged from 2.0%–10.3% when using different instruments and columns [Table 5]. In the meanwhile, the result shows that the RSDs of DRT were all lower 4.3% on the various instruments and different columns, indicating that DRT was more feasible and suitable for positioning analyte peaks.
Table 5.
Variations of RRT and RDT on different columns and instruments
Quantification of ginsenosides and the method assessment
The developed SSDMC method was applied to determine the ginsenoside Rg1, Re, Rf, Rg2, Rb1, Rc, Rb2, Rb3, Rd in 16 batches of P. ginseng. As shown in Table 6, the data derived from SSDMC method and internal standard method had high consistency. The average PDs of eight analytes were all less than 5%. The Cos (θ) (>0.9999) of all determined vectors indicated that there was no significant difference between these two methods. Therefore, SSDMC method is a technique both feasible and accurate in the simultaneous determination of nine ginsenosides in P. ginseng.
Table 6.
Contents of nine ginsenosides determined by external standard method and SSMDC method (mg g-1, n=3)
CONCLUSION
In this study, an SSDMC method has been established and applied for the simultaneous determination of nine ginsenosides in P. ginseng. Various HPLC instruments and chromatographic conditions were investigated to verify its applicability. The results indicated that SSDMC possesses high accuracy and feasibility, and other factors have not significantly influence on the robustness of the Fs. The results of the study also were conclusive proof to the application of SSDMC method for determination of multicomponents in TCMs and TCM prescriptions. Because SSDMC method can solve the problems with the absence of relevant standard substances (e.g., ginsenoside Rg2) during quantitative analysis, it will play an important role in the quality control of TCMs in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hou JJ, Wu WY, Da J, Yao S, Long HL, Yang Z, et al. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J Chromatogr A. 2011;1218:5618–27. doi: 10.1016/j.chroma.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 2.Wang ZM, Gao HM, Fu XT, Wang WH. Multi-components quantitation by one marker new method for quality evaluation of Chinese herbal medicine. Zhongguo Zhong Yao Za Zhi. 2006;31:1925–8. [PubMed] [Google Scholar]
- 3.Da J, Cheng CR, Yao S, Long HL, Wang YH, Khan IA, et al. A reproducible analytical system based on the multi-component analysis of triterpene acids in Ganoderma lucidum. Phytochemistry. 2015;114:146–54. doi: 10.1016/j.phytochem.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Gao XY, Jiang Y, Lu JQ, Tu PF. One single standard substance for the determination of multiple anthraquinone derivatives in rhubarb using high-performance liquid chromatography-diode array detection. J Chromatogr A. 2009;1216:2118–23. doi: 10.1016/j.chroma.2008.11.104. [DOI] [PubMed] [Google Scholar]
- 5.Hou JJ, Wu WY, Liang J, Yang Z, Long HL, Cai LY, et al. A single, multi-faceted, enhanced strategy to quantify the chromatographically diverse constituents in the roots of Euphorbia kansui. J Pharm Biomed Anal. 2014;88:321–30. doi: 10.1016/j.jpba.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 6.The United States Pharmacopeia Convention. The U.S. Pharmacopeia (USP 33) Baltimore, MD: United Book Press; 2010. p. 1087. [Google Scholar]
- 7.National Commission of Chinese Pharmacopoeia. Pharmacopoeia of People's Republic of China. I. Beijing: China Medical Science Press; 2015. p. 76. [Google Scholar]
- 8.Chen Z, Lu T, Yue X, Wei N, Jiang Y, Chen M, et al. Neuroprotective effect of ginsenoside Rb1 on glutamate-induced neurotoxicity: With emphasis on autophagy. Neurosci Lett. 2010;482:264–8. doi: 10.1016/j.neulet.2010.07.052. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Liu B, Dluzen DE, Jin Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J Ethnopharmacol. 2007;111:458–63. doi: 10.1016/j.jep.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Wang HP, Yang XB, Yang XW, Liu JX, Xu W, Zhang YB, et al. Ginsenjilinol, a new protopanaxatriol-type saponin with inhibitory activity on LPS-activated NO production in macrophage RAW 264.7 cells from the roots and rhizomes of Panax ginseng. J Asian Nat Prod Res. 2013;15:579–87. doi: 10.1080/10286020.2013.787992. [DOI] [PubMed] [Google Scholar]
- 11.Yamabe N, Song KI, Lee W, Han IH, Lee JH, Ham J, et al. Chemical and free radical-scavenging activity changes of ginsenoside Re by maillard reaction and its possible use as a renoprotective agent. J Ginseng Res. 2012;36:256–62. doi: 10.5142/jgr.2012.36.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian J, Fu F, Geng M, Jiang Y, Yang J, Jiang W, et al. Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci Lett. 2005;374:92–7. doi: 10.1016/j.neulet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Gong L, Li SL, Li H, Zhang L. Ginsenoside Rg1 protects primary cultured rat hippocampal neurons from cell apoptosis induced by ß-amyloid protein. Pharm Biol. 2011;49:501–7. doi: 10.3109/13880209.2010.521514. [DOI] [PubMed] [Google Scholar]
- 14.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–93. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 15.Leung KW, Wong AS. Pharmacology of ginsenosides: A literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li SP, Qiao CF, Chen YW, Zhao J, Cui XM, Zhang QW, et al. A novel strategy with standardized reference extract qualification and single compound quantitative evaluation for quality control of Panax notoginseng used as a functional food. J Chromatogr A. 2013;1313:302–7. doi: 10.1016/j.chroma.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Zhang JL, Sheng YX, Guo DA, Wang Q, Guo HZ. Simultaneous quantification of six major active saponins of Panax notoginseng by high-performance liquid chromatography-UV method. J Pharm Biomed Anal. 2005;38:45–51. doi: 10.1016/j.jpba.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 18.National Commission of Chinese Pharmacopoeia. Pharmacopoeia of People's Republic of China. I. Beijing: China Medical Science Press; 2015. p. 8. [Google Scholar]


