Abstract
Background
Shiga toxin (Stx)-producing Escherichia coli (STECs) are the most common cause of acute renal failure in children. The present study evaluated a 10-year STEC polymerase chain reaction screening regimen in children.
Methods
All routine stool culture specimens from patients below 10 years of age (n = 10 342) from May 2003 through April 2013 in the County of Jönköping, Sweden, were included. Patients were divided in 1 group where analyses of STEC were requested by the clinician (n = 2366) and 1 screening group (n = 7976). Patients who were positive for STEC were tested weekly until they were negative. Clinical data were collected through a questionnaire and by reviewing medical records.
Results
In specimens from 191 patients, stx was found (162 index cases). The prevalence was 1.8% in the requested group and 1.5% in the screening group (P = .5). Diarrhea was the most frequent symptom reported in 156 cases and of these 29 (19%) had hemorrhagic colitis (HC) and 7 children developed hemolytic uremic syndrome (HUS). No difference regarding severity of symptoms between the groups was found. Stx2 predominated in cases with HC (P < .0001) and HUS (P = .04). Median stx shedding duration was 20 days (1–256 days), and no difference in duration was seen between stx types (P = .106–1.00) and presence of eaeA (P = .72).
Conclusions
Most STEC cases were found in the screening group with comparable prevalence and disease severity as in patients where analysis was requested. Furthermore, non-O157 serotypes caused severe disease when carrying stx2, and prolonged shedding of STEC may be a risk for transmission.
Keywords: screening, serotype, shedding, Shiga toxin, STEC
Shiga toxin (Stx)-producing Escherichia coli (STEC) bacteria are causative pathogens of diarrhea, hemorrhagic colitis (HC), and hemolytic uremic syndrome (HUS), and these bacteria are also the most common cause of acute renal failure in children [1–4]. Human STEC isolates are also designated enterohemorrhagic E coli, and serotype O157:H7 is one of the predominant serotypes responsible for outbreaks worldwide [5, 6]. Enterohemorrhagic E coli O157 is the main focus for diagnostics; however, non-O157 serotypes, such as O26, O103, O111, and O145, contribute significantly to cases of HC and HUS [5]. In 2011, a large outbreak of E coli O104:H4 in Germany led to HUS in more than 800 patients and 53 deaths [7, 8]. The Stxs are divided into 2 major types, Stx1 and Stx2, and Stx2 is responsible for the most severe symptoms [9–11]. In addition, several different subtypes of each Stx have been described [12, 13], and some Stx2 subtypes seem to be associated with more severe disease [14]. In addition, the presence of the gene for the adhesion factor intimin (eaeA) is linked to disease severity [9]. The issue of STEC carriage after infection is significant, and steps should be taken to limit the spread from person-to-person. However, only limited data regarding a median duration of carriage (17–18 days) [15] are available.
Rapid STEC detection is important in outbreak management and patient treatment, including prompt parenteral hydration, monitoring for development of severe disease, and avoidance of antibiotics and antidiarrheal agents, which can exacerbate disease [16]. The detection of STEC by culture is challenging, and traditional culture methods detect mainly O157:H7 [17, 18]. The Centers for Disease Control and Prevention (CDC) has issued recommendations to test simultaneously for O157 and non-O157 STEC in all stool specimens from patients with acute community-acquired diarrhea [19]. This diagnostic regimen was also recently recommended in a study by Lefterova et al [20]. Proper clinical diagnosis and management of non-O157 STEC infections also depend on improved physician awareness [20]. Currently, serotype-independent polymerase chain reaction (PCR) assays are used to detect stxs and are widely used for accurate and rapid diagnostics [20, 21].
When comparing the prevalence of STEC in the Nordic countries, Sweden shows the highest rates, with the highest number of outbreaks occurring in 2005 on the west coast of the country [22]. The higher prevalence may also depend on differences in diagnostic regimens and the number of specimens that were tested for STEC.
In this study, we evaluated a 10-year STEC PCR screening regimen in children with diarrhea in a Swedish county. The most common serotypes were correlated with clinical symptoms, stx type, and presence of eaeA. Furthermore, our goal was to add insights regarding stx shedding to the limited data available.
MATERIALS AND METHODS
Patients
Our study comprised all routine diarrheal stool culture specimens from patients younger than 10 years of age (n = 10 342) from 1 May 2003 through April 2013 in the County of Jönköping, Sweden. All stool specimens were collected using swabs (Copan Diagnostics Inc., Brescia, Italy). Patients were divided in 1 group in which analyses of STEC were requested by the clinician (n = 2366) and 1 screening group (n = 7976). In addition, contact tracing around index cases was performed (n = 202). The STEC-positive patients were sampled weekly until they were negative, and the duration of stx shedding was defined as the time from the first positive sample to the first negative sample. Clinical data were collected from all patients (n = 191) who tested positive for STEC by PCR through a questionnaire and by reviewing medical records. Results from clinical chemistry analysis on peripheral blood done at the routine chemistry laboratory at Ryhov County Hospital were available from 60 children, mainly from patients who required hospitalization. Criteria for HUS included 3 primary symptoms: hemolytic anemia with fragmentocytes, low platelet count, and acute renal failure with a creatinine outside the reference range of normal for age.
Shiga Toxin-Producing Escherichia coli Detection and Typing
The presence of stx in diarrheal stool specimens was determined by real-time PCR on suspensions of overnight cultures on blood agar plates [21]. In total, 157 of 191 PCR-positive specimens were sent to the Karolinska University Hospital for confirmatory testing, isolation of STEC, and serotyping according to methods described by Svenungsson et al [23].
Statistical Analyses
Statistical analyses were done with Statistica version 12. Fisher's exact test and Pearsons χ2 test were used when comparing proportions. Mean values were compared by Mann-Whitney U test (nonnormal distributed) and Student t test as well as Bonferroni test (normal distributed). A P value < .05 was considered statistically significant.
RESULTS
Stx was found in specimens from 191 patients (104 boys, 87 girls), and, of these cases, 162 (85%) were index cases. Confirmatory testing at the Karolinska University Hospital, including both stx and eaeA analysis, was performed on 157 of the total 191 specimens. In 153 (97%) of these 157 specimens, STEC was confirmed by stx detection and culture was successful in 88 (56%) cases. In 115 of 157 (73%) specimens, eaeA was detected.
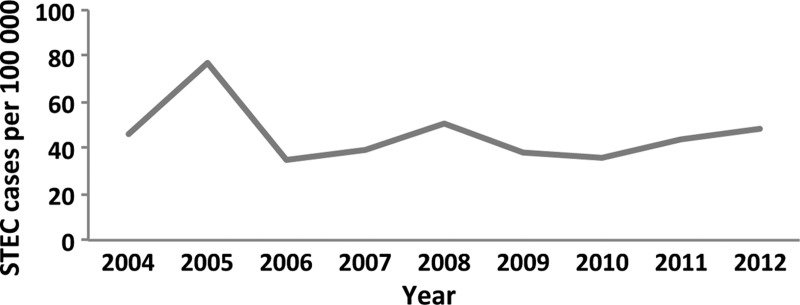
In total, 121 STEC cases were detected in the screening group, 41 in the requested group and 29 by contact tracing. The prevalence was 1.8% in the requested group and 1.5% in the screening group (P = .5), corresponding prevalence in the contact-tracing group was 14%. The numbers of children with STEC were 118 (62%), 40 (21%), and 33 (17%) in the age groups 0–3, 4–6, and 7–9 years, respectively. The annual incidence varied from 39 to 86 per 100 000 (Figure 1). Children below 10 years of age comprised 57.5% of the STEC cases in the County; however, when considering only STEC found in the requested group, children comprised approximately 20%. In comparison, culture for routine diagnostics revealed 200 cases of Campylobacter, 135 Salmonella, 76 Yersinia, and 18 Shigella, respectively.
Figure 1.
Annual incidence of Shiga toxin-producing Escherichia coli (STEC) in children below 10 years of age in the county of Jönköping, Sweden.
Clinical Characteristics and Laboratory Parameters
Diarrhea was the most frequent symptom reported in 156 (82%) cases, and, of these, 29 (19%) had HC. Abdominal pain was the second most common symptom (34%), followed by vomiting (17%) and fever (17%). Seven children developed HUS (3.6%). Stx2 predominated in cases with HC (P < .0001) and HUS (P = .04). Hospitalization was necessary in 15 (7.8%) cases with a median length of stay of 15 days. In children with HC, elevated levels of leucocytes were detected (P < .0001). In HUS cases, elevated levels of leucocytes and creatinine were observed as well as low levels of thrombocytes (P < .0001) and erythrocyte volume fraction (P = .0001).
Data Regarding stx Shedding Time, Serotypes, and Subtypes of stx
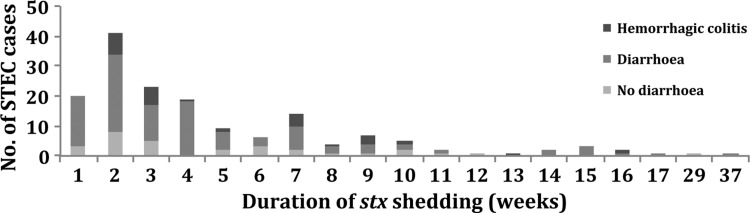
Data on stx shedding time was available for 165 (86%) children, with a median duration of 20 days (1–256 days). For children with HC (n = 29), the median duration was 29 days (8–107 days); for children with uncomplicated diarrhea (n = 127), the median duration was 20 days (1–256 days) (P = .07) (Figure 2). The HUS cases had a median duration of 23 days (18–105 days). There was no difference in mean duration of stx shedding comparing stx types (P = .11) and presence of eaeA (P = .72).
Figure 2.
Duration of stx shedding in children with no diarrhea (n = 35), diarrhea (n = 127), and hemorrhagic colitis (n = 29). STEC, Shiga toxin-producing Escherichia coli.
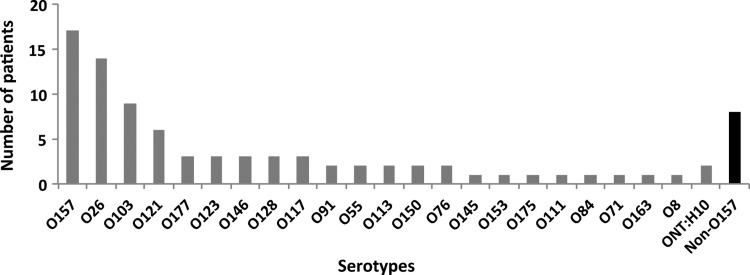
In 88 cases, STEC isolation was successful, and the most common serotypes were O157 (n = 17), O26 (n = 14), O103 (n = 9), and O121 (n = 6). In addition, 19 other serotypes were found (Figure 3). In 3 of 7 HUS cases, isolation and serotyping was successful (two O121, one O157:H7), as well as in 15 of 29 HC cases (seven O157:H7, two O121, and two O103 and some singletons).
Figure 3.
Serotype distribution of isolated Shiga toxin-producing Escherichia coli (n = 88). The black bar illustrates non-O157 strains that we were not able to serotype further.
The distribution of stx types was 49%, 33%, and 18% for stx1, stx2, and stx1 + stx2, respectively. We found no differences regarding stx types, duration of stx shedding, and severity of symptoms between the screening group and the requested group. Stx1 was most frequent in serotypes O26 and O103, whereas stx2 was more frequent in O157 and O121. Serotype O157 had a higher probability of harboring eaeA (P = .004). Patients with stx2 were more often hospitalized (P ≤ .0001) and had HC (P ≤ .0001) or HUS (P = .04) compared with patients with stx1 or stx1 + stx2. eaeA showed no significant correlation with disease severity; however, HC cases showed a trend towards significance (P = .07). No significant difference in duration of stx in feces was seen between the stx types (P = .106–1.00), eaeA presence (P = .72), age groups (P = 1.00), or gender (P = .35). We found no difference between stx type and gender (P = .68) or between age groups (P = .25). In patients infected in Sweden, stx2 was more common than in patients infected abroad (P ≤ .0001).
Contact Tracing
Contact tracing was performed in approximately 112 of 162 index cases, including 202 diarrheal stool culture specimens from children below 10 years of age. In this group, the STEC-positive rate was 14% (29 of 202), which was higher than in the other 2 groups (requested and screening) (each P < .00001). Source of transmission was determined at 5 occasions; in 4 cases, it was animal contact on a farm and in one case sausage.
DISCUSSION
In this study, we show comparable STEC prevalence and disease severity in children where the analysis was not requested and in those where STEC analysis was requested. Furthermore, we found that a high diversity of serotypes, including non-O157, caused severe disease. In addition, a great variation in the duration of stx shedding was shown, but no relation between shedding time and stx type or severity of symptoms was found.
The yearly incidence was relatively constant during the study except for 2005, which coincided with an outbreak on the west coast of Sweden [22]. The southern part of Sweden, including Jönköping, generally reports the highest annual STEC figures in the country. This may be explained by higher STEC-screening activities in these counties and more farms with a higher verotoxin-producing E coli frequency [24]. The Swedish annual STEC incidence is higher compared with other Nordic countries [25], which may be explained by differences in diagnostic regimens, screening activities, and differences in reporting of cases between countries. In Sweden, reporting of non-O157 serotypes was included in 2004, which also includes reporting based on stx detection only.
One limitation of the present study is the fact that specimens were sent to another laboratory for STEC isolation, which may explain the low STEC isolation rates. However, the vast majority of stx-positive specimens were also detected by PCR at the Karolinska University Hospital, confirming our results. Because culture of non-O157 STEC is more challenging, we may have underestimated the importance of non-O157 STEC.
The majority of STEC cases (60%) were detected in the group of children between 0 and 4 years, which is in agreement with previous findings [26]. Children in this group often wear diapers and attend daycare, a combination that enhances the risk for transmission, as recently shown in an outbreak in Germany [27]. In accordance with previous findings, Stx2 was responsible for the most severe symptoms in our study as well [9-11]. In the present study, stx shedding was usually eliminated after less than 1 month; however, sometimes stx was excreted for several months (maximal duration 256 days). Proper follow-up of children is important to avoid further STEC spread and to prevent outbreaks. Furthermore, novel therapeutic strategies in the treatment for decolonization of long-term STEC carriers should be evaluated, and recent data indicate promising results for azithromycin [28].
In the present study, the majority of STEC cases were detected by screening of diarrhoeal stool culture specimens where analysis for stx was not requested. The frequent STEC detection in screening specimens underlines the lack of STEC awareness among physicians, who do not emphasize the need for STEC analysis. In recent studies, these same conclusions were reached by the CDC [19] and Lefterova et al [20]. Undetected cases of STEC infection may also be present in older individuals, and further studies are needed to determine its presence in diarrheal stool specimens of adults. Comparing culture data for routine diagnostics revealed that STEC was the second most common pathogen detected; however, because different methods are used for detection, this result should be interpreted carefully. Hence, molecular techniques can be used to detect diarrheal pathogens and affect the figures. Nevertheless, the high prevalence of STEC underlines its importance as a common diarrheal pathogen among children.
The source of infection was only revealed in 5 cases, and the methods used presently in Sweden are cumbersome, focusing only on 5 serotypes in animals. Elimination of the source is crucial in outbreak situations, and better methods are needed to determine sources of infection.
CONCLUSIONS
In conclusion, most cases of STEC were found by PCR screening, with comparable prevalence and disease severity found in patients where STEC analysis was requested. Furthermore, a high diversity of serotypes (including non-O157) caused severe disease. Serotype-independent methods for STEC detection and improved physician awareness will more accurately detect the true number of infections and enhance patient safety. Prolonged shedding of STEC may be a risk factor for transmission, and therefore local guidelines for school-aged children should be reviewed to prevent further spread.
Acknowledgments
We thank the molecular diagnostic staff at the Department of Laboratory Services in County Council Jönköping, Sweden, for continuous support.
Financial support. This work was supported by grants from Futurum, the Academy for Healthcare, County Council Jönköping, Sweden.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005; 365:1073–86. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Ruggenenti P. The hemolytic uremic syndrome. Kidney Int Suppl 1998; 66:S54–7. [PubMed] [Google Scholar]
- 3.Karch H, Tarr PI, Bielaszewska M. Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol 2005; 295:405–18. [DOI] [PubMed] [Google Scholar]
- 4.Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev 1991; 13:60–98. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KE, Thorpe CM, Sears CL. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin Infect Dis 2006; 43:1587–95. [DOI] [PubMed] [Google Scholar]
- 6.Hauswaldt S, Nitschke M, Sayk F et al. . Lessons learned from outbreaks of Shiga toxin producing Escherichia coli. Curr Infect Dis Rep 2013; 15:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchholz U, Bernard H, Werber D et al. . German outbreak of Escherichia coli O104:H4 associated with sprouts. New Engl J Med 2011; 365:1763–70. [DOI] [PubMed] [Google Scholar]
- 8.Frank C, Werber D, Cramer JP et al. . Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. New Engl J Med 2011; 365:1771–80. [DOI] [PubMed] [Google Scholar]
- 9.Boerlin P, McEwen SA, Boerlin-Petzold F et al. . Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol 1999; 37:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matussek A, Lauber J, Bergau A et al. . Molecular and functional analysis of Shiga toxin-induced response patterns in human vascular endothelial cells. Blood 2003; 102:1323–32. [DOI] [PubMed] [Google Scholar]
- 11.Proulx F, Seidman EG, Karpman D. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr Res 2001; 50:163–71. [DOI] [PubMed] [Google Scholar]
- 12.Scheutz F, Teel LD, Beutin L et al. . Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol 2012; 50:2951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geue L, Stieber B, Monecke S et al. . Development of a rapid microarray-based DNA subtyping assay for the alleles of Shiga toxins 1 and 2 of Escherichia coli. J Clin Microbiol 2014; 52:2898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich AW, Bielaszewska M, Zhang WL et al. . Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis 2002; 185:74–84. [DOI] [PubMed] [Google Scholar]
- 15.Vonberg RP, Hohle M, Aepfelbacher M et al. . Duration of fecal shedding of Shiga toxin-producing Escherichia coli O104:H4 in patients infected during the 2011 outbreak in Germany: a multicenter study. Clin Infect Dis 2013; 56:1132–40. [DOI] [PubMed] [Google Scholar]
- 16.Davis TK, McKee R, Schnadower D, Tarr PI. Treatment of Shiga toxin-producing Escherichia coli infections. Infect Dis Clin North Am 2013; 27:577–97. [DOI] [PubMed] [Google Scholar]
- 17.Pulz M, Matussek A, Monazahian M et al. . Comparison of a shiga toxin enzyme-linked immunosorbent assay and two types of PCR for detection of shiga toxin-producing Escherichia coli in human stool specimens. J Clin Microbiol 2003; 41:4671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wylie JL, Van Caeseele P, Gilmour MW et al. . Evaluation of a new chromogenic agar medium for detection of Shiga toxin-producing Escherichia coli (STEC) and relative prevalences of O157 and non-O157 STEC in Manitoba, Canada. J Clin Microbiol 2013; 51:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould LH, Bopp C, Strockbine N et al. . Recommendations for diagnosis of shiga toxin–producing Escherichia coli infections by clinical laboratories. MMWR Recomm Rep 2009; 58:1–14. [PubMed] [Google Scholar]
- 20.Lefterova MI, Slater KA, Budvytiene I et al. . A sensitive multiplex, real-time PCR assay for prospective detection of Shiga toxin-producing Escherichia coli from stool samples reveals similar incidences but variable severities of non-O157 and O157 infections in northern California. J Clin Microbiol 2013; 51:3000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellin T, Pulz M, Matussek A et al. . Rapid detection of enterohemorrhagic Escherichia coli by real-time PCR with fluorescent hybridization probes. J Clin Microbiol 2001; 39:370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soderstrom A, Osterberg P, Lindqvist A et al. . A large Escherichia coli O157 outbreak in Sweden associated with locally produced lettuce. Foodborne Pathog Dis 2008; 5:339–49. [DOI] [PubMed] [Google Scholar]
- 23.Svenungsson B, Lagergren A, Ekwall E et al. . Enteropathogens in adult patients with diarrhea and healthy control subjects: a 1-year prospective study in a Swedish clinic for infectious diseases. Clin Infect Dis 2000; 30:770–8. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson E, Aspan A, Gunnarsson A, Vagsholm I. Prevalence of verotoxin-producing Escherichia coli (VTEC) 0157 in Swedish dairy herds. Epidemiol Infect 2005; 133:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Available at: http://www.ecdc.europa.eu/en/publications/Publications/annual-epidemiological-report-2013.pdf Accessed 12 October 2014.
- 26.Gerber A, Karch H, Allerberger F et al. . Clinical course and the role of shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997–2000, in Germany and Austria: a prospective study. J Infect Dis 2002; 186:493–500. [DOI] [PubMed] [Google Scholar]
- 27.Reida P, Wolff M, Pohls HW et al. . An outbreak due to enterohaemorrhagic Escherichia coli O157:H7 in a children day care centre characterized by person-to-person transmission and environmental contamination. Zentralbl Bakteriol 1994; 281:534–43. [DOI] [PubMed] [Google Scholar]
- 28.Nitschke M, Sayk F, Hartel C et al. . Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA 2012; 307:1046–52. [DOI] [PubMed] [Google Scholar]