Abstract
Background
Limited data are available for once-daily (QD) darunavir (DRV)/ritonavir (r) in the pediatric population. Coadministration of etravirine (ETR) may alter the pharmacokinetics (PK) of DRV. We evaluated the PK interactions between DRV/r (QD) and ETR QD or twice-daily (BID) in children, adolescents, and young adults.
Methods
Human immunodeficiency virus-infected subjects 9 to < 24 years old on optimized background therapy including DRV/r 800/100 mg QD alone or combined with ETR 200 mg BID or ETR 400 mg QD were enrolled. Protocol-defined target drug exposure ranges based on adult data were used to assess the adequacy of each regimen. Intensive 24-hour blood sampling was performed, and PK parameters were determined using noncompartmental analysis.
Results
Thirty-one subjects (14 males) completed the study; 16 received DRV/r QD alone (group 1), 6 received DRV/r plus ETR BID (group 2A), and 9 received DRV/r plus ETR QD (group 2B). The geometric mean (90% confidence interval [CI] geometric mean) for DRV area under the curve at 24 hours (AUC24) was 57.9 (49.6–67.6), 74.9 (44.4–126.5), and 66.4 (50.8–86.9) mg × h/L for patients in groups 1, 2A, and 2B, respectively. The increased DRV exposure when coadministered with ETR was not statistically significant. The geometric mean (90% CI geometric mean) of ETR AUC24 was 8.6 (4.4–16.8) and 11.9 (7.5–18.9) mg × h/L for groups 2A and 2B, respectively, with comparable C24.
Conclusions
The results suggest that DRV/r QD with ETR 400 mg QD or 200 mg BID is appropriate and support further evaluation of the safety and efficacy of the once-daily regimen in older children, adolescents, and young adults.
Keywords: antiretrovirals, darunavir, etravirine, pediatrics, pharmacokinetics, ritonavir
Darunavir (DRV) is a second-generation protease inhibitor approved by the US Food and Drug Administration (FDA) in 2006 for the treatment of human immunodeficiency virus (HIV)-infection. It is coadministered with 100 mg of ritonavir and used as part of combination antiretroviral (ARV) treatment for ARV-naive and ARV-experienced adult patients. Once- and twice-daily DRV/r dosing is also approved for children older than 3 years and weighing at least 10 kg [1].
Darunavir and ritonavir are metabolized by cytochrome P450 enzymes (CYP), in particular CYP3A, and therefore inhibition or induction of CYP3A4 by other drugs can potentially alter DRV exposure. In contrast, DRV and ritonavir inhibit CYP3A4 and can potentially increase the exposure of other drugs metabolized by this enzyme. Thus, it is important to evaluate drug-drug interactions associated with DRV/ritonavir (r) use. Etravirine (ETR) is a nonnucleoside reverse-transcriptase inhibitor approved for children ≥6 years old. Etravirine is primarily metabolized by CYP3A, CYP2C9, and CYP2C19. Etravirine is also an inducer of CYP3A and an inhibitor of CYP2C9, CYP2C19, and P-glycoprotein. Therefore, the coadministration of ETR with DRV/r could alter the plasma concentrations of either compound.
When DRV/r 600 mg/100 mg twice-daily was given with ETR 200 mg twice-daily in HIV-infected adults who had failed ARV treatment, the DRV exposure was similar to values previously measured in the absence of ETR [2]. In addition, no changes in ETR pharmacokinetics (PK) occurred in treatment-naive, HIV-infected adults receiving once-daily ETR 400 mg with and without once-daily DRV/r 800/100 mg [3]. As a result, dose modifications are not recommended for adult patients receiving these drug combinations. Although DRV/r has been extensively studied in adults, studies in the pediatric population are limited. Because age-associated physiological changes such as body composition and organ function can affect the PK of drugs [4–8], it is important to investigate the PK and potential drug-drug interactions of DRV/r in children and adolescents. A previous study suggested lower DRV and ETR exposures in adolescents and young adults receiving twice-daily DRV/r (600/100 mg) and twice-daily ETV (400 mg) [9]. Although once-daily DRV/r (800/100 mg) has been shown to be effective and well tolerated in adolescents when given with a backbone of 2 nucleoside reverse-transcriptase inhibitors [10), drug-drug interactions between once-daily DRV/r in combination with ETR have not been studied. Indeed, it has been shown that ETR concentrations are reduced by the addition of DRV, although no dose change is recommended. In this study, we present a pharmacokinetic assessment of once-daily DRV/r (800/100 mg) with or without ETR, either at 200 mg twice-daily or 400 mg once-daily in older children, adolescents, and young adults.
MATERIALS AND METHODS
Study Design
The International Maternal Pediatric and Adolescent AIDS Clinical Trial (IMPAACT) Protocol P1058A is a multicenter opportunistic trial studying the PK of common ARV combinations in children, adolescents, and young adults, who are receiving them as part of clinical care (clinicaltrials.gov, NCT00977756). In the current study, the PK of DRV/r with and without ETR were assessed. Etravirine and ritonavir PK were also determined. Eligible subjects included HIV-infected patients age 9 to < 24 years old, with a body surface area (BSA) ≥ 0.85 m2, and stable for at least 30 days (before screening/entry) on an ARV regimen containing the following: DRV/r 800/100 mg once-daily (group 1); DRV/r 800/100 mg once-daily plus ETR 200 mg twice-daily (group 2A); and DRV/r 800/100 mg once-daily plus ETR 400 mg once-daily (group 2B). All patients received optimal background regimens at their physician's discretion.
Patients receiving study drugs for 30 days before screening were enrolled in the study. Medication adherence was checked by phone calls 3 days before PK visit or at the clinic, and the visit was rescheduled if patients had missed 2 or more doses within a week. Subjects were excluded if they had clinical or laboratory toxicity that was grade 2 or higher (greater than grade 1 for total bilirubin) at screening or had hemoglobin level of <8.5 g/dL. A negative pregnancy test was required at enrollment for females of childbearing capacity. Human immunodeficiency virus-1 RNA level and CD4 were collected as part of standard clinical care. This study was performed at IMPAACT sites in the United States and was approved by the Institutional Review Board at each site.
Pharmacokinetic Design and Bioanalysis
Antiretroviral drugs were administered in an open-label fashion with food (full meal or light snack, high or low fat) at baseline. Blood samples were collected predose and at 1, 2, 4, 6, 8, 12, and 24 hours postdose. Darunavir and ritonavir concentrations were measured at the University of Alabama at Birmingham, using a validated ultraperformance liquid chromatography (UPLC) tandem mass spectrometry (MS/MS) assay. In brief, plasma samples (50 µL) were prepared using a liquid-liquid extraction with t-butylmethylether. Chromatographic separation was performed on a reverse-phase column (X Bridge C18, 2.1 × 100 mm, 3.5 micron particle size), with a mobile phase consisting of an isocratic flow of 45:55 0.1% formic acid in 20 mM ammonium acetate: 0.1% formic acid in acetonitrile. Detection and quantitation of DRV, ritonavir, and their respective stable-labeled isotopic internal standards were achieved by electrospray ([ESI]+ for ritonavir, ESI− for DRV) MS/MS detection with an assay range of 25–15 000 ng/mL for DRV and 10–15 000 ng/mL for ritonavir. Etravirine concentrations were measured at the University of Nebraska Medical Center using a validated ultrahigh-performance liquid chromatography assay method with a range of 20–20 000 ng/mL [11] and at the University of Colorado, Denver using a validated UPLC/MS/MS method with a range of 5–5000 ng/mL. For the UPLC/MS/MS method, samples (100 µL) were prepared using a liquid-liquid extraction with t-butylmethylether at basic pH. Chromatographic separation was performed on a reverse-phase column (Acquity UPLC BEH Shield RP18, 2.1 × 100 mm, column with a 1.7 micron particle size), with a mobile phase consisting of an isocratic flow of 75:25 acetonitrile/water with 0.1% formic acid (v/v) at 0.5 mL/min. Detection and quantitation of ETR and its stable-labeled isotopic internal standard were achieved by protonated ESI+ MS/MS detection. Overall, interassay/intra-assay variability was less than 20% at the lower limit of quantification and 15% at the other concentrations. Assays were validated according to the FDA guidance on bioanalytical method validation, and the laboratories participated in the clinical pharmacology quality assurance external quality control program [12].
Pharmacokinetic and Statistical Analyses
Pharmacokinetic parameters for DRV, ritonavir, and ETR were determined using noncompartmental methods (WinNonlin Phoenix version 6.30.395; Pharsight Corp., Mountain View, CA). The area under the plasma concentration-time curve (AUC) was calculated using the linear/log trapezoidal rule. For twice-daily ETR, AUC24 was calculated as the steady-state AUC12 × 2.
Based on data from adults infected with HIV, once-daily DRV/r 800/100 mg yielded a mean AUC24 of 61.1 ± 22.5 mg × h/L [13]. Assuming similar variability in AUC24 in our cohort, 15 children provided 80% power to detect, in a 1-sample, 2-sided test with 5% Type I error, a change of 30% or more relative to this reported mean. Protocol-defined AUC target range for DRV was 48.8–76.4 mg × h/L (±22% on the log scale [a factor of 1.25 on the natural scale] around the target mean AUC of 61.1 mg × h/L). Likewise, the protocol-defined AUC range for 200 mg of ETR twice-daily was 5.51 (4.41–6.89) mg × h/L [14] and for 400 mg of ETR once-daily 10.39 (8.31–13.0) mg × h/L [15]. These ranges correspond to 80%–125% of the mean, which is used to assess bioequivalence. Geometric means for specific PK parameters were compared between the treatment groups using a parametric t test on the log scale.
RESULTS
Thirty-three patients infected with HIV were enrolled. Two patients from group 2A were excluded due to a protocol deviation (1 patient was nonadherent and 1 patient received DRV 600 mg twice-daily). Demographic characteristics of the 31 participants who completed the study are shown in Table 1. Darunavir and ritonavir pharmacokinetic data were available for 16 patients who received DRV/r 800/100 mg once-daily (group 1). In group 2 (DRV/r plus ETR), 15 patients had DRV pharmacokinetic data, 6 patients in group 2A (ETR 200 mg twice-daily) and 9 patients in group 2B (ETR 400 mg once-daily). Three samples (1 in group 2A and 2 in group 2B) were not assayed for ritonavir; therefore, concentration-time data for ritonavir pharmacokinetic analysis were only available for 5 and 7 patients in group 2A and group 2B, respectively.
Table 1.
Baseline Demographic and Clinical Characteristics*
| Characteristic | Darunavir/Ritonavir 800/100 mg Once-Daily Group 1 |
Darunavir/Ritonavir 800/100 mg Plus (Group 2) |
|
|---|---|---|---|
| Etravirine 200 mg Twice-Daily Group 2A |
Etravirine 400 mg Once-Daily Group 2B |
||
| N | 16 | 6 | 9 |
| Gender | |||
| Male | 8 (50) | 3 (50) | 3 (33.3) |
| Race/Ethnicity | |||
| African American, non-Hispanic | 11 (68.8) | 2 (33.3) | 8 (89.9) |
| White, non-Hispanic | 0 | 2 (33.3) | 1 (11.1) |
| Hispanic | 5 (31.2) | 2 (33.4) | 0 |
| Tanner Stage | |||
| 1 | 0 | 0 | 1 (11.1) |
| 2 | 0 | 0 | 2 (22.2) |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 |
| 5 | 16 (100) | 6 (100) | 6 (66.7) |
| Age (years) | 20.1 (13.7–23.2) | 20.1 (18.8–22.6) | 16.6 (9.9–22.9) |
| Weight (kg) | 66.7 (40.0–92.3) | 58.7 (48.1–120.9) | 58.97 (28.1–115.5) |
| BSA (m2) | 1.8 (1.3–2.1) | 1.6 (1.5–2.3) | 1.6 (1.0–2.4) |
| HIV RNA (copies/mL) | < 40 ( < 40–1712) | 30 ( < 20–5852) | < 40 ( < 40–372) |
| Viral load > 200 copies/mL | 2 (12.5) | 2 (33.3) | 2 (22.2) |
| CD4+ T-cell count (cells/µL) | 696 (247–1540) | 485 (108–920) | 830 (259–1463) |
Abbreviations: BSA, body surface area; HIV, human immunodeficiency virus.
*Numbers are presented as median (range) or n (%).
Darunavir Pharmacokinetics
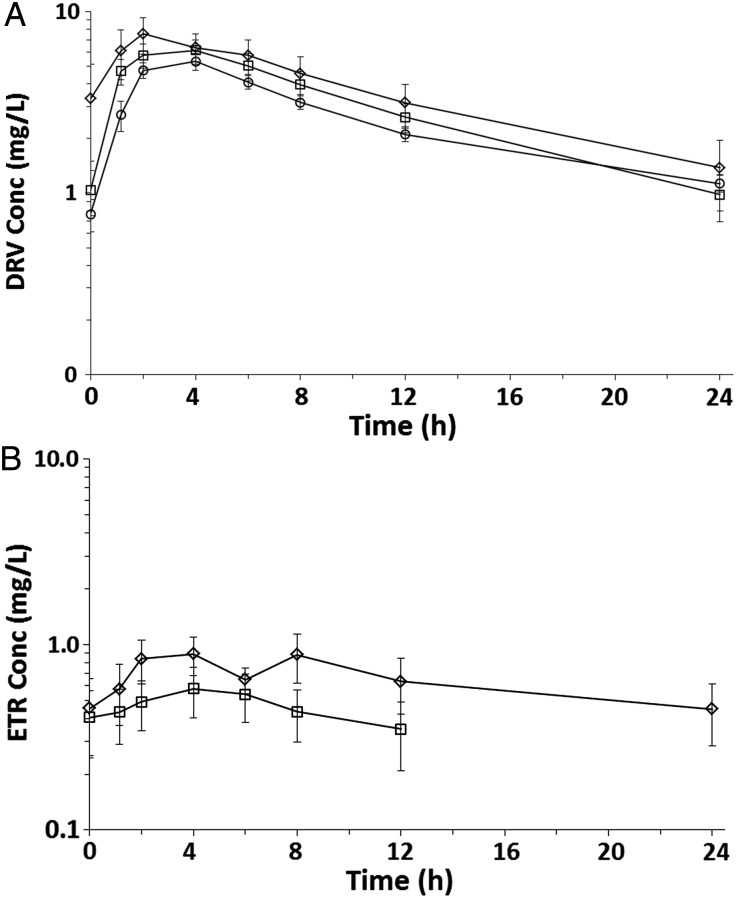
The concentration-time profiles of DRV in group 1 (DRV/r, N = 16) and in groups 2A (DRV/r plus ETR 200 mg twice-daily, N = 6) and 2B (DRV/r plus ETR 400 mg once-daily, N = 9) are shown in Figure 1A. The pharmacokinetic parameters of DRV in groups 1, 2A, and 2B are summarized in Table 2. The geometric mean ([GM] 90% confidence interval [CI GM]) for DRV AUC24, maximum plasma concentration (Cmax), and plasma concentration at 24 hours (C24) were 57.9 (49.6–67.6) mg × h/L, 5.5 (4.6–6.5) mg/L, and 1.0 (0.8–1.3) mg/L, respectively. The GM (90% CI GM) for DRV AUC24 were 74.9 (44.4–126.5) and 66.4 (50.8–86.9) mg × h/L in group 2A and group 2B, respectively, suggesting increased DRV exposure in patients receiving DRV/r plus ETR compared with those receiving DRV/r alone (group 1). This trend was more pronounced in patients receiving ETR twice-daily compared with those receiving ETR once-daily. However, these changes were not statistically significant (P > .2).
Figure 1.
Concentration-time profile of the mean (with standard error [SE]) darunavir (DRV) concentration with etravirine (ETR) 400 mg once-daily (square) or ETR 200 mg twice-daily (diamond) and without (circle) ETR (A). Mean (with SE) ETR concentration for patients who received ETR 200 mg twice-daily (square) and ETR 400 mg once-daily (diamond) (B).
Table 2.
Pharmacokinetic Parameters of Darunavir, With and Without Etravirine*
| Darunavir PK Parameter | Darunavir/Ritonavir | Darunavir/Ritonavir Plus Etravirine (Group 2) |
|
|---|---|---|---|
| (Group 1) | Etravirine 200 mg Twice-Daily (Group 2A) |
Etravirine 400 mg Once-Daily (Group 2B) | |
| N | 16 | 6 | 9 |
| AUC target range (mg × h/L) | 48.8–76.4 | 48.8–76.4 | 48.8–76.4 |
| AUC24 (mg × h/L) | 57.9 (49.6–67.6) | 74.9 (44.4–126.5) | 66.4 (50.8–86.9) |
| C24 (mg/L) | 1.0 (0.8–1.3) | 0.9 (0.4–2.2) | 0.7 (0.4–1.2) |
| Cmax (mg/L) | 5.5 (4.6–6.5) | 7.2 (4.8–10.6) | 6.7 (5.3–8.4) |
| T1/2 (h) | 10.7 (8.8–12.2) | 7.7 (5.5–10.7) | 7.4 (5.9–9.2) |
| CL/F (L/h) | 13.8 (11.8–16.1) | 10.7 (6.3–18.0) | 12.1 (9.2–15.8) |
| V/F (L) | 206.5 (161.0–264.9) | 118.3 (79.92–175.1) | 128.0 (99.5–164.5) |
Abbreviations: AUC, are under the curve; CI, confidence interval; C24, plasma concentration at 24 hours; CL/F, oral clearance; Cmax, maximum plasma concentration; T1/2, half-life; V/F, volume of distribution.
*Values are geometric mean (90% CI geometric mean).
We also observed that patients in group 2A and group 2B had lower DRV C24 and volume of distribution (Vd/F) than those in group 1. Although differences in C24 between group 1 and groups 2A (P = .658) and 2B (P = .116) were not significant, the differences between Vd/F between group 1 and groups 2A (P = .046) and 2B (P = .037) were statistically significant. Overall, in group 1, the DRV AUC24 was below the lower limit of the target range set by the P1058A protocol in 25% of patients (4 of 16) and higher than the upper limit of the target range in 2 patients. However, the GM (90% CI GM) AUC24 fell within the preset target range. In groups 2A and 2B, 26.7% of patients (4 of 15) had DRV AUC24 lower than those defined by the protocol, and the upper bound 90% CI of the GM AUC24 was outside the DRV target range, whereas 5 patients had DRV AUC24 higher than those defined by the protocol.
Etravirine Pharmacokinetics
The ETR concentration-time profiles for group 2A (200 mg twice-daily, N = 6) and group 2B (400 mg once-daily, N = 9) are shown in Figure 1B and suggest lower ETR exposure in the twice-daily group. This trend was also seen in lower ETR AUC24 and Cmax in group 2A compared with group 2B (Table 3). The ETR Vd/F GM (90% CI GM) in our analysis (1677 [90% CI GM, 747.8–3760] liters and 863.5 [90% CI GM, 566.4–1316] liters for group 2A and group 2B, respectively) were higher than previously published (422 liters) [16, 17]. Four patients in group 2A (66.7%) and 3 patients in group 2B (33.3%) had an ETR AUC12 below the lower limit of the protocol-defined target range.
Table 3.
Pharmacokinetic Parameters of Etravirine*
| Etravirine PK Parameter | Darunavir/Ritonavir Plus Etravirine (Group 2) |
|
|---|---|---|
| Etravirine 200 mg Twice-Daily (Group 2A) | Etravirine 400 mg Once-Daily (Group 2B) | |
| N | 6 | 9 |
| AUC target range (mg × h/L) | 4.4–6.9 | 8.3–13.0 |
| AUC24 (mg × h/L) | 8.6 (4.4–16.8)† | 11.9 (7.5–18.9) |
| C24 (mg/L) | 0.3 (0.1–0.7) | 0.3 (0.2–0.5) |
| Cmax (mg/L) | 0.5 (0.3–0.9) | 0.9 (0.7–1.3) |
| T1/2 (h) | 25.1 (12.1–51.8) | 17.9 (11.0–29.1) |
| CL/F (L/h) | 46.4 (23.8–90.5) | 33.5 (21.2–53.1) |
| V/F (L) | 1677 (747.8–3760) | 863.5 (566.4–1316) |
Abbreviations: AUC, are under the curve; CI, confidence interval; C24, plasma concentration at 24 hours; CL/F, oral clearance; Cmax, maximum plasma concentration; pharmacokinetics; T1/2, half-life; V/F, volume of distribution.
*Values presented as geometric mean (90% CI geometric mean).
†AUC12 × 2.
Ritonavir Pharmacokinetics
The pharmacokinetic parameters of ritonavir are summarized in Table 4. The AUC24, Cmax, and CL/F were comparable among groups 1, 2A, and 2B, and the ritonavir AUC24 and Cmax from our study were comparable to reported values in adults [18].
Table 4.
Pharmacokinetic Parameters of Ritonavir, With and Without Etravirine*
| Ritonavir PK Parameters | Darunavir/Ritonavir | Darunavir/Ritonavir Plus Etravirine (Group 2) |
|
|---|---|---|---|
| (Group 1) | Etravirine 200 mg Twice-Daily (Group 2A) |
Etravirine 400 mg Once-Daily (Group 2B) | |
| N | 16 | 5‡ | 7§ |
| AUC24 (mg × h/L) | 4.4 (3.5–5.5) | 5.2 (1.7–16.2) | 4.4 (3.4–5.8) |
| Cmax (mg/L) | 0.6 (0.4–0.7) | 0.6 (0.2–2.0) | 0.6 (0.4–0.8) |
| T1/2 (h) | 4.8 (4.2–5.4)¶ | 9.5 (2.3–40.1) | 4.7 (3.5–6.3)[dbar] |
| CL/F (L/h) | 22.8 (18.1–28.6) | 19.3 (6.2–60.6) | 22.3 (17.1–29.2) |
| V/F (L) | 135.6 (109.8–167.5) | 265.2 (139.07–505.9) | 139.6 (87.7–222.3) |
Abbreviations: AUC, are under the curve; CI, confidence interval; CL/F, oral clearance; Cmax, maximum plasma concentration; pharmacokinetics; T1/2, half-life; V/F, volume of distribution.
*Values presented as geometric mean (90% CI geometric mean).
‡ Data were not available for 1 patient.
§Data were not available for 2 patients.
¶N = 14.
[dbar]N = 6.
Effect of Age on Darunavir and Etravirine Pharmacokinetics
To assess whether age had an effect on DRV PK parameters, we divided group 1 patients into 2 subgroups: patients older than 18 years of age (N = 10) and patients less than or equal to 18 years of age (N = 6). We observed an overlap of the concentration-time profiles stratified by age (data not shown), which suggested that DRV exposure was not affected by age. In patients receiving DRV/r plus ETR, we observed a trend towards higher DRV and ETR exposures in the younger age group (N = 7) compared with the older age group (N = 8). However, this result was not clinically relevant.
DISCUSSION
In the present study, the AUC24, Cmax, and C24 of DRV were comparable to previously published results in adults receiving DRV/r once daily [13]. Yet, we observed that DRV exposure was higher when coadministered with ETR in adolescents and young adults. This effect was more pronounced when ETR was given twice-daily (200 mg/dose) than once-daily (400 mg/dose). We observed a trend towards lower ETR exposure in the twice-daily group (group 2A) compared with the once-daily group (group 2B). However, our values were similar to those reported previously in HIV-infected adults who also received DRV/r [3, 19, 20]. Of note, because the aim of the current study was to investigate the effect of ETR on DRV PK and not vice versa, enrollment to groups 2A and 2B was not dictated by statistical considerations; therefore, we may not have enough power to detect significant changes in ETR PK. The differences in DRV Vd/F between groups 1 and 2 were statistically significant and could be due to altered pharmacological properties (absorption, distribution, metabolism, and elimination) of DRV in the presence of ETR. However, because of the high variability in our data, further studies are needed to evaluate these observations.
In this study, the DRV C24 values for patients in both group 1 and 2 were higher than the 50% effective concentration (EC50) of DRV (0.055 mg/L [13]) for wild-type virus and the protein-bound corrected 95% inhibitory concentration (PBIC95, 0.025 mg/L) [21]. The clinical efficacy of DRV/r 800/100 mg QD in adolescents has been reported by others in treatment-naive adolescents, in which 92% and 83% of patients achieved favorable virologic response ( < 50 copies/mL) at 24 and 48 weeks, respectively [10]. In the case of ETR, the C24 (C12 for twice-daily dosing) values were higher than the ETR protein-binding adjusted EC50 (0.004 mg/L in cell-based assays for wild-type HIV-1) [19, 22]. Five patients in group 2A (83.3%) had an ETR C12 higher than the ETR PBIC95 (0.116 mg/L) [21], and 8 patients in group 2B (88.9%) had an ETR C24 higher than the PBIC95. In a clinical trial, treatment-experienced adults who received ETR at 200 mg BID and had ETR AUC12, and C0 in the lowest quartile ( ≤ 2.72 mg x h/L and ≤ 0.16 mg/L, respectively) also had the lowest virologic response (58.1%) [23]. Accordingly, using 0.16 mg/L as a clinically relevant trough concentration threshold, 5 patients (83.3% of group 2A) in our study had ETR Clast above the threshold for efficacy. Although our study did not investigate efficacy, these parameters suggest that the specified dosing of DRV and ETR was appropriate. Furthermore, although this study did not assess the safety of the drug combinations, no serious adverse events were reported.
Although we stratified patients by age, this analysis was not preplanned and is likely to have limited power to fully assess the effects of covariates (age, gender, weight, and BSA) on PK of DRV and ETR. Although the age of 18 generally represents adulthood, it does not represent physiological development during late childhood and adolescence. Therefore, a better stratification strategy or cutoff value (eg, Tanner) should be investigated.
CONCLUSIONS
In conclusion, our AUC data are consistent with previous reports in adults and suggest that once daily DRV/r with once- or twice-daily ETR may be appropriate in older children, adolescents, and young adults and support further evaluation of the safety and efficacy of DRV/r with ETR in this study population. More studies are needed to assess the safety and efficacy of the once-daily combination in young children ( < 9 years of age) because once-daily dosing would simplify drug regimen and may improve adherence, which is essential when treating pediatric patients.
Acknowledgments
We thank the patients, families, investigators, and trial site personnel for their contributions to the study.
Disclaimer. The views expressed in written conference materials or publications and by speakers and moderators at US Department of Health and Human Services (HHS)-sponsored conferences, do not necessarily reflect the official policies of the HHS; nor does mention of trade names, commercial practices, or organizations imply endorsement by the US Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health.
References
- 1.PREZISTA (darunavir) [prescribing information]. Available at: http://www.prezista.com/healthcare/full-prescribing-information. Accessed 4 September 2013.
- 2.Boffito M, Winston A, Jackson A et al. . Pharmacokinetics and antiretroviral response to darunavir/ritonavir and etravirine combination in patients with high-level viral resistance. AIDS 2007; 21:1449–55. [DOI] [PubMed] [Google Scholar]
- 3.Dejesus E, Lalezari J, Osiyemi O et al. . Pharmacokinetics of once-daily etravirine without and with once-daily darunavir/ritonavir in antiretroviral-naive HIV type-1-infected adults. Antivir Ther 2010; 15:711–20. [DOI] [PubMed] [Google Scholar]
- 4.Kearns GL, Abdel-Rahman SM, Alander SW et al. . Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 2003; 349:1157–67. [DOI] [PubMed] [Google Scholar]
- 5.Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect 1994; 102 (Suppl 11):107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strolin Benedetti M, Baltes EL. Drug metabolism and disposition in children. Fundam Clin Pharmacol 2003; 17:281–99. [DOI] [PubMed] [Google Scholar]
- 7.Strassburg CP, Strassburg A, Kneip S et al. . Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 2002; 50:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baheti G, King JR, Acosta EP, Fletcher CV. Age-related differences in plasma and intracellular tenofovir concentrations in HIV-1 infected children, adolescents and adults. AIDS 2013; 27:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King JR, Yogev R, Wiznia A et al. . Low darunavir (DRV) and etravirine (ETR) exposure when used in combination in HIV-infected children and adolescents. In: 19th Conference on Retroviruses and Opportunistic Infections. Seattle, Washington: March 5–8, 2012. [Google Scholar]
- 10.Flynn P, Komar S, Blanche S et al. . Efficacy and safety of darunavir/ritonavir at 48 weeks in treatment-naïve, HIV-1-infected adolescents: results from a phase 2 open-label trial (DIONE). Pediatr Infect Dis J 2014; 33:940–5. [DOI] [PubMed] [Google Scholar]
- 11.Sandkovsky U, Swindells S, Moore R et al. . Acceptable raltegravir and etravirine concentrations in plasma when administered via gastrostomy tube. Pharmacotherapy 2012; 32:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiFrancesco R, Tooley K, Rosenkranz SL et al. . Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther 2013; 93:479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boffito M, Miralles D, Hill A. Pharmacokinetics, efficacy, and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naïve and -experienced patients. HIV Clin Trials 2008; 9:418–27. [DOI] [PubMed] [Google Scholar]
- 14.Kakuda TN, Green BE, Morrish G et al. . Population pharmacokinetics of etravirine in HIV-1-infected treatment-experienced children and adolescents (6–17 years): week 24 primary analysis of the phase II PIANO trial [Abstract TULBPE026]. In: 6th International AIDS Society Conference on HIV Pathogenesis and Treatment. Rome, Italy. July 17–20, 2011. [Google Scholar]
- 15.DeJesus E, Lalezari JP, Osiyemi OO et al. . Pharmacokinetics of once-daily etravirine without and with once-daily darunavir/ritonavir in antiretroviral-naive HIV type-1-infected adults. Antivir Ther (Lond) 2010; 15:711–20. [DOI] [PubMed] [Google Scholar]
- 16.Schöller-Gyüre M, Kakuda TN, Raoof A et al. . Clinical pharmacokinetics and pharmacodynamics of etravirine. Clin Pharmacokinet 2009; 48:561–74. [DOI] [PubMed] [Google Scholar]
- 17.Tseng A, MacArthur RD. Profile of etravirine for the treatment of HIV infection. Ther Clin Risk Manag 2010; 6:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson A, Watson V, Back D et al. . Plasma and intracellular pharmacokinetics of darunavir/ritonavir once daily and raltegravir once and twice daily in HIV-infected individuals. J Acquir Immune Defic Syndr 2011; 58:450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.INTELENCE (Etravirine) [prescribing information]. Available at: http://www.intelence.com/hcp/full-prescribing-information. Accessed 9 September 2013.
- 20.Gutiérrez-Valencia A, Martin-Peña R, Torres-Cornejo A et al. . Intracellular and plasma pharmacokinetics of 400 mg of etravirine once daily versus 200 mg of etravirine twice daily in HIV-infected patients. J Antimicrob Chemother 2012; 67:681–4. [DOI] [PubMed] [Google Scholar]
- 21.Acosta EP, Limoli KL, Trinh L et al. . Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob Agents Chemother 2012; 56:5938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soon GH, Shen P, Yong EL et al. . Pharmacokinetics of darunavir at 900 milligrams and ritonavir at 100 milligrams once daily when coadministered with efavirenz at 600 milligrams once daily in healthy volunteers. Antimicrob Agents Chemother 2010; 54:2775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakuda T, Sekar V, Vis P et al. . Pharmacokinetics and pharmacodynamics of darunavir and etravirine in HIV-1-Infected, treatment-experienced patients in the Gender, Race, and Clinical Experience (GRACE) Trial. AIDS Res Treat 2012; 2012:186987. [DOI] [PMC free article] [PubMed] [Google Scholar]