Abstract
Twenty-four Ethiopian communities were randomized to receive either (1) quarterly mass azithromycin distributions for trachoma for 1 year or (2) delayed treatment. Nasopharyngeal swabs collected from separate cross-sectional population-based samples of children were processed for Streptococcus pneumoniae. Mass azithromycin did not significantly alter the pneumococcal serotype distribution, and hence it would not be expected to alter vaccine coverage.
Keywords: Africa, pneumococcal vaccines, serotype, Streptococcus pneumoniae
Millions of doses of azithromycin are distributed throughout the world each year for trachoma. These distributions reduce the ocular strains of chlamydia that cause trachoma, but they may have unintended consequences. It is possible that the intense selection pressure of mass azithromycin distributions could clear commensal bacteria such as nasopharyngeal Streptococcus pneumoniae and allow different pneumococcal strains to repopulate this anatomic niche. If newly populating strains were more likely to cause invasive disease, this could limit enthusiasm for mass azithromycin distributions. To help determine the impact of mass azithromycin distributions on pneumococcal disease, we performed population-based nasopharyngeal pneumococcal serotyping before and after mass azithromycin for trachoma [1].
METHODS
Study Design
This is an ancillary analysis of a cluster-randomized trial designed to compare treatment strategies for trachoma (clinicaltrials.gov NCT00322972). In the trial, 24 Ethiopian communities were randomized to receive either quarterly mass azithromycin distributions of children aged 1–10 years or delayed treatment [2]. We previously reported that nasopharyngeal pneumococcal resistance was significantly higher in the azithromycin-treated communities at month 12 [1]. We subsequently serotyped these pneumococci and report the results herein. We obtained ethical approval from the University of California, San Francisco, Emory University, and the Ethiopian Science and Technology Commission.
Intervention
All children aged 1–10 years in the quarterly treatment group were offered a single directly observed dose of azithromycin, 20 mg/kg according to height-based dosing, at months 0, 3, 6, and 9. Communities in the delayed treatment group received no mass azithromycin during the study.
Monitoring
We selected a random sample of 10 children aged 0–9 years from each community for monitoring, with a new random sample at each study visit. Communities in the azithromycin-treated group were monitored in May 2006 (1–2 weeks before the first mass azithromycin treatment) and May 2007 (approximately 3 months after the fourth quarterly mass azithromycin treatment). Communities in the delayed treatment group were monitored in May 2007. Both study visits occurred before the 2011 rollout of the 10-valent pneumococcal conjugate vaccine (PCV-10) in Ethiopia. We collected a nasopharyngeal swab through the right naris of each child and stored the swab in skim milk, tryptone, glycerol, glucose media. We stored swabs on ice for <8 hours while in the field, then in a −20°C freezer for several weeks, then at −4°C for <96 hours while being transported to San Francisco, and finally at −80°C until processing.
Microbiological Testing
We isolated and confirmed S pneumoniae using streptococcus-selective media and optochin and bile solubility testing (Remel); we subcultured separately any colonies that had a different morphologic appearance. Pneumococcal isolates were tested for resistance to azithromycin, tetracycline, and penicillin using Etest (bioMérieux - AB Biodisk). We determined the serotype of pneumococcal isolates with a sequential multiplex polymerase chain reaction assay and the Quellung reaction [3, 4].
Statistics
We assessed whether the pretreatment distribution of serotypes was different from the posttreatment distribution by calculating Jolley's classification index, in which a value of zero indicates identical serotype frequencies pre- and posttreatment and a value of 1 indicates no overlap [5]. We assessed whether the classification index was significantly different from zero through permutation, stratified by study community. We assessed for differences in the proportion of isolates covered by each vaccine with a mixed-effects logistic regression model with village and individual as nested random effects. The number of communities included in the study was based on the underlying trachoma trial and the number of swabs per community on the primary pneumococcal outcome [1, 2]; the present study had 80% power to detect a 25% difference in the proportion of vaccine-type serotypes between the antibiotic and control group assuming 80% pneumococcal carriage, intraclass correlation coefficient of 0.05, type-I error rate of 0.05, and 30% proportion of vaccine-type serotypes before treatment [1]. All analyses were performed with Stata 10.1 (Statacorp, College Station, TX).
RESULTS
Pretreatment
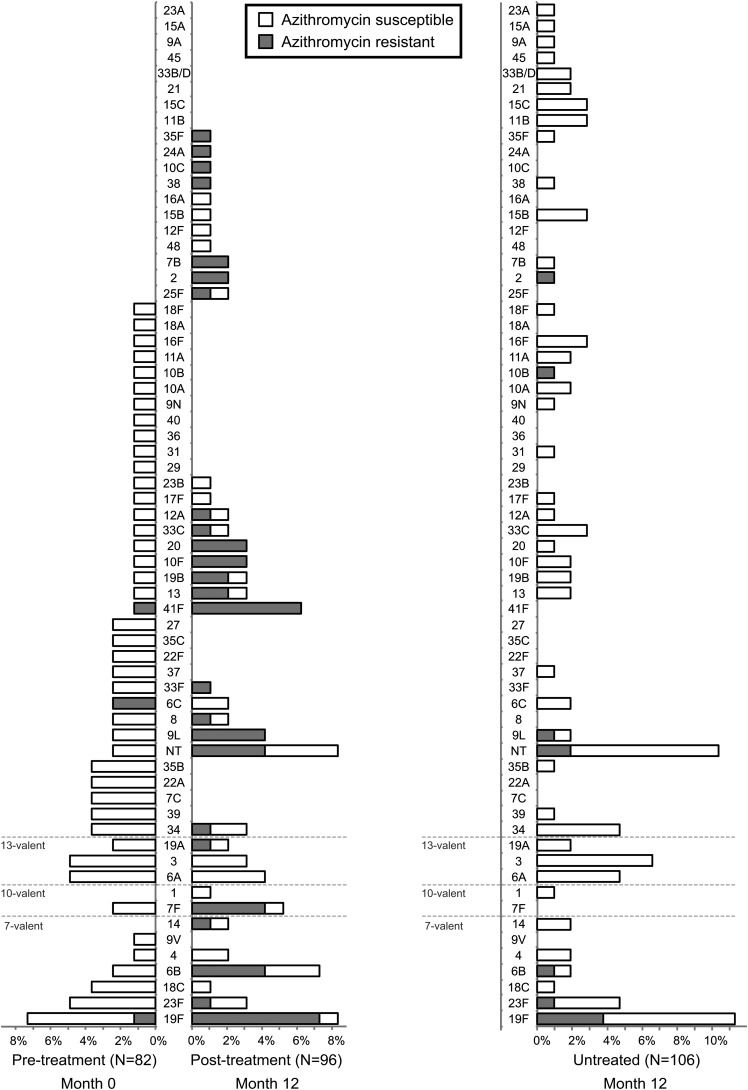
Pretreatment swabs from 1 community were lost. In the remaining 11 communities, 110 children aged 0-9 years were swabbed before mass azithromycin treatments, and S pneumoniae was isolated from 76 (pneumococcal carriage, 69.1%; 95% confidence interval [CI], 59.6%–77.6%). Multiple pneumococcal isolates were isolated from 5 children, for a total of 82 isolates. From these 82 isolates, we identified 43 unique serotypes (Figure 1). The 10 most prevalent serotypes accounted for 43.9% of isolates. The PCV-10 would have provided serotype coverage for 19 (23.2%; 95% CI, 13.9%–32.4%) isolates, whereas the 13-valent vaccine (PCV-13) would have covered 29 (35.4%; 95% CI, 25.1%–45.6%).
Figure 1.
Nasopharyngeal pneumococcal serotypes before and after mass azithromycin for trachoma. Separate random samples of children aged 0–9 years were investigated in 12 Ethiopian communities before mass azithromycin distributions (month 0; left panel) and 3 months after a fourth quarterly mass azithromycin distribution (month 12; middle panel). An analogous sample was monitored in 12 neighboring untreated communities at the 12-month visit only (right panel). Bars indicate the proportion of serotypes detected from the respective study visit: white bars represent azithromycin-susceptible isolates, and gray bars represent azithromycin-resistant isolates. Vaccine-type serotypes are grouped at the bottom of the figure.
Posttreatment
Antibiotic coverage among children aged 1–10 years exceeded 75% in each of the 12 communities at each of the 4 quarterly distributions [1, 2]. We swabbed 119 children aged 0–9 years from these 12 communities after the fourth round of mass azithromycin, and we isolated 96 total pneumococcal isolates from 93 swabs (carriage 78.2%; 95% CI, 65.2%–87.2%). From these 96 isolates, we identified 36 unique serotypes, including 13 serotypes not detected in the pretreatment isolates. The serotype distribution from the 11 communities who also had pretreatment swabs is shown in Figure 1. In these 11 communities, the distribution of serotypes after the mass azithromycin distributions was not significantly different from the distribution beforehand (classification index 0.385; P = .06). In the 11 communities with nonmissing data, PCV-10 would have covered 28 (31.1%; 95% CI, 21.8%–40.5%) isolates after mass azithromycin distributions, and PCV-13 would have increased this number to 37 (41.1%; 95% CI, 29.9%–49.3%), neither of which were different from the pretreatment estimates (P = .24 and P = .41, respectively).
Untreated Control Communities
We collected 120 nasopharyngeal swabs from children aged 0–9 years in 12 untreated communities at the 12-month visit and isolated 106 pneumococcal isolates from 98 swabs (carriage 81.7%; 95% CI, 73.6%–88.1%). We identified 43 different serotypes (Figure 1). The distribution of serotypes in the untreated communities was not significantly different from that of the children-treated communities at month 12 (classification index 0.302; permutation P = 1.0). The PCV-10 would have covered 25 isolates (23.6%; 95% CI, 15.6%–31.6%), and PCV-13 would have covered 39 (36.8%; 95% CI, 28.0%–45.4%).
Resistance
Of the 96 total pneumococcal isolates recovered in the quarterly treatment arm after mass azithromycin treatments, we detected azithromycin resistance in 56 (58.3%), tetracycline resistance in 34 (35.4%), and penicillin resistance in none. Resistant isolates had a significantly different distribution of serotypes compared with susceptible isolates: the classification index for azithromycin and tetracycline were 0.528 and 0.592, respectively (P = .02 for each; see Figure, Supplemental Digital Content 1). The proportion of the 56 azithromycin-resistant isolates covered by the vaccines was not significantly different from the proportion of the 40 azithromycin-susceptible isolates covered: 30.4% (95% CI, 17.5%–43.2%) versus 30.0% (95% CI, 16.1%–43.9%) for PCV-10 (P = .93) and 32.1% (95% CI, 19.9%–44.4%) versus 50.0% (95% CI, 35.6%–64.4%) for PCV-13 (P = .34). In contrast, a higher proportion of the 34 tetracycline-resistant isolates were covered by each vaccine than were the 62 tetracycline-susceptible isolates: 52.9% (95% CI, 34.8%–71.0%) versus 17.7% (95% CI, 8.1%–27.4%) for PCV-10 (P < .001) and 52.9% (95% CI, 35.6%–70.3%) versus 32.3% (95% CI, 19.4%–45.0%) for PCV-13 (P = .05).
DISCUSSION
We observed a highly diverse set of serotypes in this Ethiopian population, with a total of 64 different pneumococcal serotypes. Besides serotype 19F, no serotype accounted for more than 5% of isolates, and the top 10 serotypes accounted for less than half of all isolates. The most common serotypes were similar to those reported in other studies of sub-Saharan African populations (eg, 3, 6A, 6B, 19F, 23F), and, similarly to these other African studies, the vaccine-type serotypes made up a low proportion of the total isolates [6, 7].
The impact of low vaccine-type serotype coverage for nasopharyngeal pneumococci in this population is unclear. On one hand, nasopharyngeal pneumococcal carriage is important because it is thought to be a necessary step for invasive pneumococcal infection. The highly diverse population of nasopharyngeal pneumococci found in this study may (1) signify a more diverse set of serotypes causing invasive disease in Ethiopia and (2) support the effort to include a more comprehensive serotype panel in pneumococcal conjugate vaccines. On the other hand, the distribution of nasopharyngeal serotypes is not reflective of the serotypes that cause invasive disease [8]. In Africa, serotypes 14, 1, 5, 6A, 6B, 23F, and 19F are the most common causes of invasive pneumococcal disease; each of these is covered by PCV-13, and only 6B is not covered by PCV-10 [9, 10].
Multiple mass azithromycin treatments for trachoma did not significantly change the distribution of serotypes in this study, although it is important to note that the study was only powered to detect a major shift in the overall distribution. We reached the same conclusion in 2 analyses: first, a comparison of the same communities before and after antibiotics and second, a comparison of the treated communities with a group of untreated communities. In a separate analysis, the proportion of vaccine-type serotypes did not change significantly after the azithromycin distributions. This result is consistent with a study from the Gambia that found that 2 villages treated with a single mass azithromycin distribution had a similar proportion of PCV-7 vaccine-type serotypes as 6 villages treated with 3 mass distributions [11]. It is encouraging that we did not find a significant change in the distribution of vaccine-type serotypes before and after the mass antibiotic treatment, and it is also encouraging that the serotypes associated with invasive pneumococcal disease did not become more prevalent after the mass treatment. These results suggest that mass azithromycin distributions are unlikely to greatly alter pneumococcal vaccine coverage.
The distribution of azithromycin- and tetracycline-resistant serotypes was different from that of susceptible serotypes, indicating that certain serotypes were more likely to have antibiotic resistance. Moreover, vaccine-type serotypes were more common in tetracycline-resistant compared with tetracycline-susceptible isolates. This result suggests that, just as has been demonstrated in other populations, the pneumococcal conjugate vaccine could reduce the prevalence of pneumococcal antibiotic resistance by preventing resistance-prone serotypes from colonizing the nasopharynx [12].
We acknowledge limitations to this study. First, we monitored only a limited number of children in each community, and we chose a different random sample at each monitoring visit. Although a complete sample would have been ideal, this study provided an unbiased estimate of serotype diversity in each community at a fraction of the cost. Second, if the storage and transport of the swabs selectively reduced the yield of certain serotypes, this would have affected the resulting distribution. Although it is a legitimate concern, any reduction in pneumococcal yield should have affected all communities in the same way and therefore should not have biased the study.
CONCUSIONS
In summary, we found that rural Ethiopian children harbored a highly diverse set of nasopharyngeal pneumococcal serotypes before initiation of PCV-10. Mass azithromycin distributions did not significantly alter the serotype distribution, although this study was not powered to detect small changes. The ratio of pneumococcal vaccine-type serotypes recovered from nasopharyngeal swabs did not change significantly after treatment, suggesting that mass azithromycin distributions may not have a major impact on pneumococcal vaccination efforts. Further study is warranted now that PCV-10 has been instituted throughout Ethiopia.
Supplementary Data
Acknowledgments
Financial support. J. D. K. received grant support from the National Institutes of Health (National Eye Institute Grant K23EY019071 and National Center for Research Resources Grant KL2 RR024130), the University of California, San Francisco Research Evaluation and Allocation Committee, the Pratt Foundation, Jackson Medical Research, and Research to Prevent Blindness; T. M. L. received grants from the National Institutes of Health (National Eye Institute Grant U10 EY016214), That Man May See, and Research to Prevent Blindness, and gifts from the Bernard Osher Foundation, the Harper Inglis Trust, the Bodri Foundation, and the South Asia Research Fund. The azithromycin used for this study was donated by Pfizer International and managed by the International Trachoma Initiative.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Skalet AH, Cevallos V, Ayele B et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med 2010; 7:e1000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.House JI, Ayele B, Porco TC et al. Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomised trial. Lancet 2009; 373:1111–8. [DOI] [PubMed] [Google Scholar]
- 3.Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol 2006; 44:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. PCR deduction of pneumococcal serotypes. Available at: http://www.cdc.gov/ncidod/biotech/strep/pcr.htm Accessed 28 July 2013.
- 5.Jolley KA, Wilson DJ, Kriz P et al. The influence of mutation, recombination, population history, and selection on patterns of genetic diversity in Neisseria meningitidis. Mol Biol Evol 2005; 22:562–9. [DOI] [PubMed] [Google Scholar]
- 6.Adetifa IM, Antonio M, Okoromah CA et al. Pre-vaccination nasopharyngeal pneumococcal carriage in a Nigerian population: epidemiology and population biology. PLoS One 2012; 7:e30548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdullahi O, Karani A, Tigoi CC et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One 2012; 7:e30787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller JE, Yaro S, Ouedraogo MS et al. Pneumococci in the African meningitis belt: meningitis incidence and carriage prevalence in children and adults. PLoS One 2012; 7:e52464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhe L, Klugman KP. Pneumococcal and Haemophilus influenzae meningitis in a children's hospital in Ethiopia: serotypes and susceptibility patterns. Trop Med Int Health 1999; 4:421–7. [DOI] [PubMed] [Google Scholar]
- 10.Johnson HL, Deloria-Knoll M, Levine OS et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med 2010; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burr SE, Milne S, Jafali J et al. Mass administration of azithromycin and Streptococcus pneumoniae carriage: cross-sectional surveys in the Gambia. Bull World Health Organ 2014; 92:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyaw MH, Lynfield R, Schaffner W et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006; 354:1455–63. [DOI] [PubMed] [Google Scholar]