Abstract
Key points
Concomitant administration of bivalent rLP2086 (Trumenba [Pfizer, Inc] and diphtheria, tetanus, and acellular pertussis and inactivated poliovirus vaccine (DTaP/IPV) was immunologically noninferior to DTaP/IPV and saline and was safe and well tolerated. Bivalent rLP2086 elicited robust and broad bactericidal antibody responses to diverse Neisseria meningitidis serogroup B strains expressing antigens heterologous to vaccine antigens after 2 and 3 vaccinations.
Background
Bivalent rLP2086, a Neisseria meningitidis serogroup B (MnB) vaccine (Trumenba [Pfizer, Inc]) recently approved in the United States to prevent invasive MnB disease in individuals aged 10–25 years, contains recombinant subfamily A and B factor H binding proteins (fHBPs). This study evaluated the coadministration of Repevax (diphtheria, tetanus, and acellular pertussis and inactivated poliovirus vaccine [DTaP/IPV]) (Sanofi Pasteur MSD, Ltd) and bivalent rLP2086.
Methods
Healthy adolescents aged ≥11 to <19 years received bivalent rLP2086 + DTaP/IPV or saline + DTaP/IPV at month 0 and bivalent rLP2086 or saline at months 2 and 6. The primary end point was the proportion of participants in whom prespecified levels of antibodies to DTaP/IPV were achieved 1 month after DTaP/IPV administration. Immune responses to bivalent rLP2086 were measured with serum bactericidal assays using human complement (hSBAs) against 4 MnB test strains expressing fHBP subfamily A or B proteins different from the vaccine antigens.
Results
Participants were randomly assigned to receive bivalent rLP2086 + DTaP/IPV (n = 373) or saline + DTaP/IPV (n = 376). Immune responses to DTaP/IPV in participants who received bivalent rLP2086 + DTaP/IPV were noninferior to those in participants who received saline + DTaP/IPV.
The proportions of bivalent rLP2086 + DTaP/IPV recipients with prespecified seroprotective hSBA titers to the 4 MnB test strains were 55.5%–97.3% after vaccination 2 and 81.5%–100% after vaccination 3. The administration of bivalent rLP2086 was well tolerated and resulted in few serious adverse events.
Conclusions
Immune responses to DTaP/IPV administered with bivalent rLP2086 to adolescents were noninferior to DTaP/IPV administered alone. Bivalent rLP2086 was well tolerated and elicited substantial and broad bactericidal responses to diverse MnB strains in a high proportion of recipients after 2 vaccinations, and these responses were further enhanced after 3 vaccinations.
ClinicalTrials.gov identifier NCT01323270
Keywords: adolescents; diphtheria, tetanus, and acellular pertussis/inactivated poliovirus vaccine (DTaP/IPV), meningitis, rLP2086, vaccine
BACKGROUND
Neisseria meningitidis is a leading cause of bacterial meningitis and sepsis in infants, adolescents, and young adults, with mortality rates after invasive meningococcal disease of approximately 10% [1, 2]. N meningitidis serogroup B (MnB), the predominant serogroup associated with meningococcal disease in Europe [3–6], was associated with 20%–49% of meningococcal disease cases in the United States from 2003 through 2012 [7]. The incidence of MnB disease is highest in children <5 years of age, with a second peak in adolescents and young adults [3, 8].
LP2086, a human factor H binding protein (fHBP), is a conserved surface-exposed bacterial lipoprotein that has been identified as an MnB vaccine target [9–11]. Studies of >1800 invasive MnB disease isolates from reference laboratories in Europe and the United States demonstrated that all strains contained fhbp [9, 12], and fHBP was expressed by nearly all strains [13]. LP2086 protein sequences segregate into 1 of 2 immunologically distinct groups (subfamily A or B). Approximately 70% of invasive disease isolates express subfamily B LP2086 variants, and 30% of isolates express subfamily A LP2086 variants [9, 12, 14]. Bivalent rLP2086 (Trumenba [Pfizer, Inc]), a vaccine that contains equal amounts of recombinant fHBPs from subfamilies A (A05) and B (B01), was approved recently in the United States for the prevention of invasive meningococcal disease caused by MnB in individuals aged 10–25 years [15]. Previous studies have shown that bivalent rLP2086 elicits serum bactericidal antibodies capable of killing MnB strains that express vaccine-homologous and -heterologous fHBPs [16, 17].
Data demonstrating that bivalent rLP2086 can be administered concomitantly to adolescents with other licensed vaccines, including the meningococcal conjugate, human papillomavirus, or diphtheria, tetanus, and acellular pertussis and inactivated poliovirus (DTaP/IPV; Repevax [Sanofi Pasteur MSD, Ltd]) vaccines, are required [18]. The primary objective of this study was to demonstrate that the immune response induced by DTaP/IPV given with bivalent rLP2086 is noninferior to the immune response induced by DTaP/IPV alone when measured 1 month after vaccination. Additional objectives were to describe the serum bactericidal antibody responses elicited by bivalent rLP2086 against 4 MnB test strains expressing either fHBP subfamily A or B proteins different from the vaccine antigens 1 month after the second and third vaccinations with bivalent rLP2086. The safety profile is also described for participants who received DTaP/IPV and bivalent rLP2086 concomitantly and for those who received DTaP/IPV alone.
METHODS
Study Design
This was a phase II, randomized, placebo-controlled, single-blind study conducted at 34 sites in Finland, Germany, and Poland. The final protocol, any amendments, and informed-consent documents were reviewed and approved by independent ethics committees at the investigational centers (Ethics Committee of the Pirkanmaa Hospital District, Tampere, Finland; Arztekammer Schleswig-Holstein Ethics Committee, Bad Segeberg, Baden-Wurttemberg Landesarztekammer Ethics Commission, Stuttgart, Landesarztekammer Hesse Ethics Committee, Frankfurt, and Landesarztekammer Niedersachsen Ethics Committee, Hannover, and Landesaerztekammer Rhineland-Pfalz Ethics Committee, Mainz, and Bioethics Committee of the Medical University, Wroclaw, Poland). The study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Conference on Harmonisation Good Clinical Practice guidelines. All local regulatory requirements were followed. Written informed consent was obtained from each participant or the parent(s)/legal guardian(s) of each participant before enrollment and before performance of any study-related procedures. The participants were randomly assigned using an interactive voice-response system, interactive Web-based response system, or an equivalent system that was accessible at all times.
Study Participants
Included in the study were healthy adolescents ≥11 to <19 years of age at enrollment who had received the full series of DTaP/IPV vaccines per the country-specific recommendations applicable at the time of vaccination. Key exclusion criteria included previous vaccination with any MnB vaccine, vaccination with any diphtheria, tetanus, pertussis, or poliomyelitis virus vaccine within 5 years of the first study vaccination, previous vaccine-related anaphylactic reaction, contraindication to diphtheria, tetanus, pertussis, or poliomyelitis virus vaccine, bleeding disorders, known or suspected disease of the immune system or receipt of immunosuppressive therapy, any significant neurologic disorder, any neuroinflammatory or autoimmune condition, history of culture-proven disease caused by N meningitidis or Neisseria gonorrhoeae, current chronic systemic antibiotic use, or current pregnancy or breastfeeding.
Interventions
Participants in the bivalent rLP2086 + DTaP/IPV group received 1 intramuscular dose of bivalent rLP2086 (0.5 mL) in the left arm and 1 intramuscular dose of DTaP/IPV (0.5 mL) in the right arm at month 0 and 1 dose of bivalent rLP2086 at months 2 and 6. Participants in the saline +DTaP/IPV group received 1 intramuscular dose of saline (0.5 mL) in the left arm and 1 intramuscular dose of DTaP/IPV (0.5 mL) in the right arm at month 0, and 1 dose of saline at months 2 and 6. The study protocol required blood samples to be obtained before vaccination 1, 1 month (28–41 days) after vaccination 1 to assess immune responses to the DTaP/IPV antigens, and 1 month after vaccinations 2 and 3 (28–42 days) to assess bactericidal antibody responses elicited by bivalent rLP2086.
Vaccines
Each 0.5-mL dose of bivalent rLP2086 contained 60 μg each of a purified subfamily A and a purified subfamily B rLP2086 protein, a 2.8 molar ratio of polysorbate 80 to protein, and 0.25 mg of Al3+ as aluminum phosphate (AlPO4) in 10 mM histidine-buffered saline at pH 6.0. A 0.5-mL dose of DTaP/IPV contained diphtheria toxoid (not less than 2 IU [2 limits of flocculation (Lf)]), tetanus toxoid (not less than 20 IU [5 Lf]), pertussis antigens (pertussis toxoid [2.5 μg], filamentous hemagglutinin [5 μg], pertactin [3 μg], and fimbriae types 2 and 3 [5 μg]), poliovirus (inactivated) type 1 (40 D antigen units), poliovirus (inactivated) type 2 (8 D antigen units), and poliovirus (inactivated) type 3 (32 D antigen units), adsorbed onto aluminum phosphate (1.5 mg [0.33 mg of aluminum]) [19].
Immunogenicity End Points
The primary immunogenicity end point was the proportion of participants in whom prespecified levels of antibodies to DTaP/IPV antigens were achieved 1 month after the DTaP/IPV dose in both groups. These levels were the same or higher than those used in the pivotal phase III trials in adolescents, which formed the basis for Repevax licensure [20]. Immune responses to the diphtheria, tetanus, and pertussis components of DTaP/IPV were assessed by using a multiplexed Luminex assay. Immune responses to poliovirus types 1, 2, and 3 were measured by using poliovirus neutralization assays. Additional descriptive end points for the primary objective were antibody levels to DTaP/IPV antigens measured as geometric mean titers (GMTs) or geometric mean concentrations 1 month after vaccination 1. End points for assessing immune responses to bivalent rLP2086 included the GMT and the proportion of participants in whom prespecified serum bactericidal antibody titers were achieved, according to the results of serum bactericidal assays using human complement (hSBAs) 1 month after bivalent rLP2086 vaccination [13]. The hSBA was based on the assay described by the World Health Organization [21] and Borrow and Carlone [22] and was reported previously [11]. The hSBA titer is the reciprocal of the highest 2-fold dilution of a test serum that results in at least a 50% reduction in MnB bacteria (50% bacterial survival) compared to the T30 colony-forming-unit value (ie, the number of bacteria surviving after incubation in assay wells containing all assay components except test serum; 100% bacterial survival). Titers were reported as step titers (ie, 1:4, 1:8, 1:16, etc). The hSBA responses were defined as the proportion of participants with an hSBA titer greater than or equal to the lower limit of quantitation of the assay (ie, an hSBA titer of ≥1:8 for MnB test strains expressing fHBP subfamily A [A56] or B [B24 or B44] variants or an hSBA titer of ≥1:16 for the other fHBP subfamily A test strain [A22]). Serum samples collected before vaccination 1 and after vaccinations 2 and 3 were tested in hSBA; sera from approximately 50% of the randomly selected participants were evaluated in hSBAs performed with MnB test strains PMB80 [A22] and PMB2948 [B24], and the other 50% were tested in hSBAs with PMB2001 [A56] and PMB2701 [B44].
Safety Assessments
Information on local reactions (redness, swelling, and pain) at the site of bivalent rLP2086 + saline injections, systemic events, and the use of antipyretic medications was collected for 7 days after each vaccination by using an e-diary. Local reactions at the site of DTaP/IPV injection have been reported previously [23] and were not monitored by e-diary in this study. Data on unsolicited adverse events (AEs) were collected from enrollment through 1 month after the last vaccination, and data on serious AEs (SAEs) were collected throughout the study. All AEs were assessed for severity, relationship to the study drug, and seriousness. Safety was evaluated for participants who received at least 1 dose of the investigational product.
Statistical Analyses
The study sample size was based on a noninferiority test for all DTaP/IPV antigens. The noninferiority criterion was −0.1 (or −10%); the rate of response to each antigen in the bivalent rLP2086 + DTaP/IPV group was assumed to be 2% less than the rate of response in the saline + DTaP/IPV group. Assuming the response for each antigen was independent, the overall power for declaring noninferiority in this study, using an exact method, was approximately 93% with 300 evaluable participants per group (type I error rate of 2.5% [1-sided test of noninferiority]). A total of 750 participants were enrolled in the study by using a 1:1 randomization ratio and assuming a 20% dropout rate.
The proportion of participants in whom the prespecified antibody-level criteria for DTaP/IPV antigens by 1 month after vaccination 1 were achieved was computed along with the difference in proportions (bivalent rLP2086 + DTaP/IPV group – saline + DTaP/IPV group) and a 2-sided 95% exact confidence interval (CI) for the difference [24]. If the lower bound of the 95% CI on the difference is greater than −10% for all 9 antigens in the DTaP/IPV, then the study achieved the noninferiority objective. The proportions of participants with an hSBA titer of ≥1:8 (A56, B24, B44) or ≥1:16 (A22) were summarized with exact 2-sided 95% CIs (or Clopper–Pearson confidence limits). Local and systemic reactions are presented descriptively and show the proportions of participants and severity of the reactions. Summaries of AEs are presented descriptively.
RESULTS
Participant Disposition and Demographic Characteristics
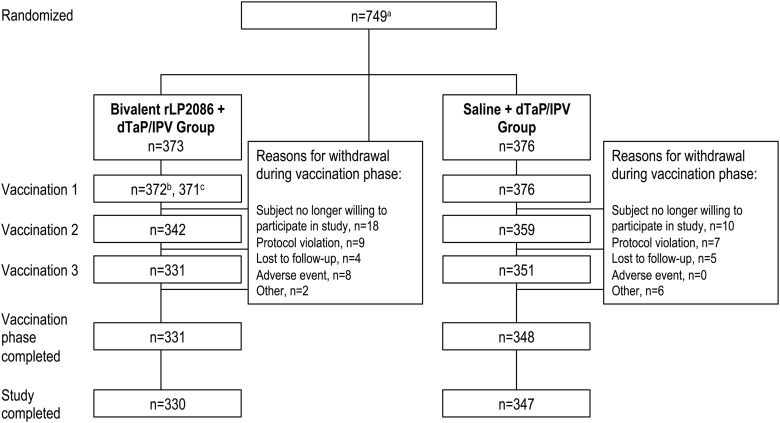
A total of 753 participants were enrolled, and 749 were randomly assigned (Figure 1). A total of 752 participants received at least 1 dose of the study vaccine, 685 of whom were evaluated for concomitant vaccine immunogenicity. Sera from approximately 50% of the participants in each vaccination group were tested in hSBAs for strains A22 and B24, and sera from the remaining 50% were tested for strains A56 and B44. The mean age at enrollment was 13.9 years (Table 1). During the vaccination phase (from randomization to 1 month after the last vaccination), 69 participants (41 [11.0%] in the bivalent rLP2086 + DTaP/IPV group, 28 [7.4%] in the saline + DTaP/IPV group) were withdrawn. A total of 677 participants (330 [88.5%] in the bivalent rLP2086 + DTaP/IPV group, 347 [92.3%] in the saline + DTaP/IPV group) completed the study (vaccination phase and 6-month follow-up contact).
Figure 1.
Study design. a A total of 753 participants were enrolled, but only 749 participants were randomly assigned; 4 participants were not randomly assigned but received the first vaccination because of a site error (2 received bivalent rLP2086 + DTaP/IPV, and 2 received saline + DTaP/IPV)b; total vaccinatedc; vaccinated with bivalent rLP2086 + DTaP/IPV (1 participant received only bivalent rLP2086). Abbreviation: DTaP/IPV, diphtheria, tetanus, and acellular pertussis and inactivated poliovirus vaccine.
Table 1.
Participant Demographics
| Demographic | Bivalent rLP2086 + DTaP/IPV (n = 374) | Saline + DTaP/IPV (n = 378) |
|---|---|---|
| Sex, male (n [%]) | 191 (51.1) | 193 (51.1) |
| Race, white (n [%]) | 370 (98.9) | 374 (98.9) |
| Age at first vaccination | ||
| ≥11 to <14 y (n [%]) | 218 (58.3) | 217 (57.4) |
| ≥14 to <19 y (n [%]) | 156 (41.7) | 161 (42.6) |
| Mean (SD) (y) | 13.8 (2.54) | 13.9 (2.60) |
| Median (y) | 13.0 | 13.0 |
| Range (y) | 11–18 | 11–18 |
Abbreviation: DTaP/IPV, diphtheria, tetanus, and acellular pertussis and inactivated poliovirus vaccine.
Immunogenicity
Response to DTaP/IPV Antigens
The primary objective of the study, to demonstrate the noninferiority of immune responses to DTaP/IPV coadministered with bivalent rLP2086 to those with DTaP/IPV administered alone, was achieved. The lower bound of the 2-sided 95% CI for the difference in the proportion of responders between the bivalent rLP2086 + DTaP/IPV and the saline + DTaP/IPV groups 1 month after the DTaP/IPV dose was greater than –0.10 (–10%) for the 9 DTaP/IPV antigens (Table 2). Levels of antibodies to the DTaP/IPV antigens, measured as geometric means, were similar between the vaccine groups for each DTaP/IPV antigen (see Supplementary Table 1).
Table 2.
Participants Who Achieved a Prespecified Antibody Level for Concomitant Vaccine Antigens 1 Month After Vaccination 1
| Concomitant Vaccine Antigen | DTaP/IPV Antigen Criteria for Antibody Levels | Bivalent rLP2086 + DTaP/IPV (n = 337)a (% [95% CI])b |
Saline + DTaP/IPV (n = 348)a (% [95% CI])b |
Differencec (% [95% CI])d |
|---|---|---|---|---|
| Diphtheria | ≥0.1 IU/mL | 99.4 (97.9 to 99.9) | 99.4 (97.9 to 99.9) | 0.0 (−1.6 to 1.5) |
| Tetanus | ≥0.1 IU/mL | 100.0 (98.9 to 100.0) | 100.0 (98.9 to 100.0) | 0.0 (−1.1 to 1.1) |
| Pertussis toxoid | ≥5 EU/mL | 94.7 (91.7 to 96.8) | 96.0 (93.3 to 97.8) | −1.3 (−4.7 to 1.9) |
| Pertussis FHA | ≥5 EU/mL | 100.0 (98.9 to 100.0) | 100.0 (98.9 to 100.0) | 0.0 (−1.1 to 1.1) |
| Pertussis PRN | ≥5 EU/mL | 100.0 (98.9 to 100.0) | 100.0 (98.9 to 100.0) | 0.0 (−1.1 to 1.1) |
| Pertussis FIM types 2 and 3 | ≥10.6 EU/mLe | 97.6 (95.4 to 99.0) | 98.9 (97.1 to 99.7) | −1.2 (−3.6 to 0.8) |
| Poliovirus type 1 | ≥1:8 titer | 100.0 (98.9 to 100.0) | 100.0 (98.9 to 100.0) | 0.0 (−1.1 to 1.1) |
| Poliovirus type 2 | ≥1:8 titer | 100.0 (98.9 to 100.0) | 100.0 (98.9 to 100.0) | 0.0 (−1.1 to 1.1) |
| Poliovirus type 3 | ≥1:8 titer | 100.0 (98.9 to 100.0) | 100.0 (98.9 to 100.0) | 0.0 (−1.1 to 1.1) |
Abbreviations: CI, confidence interval; DTaP/IPV, diphtheria, tetanus, and acellular pertussis and inactivated poliovirus vaccine; FHA, filamentous hemagglutinin; FIM, fimbrial agglutinogen; PRN, pertactin.
an = number of participants with a valid and determinate assay result for the given antigen.
bExact 2-sided confidence interval (Clopper and Pearson) based on the observed proportion of participants.
cDifference in proportions (rLP2086 + DTaP/IPV group – saline + DTaP/IPV group), expressed as a percentage.
dCIs for the ratio are back transformations of a CI based on the Student t test distribution for the mean difference of the logarithms of the measures (rLP2086 + DTaP/IPV group – saline + DTaP/IPV group).
eLower limit of quantitation for the assay.
Response to MnB Test Strains Expressing fHBPs Different From Vaccine Antigens
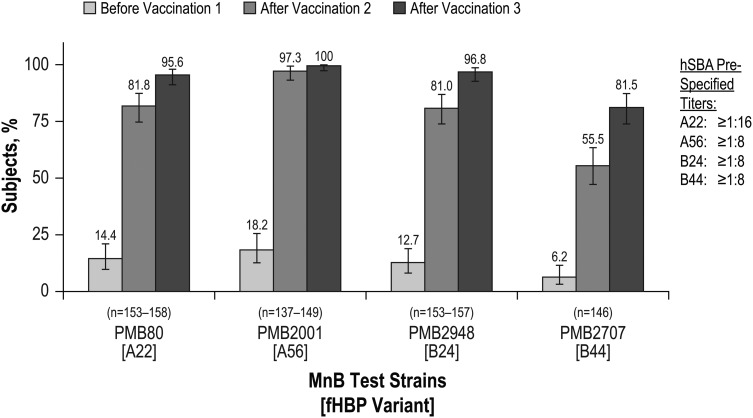
Substantial hSBA responses were observed in the bivalent rLP2086 + DTaP/IPV group 1 month after vaccination 2 that increased further after vaccination 3 (Figure 2). The proportions of participants in the bivalent rLP2086 + DTaP/IPV group in whom hSBA titers greater than or equal to the lower limit of quantitation after vaccinations 2 and 3 were achieved were >81% and >95%, respectively, for PMB80 (A22), PMB2001 (A56), and PMB2948 (B24) and 55.5% and 81.5% for PMB2707 (B44). For participants in the saline + DTaP/IPV group, the proportion of participants with an hSBA titer greater than the respective cutoffs after vaccination 3 was similar to that at baseline. hSBA GMTs for each of the 4 MnB test strains were substantially higher in the bivalent rLP2086 + DTaP/IPV group than in the saline + DTaP/IPV group after vaccination 2, and additional increases were noted after vaccination 3 (Table 3).
Figure 2.
Percentage of participants in the bivalent rLP2086 + DTaP/IPV group with a serum bactericidal antibody assay using human complement (hSBA) response based on prespecified hSBA titers before vaccination 1 and 1 month after vaccinations 2 and 3. Abbreviations: DTaP/IPV, diphtheria, tetanus, and acellular pertussis and inactivated poliovirus vaccine; fHBP, factor H binding protein; MnB, Neisseria meningitidis serogroup B.
Table 3.
hSBA Geometric Mean Titers to MnB Test Strains
| Strain (fHBP Variant) and Time Point | Bivalent rLP2086 + DTaP/IPV |
Saline + DTaP/IPV |
||
|---|---|---|---|---|
| na | hSBA GMT (95% CI)b,c | na | hSBA GMT (95% CI)b,c | |
| PMB80 (A22) | ||||
| Vaccination 2 | 154 | 35.5 (30.3–41.6) | 166 | 11.2 (10.0–12.5) |
| Vaccination 3 | 158 | 63.4 (55.3–72.8) | 166 | 11.0 (9.9–12.3) |
| PMB2001 (A56) | ||||
| Vaccination 2 | 149 | 91.1 (78.0–106.5) | 151 | 8.3 (6.8–10.3) |
| Vaccination 3 | 148 | 151.5 (131.5–174.6) | 152 | 8.5 (6.9–10.5) |
| PMB2948 (B24) | ||||
| Vaccination 2 | 153 | 15.9 (13.6–18.6) | 167 | 4.8 (4.4–5.2) |
| Vaccination 3 | 157 | 28.3 (24.5–32.7) | 170 | 4.8 (4.4–5.2) |
| PMB2707 (B44) | ||||
| Vaccination 2 | 146 | 14.6 (11.6–18.4) | 159 | 4.7 (4.2–5.1) |
| Vaccination 3 | 146 | 36.5 (28.9–46.2) | 159 | 4.7 (4.3–5.2) |
Abbreviations: CI, confidence interval; DTaP/IPV, diphtheria, tetanus, and acellular pertussis and inactivated poliovirus vaccine; GMT, geometric mean titer; hSBA, serum bactericidal assay using human complement.
an = number of participants with a valid and determinate hSBA titer result for the given strain. There were 637 participants in the bivalent rLP2086 immunogenicity population, including 307 participants in the bivalent rLP2086 + DTaP/IPV group and 330 participants in the saline + DTaP/IPV group.
bCIs are back transformations of CIs based on the Student t test distribution for the mean logarithm of the hSBA titers.
cSera from approximately 50% of the participants from each vaccination group were tested in hSBAs for strains A22 and B24, and sera from the remaining 50% of the participants were tested for strains A56 and B44.
Safety
Data on local reactions at the bivalent rLP2086 injection site were collected from participants in the bivalent rLP2086 + DTaP/IPV group and reactions at the saline injection site from participants in the saline + DTaP/IPV group, which allowed for comparison of local reactions associated with bivalent rLP2086 + saline injections (Supplementary Table 2). After vaccinations 1, 2, and 3, local reactions were reported by a higher proportion of participants who received the bivalent rLP2086 vaccine than those who received saline. Injection-site pain was the most commonly reported local reaction after each injection. Most cases of injection-site pain, swelling, and redness were mild or moderate in severity. The median durations of injection-site pain, redness, and swelling in both groups were comparable. One participant in the rLP2086 + DTaP/IPV group withdrew from the study after vaccination 1 because of moderate pain and swelling at the injection site. There was a trend toward lower rates of local reactions with each subsequent dose of the bivalent rLP2086 vaccine. Across all 3 injections, 16.4%–26.1% and 8.0%–18.3% of the participants used antipyretic medications after the bivalent rLP2086 + DTaP/IPV and saline + DTaP/IPV vaccines, respectively.
After vaccination 1, fever (temperature ≥ 38.0°C) was reported in 12.1% of participants in the bivalent rLP2086 + DTaP/IPV group and 5.3% of participants in the saline +DTaP/IPV group. No temperature of ≥40.0°C was reported after vaccination 1 or throughout the study. Headache and fatigue were the most commonly reported systemic events after any vaccination. Severe occurrences of headache were reported in 1.1% of participants in the bivalent rLP2086 + DTaP/IPV group and in 2.1% of participants in the saline + DTaP/IPV group. Severe occurrences of fatigue were reported in 4.0% of the participants in the bivalent rLP2086 + DTaP/IPV group and 2.9% of participants in the saline + DTaP/IPV group. Three participants in the bivalent rLP2086 + DTaP/IPV group and no participants in the saline + DTaP/IPV group withdrew from the study after vaccination 1 because of systemic events.
After vaccinations 2 and 3, participants in the bivalent rLP2086 + DTaP/IPV group reported a slightly higher incidence of fever (temperature ≥ 38.0°C), headache, and fatigue than participants in the saline + DTaP/IPV group. In general, the frequencies of other systemic events after vaccinations 2 and 3 were similar between the vaccination groups.
The mean duration for any fever (temperature ≥ 38.0°C) ranged from 1.2 to 1.6 days in the bivalent rLP2086 + DTaP/IPV group and 1.1 to 3.3 days in the saline +DTaP/IPV group. No significant potentiation of systemic events, defined as an increase in the severity of a systemic event after subsequent vaccination, was seen with bivalent rLP2086 vaccination.
During the vaccination phase, 37.4% of participants in the bivalent rLP2086 + DTaP/IPV group and 40.2% of participants in the saline + DTaP/IPV group reported ≥1 AE. The most frequently observed AEs were nasopharyngitis, pharyngitis, and upper respiratory tract infection. During the vaccination phase, 2.7% of participants in the bivalent rLP2086 + DTaP/IPV group and 0.5% of participants in the saline + DTaP/IPV group reported severe AEs, and related AEs were reported by 3.2% of participants in the bivalent rLP2086 + DTaP/IPV group (mostly reactogenicity events) and 1.1% of participants in the saline + DTaP/IPV group.
Throughout the study, the most common SAE was appendicitis, which was reported by 3 participants (1 in the rLP2086 + DTaP/IPV group, 2 in the saline + DTaP/IPV group). Each of the other SAEs was reported by 1 participant in either group. No participant reported an SAE related to the investigational product. One participant in the bivalent rLP2086 + DTaP/IPV group died as a result of a motor vehicle crash.
DISCUSSION
Meningococcal B infection and disease remain major health concerns in many parts of the world, and successful implementation of a safe and effective vaccine would lead to substantial reductions in the occurrence of this devastating disease. Concurrent administration of approved vaccines to adolescents could increase adherence to the recommended vaccination schedules in this population. Data from this study support the concomitant administration to adolescents of the investigational vaccine, bivalent rLP2086 + DTaP/IPV. The primary objective of this study was met. Immune responses to DTaP/IPV antigens induced after concurrent DTaP/IPV and bivalent rLP2086 vaccination were noninferior to the immune response induced by DTaP/IPV alone.
In this study, 2 doses of bivalent rLP2086 generated substantial and broadly protective serum bactericidal antibody responses, as measured in hSBAs against 4 diverse MnB test strains. Three doses of the vaccine elicited the most robust hSBA response (ie, responder rates). The MnB test strains used in this study express fHBP variants that are different (heterologous) from the protein variants present in the vaccine and represent the epidemiologically relevant diversity of fHBP variants identified in disease-causing isolates. On the basis of fHBP subgroup, these 4 MnB test strains are representative of >90% of the invasive MnB disease-causing isolates in the United States and Europe combined [17].
Although an hSBA titer of ≥1:4 is the recognized immunologic correlate of protection from meningococcal disease [25, 26], the hSBA titers used in this study to measure a protective level were ≥1:8 for 3 of the 4 MnB test strains and ≥1:16 for the fourth and thus assessed protective bivalent rLP2086 immune responses according to a more stringent measure. More than 80% of the participants had an hSBA titer that exceeded these levels for 3 of the test strains after the second vaccination and exceeded these levels for all 4 of the test strains after the third vaccination (Figure 2). In addition, GMTs for all the test strains were substantially greater than 1:4 after bivalent rLP2086 vaccinations 2 and 3. Thus, as shown in this study, both 2 and 3 vaccinations with bivalent rLP2086 elicited broadly protective immune responses against MnB in a high proportion of the participants according to a measure well above the correlate of protection. In contrast, baseline responses in adolescent participants were generally low, which is expected for a population vulnerable to MnB infection and disease. The results of this study are consistent with the results of a phase II dosing study of bivalent rLP2086 in which bivalent rLP2086 elicited a substantial and broad immune response after 2 doses and a more robust response after 3 doses across a range of dosing schedules (our unpublished data). Safety data suggest that the bivalent rLP2086 vaccine was generally safe and well tolerated when given in 3 doses and when given concomitantly with DTaP/IPV. These safety and noninferiority data suggest that the administration of bivalent rLP2086 + DTaP/IPV can be completed during the same office or clinic visit. This result is important because the simultaneous administration of multiple vaccines is not only more convenient but also increases the probability that recommended immunization schedules will be followed [27]. Additional studies of the bivalent rLP2086 vaccine administered concurrently with other vaccines commonly used in adolescent and young adult populations are ongoing.
CONCLUSIONS
Immune responses to DTaP/IPV administered with bivalent rLP2086 to adolescents were noninferior to DTaP/IPV administered alone. Bivalent rLP2086 was well tolerated and elicited substantial and broad bactericidal responses to diverse MnB strains in a high proportion of recipients after 2 vaccinations, and these responses were further enhanced after 3 vaccinations.
Acknowledgments
Writing support was provided by Daniel E. McCallus, PhD, of Complete Healthcare Communications, Inc. We thank Javier Diez-Domingo, MD, of Centro Superior de Investigación en Salud Pública, Valencia, Spain, and Annaliesa S. Anderson of Pfizer, Inc, for their critical review of the manuscript.
Financial support. This work was supported by Pfizer Inc.
Potential conflicts of interest. J. W. was the principal investigator of clinical trials sponsored by GlaxoSmithKline, Novartis, Wyeth, and Pfizer, received travel grants from these companies to participate in scientific conferences, and was paid for lectures. T. V. received consulting fees and support for meetings and travel or accommodation expenses from GlaxoSmithKline and is a consultant and speaker for Merck, Sanofi Pasteur-Merck Sharp & Dohme (SPMSD), MedImmune, Novartis, and Pfizer. J. B., J. E., Q. J., K. U. J., T. R. J., S. L. H., R. E. O., L. J. Y., and J. L. P. are employees of Pfizer Inc.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 2007; 369:2196–210. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics, Committee on Infectious Diseases. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 2005; 116:496–505. [DOI] [PubMed] [Google Scholar]
- 3.European Union Invasive Bacterial Infections Surveillance Network. Invasive Neisseria meningitidis in Europe 2006. Available at: http://www.hpa-bioinformatics.org.uk/euibis/documents/2006_meningo.pdf. Accessed April 30, 2014.
- 4.Public Health England. Invasive meningococcal infections laboratory reports, England and Wales by capsular group & epidemiological year, 1998/99–2012/13* (*provisional). Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/401058/Table_1_Invasive_meningococcal_infections_lab_reports__E_W_by_capsular_group___epi_year.pdf. Accessed June 9, 2014.
- 5.Skoczynska A, Wasko I, Kuch A et al. A decade of invasive meningococcal disease surveillance in Poland. PLoS One 2013; 8:e71943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe 2008/09. Available at: http://www.ecdc.europa.eu/en/publications/Publications/1107_SUR_IBD_2008-09.pdf. Accessed June 9, 2014.
- 7.Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs). Available at: http://www.cdc.gov/abcs/reports-findings/surv-reports.html. Accessed August 4, 2014.
- 8.Cohn AC, MacNeil JR, Harrison LH et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010; 50:184–91. [DOI] [PubMed] [Google Scholar]
- 9.Hoiseth SK, Murphy E, Andrew L et al. A multi-country evaluation of Neisseria meningitidis serogroup B factor H-binding proteins and implications for vaccine coverage in different age groups. Pediatr Infect Dis J 2013; 32:1096–101. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher LD, Bernfield L, Barniak V et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 2004; 72:2088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richmond PC, Marshall HS, Nissen MD et al. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012; 12:597–607. [DOI] [PubMed] [Google Scholar]
- 12.Murphy E, Andrew L, Lee KL et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis 2009; 200:379–89. [DOI] [PubMed] [Google Scholar]
- 13.McNeil LK, Murphy E, Zhao XJ et al. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine 2009; 27:3417–21. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Cohn A, Comanducci M et al. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine 2011; 29:4739–44. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration. First vaccine approved by FDA to prevent serogroup B meningococcal disease. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm420998.htm. Accessed October 29, 2014.
- 16.Jiang HQ, Hoiseth SK, Harris SL et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 2010; 28:6086–93. [DOI] [PubMed] [Google Scholar]
- 17.Zlotnick GW, Jones TR, Liberator P et al. The discovery and development of a novel vaccine to protect against Neisseria meningitidis serogroup B disease. Hum Vaccin Immunother 2015; 11:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broder KR, Cortese MM, Iskander JK et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–34. [PubMed] [Google Scholar]
- 19.Sanofi Pasteur MSD. Repevax patient information leaflet. Available at: http://www.medicines.org.uk/emc/medicine/17377/PIL/repevax/. Accessed March 31, 2014.
- 20.Electronic Medicines Compendium. Repevax: summary of product characteristics. Available at: http://www.medicines.org.uk/emc/medicine/15256. Accessed June 16, 2014.
- 21.World Health Organization. Standardization and validation of serological assays for the evaluation of immune responses to Neisseria meningitidis serogroup A/C vaccines. Geneva, March 8–9, 1999. Available at: https://extranet.who.int/iris/restricted/handle/10665/66298. Accessed October 8, 2014. [DOI] [PubMed]
- 22.Borrow R, Carlone GM. Serogroup B and C serum bactericidal assays. Methods Mol Med 2001; 66:289–304. [DOI] [PubMed] [Google Scholar]
- 23.Dominicus R, Galtier F, Richard P, Baudin M. Immunogenicity and safety of one dose of diphtheria, tetanus, acellular pertussis and poliomyelitis vaccine (Repevax) followed by two doses of diphtheria, tetanus and poliomyelitis vaccine (Revaxis) in adults aged ≥ 40 years not receiving a diphtheria- and tetanus-containing vaccination in the last 20 years. Vaccine 2014; 32:3942–9. [DOI] [PubMed] [Google Scholar]
- 24.Chan IS, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics 1999; 55:1202–9. [DOI] [PubMed] [Google Scholar]
- 25.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 1969; 129:1307–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration. Use of serum bactericidal antibody as an immunological correlate for demonstrating effectiveness of meningococcal conjugate vaccines (serogroups A, C, Y, W-135) adminstered to children less than 2 years of age. Available at: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/bloodvaccinesandotherbiologics/vaccinesandrelatedbiologicalproductsadvisorycommittee/ucm248585.pdf. Accessed June 16, 2014. [Google Scholar]
- 27.National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–64. [PubMed] [Google Scholar]