Abstract
Background
Viruses from 2 influenza B lineages co-circulate, leading to suboptimal protection with trivalent influenza vaccines (TIV). Quadrivalent influenza vaccines (QIV) containing both lineages offer broader protection.
Methods
We compared inactivated seasonal QIV versus TIV (15 and 7.5 μg hemagglutinin [HA] for each influenza strain, respectively) in a phase II randomized (1 : 1), observer-blind trial in US children 6–35 months of age (identifier NCT01974895). The primary objective was to evaluate immune responses induced by QIV for the 4 vaccine strains 28 days after completion of vaccination. A secondary objective was to demonstrate superiority of QIV versus TIV for the B/Victoria strain contained in QIV but not TIV. Immunogenicity was evaluated in the per-protocol cohort (N = 280), and safety was evaluated in the intent-to-treat cohort (N = 314).
Results
Seroconversion rates (SCRs) for QIV were 80.4% (95% confidence interval [CI], 73.0%–86.6%), 72.0% (95% CI, 63.9%–79.2%), 86.0% (95% CI, 79.2%–91.2%), and 66.4% (95% CI, 58.1%–74.1%) for A/H1N1, A/H3N2, B/Yamagata, and B/Victoria, respectively. Quadrivalent influenza vaccines demonstrated immunogenic superiority over TIV for B/Victoria with a geometric mean titer ratio of 4.73 (95% CI, 3.73%–5.99%) and SCR difference of 54.02% (95% CI, 43.88%–62.87%). Safety was similar between the vaccine groups despite the QIV's higher antigen content. No serious adverse events were reported related to vaccination.
Conclusions
Quadrivalent influenza vaccine (15 µg HA/strain) was immunogenic with an acceptable safety profile. The next phase of its development in children 6–35 months of age is a phase III trial in countries where it is not yet licensed. In countries where it is already licensed, a switch from TIV to QIV would provide broader protection in this vulnerable group.
Keywords: children, immunogenicity, influenza, quadrivalent, vaccine
Influenza infection creates a high disease burden among children [1–4], with routine vaccination recommended in the United States [5] and elsewhere. Until recently, most programs used trivalent influenza vaccines (TIV) containing 2 influenza A strains (H1N1 and H3N2) and 1 B strain. Two antigenically distinct lineages of influenza B (Yamagata and Victoria) have cocirculated worldwide since 2000, and in the past decade, a substantial proportion of influenza B isolates from patients have been mismatched to the influenza B strain in the seasonal TIV. For example, during the 2012–2013 season, one third of influenza B viruses tested by the US Centers for Disease Control and Prevention were of the lineage absent from the seasonal vaccine [6].
During seasons where the predominant circulating influenza B virus is from the alternate lineage than the B strain included in the TIV, suboptimal vaccine protection can be expected [7–9]. A quadrivalent influenza vaccine (QIV) containing B strains derived from both lineages could offer broader protection by eliminating B lineage mismatch. This may be particularly important in children because, although vaccinated adults show moderate cross-reactive antibody responses against the alternative B lineage [10], children show poor cross-reactivity [11, 12]. Indeed, a meta-analysis of vaccine trials in young children found that efficacy was substantially reduced against influenza B strains of the alternative lineage to that contained in the vaccine [9].
Currently, the licensed dose in the United States for children between 6 and 35 months of age is 0.25 mL with 7.5 μg hemagglutinin (HA) of each influenza strain. This represents half of the 0.5 mL dose with 15 μg HA per strain that is licensed for older children and adults. The practice of administering the lower dose to young children began more than 30 years ago to reduce the fever and febrile convulsions associated with the whole virus vaccines available at the time [13]. However, studies have shown that children of this age mount a variable immune response to the 7.5 μg HA dose. Current split virus vaccines are better tolerated compared with whole virus vaccines and are associated with substantially lower rates of fever and febrile convulsions. Several studies have now evaluated these vaccines at the 15 μg HA dose in children 6–35 months of age. The higher dose elicits an increased immune response, particularly in children younger than 18 months, without increasing reactogenicity compared with the 7.5 μg HA dose [14–16].
The QIV manufactured by GSK Vaccines has been developed for use at the 15 μg HA dose regardless of age. It is licensed in Canada and Mexico for children from the age of 6 months. However, in the United States, it is only licensed from the age of 3 years. Herein, we describe a phase II study evaluating immunogenicity and safety of the QIV at a 15 μg HA dose in children between 6 and 35 months of age in the United States. Ideally, we would have compared the investigational QIV with a licensed QIV at the same 15 μg HA dose. Unfortunately, this was not possible. The TIV and QIV manufactured by Sanofi Pasteur are the only influenza vaccines licensed in the United States in this age group, both of which are used at a dose of 7.5 μg HA. Trial access to the QIV could not be guaranteed. Hence, the TIV at a 7.5 μg dose was the only available choice of comparator for the study.
METHODS
This study was a phase II randomized, controlled, observer-blind, multicenter trial comparing the immunogenicity and safety profiles of an inactivated QIV versus TIV in children 6–35 months of age in the United States (identifier NCT01974895). The primary objective was to evaluate immunogenicity of the QIV according to the US Center for Biologics Evaluation and Research (CBER) criteria for seroconversion. This criterion, rather than the seroprotection rate, was selected because it is more relevant for children who may be both unvaccinated and unexposed previously to either or both influenza A and B infection.
The trial was sponsored by GlaxoSmithKline Biologicals SA, and it was approved by independent ethics committees and/or institutional review boards, conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and US regulatory requirements. Parents or legally acceptable representatives provided written informed consent prior to participation of their child.
Participants, Vaccines, and Study Design
Healthy children and those with chronic illness who were not acutely ill at the time of enrollment (determined by the investigator's clinical examination and assessment of the child's medical history) were enrolled from 12 centers across the United States during the fall and winter of 2013–2014. Children were excluded if they were febrile (temperature ≥38.0°C), acutely ill, immunocompromised, allergic to any vaccine component, had a history of Guillain-Barré syndrome within 6 weeks of prior influenza vaccination, had a known coagulation disorder, had received any influenza vaccine within the last 6 months, had received any immunoglobulin or blood product within the last 3 months, or had received an investigational product within the last 30 days.
In accordance with the US Advisory Committee on Immunization Practices definition, children were considered vaccine-primed if they had received 2 or more doses of seasonal influenza vaccine since July 1, 2010; all others were considered vaccine-unprimed.
The investigational QIV (GSK Vaccines, dba ID Biomedical Corporation, Sainte-Foy, Quebec, Canada) contained 15 μg HA of each of 4 strains (A/California/7/2009 [A/H1N1], A/Texas/50/2012 [A/H3N2], B/Brisbane/60/2008 [B/Victoria], and B/Massachusetts/2/2012 [B/Yamagata]), in a 0.5 mL dose. The HA content was measured by a validated Single Radial Immunodiffusion assay. The licensed comparator TIV (Sanofi Pasteur, Swiftwater, PA) contained 7.5 μg of each of the same A/H1N1, A/H3N2, and B Yamagata strains in a 0.25 mL dose. Vaccine-primed children received 1 vaccine dose on study day 0. Vaccine-unprimed children received a vaccine dose on days 0 and 28. Administration was via intramuscular injection in the deltoid muscle of the nondominant arm for children ≥12 months of age or the anterolateral region of the left thigh for children <12 months of age.
Children were randomized 1:1 to receive either QIV or TIV according to an internet-based randomization system. A minimization algorithm was used to balance enrollment by group accounting for age (6–17 and 18–35 months), center, and priming status. The randomization system provided the unique treatment number to be used for each dose. GSK Vaccines performed the randomization.
Study Endpoints and Procedures
Each child had a blood sample taken at baseline and again 28 days after completion of the 1- or 2-dose vaccine series (depending on vaccine-priming status). Hemagglutination inhibition (HI) titers were determined from serum obtained pre- and postvaccination. Results were reported for each vaccine group as (1) geometric mean titers (GMTs), (2) seropositivity rates, (3) seroconversion rates (SCRs), (4) seroprotection rates (SPRs), and (5) mean geometric increases (MGIs). The limit of quantitation for the HI assay was 1:10; samples <1:10 were considered seronegative and were given an arbitrary value of 5 for the GMT calculation.
Seropositivity rate was defined as the percentage of participants with reciprocal HI titer ≥1:10. Seroconversion rate was defined as the percentage of participants with either (a) prevaccination reciprocal HI titer <1:10 and postvaccination reciprocal titer ≥1:40 or (b) prevaccination reciprocal titer ≥1:10 and at least a 4-fold increase in postvaccination reciprocal titer. Seroprotection rate was defined as the percentage of participants who attained reciprocal HI titers of ≥1:40. Mean geometric increase was defined as the geometric mean of the within-subject ratios of the postvaccination/prevaccination reciprocal HI titer.
Solicited injection site and general adverse events (AEs) were recorded in diary cards on the day of vaccination and for 6 days afterwards. Spontaneously reported symptoms were recorded until 28 days after vaccination. Serious AEs (SAEs), potential immune-mediated disorders (pIMDs), and medically attended AEs (MAEs) were recorded until the final telephone contact on day 180. Fever was defined as temperature ≥38.0°C by any route (axillary, rectal, oral, or tympanic).
Study Objectives
The primary objective was to evaluate whether the QIV elicited an immune response against each vaccine strain that met the CBER target for SCR 28 days after completion of the vaccine course, ie, lower limit (LL) of the 95% confidence interval (CI) ≥40%. Secondary objectives included: (1) demonstrating superior immunogenicity of QIV versus TIV against the B/Victoria strain 28 days after completion of the vaccine series (criteria: LL of the 95% CI for GMT ratio [QIV/TIV] >1.5 and for SCR difference [QIV minus TIV] >10%); (2) describing GMT, SPR, SCR, and MGI; (3) describing safety and reactogenicity; and (4) evaluating the relative risk of fever (≥38.0°C) in the QIV group versus the TIV group over the 4-day postvaccination period. The tertiary objective was to describe the GMT ratio (TIV/QIV) and SCR difference (TIV minus QIV) for the H1N1, H3N2, and B/Yamagata strains.
Statistics
The primary immunogenicity analysis was based on the per-protocol cohort, which included children who met all eligibility criteria, complied with the protocol and vaccine schedule, and had data available for antibodies against at least 1 vaccine strain postvaccination. Immunogenicity analyses excluded participants with missing or nonevaluable measurements. The safety analysis was based on the intent-to-treat cohort.
A point estimate and its 2-sided exact 95% CI were calculated using Proc StatXact for GMT, SCR, SPR, and MGI by vaccine group for all children. In addition, subgroup analyses were conducted to explore potential differences between 2 age strata (6–17 and 18–35 months). The 95% CIs for the mean of log-transformed antibody titers were first obtained assuming that log-transformed values were normally distributed with unknown variance. The 95% CIs for GMT were then computed by exponential-transformation of the 95% CI for the mean of log-transformed titer. The GMT ratio (QIV/TIV) was computed using an analysis of covariance model on the log-transformed titers, including the vaccine group as a fixed effect and prevaccination HI titers, age, and vaccine-priming status as covariates. The asymptotic standardized 95% CI for the difference in SCR between QIV and TIV was computed using Proc StatXact [17].
Study power was calculated using PASS 2005. Assuming 50 evaluable children in the QIV group, the overall power of the study was 99.34% to meet CBER criteria for SCR simultaneously for all 4 strains (primary objective). Assuming 50 evaluable children in each vaccine group, the power to demonstrate superiority of the QIV over the TIV was 99.17% in terms of GMT ratio and >99.99% in terms of SCR difference (a secondary objective). No power calculation was performed for other secondary and tertiary objectives. However, the study planned to enroll 250 participants per group to assess the SCR difference for the common strains between the 2 groups with reasonably adequate power and to increase the probability of detecting fever, a potential consequence of the increased antigen content in the QIV.
RESULTS
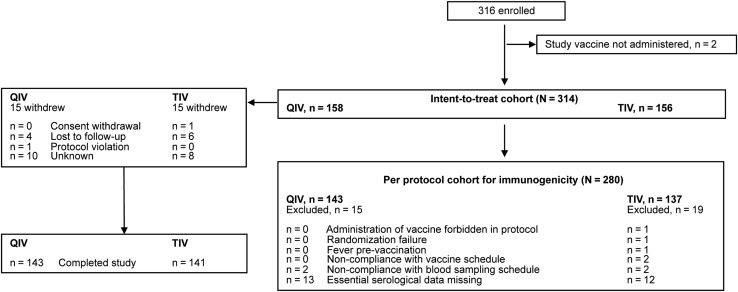
The first participant was enrolled in October 2013 and the last study contact was in July 2014. The initial enrollment target was 500 children, but 1 major planned center was unable to participate; thus, 316 children were enrolled. Although the planned enrollment of 500 children was not attained, the 316 children enrolled assured adequate power for the analysis of the primary and secondary confirmatory objectives. A total of 314 and 280 children were included in the intent-to-treat cohort (Total Vaccinated Cohort) and per-protocol cohort for immunogenicity, respectively (Figure 1). Demographics were similar between groups (Table 1). In the QIV group, 64 (40.5%) children received 1 vaccine dose, whereas 94 (59.5%) received 2 doses. Corresponding values in the TIV group were 64 (41.0%) and 92 (59.0%).
Figure 1.
Participant disposition. QIV, quadrivalent influenza vaccine; TIV, trivalent influenza vaccine.
Table 1.
Participant Demographics (Intent-to-Treat Cohort)
| Characteristic | QIV N = 158 |
TIV N = 156 |
Total N = 314 |
|---|---|---|---|
| Mean age (SD), months | 19.6 (8.8) | 19.8 (8.9) | 19.7 (8.9) |
| Female gender, n (%) | 74 (46.8) | 82 (52.6) | 156 (49.7) |
| Geographic ancestry, n (%) | |||
| White (Caucasian or European heritage) | 86 (54.4) | 88 (56.4) | 174 (55.4) |
| African/African American | 56 (35.4) | 56 (35.9) | 112 (35.7) |
| American Indian or Alaskan Native | 1 (0.6) | 0 (0) | 1 (0.3) |
| Asian | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| White (Arabic or North African) | 1 (0.6) | 0 (0) | 1 (0.3) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 0 (0) | 0 (0) |
| Other | 13 (8.2) | 11 (7.1) | 24 (7.6) |
Abbreviations: QIV, quadrivalent influenza vaccine; SD, standard deviation; TIV, trivalent influenza vaccine.
Immunogenicity
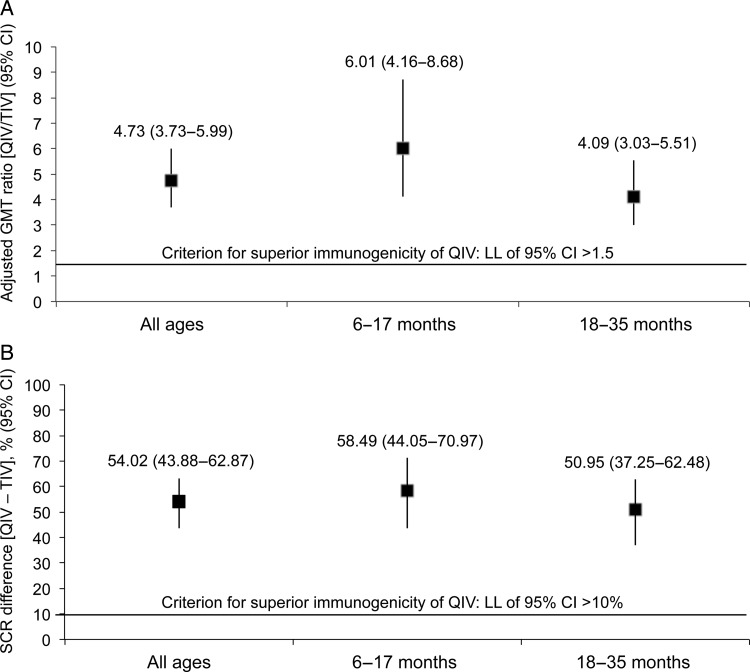
Seroconversion rates at 28 days after vaccination with the QIV were 80.4% for A/H1N1, 72.0% for A/H3N2, 86.0% for B/Yamagata, and 66.4% for B/Victoria (Table 2). The LL of the 95% CI for SCR was >40% for each vaccine strain in the overall population and in both age groups (Table 2), demonstrating that QIV met CBER criterion for seroconversion. In addition, QIV immunogenicity was superior to TIV for the B/Victoria strain overall and in both age strata: the LL of the 95% CI was >1.5 for adjusted GMT ratio and >10% for the difference in SCR (Figure 2A and B).
Table 2.
SCR, SPR, and MGI Against Each Vaccine Strain Overall and According to Age Stratum at 28 Days After Completion of Vaccination Series (Per-Protocol Cohort for Immunogenicity)
| Variable | All Ages |
6–17 Months |
18–35 Months |
|||
|---|---|---|---|---|---|---|
| QIV N = 143 |
TIV N = 137 |
QIV N = 53 |
TIV N = 53 |
QIV N = 90 |
TIV N = 84 |
|
| SCR, % (95% CI) | ||||||
| A/H1N1 | 80.4 (73.0–86.6) | 71.5 (63.2–78.9) | 64.2 (49.8–76.9) | 66.0 (51.7–78.5) | 90.0 (81.9–95.3) | 75.0 (64.4–83.8) |
| A/H3N2 | 72.0 (63.9–79.2) | 68.6 (60.1–76.3) | 56.6 (42.3–70.2) | 67.9 (53.7–80.1) | 81.1 (71.5–88.6) | 69.0 (58.0–78.7) |
| B/Yamagata | 86.0 (79.2–91.2) | 83.9 (76.7–89.7) | 71.7 (57.7–83.2) | 73.6 (59.7–84.7) | 94.4 (87.5–98.2) | 90.5 (82.1–95.8) |
| B/Victoria | 66.4 (58.1–74.1) | 12.4 (7.4–19.1) | 60.4 (46.0–73.5) | 1.9 (0.0–10.1) | 70.0 (59.4–79.2) | 19.0 (11.3–29.1) |
| Post-vaccination SPR, % (95% CI) | ||||||
| A/H1N1 | 87.4 (80.8–92.4) | 81.0 (73.4–87.2) | 67.9 (53.7–80.1) | 69.8 (55.7–81.7) | 98.9 (94.0–100) | 88.1 (79.2–94.1) |
| A/H3N2 | 82.5 (75.3–88.4) | 80.3 (72.6–86.6) | 60.4 (46.0–73.5) | 69.8 (55.7–81.7) | 95.6 (89.0–98.8) | 86.9 (77.8–93.3) |
| B/Yamagata | 94.4 (89.3–97.6) | 90.5 (84.3–94.9) | 84.9 (72.4–93.3) | 79.2 (65.9–89.2) | 100 (96.0–100) | 97.6 (91.7–99.7) |
| B/Victoria | 70.6 (62.4–77.9) | 19.7 (13.4–27.4) | 60.4 (46.0–73.5) | 3.8 (0.5–13.0) | 76.7 (66.6–84.9) | 29.8 (20.3–40.7) |
| MGI (95% CI) | ||||||
| A/H1N1 | 13.7 (11.1–17.0) | 9.1 (7.3–11.3) | 11.2 (7.3–17.1) | 8.3 (5.7–12.2) | 15.5 (12.4–19.5) | 9.6 (7.4–12.6) |
| A/H3N2 | 9.1 (7.7–10.8) | 7.5 (6.4–8.9) | 7.3 (5.4–9.8) | 8.6 (6.5–11.3) | 10.4 (8.5–12.7) | 6.9 (5.6–8.6) |
| B/Yamagata | 14.6 (11.7–18.2) | 11.4 (9.1–14.2) | 9.8 (6.2–15.5) | 8.4 (5.5–12.9) | 18.4 (14.9–22.9) | 13.7 (10.8–17.5) |
| B/Victoria | 8.9 (7.3–10.9) | 1.9 (1.7–2.2) | 8.2 (5.8–11.6) | 1.4 (1.2–1.6) | 9.4 (7.4–12.0) | 2.4 (2.0–2.8) |
Abbreviations: CI, confidence interval; MGI, mean geometric increase; QIV, quadrivalent influenza vaccine; SCR, seroconversion rate; SPR, seroprotection rate; TIV, trivalent influenza vaccine.
Figure 2.
Adjusted geometric mean titer (GMT) ratio and difference in seroconversion rate (SCR) for quadrivalent influenza vaccine (QIV) versus trivalent influenza vaccine (TIV) against B/Victoria overall and according to age stratum at 28 days after completion of vaccination series (per-protocol cohort for immunogenicity). (A) GMT ratio (QIV/TIV); (B) difference in SCR (QIV minus TIV). CI, confidence interval; LL, lower limit.
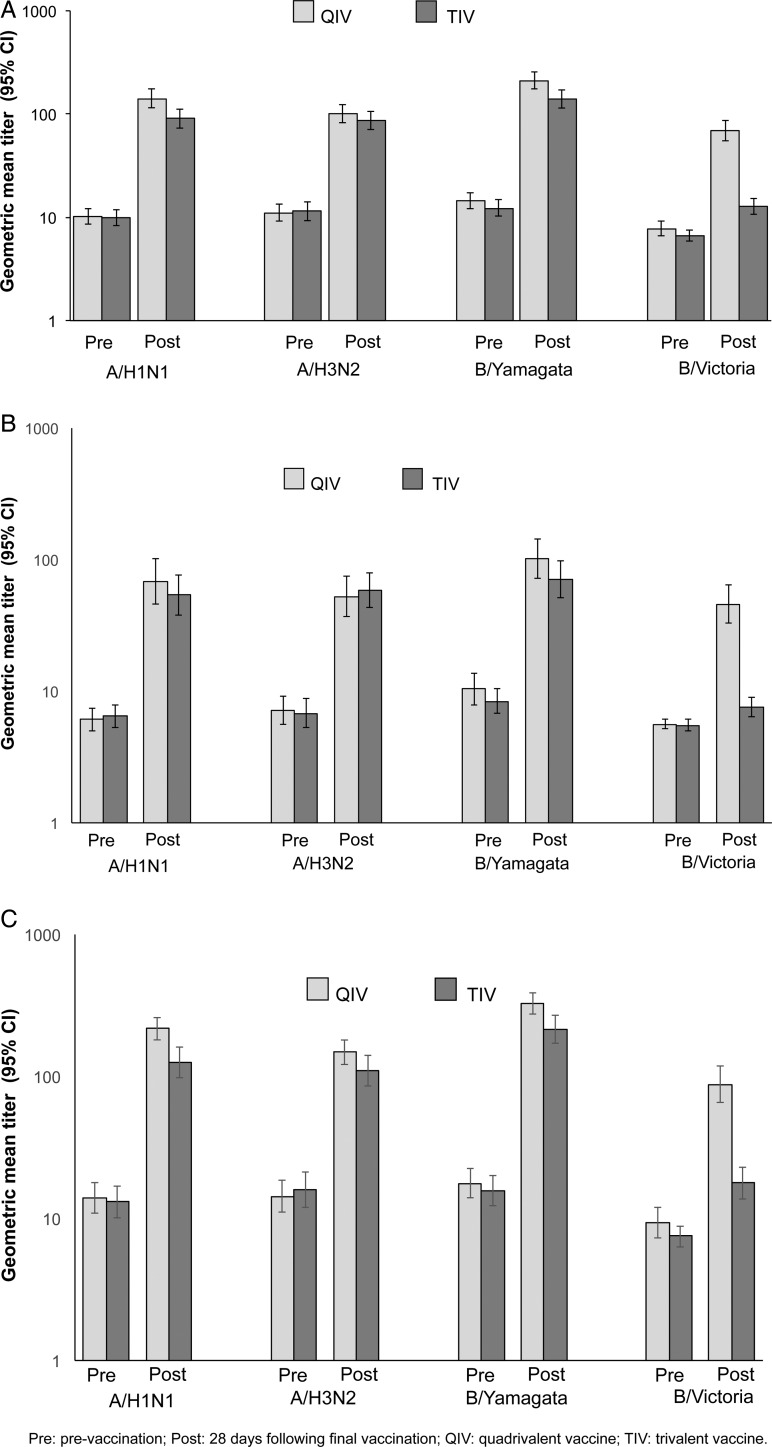
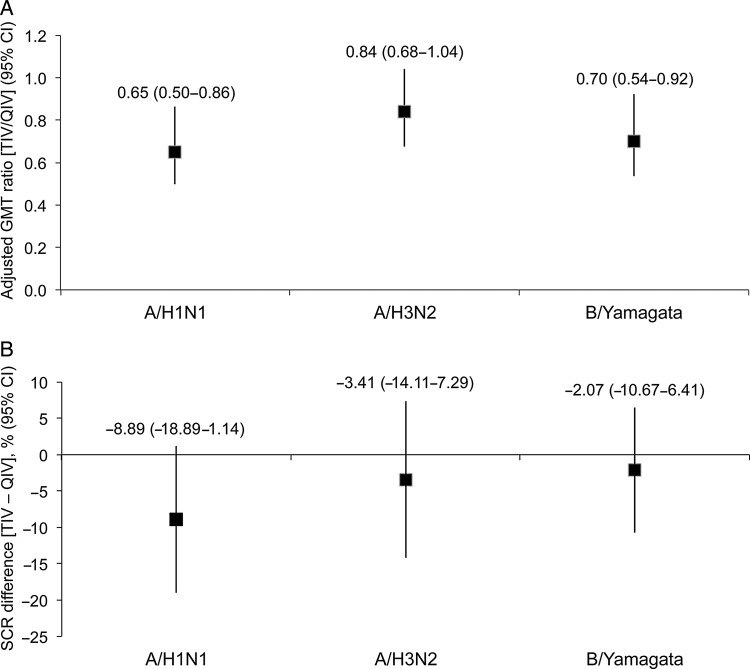
Quadrivalent influenza vaccine and TIV had similar immunogenicity expressed as postvaccination GMT against the A/H1N1, A/H3N2, and B/Yamagata strains (Figure 3A). Quadrivalent influenza vaccine, but not TIV, was immunogenic against the B/Victoria strain. Geometric mean titers were numerically higher in children 18–35 months of age compared with children 6–17 months of age, but with no notable treatment group differences within each age stratum for the 3 common strains (Figure 3B and C). Robust immunogenicity against all 4 strains was shown for QIV in terms of SPR and MGI, and TIV was immunogenic against the A/H1N1, A/H3N2, and B/Yamagata strains (Table 2). In a planned exploratory analysis, QIV and TIV were similarly immunogenic with regard to GMT ratio and SCR difference for the 3 common strains (Figure 4A and B).
Figure 3.
Geometric mean titer (GMT) overall and according to age stratum prevaccination and 28 days after completion of vaccination series (per-protocol cohort for immunogenicity). (A) All ages; (B) 6–17 months; (C) 18–35 months. CI, confidence interval; Pre, pre-vaccination; Post, 28 days following final vaccination; QIV, quadrivalent influenza vaccine; TIV, trivalent influenza vaccine.
Figure 4.
Adjusted geometric mean titer (GMT) ratio and difference in seroconversion rate (SCR) for quadrivalent influenza vaccine (QIV) versus trivalent influenza vaccine (TIV) against the common vaccine strains (A/H1N1, A/H3N2, and B/Yamagata) at 28 days after completion of vaccination series (per-protocol cohort for immunogenicity). (A) Adjusted GMT ratio (TIV/QIV); (B) SCR difference (TIV minus QIV).
Safety and Reactogenicity
Pain at the injection site was the most common AE, occurring in approximately one third of both QIV and TIV recipients (Table 3). Irritability or fussiness was the most common solicited general symptom, occurring in 50.3% and 45.3% of children receiving QIV and TIV, respectively. Ten children in each group experienced fever ≥38.0°C during the 7-day postvaccination period (Table 3), of whom 7 and 8 children experienced fever within 4 days postvaccination in the QIV and TIV groups, respectively. There was no difference between groups in relative risk of fever ≥38.0°C (0.86 [95% CI, 0.33–2.23]) over 4 days postvaccination.
Table 3.
Safety Outcomes Reported Throughout the Study (Intent-to-Treat Cohort)
| Variable | Number (%) Children Reporting Outcome |
|
|---|---|---|
| QIV | TIV | |
| Soliciteda injection site symptoms during 7-day postvaccination period (N = 151 QIV, N = 148 TIV)b | ||
| Pain | 48 (31.8) | 48 (32.4) |
| Redness | 2 (1.3) | 0 (0) |
| Swelling | 0 (0) | 1 (0.7) |
| Solicited general symptoms during 7-day postvaccination period (N = 151 QIV, N = 148 TIV)b | ||
| Drowsiness | 60 (39.7) | 56 (37.8) |
| Fever (≥38.0°C) | 10 (6.6) | 10 (6.8) |
| Irritability/fussiness | 76 (50.3) | 67 (45.3) |
| Loss of appetite | 49 (32.5) | 46 (31.1) |
| Unsolicited (spontaneously reported) symptoms during 28-day postvaccination period (N = 158 QIV, N = 156 TIV) | ||
| All | 77 (48.7) | 75 (48.1) |
| Related to vaccine | 11 (7.0) | 7 (4.5) |
| Serious adverse event during entire study period (N = 158 QIV, N = 156 TIV) | ||
| All | 5 (3.2) | 4 (2.6) |
| Medically attended eventc during entire study period (N = 158 QIV, N = 156 TIV) | ||
| All | 77 (48.7) | 89 (57.1) |
Abbreviations: AEs, adverse events; QIV, quadrivalent influenza vaccine; TIV, trivalent influenza vaccine.
aAll solicited injection site symptoms were considered related to vaccination.
bOnly subjects who have documented safety data were included in the calculation of solicited AEs.
cHospitalization, emergency room visit, medical practitioner visit.
Spontaneously reported AEs considered related to vaccination occurred in 7.0% and 4.5% of children in the QIV and TIV group, respectively (Table 3). Two grade 3 AEs (defined as severe enough to prevent everyday activity) considered possibly related to vaccination occurred in the QIV group (diarrhea and upper respiratory tract infection) and 1 in the TIV group (nasopharyngitis). Medically attended AEs occurred in 48.7% of children in the QIV group and 57.1% in the TIV group (Table 3).
Five children in the QIV group experienced an SAE: unspecified viral infection (1), respiratory syncytial virus infection (2), dehydration (1), and sleep apnea syndrome (1). Four children in the TIV group experienced SAEs: respiratory syncytial virus bronchiolitis (1), convulsion (1), failure to thrive (1), and neck abscess (1). None of the SAEs reported from either group was considered related to vaccination. No children experienced a pIMD, and there were no deaths during the study.
DISCUSSION
The 2013–2014 influenza season was the first time the World Health Organization selected a second influenza B virus for inclusion in QIV formulations, reflecting their recognition of the potential benefit of QIV for reducing the risk of influenza B disease [18, 19]. Although QIV eliminates the risk of reduced vaccine effectiveness due to influenza B virus lineage mismatch for all ages, children lacking prior exposure to both influenza B lineages may especially benefit from QIV. The QIV manufactured by GSK Vaccines and studied in the present trial is licensed for children from 6 months of age in Mexico and Canada but only from 3 years of age in the United States. To license GSK's QIV in younger children in the United States, a study demonstrating immunological noninferiority and an acceptable safety profile versus a licensed product is required. After the positive results of this phase II trial, a phase III trial (identifier NCT02242643) comparing GSK's QIV at a dose of 15 μg HA with the licensed QIV manufactured by Sanofi Pasteur is now underway at more than 60 sites in the United States and Mexico in children 6–35 months of age.
The present study showed that the QIV is immunogenic in children 6–35 months of age in stable health, with SCRs of 80.4%, 72.0%, 86.0%, and 66.4% against the A/H1N1, A/H3N2, B/Yamagata and B/Victoria strains, respectively. Because the LL of the 95% CI was ≥40% for each strain, these results met CBER's SCR licensure criterion. In addition, the QIV had superior immunogenicity against the B/Victoria strain compared with TIV. Immunogenicity against the 3 strains common to both vaccines was similar, indicating that addition of the second B strain does not affect immunogenicity of the other influenza strains. A study of Sanofi Pasteur's QIV in young children also supported that the addition of a second B strain has no negative impact on the immune responses to other strains, albeit at a dose of 7.5 μg HA [20]. There were no treatment group differences within either age strata. This is an important finding because in a previous study of an investigational inactivated TIV given at doses of either 7.5 μg or 15 μg HA, the investigational TIV elicited a lower immune response in children <18 months of age than Sanofi Pasteur's TIV [14], the same comparator as used in our study. In contrast, in our study, the immune response against the common vaccine strains was similar with the QIV and TIV in children 6–17 months of age.
Immunogenicity and safety of inactivated QIVs have been evaluated in adults [21–24] and children [20, 25–27]. In all studies, the QIVs produced superior immune responses to the B lineage not contained in the control TIV and a comparable response to the common vaccine strains. One open-label [25] and 1 TIV-controlled study [27] evaluated GSK's QIV in children 6–35 months of age during the 2010–2011 and 2012–2013 influenza seasons. The results of those studies, taken together with the results described here, show that the QIV produces comparable immunogenicity across seasons. The GMT ratio and SCR difference versus TIV for the B/Victoria strain were of similar magnitude in the present study to those in the above-mentioned TIV-controlled study of GSK's QIV (GMT ratio 6.3 and SCR difference 64.2%) [27] and a study of Sanofi Pasteur's QIV (GMT ratio 4.4 and SCR difference 51.8%) [20].
The QIV was given at the full adult dose of 15 μg HA per influenza strain in the present study, rather than the lower 7.5 μg HA dose traditionally recommended for infants in the United States. Historically, the lower dose was recommended because of the increase in fever and febrile convulsions observed in young children given a full dose of the whole virus vaccines available at the time [13]. However, studies of the TIV have shown variable immune responses in young children to the 7.5 μg HA dose, particularly to the vaccine B strain [11, 28, 29]. In contrast, studies using the full 15 μg HA dose as a split virion vaccine in young children have shown a consistently robust immune response with no increase in reactogenicity or fever [14–16].
Importantly, our study showed that the QIV and the TIV have similar reactogenicity and AE profiles, with no apparent adverse effect on tolerability of the higher antigen content in the QIV (60 μg HA for 4 strains compared with 22.5 μg for 3 strains in the TIV). Some previous studies have suggested that pain at the injection site may be modestly increased with QIV compared with TIV or hepatitis A vaccine [25, 30], whereas others report similar levels of pain [21, 23, 26, 27]. The incidence of fever was similar with both vaccines, consistent with previous published studies [25, 30].
Quadrivalent influenza vaccines in young children are expected to be particularly valuable during seasons in which both B lineages are cocirculating or there is an unexpected shift from one lineage to another. Influenza B is reported to cause a disproportionate number of influenza-related deaths in children [31]. Furthermore, it is well recognized, particularly in children, that vaccine efficacy and immunogenicity against influenza B is reduced if the B strain in the TIV is of a different lineage to the circulating B strain [7–9, 11, 12]. Mismatching of the vaccine and circulating B strain has occurred frequently. In the United States, the B strain in the seasonal TIV was mismatched to the circulating strain in 6 of the last 14 seasons [32]. Use of QIV in place of TIV is predicted to reduce the number of influenza cases, influenza-related hospitalizations, and influenza-related deaths in the overall population [33–35].
CONCLUSIONS
In conclusion, the investigational QIV was immunogenic with an acceptable safety profile in children 6–35 months of age. Compared with the licensed TIV, QIV had superior immunogenicity against the B/Victoria strain and comparable immunogenicity against the 3 strains common to both vaccines. The next phase of the QIV's development in children 6–35 months of age is a phase III trial in countries where it is not yet licensed. In countries where it is already licensed for this age group, a switch from TIV to QIV would provide broader protection in this vulnerable group.
Acknowledgments
We are indebted to the participating study volunteers and their parents, clinicians, nurses, and laboratory technicians at the study sites, as well as to the sponsor's project staff for their support and contributions throughout the study. In particular, we thank W. Seger, A. Moskow, S. Moskow, R. Hines, and E. Zissman (investigators); and W. Jiang (Central Safety Contact), C. Probst (Regulatory Affairs representative), B. Corsaro (Project Manager), E. Praet (Study Delivery Lead), C. Stalens (Study Manager), S. Ravault (Clinical Regulatory Affairs), and B. Pereira (Scientific Writer) from GSK Vaccines. Finally, we thank M. L. Greenacre (An Sgriobhadair, UK, on behalf of GSK Vaccines) for providing medical writing services and B. Dumont (Business & Decision Life Sciences, on behalf of GSK Vaccines) for editorial assistance and manuscript coordination.
Author contributions. V. K. J. acquired the funding and participated in the choice of centers and recruitment of the investigators. All authors participated in the conception, design, and planning of the study. J. B. D. and V. K. J. provided subjects or study materials. L. W., V. C., J. B. D., and P. L. participated to the acquisition or the assembling of the data. L. W., V. C., P. L., and V. K. J. performed or supervised the analysis, and L. W., V. C., B. L. I., and V. K. J. performed the quality check. All authors contributed to the interpretation of results. V. C. and P. L. provided statistical expertise. L. W., P. L., B. L. I., and V. K. J. supervised of the study group. All authors read and approved the final manuscript.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA paid for all costs associated with the development of this manuscript.
Potential conflicts of interest. L. W., V. C., P. L., B. L. I., and V. K. J. are employees of the GSK group of companies. L. W., P. L., B. L. I., and V. K. J. report ownership of stock options and/or restricted shares. J. B. D. reports payments from the GSK group of companies for the conduct of the study; and grants from Pfizer and from the GSK group of companies, outside the submitted work. J. B. D. also provides consulting in the area of immunizations for Sanofi Pasteur, Novartis, Merck, the GSK group of companies, and Medimmune; he has also served on advisory boards for the GSK group of companies and Medimmune.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.O'Brien MA, Uyeki TM, Shay DK et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 2004; 113:585–93. [DOI] [PubMed] [Google Scholar]
- 2.Izurieta HS, Thompson WW, Kramarz P et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232–9. [DOI] [PubMed] [Google Scholar]
- 3.Molinari NA, Ortega-Sanchez IR, Messonnier ML et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–96. [DOI] [PubMed] [Google Scholar]
- 4.Bourgeois FT, Valim C, Wei JC et al. Influenza and other respiratory virus-related emergency department visits among young children. Pediatrics 2006; 118:e1–8. [DOI] [PubMed] [Google Scholar]
- 5.Advisory Committee on Immunization Practices. Prevention and control of influenza with vaccines: interim recommendations of the Advisory Committee on Immunization Practices (ACIP), 2013. MMWR Morb Mortal Wkly Rep 2013; 62:356. [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Influenza activity--United States, 2012–13 season and composition of the 2013–14 influenza vaccine. MMWR Morb Mortal Wkly Rep 2013; 62:473–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Skowronski DM, Masaro C, Kwindt TL et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005–2006 season of dual A and B vaccine mismatch in Canada. Vaccine 2007; 25:2842–51. [DOI] [PubMed] [Google Scholar]
- 8.Belongia EA, Kieke BA, Donahue JG et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis 2009; 199:159–67. [DOI] [PubMed] [Google Scholar]
- 9.Belshe RB, Coelingh K, Ambrose CS et al. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 2010; 28:2149–56. [DOI] [PubMed] [Google Scholar]
- 10.Barr IG, Komadina N, Durrant C et al. Circulation and antigenic drift in human influenza B viruses in SE Asia and Oceania since 2000. Commun Dis Intell Q Rep 2006; 30:350–7. [DOI] [PubMed] [Google Scholar]
- 11.Englund JA, Walter EB, Gbadebo A et al. Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers. Pediatrics 2006; 118:e579–85. [DOI] [PubMed] [Google Scholar]
- 12.Levandowski RA, Regnery HL, Staton E et al. Antibody responses to influenza B viruses in immunologically unprimed children. Pediatrics 1991; 88:1031–6. [PubMed] [Google Scholar]
- 13.Wright PF, Dolin R, La Montagne JR. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Center for Disease Control, and the Bureau of Biologics of the Food and Drug Administration. Summary of clinical trials of influenza vaccines - II . J Infect Dis 1976; 134:633–8. [DOI] [PubMed] [Google Scholar]
- 14.Pavia-Ruz N, Angel Rodriguez, Weber M et al. A randomized controlled study to evaluate the immunogenicity of a trivalent inactivated seasonal influenza vaccine at two dosages in children 6 to 35 months of age. Hum Vaccin Immunother 2013; 9:1978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skowronski DM, Hottes TS, Chong M et al. Randomized controlled trial of dose response to influenza vaccine in children aged 6 to 23 months. Pediatrics 2011; 128:e276–89. [DOI] [PubMed] [Google Scholar]
- 16.Langley JM, Vanderkooi OG, Garfield HA et al. Immunogenicity and safety of 2 dose levels of a thimerosal-free trivalent seasonal influenza vaccine in children aged 6–35 months: a randomized, controlled trial. J Pediatr Infect Dis Soc 2012; 1:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CJ Clopper, Pearson E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26:404–13. [Google Scholar]
- 18.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2013–14 northern hemisphere influenza season. Available at: www.who.int/influenza/vaccines/virus/recommendations/2013_14_north/en. Accessed 21 January 2015.
- 19.U.S. Food and Drug Administration. February 27, 2013: vaccines and related biological products advisory committee meeting summary minutes. Available at: www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm343796. Accessed 21 January 2015.
- 20.Greenberg DP, Robertson CA, Landolfi VA et al. Safety and immunogenicity of an inactivated quadrivalent influenza vaccine in children 6 months through 8 years of age. Pediatr Infect Dis J 2014; 33:630–6. [DOI] [PubMed] [Google Scholar]
- 21.Tinoco JC, Pavia-Ruz N, Cruz-Valdez A et al. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged ≥18 years: a phase III, randomized trial. Vaccine 2014; 32:1480–7. [DOI] [PubMed] [Google Scholar]
- 22.Pepin S, Donazzolo Y, Jambrecina A et al. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine 2013; 31:5572–8. [DOI] [PubMed] [Google Scholar]
- 23.Kieninger D, Sheldon E, Lin WY et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years. BMC Infect Dis 2013; 13:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg DP, Robertson CA, Noss MJ et al. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine 2013; 31:770–6. [DOI] [PubMed] [Google Scholar]
- 25.Langley JM, Carmona Martinez A, Chatterjee A et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate: a phase III randomized controlled trial in children. J Infect Dis 2013; 208:544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domachowske JB, Pankow-Culot H, Bautista M et al. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3–17 years. J Infect Dis 2013; 207:1878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langley JM, Wang L, Aggarwal N et al. Immunogenicity and reactogenicity of an inactivated quadrivalent influenza vaccine administered intramuscularly to children 6 to 35 months of age in 2012–2013: a randomized, double-blind, controlled, multicenter, multi-country, clinical trial. J Pediatr Infect Dis Soc 2015; 4:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter EB, Rajagopal S, Zhu Y et al. Trivalent inactivated influenza vaccine (TIV) immunogenicity in children 6 through 23 months of age: do children of all ages respond equally? Vaccine 2010; 28:4376–83. [DOI] [PubMed] [Google Scholar]
- 29.Walter EB, Neuzil KM, Zhu Y et al. Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming? Pediatrics 2006; 118:e570–8. [DOI] [PubMed] [Google Scholar]
- 30.Jain VK, Rivera L, Zaman K et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med 2013; 369:2481–91. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC). Influenza-associated pediatric deaths--United States, September 2010–August 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1233–8. [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Past weekly surveillance reports. Available at: www.cdc.gov/flu/weekly/pastreports.htm. Accessed 21 January 2015. [Google Scholar]
- 33.Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–8. [DOI] [PubMed] [Google Scholar]
- 34.Van Bellinghen LA, Meier G, Van Vlaenderen I. The potential cost-effectiveness of quadrivalent versus trivalent influenza vaccine in elderly people and clinical risk groups in the UK: a lifetime multi-cohort model. PLoS One 2014; 9:e98437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clements KM, Meier G, McGarry LJ et al. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum Vaccin Immunother 2014; 10:1171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]