Abstract
Qualitative polymerase chain reaction (PCR) was used to determine the prevalence of fecal excretion of BK virus, JC virus, and simian virus 40 in 1-year-old infants. Overall, 17.8% of 321 specimens from 64.1% of 39 infants were polyomavirus positive. These data suggest that the gastrointestinal tract may be a site of polyomavirus persistence in humans.
Keywords: BK virus, infancy, polyomavirus
Polyomaviruses are ubiquitous in the human population, and acquisition, especially of BK virus (BKV), is believed to occur primarily in childhood [1]. Several anatomic sites of BKV persistence have been identified, including the urinary tract, the gastrointestinal tract, the tonsils, and leukocytes, but the mode of transmission has not been confirmed. Several reports have suggested that the gastrointestinal tract is a common site of polyomavirus excretion, and it is logical to hypothesize that it may also be the initial site of infection. Environmental virology studies have detected the presence of BKV and JC virus (JCV) in sewage samples and polluted waters from sites throughout the world, a finding that is consistent with urinary and/or fecal excretion [2–5]. These studies also provided estimates of the extended stability of JCV and BKV in the environment, a potentially important factor in the ubiquity of infection and probability of recurrent exposure to polyomaviruses throughout life. Point-prevalence studies have detected BKV infrequently in urine specimens from healthy children and adults [6–8], whereas fecal excretion of BKV and JCV has been detected in both children and adults [9–12]. The aims of this study were to confirm polyomavirus fecal excretion by young children and to determine the prevalence and duration of BKV shedding in the stool of healthy 1-year-old children by using a unique and well-characterized specimen set that was collected and maintained under rigorous standards.
MATERIALS AND METHODS
Specimens
Stool specimens collected prospectively between 1999 and 2001 from infants in San Pedro Mártir, Mexico City, who had enrolled in a long-term study were available from archival storage at the Cincinnati Children's Hospital Medical Center (Cincinnati, OH). The specimens had been collected weekly from each study participant and analyzed previously for the presence of diarrheal pathogens and the effects of breastfeeding [13]. After approval from the Baylor College of Medicine Institutional Review Board for Human Subjects Research (Houston, TX), a subset of deidentified specimens was aliquoted for this study. The original specimens (∼10% vol/vol in phosphate-buffered saline [PBS] at –70°C) were thawed at 4°C, and 1:10 dilutions (coded and deidentified) were prepared in PBS and stored at –20°C before being transferred to the Baylor College of Medicine.
Polymerase Chain Reaction Testing of Stool Samples
All specimen testing was performed in a laboratory that had not previously performed virology research and had not used simian virus 40 (SV40)-containing plasmids. The specimens were thawed at 4°C, and serial 10-fold dilutions ranging from 1:100 through 1:1 000 000 were prepared in PBS; negative controls were carried in parallel. Specimens were incubated at 95°C for 10 minutes, vortexed, and clarified by low-speed centrifugation before use as templates for polymerase chain reaction (PCR). Qualitative PCR using the primers PYV-for and PYV-rev was performed as previously described [10, 11]. These primers detect a sequence in the early region common to BKV, JCV, and SV40 with a limit of detection of approximately 250 genomes/mL. PCR amplicons of the appropriate size (∼187 bp) were cleaned using the Qiagen PCR clean-up kit before DNA sequence analysis by the LoneStar Laboratory (Houston, TX) using BigDye Terminator technology. Only the PCR amplicons with a definitive polyomavirus sequence were considered positive. Differences in polyomavirus DNA sequences in this region were compared to distinguish among BKV, JCV, and SV40 [10, 11].
Patterns of Viral Shedding
Aliquots of 321 stool specimens that had been collected weekly from 39 infants between 11 and 13 months of age were tested by PCR. The average number of specimens per subject was 8.2 (range, 4 to 9). Overall, 57 (17.8%) specimens were positive for human polyomaviruses; 48 (84.2%) were positive for BKV, and 9 (15.8%) were positive for SV40. JCV was not detected. Thirty-six (63.2%) virus-positive stool specimens were detected at a dilution of 1:100 000, the most common positive stool dilution. Eleven (19.3%) of the 57 virus-positive specimens were positive at more than 1 test dilution.
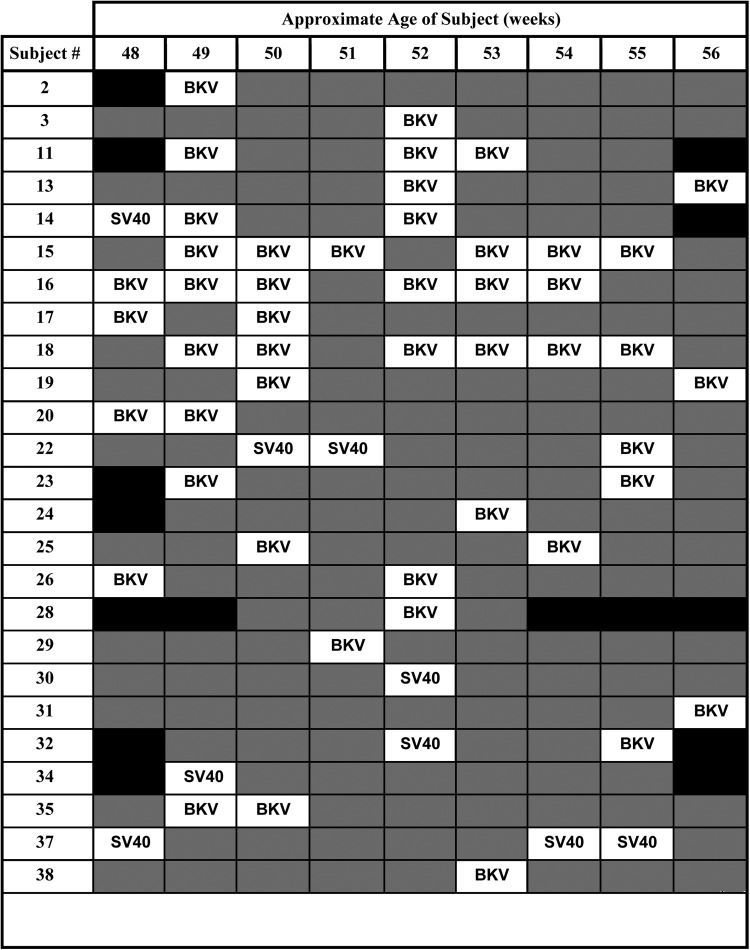
Twenty-five (64.1%) of 39 subjects provided at least 1 polyomavirus-positive specimen during the period of study, whereas 16 (41% of all subjects, 64% of virus-positive subjects) provided 2 or more positive samples (Figure 1). Twenty-one (53.8%) infants tested positive for BKV, and 6 (15.4%) tested positive for SV40; 3 infants tested positive for both SV40 and BKV. Among virus-shedding subjects, the frequency of positive samples averaged 27% (range, 11% to 67%). Some subjects shed BKV intermittently over a 7-week period, with 3 subjects testing positive for BKV in 6 of 9 specimens. There was no association of polyomavirus shedding with diarrheal disease or breastfeeding (data not shown).
Figure 1.
Timing of polyomavirus shedding in fecal specimens from 25 subjects between 48 and 56 weeks of age with 1 or more positive specimens. Viruses detected in individual fecal specimens are indicated (BKV and SV40). Shaded boxes indicate that no viruses were detected; solid black boxes indicate that no specimens were available.
This longitudinal multispecimen prevalence study demonstrated that polyomavirus excretion, especially BKV excretion, is common in early childhood and may be even more common than predicted on the basis of some seroprevalence data [14–16]. Our earlier study of fecal polyomavirus excretion in a pediatric population in Houston revealed that BKV was commonly excreted in childhood, even among infants. This study, based on a series of stool specimens collected frequently from Mexican infants, revealed that the analysis of a single specimen per subject would significantly underestimate the true prevalence of fecal polyomavirus shedding. This study also demonstrated that some infants are consistent shedders of detectable levels of BKV (>50% virus-positive specimens), whereas most infants are sporadic shedders (<30% virus-positive specimens). These differences might represent recent versus more remote BKV infections, respectively, or different viral loads, with lower levels escaping detection. It is also possible that the viruses are not uniformly distributed within the bulk of a stool specimen, so that an inadvertent sampling bias might explain the intermittent detection of particular viruses. The lack of detection of JCV in this study is not unexpected because of the young age of the subjects. Seroprevalence data indicate that JCV infections commonly occur later than in the first year of life, and our previous study of fecal polyomavirus shedding by children also failed to detect JCV. We have identified JCV in fecal specimens from adult subjects, indicating that the failure to detect JCV in pediatric populations is not a methodological flaw in the assay [10]. The developmentally regulated expression of sialic acid residues in the gastrointestinal tract, which serve as receptors for BKV, likely contributes to the finding of BKV in the feces of young infants [17, 18].
There are several limitations to this study. The question of whether these results reflect, at least in part, urinary contamination of fecal specimens cannot be answered here. However, urinary shedding of BKV is infrequent, and viral loads are generally low in immunocompetent subjects (even in pregnant women [19, 20]). As such, a significant contribution of urinary excretion is inconsistent with the results reported here, because the majority of the specimens tested positive at a dilution of 1:100 000. It is not known how closely the pattern of frequent early infection in these infants from Mexico reflects patterns that occur in other populations. A larger prospective study of longer duration will be necessary to address such issues, to evaluate differences in the natural histories of BKV and JCV, and to ascertain the presence of infectious viruses in feces. It is possible that the interval between collection and analysis of the specimens (and the possible degradation of viral DNA during prolonged cryopreservation) resulted in an underestimation of the true prevalence of BKV infection and excretion. Despite the fact that extreme precautions were taken to avoid potential contamination, the detection of SV40 T-antigen DNA sequences does not provide definitive proof of SV40 infection. As such, more sensitive methods will be required to validate this finding.
Fecal shedding of polyomaviruses has been detected in humans and other species, including chimpanzees and geese, and in cage waste of SV40-infected rhesus macaques [21–25]. In addition, we have detected squirrel monkey polyomavirus in a high proportion of stool specimens from Bolivian squirrel monkeys (J.A.V., unpublished data). The common finding of fecal polyomavirus excretion among species known to harbor polyomaviruses suggests that gastrointestinal tract persistence may be a feature of the natural history of polyomaviruses. This idea is supported by the detection of the cynomolgus polyomavirus in smooth muscle of the gastrointestinal tract in an immune-suppressed cynomolgus macaque with diarrhea [26].
In summary, this study demonstrates the high frequency of fecal BKV shedding in infancy and indicates that BKV infection occurs during the first year of life for the majority of infants in the Mexican population analyzed. These observations provide additional evidence that stool, especially from infants, should be considered a source of BKV exposure in humans and suggest that fecal–oral transmission is a possible explanation for the ubiquity of BKV in the human population.
Acknowledgments
We thank Jareen Meinzen-Derr for assistance with specimen tracking and data analysis. The authors declare that they have no conflict of interest related to the conduct, outcome, or publication of this manuscript.
Financial support. This work was supported by a Junior Faculty Seed Funding Grant from the Baylor College of Medicine (to J.A.V.) and research grant R01 CA104818 (to J.S.B.) from the National Cancer Institute. Sample acquisition and storage was made possible through NIH grant P01 HD 13021 (to A.L.M., G.M.R.-P., and X.J.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Knowles WA. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV). Adv Exp Med Biol 2006; 577:19–45. [DOI] [PubMed] [Google Scholar]
- 2.Bofill-Mas S, Formiga-Cruz M, Clemente-Casares P et al. . Potential transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA. J Virol 2001; 75:10290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bofill-Mas S, Pina S, Girones R. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl Environ Microbiol 2000; 66:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQuaig SM, Scott TM, Harwood VJ et al. . Detection of human-derived fecal pollution in environmental waters by use of a PCR-based human polyomavirus assay. Appl Environ Microbiol 2006; 72:7567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McQuaig SM, Scott TM, Lukasik JO et al. . Quantification of human polyomaviruses JC virus and BK virus by TaqMan quantitative PCR and comparison to other water quality indicators in water and fecal samples. Appl Environ Microbiol 2009; 75:3379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang D, Wang M, Ou WC et al. . Genotypes of human polyomaviruses in urine samples of pregnant women in Taiwan. J Med Virol 1996; 48:95–101. [DOI] [PubMed] [Google Scholar]
- 7.Jin L, Gibson PE, Booth JC, Clewley JP. Genomic typing of BK virus in clinical specimens by direct sequencing of polymerase chain reaction products. J Med Virol 1993; 41:11–7. [DOI] [PubMed] [Google Scholar]
- 8.Vanchiere JA, White ZS, Butel JS. Detection of BK virus and simian virus 40 in the urine of healthy children. J Med Virol 2005; 75:447–54. [DOI] [PubMed] [Google Scholar]
- 9.Bialasiewicz S, Whiley DM, Lambert SB et al. . Detection of BK, JC, WU, or KI polyomaviruses in faecal, urine, blood, cerebrospinal fluid and respiratory samples. J Clin Virol 2009; 45:249–54. [DOI] [PubMed] [Google Scholar]
- 10.Vanchiere JA, Abudayyeh S, Copeland CM et al. . Polyomavirus shedding in the stool of healthy adults. J Clin Microbiol 2009; 47:2388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanchiere JA, Nicome RK, Greer JM et al. . Frequent detection of polyomaviruses in stool samples from hospitalized children. J Infect Dis 2005; 192:658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong AS, Cheng VS, Yuen KY et al. . High frequency of polyoma BK virus shedding in the gastrointestinal tract after hematopoietic stem cell transplantation: a prospective and quantitative analysis. Bone Marrow Transplant 2009; 43:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meinzen-Derr JK, Guerrero ML, Altaye M et al. . Risk of infant anemia is associated with exclusive breast-feeding and maternal anemia in a Mexican cohort. J Nutr 2006; 136:452–58. [DOI] [PubMed] [Google Scholar]
- 14.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog 2009; 5:e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowles WA, Pipkin P, Andrews N et al. . Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 2003; 71:115–23. [DOI] [PubMed] [Google Scholar]
- 16.Viscidi RP, Rollison DE, Sondak VK et al. . Age-specific seroprevalence of Merkel cell polyomavirus, BK virus, and JC virus. Clin Vaccine Immunol 2011; 18:1737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbe-Masselot C, Maes E, Rousset M et al. . Glycosylation of human fetal mucins: a similar repertoire of O-glycans along the intestinal tract. Glycoconj J 2009; 26:397–413. [DOI] [PubMed] [Google Scholar]
- 18.Varki A. Sialic acids in human health and disease. Trends Mol Med 2008; 14:351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kling CL, Wright AT, Katz SE et al. . Dynamics of urinary polyomavirus shedding in healthy adult women. J Med Virol 2012; 84:1459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClure GB, Gardner JS, Williams JT et al. . Dynamics of pregnancy-associated polyomavirus urinary excretion: a prospective longitudinal study. J Med Virol. 2012; 84:1312–22. [DOI] [PubMed] [Google Scholar]
- 21.Bofill-Mas S, Albinana-Gimenez N, Pipkin PA et al. . Isolation of SV40 from the environment of a colony of cynomolgus monkeys naturally infected with the virus. Virology 2004; 330:1–7. [DOI] [PubMed] [Google Scholar]
- 22.Johne R, Enderlein D, Nieper H, Muller H. Novel polyomavirus detected in the feces of a chimpanzee by nested broad-spectrum PCR. J Virol 2005; 79:3883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim ES, Reyes A, Antonio M et al. . Discovery of STL polyomavirus, a polyomavirus of ancestral recombinant origin that encodes a unique T antigen by alternative splicing. Virology 2013; 436:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palya V, Ivanics E, Glavits R et al. . Epizootic occurrence of haemorrhagic nephritis enteritis virus infection of geese. Avian Pathol 2004; 33:244–50. [DOI] [PubMed] [Google Scholar]
- 25.Siebrasse EA, Reyes A, Lim ES et al. . Identification of MW polyomavirus, a novel polyomavirus in human stool. J Virol 2012; 86:10321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Gorder MA, Della PP, Henson JW et al. . Cynomolgus polyoma virus infection: a new member of the polyoma virus family causes interstitial nephritis, ureteritis, and enteritis in immunosuppressed cynomolgus monkeys. Am J Pathol 1999; 154:1273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]