We showed that the right ventricle unloaded by venoarterial extracorporeal membrane oxygenation (VA-ECMO) has diminished capacity to alter substrate utilization compared with the left ventricle. This decrease in metabolic flexibility contributes to the inability to increase high-energy phosphate reserves during myocardial rest by VA-ECMO.
Keywords: cardiac metabolism, extracorporeal circulation, thyroid hormone, pediatrics
Abstract
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) provides hemodynamic rescue for patients encountering right or left ventricular (RV or LV) decompensation, particularly after surgery for congenital heart defects. ECMO, supported metabolically by parenteral nutrition, provides reductions in myocardial work and energy demand and, therefore, enhances functional recovery. The RV must often assume systemic ventricular pressures and function on weaning from VA-ECMO. However the substrate utilization responses of the RV to VA-ECMO or stimulation are unknown. We determined RV and LV substrate utilization response to VA-ECMO in immature swine heart. Mixed-breed male Yorkshire pigs (33–49 days old) underwent normal pressure volume loading (control, n = 5) or were unloaded by VA-ECMO (ECMO, n = 10) for 8 h. Five pigs with ECMO received intravenous thyroid hormone [triiodothyronine (T3)] to alter substrate utilization. Carbon 13 (13C)-labeled substrates (lactate and medium-chain and long-chain fatty acids) were systemically infused as metabolic tracers. Analyses by nuclear magnetic resonance showed that both ventricles have similar trends of fractional 13C-labeled substrate contributions to the citric acid cycle under control conditions. VA-ECMO produced higher long-chain fatty acids and lower lactate contribution to the citric acid cycle via inhibition of pyruvate dehydrogenase, whereas T3 promoted lactate metabolism in both ventricles. However, these metabolic shifts were smaller in RV, and RV fatty acid contributions showed minimal response to perturbations. Furthermore, VA-ECMO and T3 also achieved high [phosphocreatine]/[ATP] and low [NADH]/[NAD+] in LV but not in RV. These data suggest that the RV shows decreased ability to modify substrate utilization and achieve improvements in energy supply/demand during VA-ECMO.
NEW & NOTEWORTHY We showed that the right ventricle unloaded by venoarterial extracorporeal membrane oxygenation (VA-ECMO) has diminished capacity to alter substrate utilization compared with the left ventricle. This decrease in metabolic flexibility contributes to the inability to increase high-energy phosphate reserves during myocardial rest by VA-ECMO.
venoarterial extracorporeal membrane oxygenation (VA-ECMO) provides a method of hemodynamic rescue for infants and children encountering acute right (RV) or left ventricular (LV) decompensation, particularly after surgery for congenital heart defects (18, 19, 27). A basic premise behind this type of therapy is that ECMO unloads the heart and provides myocardial rest and recovery, while maintaining systemic circulation. During recent studies, our laboratory has explored this myocardial recovery period and if metabolic manipulation during VA-ECMO could alter the baseline energy state (5, 8, 9, 11, 12, 14, 22). Presumably, nutritional or hormonal modulation of metabolism could shorten the recovery period, and prompt earlier weaning from the circuit, and thus yield fewer adverse events. Unloading through VA-ECMO produces overall decreases in LV pyruvate flux and amino acid flux, while increasing relative long-chain free fatty acid (LCFA) flux (12). Thyroid hormone [triiodothyronine (T3)] supplementation increases flux through pyruvate decarboxylation (9, 11), whereas, in contrast, excess pyruvate provision increases its carboxylation and elevates citric acid cycle (CAC) intermediates without altering pyruvate decarboxylation (14). Furthermore, the LV responds differently to odd-numbered and even-numbered medium-chain fatty acids (MCFAs), which are frequently used as parenteral nutritional supplements during ECMO (8). Thus we have noted substantial metabolic flexibility within the LV, which can be manipulated and improve cardiac function during weaning from mechanical circulatory support.
VA-ECMO also yields unloading of the RV, which undergoes considerable stress particularly after palliative surgery for congenital heart defects. In these scenarios, the RV is exposed to pressure overload and may even be required to maintain systemic circulation. Perioperative RV failure that fails to respond to aggressive medical therapy occurs often and can require ECMO support as a bridge to recovery or transplant (4). VA-ECMO can also be used to rescue the RV after LV assist device-induced initiation or exacerbation of RV dysfunction (23). Thus VA-ECMO has been indicated in patients who suffered RV failure as well as LV or biventricular failure. However, we know little regarding the RV metabolic response to VA-ECMO. We need to determine this response to develop metabolic therapies, which can manipulate the RV energy state during ECMO. In the present studies, we have evaluated relative flux of multiple substrates through RV myocardium and determined response compared with the LV. We supplied lactate, MCFA and LCFAs, typical components of nutritional supply during ECMO, to both LV and RV. Because of physiological workload differences, difficulties exist in comparing metabolic responses between the two ventricles. We have obviated this difference by unloading both ventricles through VA-ECMO. We used an established porcine model, which emulates conditions during VA-ECMO in the human infant.
MATERIALS AND METHODS
Animals.
All experimental procedures were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by Seattle Children’s Institutional Animal Use and Care Committee. Fifteen male mixed-breed Yorkshire piglets (body weight 9.9–14.5 kg, age 33–49 days) were used for this study and were divided into three groups: normal circulation without ECMO as control (Ctrl) group, 8 h VA-ECMO (ECMO), and VA-ECMO with intravenous T3 supplement (ECMO-TH), as shown in Fig. 1A. Our laboratory’s published data (9, 11) showed that T3 infusion showed stimulation of relative LV pyruvate dehydrogenase (PDH) flux. We used this modality to determine response in RV compared with LV under ECMO and ECMO-TH. Piglets were fasted overnight, with free access to water. They were premedicated with an intramuscular injection of ketamine (33 mg/kg) and xylazine (2 mg/kg). After intubation through surgical tracheostomy, the piglets were mechanically ventilated with 40–60% inhaled oxygen and isoflurane (1–2%) to maintain general anesthesia.
Fig. 1.
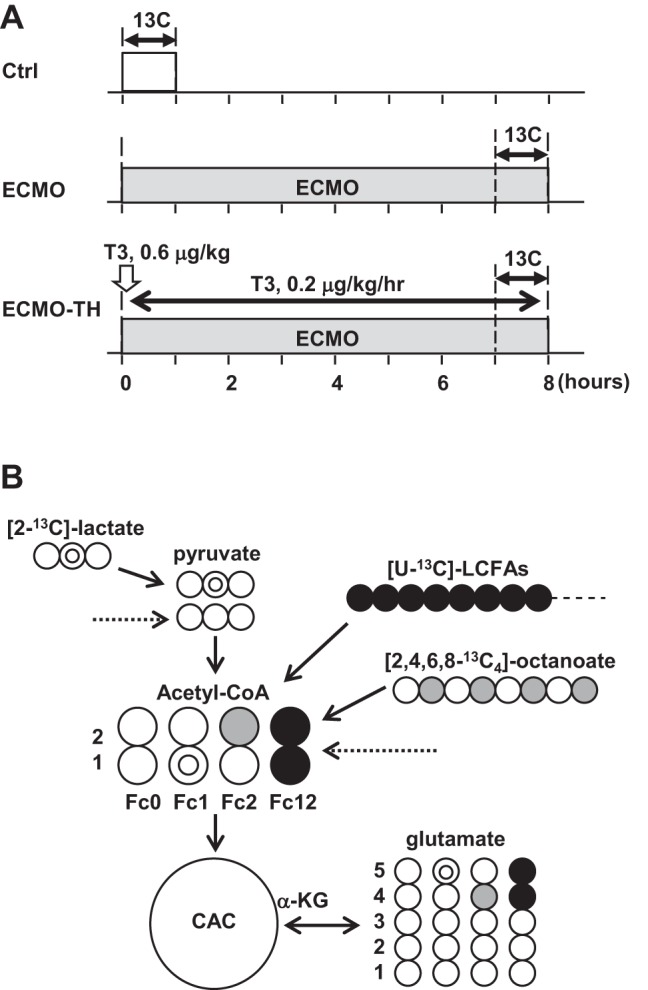
Diagram of experimental protocols and metabolic fate of 13C-labeled substrates. A: baseline hemodynamic and plasma data were performed before time 0. The duration of labeled substrate infusion was 60 min, and VA-ECMO duration was 8 h. Triiodothyronine (T3) was intravenously given in the ECMO-TH (VA-ECMO with intravenous T3 supplement) group. B: schematic demonstrating the 13C-labeling of acetyl-CoA and glutamate with [2-13C]lactate, [U-13C]LFCAs, and [2,4,6,8-13C4]octanoate as substrates at the end of first turn of the CAC. Double circles, 13C from [2-13C]lactate; shaded circles, 13C from [2,4,6,8-13C4]octanoate; solid circles, 13C from [U-13C]LFCAs; open circles, 12C. Fc, fraction of acetyl-CoA enriched in 13C; Ctrl, normal circulation without ECMO; ECMO, 8 h-VA-ECMO. Arrow with a dotted line is coming from endogenous substrates.
An arterial line for systemic blood pressure monitoring and blood sampling was placed in the femoral artery. A saline-filled catheter was inserted into the internal jugular vein for continuous heparin infusion and recording of the central venous pressure. Blood glucose, pH, Pco2, Po2, and hemoglobin were measured at regular intervals until the end of experiment by Radiometer ABL 800 (Radiometer America, Westlake, OH). After median sternotomy, a flow probe (Transonic Systems, Ithaca, NY) around ascending aorta was attached for measurement of cardiac output. The flow probe was placed distal to the insertion of the aortic ECMO cannula. Therefore, flow probe output during ECMO conditions reflected the sum of minimal LV ejection volume and pump flow. Both ventricular pressure catheters (Millar Instruments, Houston, TX) were directly inserted for RV and LV pressure measurement. The ascending aorta and right atrium were cannulated to create a VA-ECMO circuit for piglets in the ECMO and ECMO-TH groups. The ECMO circuit consisted of the following: a roller peristaltic pump console (Sarns 8000, Terumo, Tokyo, Japan); a hollow fiber membrane oxygenator (CX-RX05RW, Terumo). Management during VA-ECMO maintained pump flow rates of 80–100 ml·kg−1·min−1, a pH of 7.35–7.45, an arterial Pco2 of 35–45 mmHg, Po2 of >100 mmHg, and a rectal temperature of 36–37.5°C.
Infusion of labeled substrates and T3.
[2-13C]lactate and [2,4,6,8-13C]octanoate (MCFA) were obtained from Sigma, and [U-13C]LCFAs were obtained from Cambridge Isotope Laboratories (all 99% enriched). LCFAs consist of palmitic acid (45–55%), palmitoleic acid (10–15%), oleic acid (20–30%), and linoleic acid (10–15%). In this study, both LCFA and MCFA were used because these are major myocardial energy substrates provided to infants on ECMO (7), and they have different access to the oxidation pathways. Octanoate is an even MCFA with an eight-carbon and has been used as a typical MCFA for metabolic studies (8, 12, 13). [2-13C]lactate, [2,4,6,8-13C]octanoate, and [U-13C]LCFAs were used at 2.6, 0.8, and 0.8 μmol·kg body wt−1·min−1, respectively, and were delivered into the left atrium (Ctrl) or the aortic return cannula (ECMO and ECMO-TH) for the final 60 min of the protocol. Immediately on completion of the 13C-labeled substrates infusion, both ventricle free walls were quickly freeze-clamped, put in liquid nitrogen, and stored at −80°C for later extraction. The ECMO-TH group was received an intravenous bolus of T3 (3,3′,5-triiodo-l-thyronine; Sigma, St. Louis, MO) at a dose of 0.6 µg/kg just after starting VA-ECMO and then continuous intravenous infusion of T3 for 8 h at 0.2 µg·kg−1·h−1. Arterial plasma T3 concentration was measured using a commercial kit (Endocrine Technology, Newark, CA).
Metabolic analyses by NMR.
13C-NMR was performed on the myocardium as previously described for determination of specific carbon glutamate labeling (3, 11, 12). Briefly, freeze-clamped hearts were ground into fine powder under liquid nitrogen and extracted with methanol/chloroform. The final supernatant was lyophilized overnight at −50°C. Lyophilized heart extracts were dissolved in 99.8% 2H2O for NMR spectral acquisition. 13C-NMR spectra were acquired on a Varian Direct Drive (VNMRS) 600-MHz spectrometer (Varian, Palo Alto, CA) equipped with a Dell Precision 390 Linux workstation running VNMRJ 2.2C. The spectrometer system was outfitted with a Varian triple-resonance salt-tolerant cold probe with a cold carbon preamplifier. Protons were decoupled with a Waltz decoupling scheme. Final spectra (~6,000 scans, ~6 h) were obtained using a 45° excitation pulse (7.05 µs at 58 dB), with an acquisition time of 1.3 s, a recycle delay of 3 s, and a spectral width of 224.1 ppm. Fourier-transformed spectra were fitted with commercial software (NUTS, Acorn NMR, Livermore, CA). All of the labeled carbon resonances (C1–C5) of glutamate were integrated using the Lorentzian peak-fitting subroutine in the acquisition program, NUTS, as previously described (3, 11, 12). The individual integral values were used as starting parameters for the CAC analysis-fitting algorithm tcaCALC (kindly provided by the Advanced Imaging Research Center at the University of Texas Southwestern). This algorithm provided the fractional enrichment for each substrate to the acetyl-CoA pool entering the CAC (17). Label from exogenous [2-13C]lactate gives rise to label in the carbon 1 of acetyl-CoA (Fc1), while [2,4,6,8-13C]octanoate labels carbon 2 (Fc2), and [U-13C]LCFAs labels both carbons 1 and 2 (Fc12), allowing for determination of each substrate’s fractional contribution to the CAC (Fig. 1B).
Myocardial energy metabolites were measured by 1H-NMR spectra from extracted RV and LV tissues, as previously described (11, 12). Collected spectra were analyzed using Chenomx software (version 7.1, Chenomx) with quantifications based on spectral intensities relative to 0.5 mM 2,2-dimethyl-2-silapentane-5-sulfonate, which was added as a spike to each sample.
Immunoblot analysis.
Fifty micrograms of total protein extract from heart tissue were electrophoresed through 4.5% stacking and 10% running SDS-polyacrylamide gels and electroblotted onto PVDF-plus membranes. Equal protein loading of samples was determined by a protein assay (Bio-Rad, Hercules, CA), confirmed by reversible protein stain kit for PVDF membranes (Thermo Scientific, Rockford, IL) and probed with antibodies against α-tubulin (Cell Signaling Technology, Danvers, MA). Membranes were probed overnight at 4°C with primary antibodies dissolved in PBS-Tween 20 containing 5% milk or bovine serum albumin. The primary antibodies used in this study were 5′ adenosine monophosphate-activated protein kinase-α (AMPK-α), phospho-AMPK-α-Thr172, acetyl-CoA carboxylase (ACC), phospho-ACC-Ser79, and PDH, obtained from Cell Signaling Technology (Danvers, MA) and phospho-PDH-Ser293 obtained from Millipore (Billerica, MA). Blots were incubated at room temperature for 1 h with the appropriate secondary antibody conjugated to horseradish peroxidase. The blots were visualized with enhanced chemiluminescence after exposure to Kodak Biomax light ML-2 film. The densitometric intensities were determined using Image J analysis software (National Institutes of Health, Bethesda, MD). Western blots were repeated in triplicate to confirm the findings.
Statistical analyses.
Reported values are means ± SE in Figs. 1–4, text, and Table 1. The statistical differences between RV and LV were determined using paired t-test. Other statistical analyses were performed by one-way ANOVA for multiple comparisons with Tukey’s post hoc test. Criterion for significance was P < 0.05 for all comparisons.
Table 1.
Hemodynamic data at the end of the protocol
| Ctrl | ECMO | ECMO-TH | |
|---|---|---|---|
| Hemoglobin, g/dl | 8.9 ± 0.3 | 7.9 ± 0.5 | 8.2 ± 0 0.6 |
| Glucose, mg/dl | 96 ± 11 | 103 ± 11 | 87 ± 15 |
| HR, beats/min | 102 ± 9 | 115 ± 9 | 116 ± 7 |
| LVSP, mmHg | 75 ± 5 | 62 ± 2* | 61 ± 1* |
| LVEDP, mmHg | 8 ± 1 | 4 ± 1* | 4 ± 1* |
| RVSP, mmHg | 25 ± 2 | 16 ± 1* | 16 ± 1* |
| RVEDP, mmHg | 7 ± 1 | 5 ± 1 | 5 ± 2 |
| AoF, l/min | 1.17 ± 0.05 | 1.20 ± 0.06 | 1.13 ± 0.04 |
Values are means ± SE. HR, heart rate; LVSP, left ventricular systolic blood pressure; LVEDP, left ventricular end-diastolic pressure; RVSP, right ventricular systolic blood pressure; RVEDP, right ventricular end-diastolic pressure; AoF, aortic flow. AoF is equivalent to total systemic output from both ECMO pump and LV ejection.
P < 0.05 vs. Ctrl.
RESULTS
Fifteen pigs had no operative or technical complications during experiments. Table 1 showed hemodynamic data at the end of protocol just before heart extraction under ECMO support. After verifying stability of ECMO for around 30 min, these data were stable until completion of protocol (data not shown). The LV end-diastolic pressure decreased to <4 mmHg in all animals supported by ECMO. RV and LV systolic blood pressures were significantly lower in ECMO and ECMO-TH groups compared with the Ctrl group. Additionally, both RV and LV were unloaded well by palpation and visualization. Arterial plasma T3 concentrations at the end of protocol were 2.2 ± 0.3, 1.2 ± 0.1, and 2.4 ± 0.6 ng/ml in Ctrl, ECMO, and ECMO-TH, respectively. VA-ECMO significantly decreased circulating T3 levels, and our continuous T3 infusion maintained T3 levels dropped by VA-ECMO, as in our laboratory’s previous studies (5, 9, 11).
Substrate fractional contributions under control conditions.
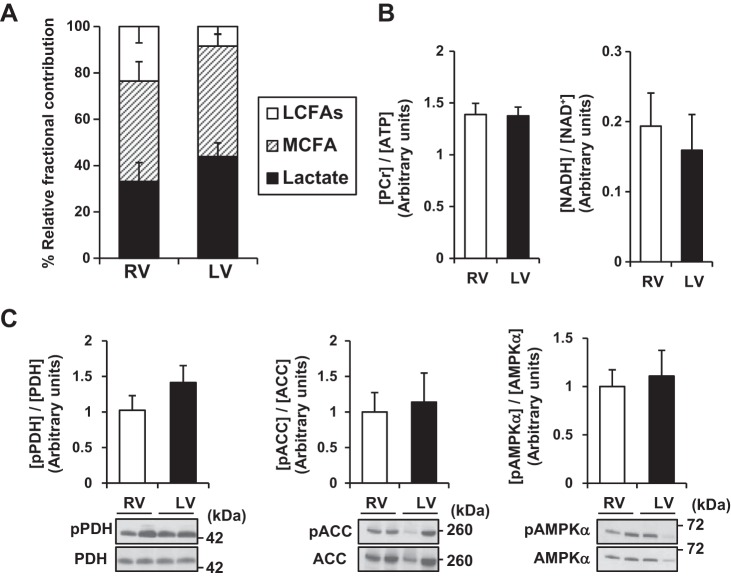
The first set of experiments provided metabolic comparisons between the RV and LV under control conditions. At baseline, we identified no differences in fractional substrate contributions of acetyl-CoA to the CAC between the two ventricles (Fig. 2A). With our protocol, lactate and MCFA provided the major labeled fraction of acetyl-CoA to the CAC. The ratio of myocardial phosphocreatine (PCr) to ATP (PCr concentration to ATP concentration; [PCr]/[ATP]), which reflects the balance of energy consumption and energy supply in the heart, was similar between LV and RV (Fig. 2B). The reduced form of nicotinamide adenine dinucleotide (NADH) ultimately donates electrons to the mitochondrial electron transport chain. Therefore, the reduced/oxidized ratio of nicotinamide adenine dinucleotide (NADH concentration to NAD+ concentration; [NADH]/[NAD+]) reflects the redox status and the activity of the mitochondrial electron transport chain (15). [NADH]/[NAD+] was also similar between LV and RV under control conditions. Western blotting was used so that phosphorylation statuses of three major metabolic enzymes, which serve as surrogates for enzyme activity, were similar between the two ventricles (Fig. 2C). Thus these data suggest that, under control conditions, no general differences between LV and RV occur in flux or in regulation of these major substrates. However, the RV sustains much lower pressure and thus lower energy requirement than the LV under control condition.
Fig. 2.
Metabolic profile in RV and LV under normal heart without ECMO support. Compared with LV, RV showed similar relative substrate fractional contributions (A), energy component ratios (B), and activities of metabolic enzymes (C) in Ctrl (normal circulation without ECMO) group. Relative fractional contributions represent percentage of total contribution of labeled substrate. Values are means ± SE; n = 5. LCFAs, long-chain fatty acids; MCFA, medium-chain fatty acid; PCr, phosphocreatine; NADH, the reduced form of nicotinamide adenine dinucleotide; NAD+, the oxidized form of nicotinamide adenine dinucleotide; pPDH, phosphorylated pyruvate dehydrogenase; pACC, phosphorylated acetyl-CoA carboxylase; pAMPK-α, phosphorylated 5′ adenosine monophosphate-activated protein kinase-α.
Metabolic response to ECMO and ECMO with T3 supplementation.
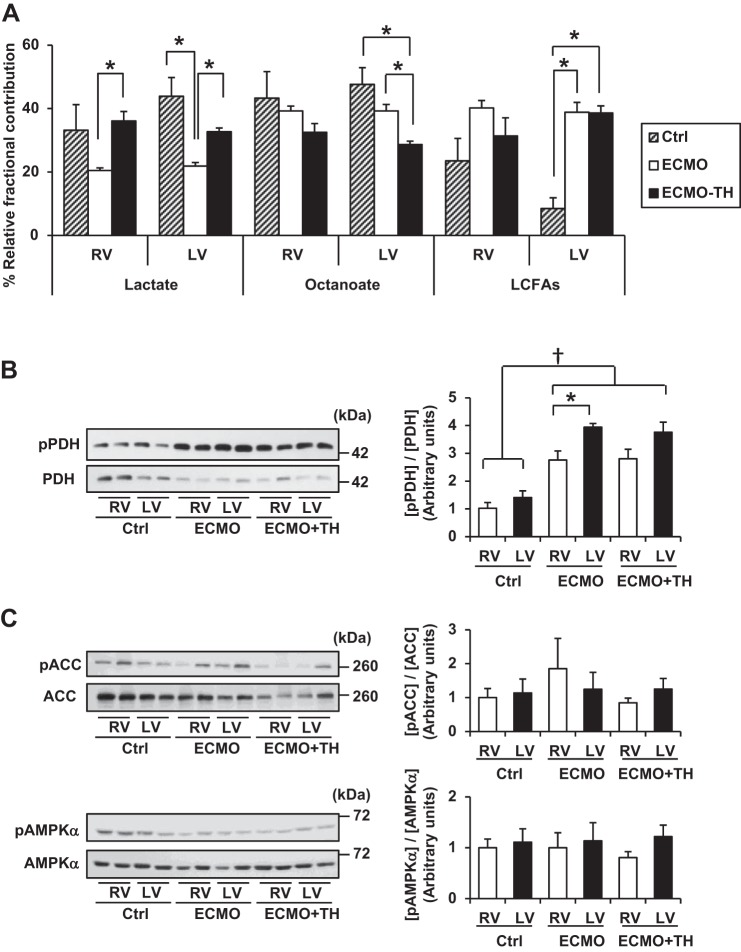
ECMO induces volume unloading of both ventricles. Thus with total volume unloading, as previously shown in our protocol, we effect a marked reduction in myocardial oxygen consumption (10, 12, 22). Under a similar workload, we did note some differences in metabolic responses between the two ventricles. The RV under ECMO shows no difference in fractional contribution in substrates compared with control conditions (Fig. 3A). However, the LV responds to volume unloading with a significant shift: relative lactate contribution decreases, whereas, through the Randle mechanism, LCFA contribution shows a marked and significant increase. These reciprocal changes in substrate contribution are associated with an increase in PDH phosphorylation, an indicator of deactivation or inhibition of this enzyme. Phosphorylated PDH is significantly greater in LV than RV (Fig. 3B). Neither AMPK nor ACC, also considered primary regulators of substrate flux, changes with volume unloading in either ventricle (Fig. 3C). Thus the data suggest that, with these levels of work, PDH is the primary regulator in the LV, but the RV lacks this mechanism.
Fig. 3.
Relative fractional contribution from each 13C-labeled substrate to acetyl-CoA by 13C-NMR and representative immunoblots of PDH, ACC, and AMPK-α with densitometric analysis. A: compared with LV, RV showed similar shift of substrate utilizations to CAC, according to ventricular unloading and T3 supplementation. However, RV had a weaker response to these stimulations than LV. B: [pPDH]/[PDH], ratio of inactivated PDH, increased in both RV and LV under ECMO (8 h-VA-ECMO) and ECMO-TH (VA-ECMO with intravenous T3 supplement) compared with Ctrl (normal circulation without ECMO). When RV and LV were compared, PDH in LV was more strongly inhibited under ECMO. C: [pACC]/[ACC] and [pAMPK-α]/[AMPK-α] were not different between RV and LV under any conditions. Phosphorylation of each protein was detected on the same gel of each protein following reprobing of membranes. Values are means ± SE; n = 5/group. *P < 0.05. †P < 0.01. pPDH, phosphorylated pyruvate dehydrogenase; pACC, phosphorylated acetyl-CoA carboxylase; pAMPK-α, phosphorylated 5′ adenosine monophosphate-activated protein kinase-α.
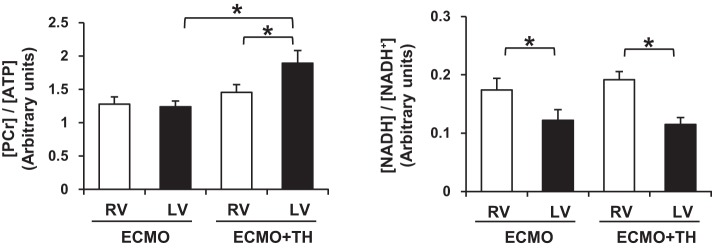
Previous studies have shown that systemic thyroid hormone (T3) levels drop during ECMO (5, 9, 11). T3 supplementation specifically stimulates acetyl-CoA flux through PDH and increases contribution lactate to the CAC in both ventricles, although there is no coexisting decrease in PDH phosphorylation. CAC contribution through octanoate decreases in both ventricles, although not significantly in RV (Fig. 3A). Again, phosphorylation of AMPK and ACC does not respond to this stimulation (Fig. 3C). T3 supplementation has been previously shown to increase LV [PCr]/[ATP], a surrogate for phosphorylation potential (Fig. 4). Similarly, T3 increased [PCr]/[ATP] in LV but not in RV. In addition, [NADH]/[NAD+] was significantly higher in RV than in LV under ECMO and ECMO-TH.
Fig. 4.
Energy metabolite ratios by 1H-NMR spectra from RV and LV tissues at the end of the protocol in ECMO (8 h-VA-ECMO) and ECMO-TH (VA-ECMO with intravenous T3 supplement) groups. [PCr]/[ATP] was not different between RV and LV in ECMO group, whereas [PCr]/[ATP] in ECMO-TH group was significantly higher in LV than in RV. Moreover, ECMO with T3 increased [PCr]/[ATP] in LV but not in RV compared with ECMO only. [NADH]/[NAD+] in ECMO and ECMO-TH group was significantly higher in RV than in LV. Values are means ± SE; n = 5 per group. *P < 0.05. PCr, phosphocreatine; NADH, the reduced form of nicotinamide adenine dinucleotide; NAD+, the oxidized form of nicotinamide adenine dinucleotide.
DISCUSSION
The principal objective of this study was to determine whether the RV exhibits metabolic flexibility during changes in loading condition, either with or without further stimulation by T3 infusion. Some have postulated that inability of RV metabolism to respond to pressure overload leads to acute failure (20, 26). T3 supplementation during VA-ECMO and during reperfusion produces substantial increases in flux through PDH in the LV (5, 9, 11). In summary, we did not identify substantial differences in baseline metabolism between the two ventricles under control ambient conditions. Assessment of RV metabolism and comparisons to the LV pose several challenges. To start, these ventricles work with different pressure-loading conditions, as under control conditions RV systolic pressure is less than one-third LV (6). This difference in loading creates a quagmire for interpreting results from comparative studies. However, unloading through ECMO does yield fairly similar hemodynamic conditions for both ventricles simultaneously, but workload is minimal. Under these unloading conditions, we could not find substantial differences in phosphorylation potential between the LV and RV (Fig. 4). Furthermore, comparisons of fractional contributions to the CAC between the two ventricles during VA-ECMO or during T3 stimulation were not different (Fig. 3A).
Measurement of metabolic flux using our techniques requires direct sampling of tissue from myocardium. Therefore, we could not perform serial measures from each heart for various conditions. Accordingly, we could not perform accurate statistical comparisons for changes in substrate fractional contributions by each ventricle. Simple observation of the data suggests that fractional contribution of LCFAs increases mores substantially when the LV transitions from control to VA-ECMO support than when the RV does (Fig. 3A). These data imply that the LV shows more metabolic flexibility, at least with regard to LCFAs. However, the dynamic range for pressure change in the LV is substantially more than with the RV, and LCFA flux might be responding to the greater decrease in workload in LV. The metabolic flux data in LV do correspond to the significant increase in PDH phosphorylation during VA-ECMO, which generally accompanies a decrease in pyruvate decarboxylation and an increase in LCFA oxidation. Thus these data suggest that, with unloading, the PDH pathway changes more in LV than RV, although again the dynamic range in the RV is fairly low (Fig. 3B). However, the response to T3 with regard to relative lactate oxidation appeared to be similar in RV.
The robust response of the LV to T3 stimulation results in an increase in [PCr]/[ATP] (Fig. 4). Prior studies have also shown that RV exhibits reduced mitochondrial capacity, and minor increases in pressure loading workload produce decreases in phosphorylation potential (25, 28). This response contrasts to the mature LV, which maintains phosphorylation potential until very high workloads are attained (1, 16, 21, 29). Few comparisons between porcine RV and LV oxygen consumption are provided in the literature. Schwartz et al. (24) showed that, under control conditions, RV free wall oxygen consumption is ~70% of LV anterior wall. Although those investigators did not directly measure substrate fractional contributions to the CAC, they did show that substrate uptake did not keep pace with increases in oxygen consumption. Accordingly, they posed one hypothesis that decreases in [PCr]/[ATP] with increases in pressure loading and oxygen consumption were due to limitations in the RV ability to extract substrate. Although we did not measure myocardial oxygen consumption in these experiments, our data show decreased [PCr]/[ATP] in RV compared with LV and inability to raise phosphorylation potential through T3 stimulation. These data suggest then that the RV shows limited ability to adapt substrate utilization to increase high-energy phosphate reserve during myocardial rest through volume unloading. Carbohydrates are more oxygen efficient in producing ATP than are FAs, but this is only an important consideration during ischemia. In fact, FAs produce more ATP per carbon unit than carbohydrates (2). Thus the RV inability to increase relative FA oxidation could explain the limitations in increasing [PCr]/[ATP]. We did not specifically measure mitochondrial complex activities. However, we did show that the total RV [NADH]/[NAD+] values during VA-ECMO conditions were lower than LV values. [NADH]/[NAD+] responds to a variety of perturbations, such as altered NAD+ supply, and it also increases substantially with hypoxia. Higher [NADH]/[NAD+] has been noted in mouse models of mitochondrial complex I dysfunction (15). Therefore, under our fully aerobic conditions, we believe this ratio reflects some deficit in mitochondrial function, such impaired transport of NAD+ into the mitochondria or inability to oxidize NADH by complex I.
This study has some limitations. First, in an effort to avoid extensive instrumentation in these small pigs cannulated for ECMO, we did not measure myocardial oxygen consumption in RV and LV. For this parameter, exact coronary flow and coronary venous blood gas data for each ventricle were required; however, it was technically difficult to conduct these measurements for small piglets. As the heart was near empty and ejecting minimal blood during our ECMO conditions, it was difficult to accurately determine pressure-volume workload on both ventricles. Second, as noted, the nature of the experiment did not allow direct measurement of metabolic changes during perturbations in individual hearts.
In summary, the RV appears slightly less responsive metabolically to pressure-volume loading changes than the LV, although this appearance could be tempered by the dynamic range of the hemodynamic parameters. This finding in RV is supported by a seemingly lower response to T3 hormone, which promotes flux through PDH. Overall, the data suggest that metabolic perturbations, such as switches in substrate supply during VA-ECMO, would affect the RV to a lesser extent than LV. This finding would have clinical indications regarding metabolic preparation by modifying substrate in nutrition supply, or training for weaning through intermittent loading, when the RV is the primary site of insult during surgery for congenital heart defects. However, our assessments do not accommodate fully for differences in hemodynamics and when the RV is exposed to pressure-loading often occurring with congenital heart defects. Metabolic studies under increased RV pressure-overloading conditions through pulmonary artery banding are underway to continue these evaluations.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-60666 (to M. A. Portman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.K. and M.A.P. conceived and designed research; M.K., D.R.L., and N.G.I. performed experiments; M.K., N.G.I., and M.A.P. analyzed data; M.K., D.R.L., N.G.I., and M.A.P. interpreted results of experiments; M.K. and M.A.P. prepared figures; M.K. and M.A.P. drafted manuscript; M.K., D.R.L., N.G.I., and M.A.P. edited and revised manuscript; M.K., D.R.L., N.G.I., and M.A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
A portion of the research was performed using Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
REFERENCES
- 1.Balaban RS, Kantor HL, Katz LA, Briggs RW. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science 232: 1121–1123, 1986. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- 2.Darvey IG. What factors are responsible for the greater yield of ATP per carbon atom when fatty acids are completely oxidised to CO2 and water compared with glucose? Biochem Educ 27: 209–210, 1999. doi: 10.1016/S0307-4412(98)00269-6. [DOI] [Google Scholar]
- 3.Des Rosiers C, Lloyd S, Comte B, Chatham JC. A critical perspective of the use of (13)C-isotopomer analysis by GCMS and NMR as applied to cardiac metabolism. Metab Eng 6: 44–58, 2004. doi: 10.1016/j.ymben.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Di Nardo M, MacLaren G, Marano M, Cecchetti C, Bernaschi P, Amodeo A. ECLS in pediatric cardiac patients. Front Pediatr 4: 109, 2016. doi: 10.3389/fped.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Files MD, Kajimoto M, O’Kelly Priddy CM, Ledee DR, Xu C, Des Rosiers C, Isern N, Portman MA. Triiodothyronine facilitates weaning from extracorporeal membrane oxygenation by improved mitochondrial substrate utilization. J Am Heart Assoc 3: e000680, 2014. doi: 10.1161/JAHA.113.000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 117: 1436–1448, 2008. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 7.Jaksic T, Hull MA, Modi BP, Ching YA, George D, Compher C; American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors . A.S.P.E.N. Clinical guidelines: nutrition support of neonates supported with extracorporeal membrane oxygenation. JPEN J Parenter Enteral Nutr 34: 247–253, 2010. doi: 10.1177/0148607110369225. [DOI] [PubMed] [Google Scholar]
- 8.Kajimoto M, Ledee DR, Olson AK, Isern NG, Des Rosiers C, Portman MA. Differential effects of octanoate and heptanoate on myocardial metabolism during extracorporeal membrane oxygenation in an infant swine model. Am J Physiol Heart Circ Physiol 309: H1157–H1165, 2015. doi: 10.1152/ajpheart.00298.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kajimoto M, Ledee DR, Xu C, Kajimoto H, Isern NG, Portman MA. Triiodothyronine activates lactate oxidation without impairing fatty acid oxidation and improves weaning from extracorporeal membrane oxygenation. Circ J 78: 2867–2875, 2014. doi: 10.1253/circj.CJ-14-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajimoto M, O’Kelly Priddy CM, Ledee DR, Xu C, Isern N, Olson AK, Des Rosiers C, Portman MA. Myocardial reloading after extracorporeal membrane oxygenation alters substrate metabolism while promoting protein synthesis. J Am Heart Assoc 2: e000106, 2013. doi: 10.1161/JAHA.113.000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajimoto M, O’Kelly Priddy CM, Ledee DR, Xu C, Isern N, Olson AK, Portman MA. Effects of continuous triiodothyronine infusion on the tricarboxylic acid cycle in the normal immature swine heart under extracorporeal membrane oxygenation in vivo. Am J Physiol Heart Circ Physiol 306: H1164–H1170, 2014. doi: 10.1152/ajpheart.00964.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajimoto M, O’Kelly Priddy CM, Ledee DR, Xu C, Isern N, Olson AK, Portman MA. Extracorporeal membrane oxygenation promotes long chain fatty acid oxidation in the immature swine heart in vivo. J Mol Cell Cardiol 62: 144–152, 2013. doi: 10.1016/j.yjmcc.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labarthe F, Gélinas R, Des Rosiers C. Medium-chain fatty acids as metabolic therapy in cardiac disease. Cardiovasc Drugs Ther 22: 97–106, 2008. doi: 10.1007/s10557-008-6084-0. [DOI] [PubMed] [Google Scholar]
- 14.Ledee DR, Kajimoto M, O’Kelly Priddy CM, Olson AK, Isern N, Robillard-Frayne I, Des Rosiers C, Portman MA. Pyruvate modifies metabolic flux and nutrient sensing during extracorporeal membrane oxygenation in an immature swine model. Am J Physiol Heart Circ Physiol 309: H137–H146, 2015. doi: 10.1152/ajpheart.00011.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE, Tian R. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 134: 883–894, 2016. doi: 10.1161/CIRCULATIONAHA.116.022495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Wang C, Murakami Y, Gong G, Ishibashi Y, Prody C, Ochiai K, Bache RJ, Godinot C, Zhang J. Mitochondrial ATPase and high-energy phosphates in failing hearts. Am J Physiol Heart Circ Physiol 281: H1319–H1326, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Malloy CR, Sherry AD, Jeffrey FM. Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers. Am J Physiol Heart Circ Physiol 259: H987–H995, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Mascio CE, Austin EH 3rd, Jacobs JP, Jacobs ML, Wallace AS, He X, Pasquali SK. Perioperative mechanical circulatory support in children: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg 147: 658–664, 2014. doi: 10.1016/j.jtcvs.2013.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merrill ED, Schoeneberg L, Sandesara P, Molitor-Kirsch E, O’Brien J Jr, Dai H, Raghuveer G. Outcomes after prolonged extracorporeal membrane oxygenation support in children with cardiac disease--Extracorporeal Life Support Organization registry study. J Thorac Cardiovasc Surg 148: 582–588, 2014. doi: 10.1016/j.jtcvs.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, Lopaschuk GD, Archer SL. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl) 88: 47–60, 2010. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portman MA, Qian K, Krueger J, Ning XH. Direct action of T3 on phosphorylation potential in the sheep heart in vivo. Am J Physiol Heart Circ Physiol 288: H2484–H2490, 2005. doi: 10.1152/ajpheart.00848.2004. [DOI] [PubMed] [Google Scholar]
- 22.Priddy CM, Kajimoto M, Ledee DR, Bouchard B, Isern N, Olson AK, Des Rosiers C, Portman MA. Myocardial oxidative metabolism and protein synthesis during mechanical circulatory support by extracorporeal membrane oxygenation. Am J Physiol Heart Circ Physiol 304: H406–H414, 2013. doi: 10.1152/ajpheart.00672.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherer M, Sirat AS, Moritz A, Martens S. Extracorporeal membrane oxygenation as perioperative right ventricular support in patients with biventricular failure undergoing left ventricular assist device implantation. Eur J Cardiothorac Surg 39: 939–944, 2011. doi: 10.1016/j.ejcts.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz GG, Greyson CR, Wisneski JA, Garcia J, Steinman S. Relation among regional O2 consumption, high-energy phosphates, and substrate uptake in porcine right ventricle. Am J Physiol Heart Circ Physiol 266: H521–H530, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GG, Steinman SK, Weiner MW, Matson GB. In vivo 31P-NMR spectroscopy of right ventricle in pigs. Am J Physiol Heart Circ Physiol 262: H1950–H1954, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, Michelakis ED. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 91: 1315–1327, 2013. doi: 10.1007/s00109-013-1059-4. [DOI] [PubMed] [Google Scholar]
- 27.Whitman GJ. Extracorporeal membrane oxygenation for the treatment of postcardiotomy shock. J Thorac Cardiovasc Surg 153: 95−101, 2017. doi: 10.1016/j.jtcvs.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Yi KD, Downey HF, Bian X, Fu M, Mallet RT. Dobutamine enhances both contractile function and energy reserves in hypoperfused canine right ventricle. Am J Physiol Heart Circ Physiol 279: H2975–H2985, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Duncker DJ, Xu Y, Zhang Y, Path G, Merkle H, Hendrich K, From AH, Bache RJ, Uğurbil K. Transmural bioenergetic responses of normal myocardium to high workstates. Am J Physiol Heart Circ Physiol 268: H1891–H1905, 1995. [DOI] [PubMed] [Google Scholar]