Vaccination against apolipoprotein B (ApoB), the protein of LDL, attracts attention as a novel approach to prevent atherosclerosis. We discovered major histocompatibility protein class II-restricted ApoB peptides, which reduce atherosclerosis and induce IL-10-producing CD4+ T cells and chemokine (C-C motif) receptor 5 expression on regulatory T cells, suggesting that immunization with ApoB peptides inhibits atherosclerosis by inducing anti-inflammatory cytokines.
Keywords: atherosclerosis, immunization, apolipoprotein B-100, MHC class II, IL-10, FoxP3, CCR5
Abstract
Although immunization with major histocompatibility complex (MHC) class II-restricted apolipoprotein B (ApoB) peptides has been shown to be atheroprotective, the mechanism is unclear. Here, we investigated CD4+ T cell populations in immunized atherosclerotic mice. Peptides (16-mers) from mouse ApoB, the core protein of low-density lipoprotein (LDL), were screened for binding to I-Ab by computer prediction and confirmed by radiolabeled peptide competition. Three new peptides, P101 (FGKQGFFPDSVNKALY, 5.5 nM IC50), P102 (TLYALSHAVNSYFDVD, 6.8 nM), and P103 (LYYKEDKTSLSASAAS, 95 nM), were tested in an atherosclerosis model (Apoe−/− mice on Western diet). Immunization with each of the three peptides (1 time in complete Freund’s adjuvant subcuntaneously and 4 time in incomplete Freund’s adjuvant intraperitoneally) but not with adjuvant alone showed significantly reduced atherosclerotic plaques in the aortic root by serial sections and in the whole aorta by en face staining. There were no differences in body weight, LDL cholesterol, or triglycerides. Peritoneal leukocytes from ApoB peptide-immunized mice, but not control mice, secreted significant amounts of IL-10 (150 pg/ml). Flow cytometry showed that peptide immunization induced IL-10 in 10% of peritoneal CD4+ T cells, some of which also expressed chemokine (C-C motif) receptor 5 (CCR5). Vaccination with ApoB peptides expanded peritoneal FoxP3+ regulatory CD4+ T cells and more than tripled the number of CCR5+FoxP3+ cells. Similar trends were also seen in the draining mediastinal lymph nodes but not in the nondraining inguinal lymph nodes. We conclude that vaccination with MHC class II-restricted autologous ApoB peptides induces regulatory T cells (Tregs) and IL-10, suggesting a plausible mechanism for atheroprotection.
NEW & NOTEWORTHY Vaccination against apolipoprotein B (ApoB), the protein of LDL, attracts attention as a novel approach to prevent atherosclerosis. We discovered major histocompatibility complex class II-restricted ApoB peptides, which reduce atherosclerosis and induce IL-10-producing CD4+ T cells and chemokine (C-C motif) receptor 5 expression on regulatory T cells, suggesting that immunization with ApoB peptides inhibits atherosclerosis by inducing anti-inflammatory cytokines.
both innate (26) and adaptive (21, 29) immunity play significant roles in the development and progression of atherosclerosis (15). Based on epidemiological (46) and experimental (34) studies, autoantibodies to oxidized low-density lipoprotein (oxLDL) are thought to be atheroprotective. However, a study showing that immunization with oxidized LDL reduced atherosclerosis despite the lack of antibody induction (13) suggests that T cell adaptive immunity plays a role in atheroprotection by vaccination.
Apolipoprotein B (ApoB) is the main protein component of LDL and a known autoantigen in atherosclerosis (18, 29). Several studies have reported that immunization with human ApoB peptides (p210 and p240) reduces atherosclerosis in Apoe−/− mice, although the mechanism was not addressed (16, 50). We have recently reported that vaccination with major histocompatibility complex (MHC) class II-restricted ApoB peptides reduces aortic plaque of atherosclerotic mice, but the atheroprotective mechanism remains unclear (44).
The role of T cells in atherosclerosis is receiving increased attention. Transfer of CD4+ T cells from Apoe−/− to immunodeficient Apoe−/− mice aggravated atherosclerosis (53), which suggests involvement of CD4+ T cells in the development of atherosclerosis. CD4+ T cells play an important role in adaptive immune response by recognizing antigenic peptides in a MHC class II-restricted manner. These cells are categorized into several functional subsets, such as Th1, Th2, Th17, regulatory T cell (Treg), and follicular helper T cell (TFH). Each of these CD4+ T cell subsets probably has a role in pathogenesis of atherosclerosis (21, 45). Th1 cells and associated cytokines such as IFN-γ have been shown to play a pathogenic role in development of atherosclerosis based on studies of inhibited Th1 polarization (24) and IFN-γ deficiency (4, 49) in atherosclerotic mice.
Tregs are CD25+FoxP3+CD4+ T cells that work as negative regulators of immune effector T cells (19, 39). Tregs can produce inhibitory cytokines such as IL-10 and TGF-β, which have been reported to be atheroprotective (21). The involvement of other T cells including Th2, Th17, and TFH in atherogenesis remains to be elucidated (21). We (28) and others (5) recently showed that Tregs switch their phenotype to Th1 and Th17, thus becoming proatherogenic.
This study was undertaken to test the hypothesis that vaccination with MHC-II-restricted ApoB peptides induces a Treg response. We monitored changes in T cell populations, transcription factors, and cytokines in mice immunized with ApoB peptides or no peptide (adjuvant only). To test whether the binding affinity of ApoB peptides to mouse MHC class II (I-Ab in C57BL/6 mice) might affect their effectiveness, we compared two new high affinity ApoB peptides (5.5 and 6.8 nM IC50, respectively) with one new intermediate affinity ApoB peptide (95 nM). Here, we report the discovery of three novel MHC class II-restricted antigenic peptides identified in the murine ApoB molecule. Immunization of Apoe−/− mice with each of these three peptides but not adjuvant alone reduces atherosclerosis, and this is associated with induction of Tregs and IL-10 production.
MATERIALS AND METHODS
Mice.
Eight-week-old female Apoe−/− mice on C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in a specific pathogen-free environment and fed chow diet until 10 wk of age. At 10 wk of age, mice were started on Western diet (WD; adjusted calories diet with 42% from fat; cat no. TD.88137; Harlan Laboratories) and remained on WD until death. Nur77-GFP reporter mice were purchased from Jackson Laboratories. All animal protocols were approved by the Animal Care Committee of La Jolla Institute for Allergy and Immunology.
Peptides.
All peptides (Fig. 1A) were custom synthesized as purified material by A&A Laboratories (San Diego, CA) and their I-Ab binding affinity was measured using a competitive inhibition assay utilizing purified MHC and high affinity radiolabeled I-Ab ligand, as previously described (42).
Fig. 1.
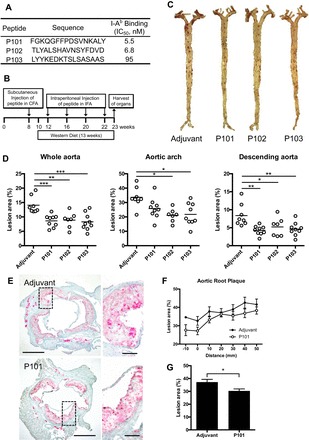
Aortic atherosclerosis is decreased in Apoe−/− mice vaccinated with major histocompatibility complex (MHC) II-restricted apolipoprotein B (ApoB) peptide P101, P102, or P103. A: peptide sequence and binding affinity to I-Ab. B: immunization scheme; female Apoe−/− mice were immunized once with either adjuvant only or peptide in complete Freund’s adjuvant (CFA) and then boosted four more times with adjuvant only or peptide incomplete Freund’s adjuvant (IFA). Western diet was maintained for 13 wk. Mice were killed and organs harvested at 23 wk of age. C: representative images of whole aorta stained with Sudan IV. D: atherosclerotic plaque was quantified as percentage of whole aorta, arch, or descending aorta. E: aortic roots were stained with Oil Red O and counterstained with hematoxylin. Representative photomicrographs and each image of high magnification are shown at right. Scale bar = 500 µm (low magnification) and 100 µm (high magnification). F: plaque as percentage of total area at each distance from the trileaflet valve obtained from serial sections. G: mean percentage of plaque lesion. Data are means ± SE; n = 7–9 in each group. *P < 0.05; **P < 0.01; ***P < 0.001. Significance was assessed by one-way ANOVA with Dunnett’s multiple comparison test (D) or Mann-Whitney test (G).
Atheroprotective immunization.
Previous work (13) has shown that atheroprotection through immunization could be achieved by injecting mice with LDL (or ox-LDL) with a combination of complete Freund’s adjuvant (CFA) initially, followed by antigen in incomplete Freund’s adjuvant (IFA) for booster immunizations. With the use of the same immunization model, 50 μg of P101, P102, or P103 were emulsified in CFA (BD Difco, Sparks, MD) and injected into the subcutaneous inguinal area at 8 wk of age. Repeated boosters with 50 μg of the same peptide emulsified in IFA (BD Difco) were administered intraperitoneally at age 12, 16, 20, and 22 wk. A WD was started at 10 wk of age. This immunization scheme will be referred to as 1xCFA + 4xIFA for the remainder of this manuscript (Fig. 1B). Mice were killed at age 23 wk and organs were harvested for analysis. Control immunizations with DMSO emulsified in CFA and IFA were also performed. Some experiments were done by subcutaneous injection of peptide with CFA at 8 wk and intraperitoneal injection of peptide with IFA at 10 wk, which is referred to as 1xCFA + 1xIFA for the remainder of this manuscript.
Atherosclerosis quantification.
Aortic root sections were examined as follows. Hearts were harvested, placed in optimal cutting temperature medium (OCT; Electron Microscopy Sciences, Hatfield, PA), and frozen at −80°C. Beginning at the first appearance of the trileaflet aortic valve, successive 5-μm transverse sections were made for a distance of 100 μm. From these, we analyzed every other section, for a total of 10 sections per root. Sections were then stained with Oil Red O and counterstained with hematoxylin. The extent of atherosclerosis was then determined as the area involved on each section. To measure en face lesion formation, the whole aorta was carefully cleaned in situ and then the whole aorta pinned out after paraformaldehyde incubation at room temperature for at least 2 h. Staining for atherosclerotic plaque was performed by incubating samples in Sudan IV. The area of whole aorta was determined using Photoshop CS (Adobe Systems). The quantification of plaque lesion was performed using Image-Pro Premier software (Media Cybernetics, Rockville, MD). The atherosclerotic lesion was determined independently by two investigators blinded as to the experiment groups.
Immunofluorescence.
Frozen sections of aortic root were thawed and fixed for 10 min in isopropanol on ice. Sections were blocked by 10% normal goat serum (G9023-10ML; Sigma) for 1 h at room temperature and then stained by rat anti-mouse chemokine (C-C motif) receptor (CCR)5-FITC (HEK/1/85a; abcam) and biotin rat anti-mouse CD4 (RM4–5; BD Biosciences) overnight followed by staining with secondary antibody: anti-FITC Alexa Fluor 488 (Molecular Probes) and Streptavidin Alexa Fluor 555. Nuclei were stained with Hoechst 33342 (Invitrogen). Images were acquired with slide scanner Axio Scan.Z1 (Zeiss) using ×40 0.95 NA objective and analyzed with imaging software ZEN (Zeiss).
Blood analysis.
Mouse whole blood was collected in EDTA tubes by cardiac heart puncture during organ harvest. The blood was spun at 3,000 g for 15 min at 4°C. The supernatant was collected and frozen at −80°C until analysis to reduce multiple freeze/thaw cycles. Lipid analysis was performed by IDEXX BioResearch.
Measurement of antibody titers to ApoB peptides.
Antibody titers in plasma were determined by chemiluminescent enzyme immunoassay as previously described (2). In brief, white flat-bottom plates (Fisher Scientific) were coated with various antigens at 5 μg/ml in PBS for overnight incubation. Following blocking with 1% BSA-TBS serum was added in increasing dilutions and incubated at room temperature for 60 min. Bound antibodies levels were detected using appropriate alkaline phosphatase-conjugated secondary IgG1 or IgG2c antibodies (Jackson ImmunoResearch) and a 50% aqueous solution of LumiPhos 530 (Lumigen). Data are expressed as relative light units counted per 100 ms (RLU/100 ms).
Flow cytometry.
Cell suspension was prepared from lymph nodes and peritoneal lavage through a 70-μm filter, incubated for 5 min with Fc Block (1:200), and subsequently stained with primary antibodies [CD45-Alexa Fluor 700 (30-F11; BioLegend), CD4-BV570 (RM4–5; BioLegend), TCRβ-APC-eFluor 780 (H57–597; eBioscience), CD25-PE (PC61; BD Bioscience), CCR5-Alexa Fluor 488 (HM-CCR5; BioLegend), and Ghost Dye UV 450 (Tonbo)]. For transcription factor staining, cells were fixed and permeabilized using the FoxP3/Transcription Factor Staining Buffer Set (eBioscience) and stained with FoxP3-PerCP-Cy5.5 (FJK-16s; eBioscience) for 30 min. For cytoplasmic cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate and ionomycin in the presence of monensin for 5 h, fixed, and permeabilized using the Intracellular Fixation and Permeabilization Buffer Set (eBioscience) and stained with IFN-γ-BV605 (XMG1.2; BioLegend), IL-10-PerCP-Cy5.5 (eBioscience), and IL-17A-PE-Cy7 (eBio1787; eBioscience). Samples were analyzed by LSR-II (BD Biosciences). Data were acquired on FACSDiva software (BD Biosciences) and analyzed by FlowJo.
Cytometric bead array.
Cell suspension was prepared from peritoneal lavage and lymph nodes of Apoe−/− mice vaccinated with each ApoB peptide (1xCFA + 1xIFA). Cells were incubated for 48 h in complete RPMI 1640 media containing 10% FBS, penicillin/streptomycin, l-glutamine, NEAA, HEPES, and sodium pyruvate in the presence or absence of the corresponding peptide. Cytokines secreted in culture supernatant were measured with mouse Th1/Th2/Th17 cytometric bead array (BD Biosciences). Secretion of IFN-γ, IL-2, IL-4, IL-6, IL-17A, TNF, and IL-10 was measured according to the manufacturer’s instructions.
Statistical analysis.
Between groups analysis was performed by one-way ANOVA with Dunnett’s multiple comparison test for multiple groups and Mann-Whitney test for two groups. Normality was verified using Shapiro-Wilk normality test. Homogeneity of variance was verified using Bartlett’s test. Data are expressed as means ± SE. P < 0.05 was considered significant.
RESULTS
Vaccination with I-Ab binding ApoB peptides, but not adjuvant alone, protects mice from atherosclerosis.
To examine the effect of three novel ApoB peptides, we vaccinated female Apoe−/− mice with three new ApoB peptides (Fig. 1A) using the same 1xCFA + 4xIFA protocol as previously reported (Fig. 1B) (44). Analysis of whole aorta showed significant reduction of atherosclerosis in P101-, P102-, and P103-immunized groups (39, 37, and 40% reduction, respectively, Fig. 1, C and D). Significant reduction of lesions was also observed in the aortic arch (P102 and 103) and descending aorta (P101, P102, and P103, Fig. 1D). This level of atheroprotection is similar to that found with two other MHC class II-restricted ApoB peptides tested in a previous study (44). There were no significant differences in the number of leukocytes, T cells, B cells, neutrophils, and macrophages as assessed by flow cytometry (n = 9 aortas each, data not shown).
Serial sections of aortic root (Fig. 1E) were analyzed to evaluate plaque. The percentage of atherosclerotic lesion tended to be decreased in P101-treated mice compared with adjuvant treated mice in most distance-matched sections (Fig. 1F). The mean percentage of plaque lesion area on each aortic root section was significantly decreased compared with the adjuvant-immunized group (Fig. 1G). There was no correlation between the I-Ab binding affinity and protection from atherosclerosis within the range (5.5 to 95 nM) tested.
Treatment with peptide did not affect body weight (data not shown). There were no significant differences in plasma levels of total cholesterol, triglyceride, LDL, and HDL among the groups (Table 1).
Table 1.
Lipid levels of Apoe−/− mice immunized with ApoB peptide
| Adjuvant | P101 | P102 | P103 | |
|---|---|---|---|---|
| TC, mg/dl | 1,125 ± 277 | 959 ± 145 | 993 ± 271 | 1,106 ± 221 |
| TG, mg/dl | 139 ± 79 | 121 ± 37 | 87 ± 12 | 131 ± 45 |
| HDL, mg/dl | 16 ± 4.9 | 13 ± 4.6 | 19 ± 10 | 15 ± 6.4 |
| LDL, mg/dl | 336 ± 97 | 276 ± 70 | 301 ± 97 | 332 ± 62 |
Values represent means ± SD; n = 4 in each group. TC, total cholesterol; TG, triglyceride. Plasma samples were collected from Apoe−/− mice on Western diet for 13 wk and lipid profile was analyzed.
Vaccination with ApoB peptides induces IL-10 in peritoneal CD4+ T cells.
To screen for changes in T cell populations, we looked for Tregs (FoxP3+CD25+), Th1 (IFN-γ+), and Th17 cells (IL-17A+RORγt+) in spleen, inguinal lymph nodes, and para-aortic lymph nodes in mice receiving adjuvant alone or ApoB peptide. None of the interventions caused significant changes in any of these populations (data not shown). Cytokine levels (IL-6, TNF, IL-10 and IFN-γ) measured by Cytometric Bead Array were also unchanged in supernatant from inguinal lymph nodes and para-aortic lymph nodes, whether unstimulated or restimulated with peptide (data not shown). IL-2 and IL-17A were undetectable (data not shown).
We reasoned that peritoneal T cells might show significant responses, because the boost injections were given intraperitoneally. Indeed, we found a significant two- to threefold expansion of IL-10-producing CD4+ T cells (gating scheme in Fig. 2A, all gates verified by fluorescence minus one) in the peritoneal cavity of mice vaccinated with P101 compared with adjuvant only controls (Fig. 2B). We also tested CCR5 expression on CD4 T cells because CCR5 has recently reported to be a chemokine receptor important for homing to atherosclerotic plaque (28). CCR5+CD4+ T cells were also expanded (Fig. 2C), and some of the IL-10-producing T cells expressed CCR5 (Fig. 2D). P102 and P103 vaccination showed similar results to P101, i.e., increased CCR5 expression and IL-10 production in peritoneal CD4 T cells (data not shown). There was no significant difference in other cytokines (IFN-γ and IL-17A, data not shown).
Fig. 2.
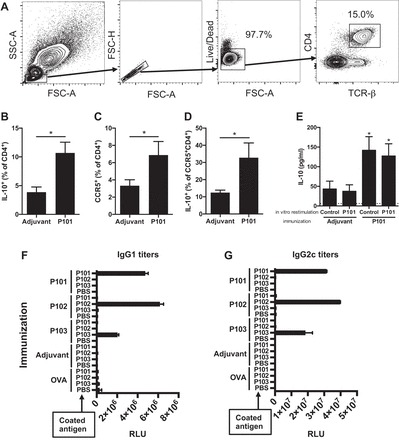
Immune response to vaccination with ApoB peptide. A: peritoneal cells were harvested from Apoe−/− mice immunized with ApoB peptide (1xCFA + 1xIFA) and analyzed by multicolor flow cytometry. All samples were stained with anti-IL-10 antibody after stimulation, staining of surface marker, and fixation. The analyzed populations are gated on live singlet TCRβ+CD4+ T cells. Percentage of IL-10-producing cells among CD4+ T cells (B), percentage of CCR5+ cells among CD4+ T cells (C), and percentage of IL-10-producing cells among CCR5+CD4+ T cells (D). E: peritoneal cells were harvested from peptide treated Apoe−/− mice and cultured with antigen-presenting cells in the presence or absence of the corresponding peptide. Secreted IL-10 level of culture supernatant was quantified with cytometric beads array. n = 6 (B–D) and n = 4 (E) in each group. *P < 0.05 vs. adjuvant group in B–E. Data are means ± SE. Significance was assessed by Mann-Whitney test (B–E). Plasma samples were collected from Apoe−/− mice on Western diet for 13 wk and the titer of IgG1 (F) and IgG2c (G) antibody binding to each ApoB peptide was measured by chemiluminescent enzyme immunoassay. The values represent relative light units (RLU); data are means ± SE. FSC, forward scatter; SSC, side scatter; TCR, T cell receptor.
To test whether IL-10 secretion was antigen specific, we restimulated immunized mice with the peptide with which they had been immunized. We found IL-10, as measured by bead-based immunoassay, was threefold elevated in the supernatants of cells from vaccinated mice, whether or not peptide was added (Fig. 2E). These experiments show that IL-10 production is vaccination specific and is induced by ApoB peptide vaccination without requirement for restimulation, i.e, secreted under baseline conditions.
Immunization with ApoB peptides induces peptide-specific IgG antibodies.
We have previously shown that vaccination with ApoB peptides induces peptide-specific antibodies, a convenient biomarker of antigen specificity (44). The production of IgG requires antigen-specific help by TFH cells and gives insight into antigen-specific T cell activation and lineage bias. IgG1 and IgG2c reflect Th2 and Th1 activity, respectively, in C57BL/6 mice, which do not express IgG2a (13). Pooled plasma samples from each group were analyzed for IgG titers by formal antibody dilution curves using chemiluminescent ELISA as previously described (44). As expected, IgG1 and IgG2c titers to P101, P102, and P103 peptides were increased in P101, P102, and P103 immunized mice, respectively (Fig. 2, F and G). These responses showed complete peptide specificity, with strong responses in both the Th1 and Th2 helper T cell compartments. In mice immunized with adjuvant only or OVA, IgG1 and IgG2c antibodies against ApoB peptides were undetectable, demonstrating exquisite and complete specificity.
FoxP3+ regulatory CD4+ T cells are modulated by vaccination.
We hypothesized that some of the IL-10 might be secreted by Tregs. Indeed, the percentage of FoxP3+CD4+ T cells was significantly increased in vaccinated mice (Fig. 3, A and C). In addition, CCR5 expression (Fig. 3, A and B) was also significantly increased among all CD4+ T cells (Fig. 3D) and among FoxP3+CD4+ T cells (Fig. 3, A, B, and E).
Fig. 3.
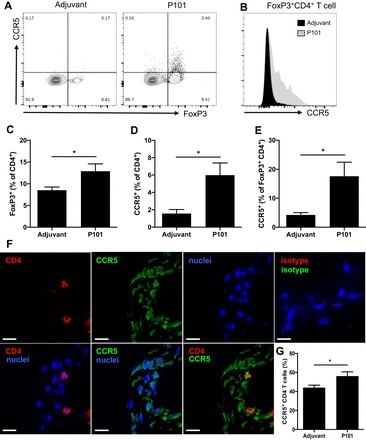
CCR5 expression in peritoneal FoxP3+CD4+ T cells in immunized mice allows homing to aorta. A: peritoneal cells were harvested from Apoe−/− mice immunized with P101 (1xCFA + 1xIFA) and analyzed by multicolor flow cytometry. All samples were stained with anti-FoxP3 antibody after staining of surface markers and fixation. Representative plots of CCR5 and FoxP3 expression are shown. Gating is based on fluorescence minus one controls. B: representative histograms of CCR5 expression on FoxP3+CD4+ T cells. Percentage of FoxP3+ (C) and CCR5+ (D) cells among CD4+ T cells and percentage of CCR5+ cells among FoxP3+CD4+ T cells (E). Frozen aortic root sections from Apoe−/− mice immunized with P101 on Western diet for 13 wk were fixed and stained with fluorescently labeled antibodies. (F) Representative immunofluorescence images of aortic roots from P101-immunized mouse stained with CD4 (red), CCR5 (green), and nuclei (blue). Top right: isotype control for CD4 and CCR5. Scale bars = 10 µm. G: Percentage of CCR5+ cells among CD4+ T cells is shown. n = 6 in each group (C, D, E, and G). *P < 0.05. Data are means ± SE. Significance was assessed by Mann-Whitney test (C, D, E, and G).
Immunofluorescence images showed infiltration of CCR5+CD4+ T cells into aortic plaques from Apoe−/− mice immunized with P101 was significantly increased (Fig. 3, F and G). These findings suggest that immunization with ApoB peptide modulates CCR5 expression in CD4+ T cells and especially in Tregs and that these CCR5+CD4+ T cells can migrate into atherosclerotic plaques.
T cell receptor activation in peritoneal cavity and mediastinal lymph nodes.
To visualize the T cell subset that responds to administered ApoB peptide, we examined activation of T cell receptor after immunization with P101 peptide (1xCFA + 2xIFA) using Nur77GFP mice. Nur77 (also known as Nr4a1), an orphan nuclear receptor whose function in T cell is not completely understood, is known as an immediate early gene upregulated by T cell receptor stimulation in thymocytes and T cells (33). In Nur77GFP mice, GFP is transiently upregulated in T cells after antigen receptor stimulation but not by inflammatory stimuli (31). As expected, Nur77 expression was significantly increased in peritoneal CD4 T cells (gating scheme in Fig. 4A) from mice immunized with P101 peptide (Fig. 4, B and D). The increase in Nur77 expression was observed mainly in FoxP3+ peritoneal T cells (Fig. 4, B and C). Indeed, FoxP3+Nur77+CD4+ double-positive T cells were also significantly increased in P101-immunized mice compared with adjuvant control (Fig. 4E). Nur77 expression was also increased in FoxP3+ and FoxP3– CD4+ T cells in the draining mediastinal lymph nodes from P101-treated mice compared with adjuvant control (Fig. 4B, bottom). These findings suggest that ApoB peptide activates T cell receptor in peritoneal cells and some of these activated T cells migrate into the draining mediastinal lymph nodes.
Fig. 4.
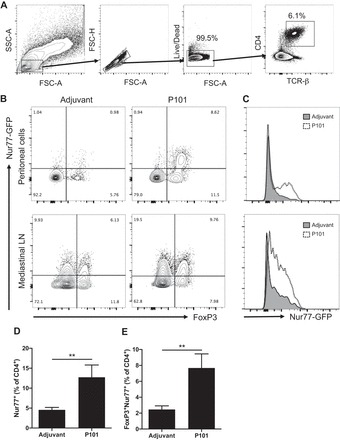
Activation of regulatory T cells (Tregs) and other CD4+ T cells in mice vaccinated with P101 peptide. A: peritoneal cavity and mediastinal lymph nodes were harvested from Nur77GFP mice immunized with P101 or adjuvant only and analyzed by multicolor flow cytometry at 24 hrs. The analyzed populations were gated on live singlet TCRβ+CD4+ T cells. B: representative plots of Nur77 (by GFP) and FoxP3 (by PerCP-Cy5.5 labeled antibody) expression on peritoneal CD4+ T cells (top) and mediastinal lymph node CD4+ T cells (bottom). C: histogram of Nur77-GFP expression among FoxP3+CD4+ T cell from peritoneal cavity (top) and mediastinal lymph node (bottom). Each plot is representative of 4 independent experiments. Percentage of Nur77+ cells (D) and FoxP3+Nur77+ cells (E) among peritoneal CD4+ T cells. n = 5 in each group. **P < 0.01. Data are means ± SE. Significance was assessed by Mann-Whitney test.
DISCUSSION
Here we show that vaccination with novel MHC class II-restricted ApoB peptides reduces atherosclerosis. These findings add three new atheroprotective ApoB peptides to the known two (44). As expected, the peptide with intermediate affinity (95 nM) was as effective as the peptides with high affinity (~6 nM). This suggests that the intermediate affinity peptide binds I-Ab sufficiently to induce an effective CD4 T cell response in our immunization protocol. In Table 2, we show all mouse ApoB peptides that bind I-Ab with affinities greater than 1 μM. When we studied the responses to one of these peptides, P101, in depth, we found that immunization increases IL-10, CCR5, and Foxp3 expression in CD4 T cells in the peritoneal cavity and mediastinal lymph node, suggesting a plausible mechanism for the atheroprotective effect.
Table 2.
Mouse ApoB peptide sequence and their I-Ab binding capacity
| Peptide | Sequence | ApoB100 Position | I-Ab (IC50 nM) | Peptide | Sequence | ApoB100 Position | I-Ab (IC50 nM) |
|---|---|---|---|---|---|---|---|
| ApoB3501–3516* | SQEYSGSVANEANVY | 3,501 | 4.3 | P103 | LYYKEDKTSLSASAAS | 3,953 | 95.1 |
| P101 | FGKQGFFPDSVNKALY | 705 | 5.5 | QYKYNQNFSAINNEHN | 3,089 | 101.7 | |
| P102 | TLYALSHAVNSYFDVD | 441 | 6.8 | QNHGYTIPVVNIEVSP | 3,241 | 122.8 | |
| ApoB978–993* | TGAYSNASSTESASY | 978 | 7.3 | EGSSVPIFEATIPEIH | 3,769 | 146.6 | |
| IDNIYIPAMGNFTYDF | 3,321 | 7.3 | WSASYTGGNTSRDHFS | 1,369 | 150.9 | ||
| QVLGKLLLSGAQTLQG | 793 | 7.3 | GSWNWACPNFSDEGIH | 2,921 | 184.0 | ||
| QSEQVKNFVASHIANI | 585 | 8.2 | VELAHRYSLSEPLQKL | 2,361 | 199.7 | ||
| SKGLLTFETSSALGPQ | 1,449 | 8.4 | STPSVTIPGPNIMVPS | 3,273 | 205.0 | ||
| INMVFKIQVPYAFKSL | 4,273 | 11.3 | KLQVATANNVSPYIKL | 4,481 | 230.0 | ||
| FAVETLASSHVIPTAI | 3,257 | 13.5 | VYSEYSVTAADFASKM | 4,361 | 266.7 | ||
| DLHIPEFQLPHLSHTI | 2,729 | 16.4 | VELNVYFHPQSPPEKK | 3,985 | 282.4 | ||
| NLQLQPYSFITTLSND | 1,785 | 18.4 | HDYKGSTSHSLPYESS | 1,945 | 301.6 | ||
| QLIEVSSPITLQALVQ | 369 | 20.5 | SLSASAASSTIGTVGL | 3,961 | 302.7 | ||
| QTFLWTMPAFEVSLRA | 2,585 | 21.0 | LQQCDGFQPISTSVSP | 209 | 324.8 | ||
| SHVIPTAISTPSVTIP | 3,265 | 21.4 | HSLPYESSISTALEHT | 1,953 | 350.1 | ||
| LKLLVEDFKTTVSNSL | 2,465 | 21.6 | RFNSTYFQGTNQIVGM | 1,521 | 364.8 | ||
| MGNFTYDFSFKSSVIT | 3,329 | 26.2 | LALIKGLVHPLSTLIS | 225 | 401.0 | ||
| LNNYALFLSPRAQQAS | 3,065 | 32.5 | KKLSTSPFALNLTMLP | 3,737 | 422.2 | ||
| VASYRADTVAKVQGVE | 1,849 | 34.2 | RQYLQASTSLLYTKNP | 3,689 | 427.3 | ||
| LPFFYSEPVNVLNGLE | 2,025 | 37.2 | QTIVIPPLEFSVPAGI | 3,825 | 523.6 | ||
| SSQISFTVDGPIAFVG | 2,937 | 39.7 | GNTKSKPTVSSSIELN | 3,441 | 532.9 | ||
| LLTQYSTPEGSSVPIF | 3,761 | 49.9 | GEVEQYSATATYELLK | 1,009 | 547.2 | ||
| LTVSQFTLPKSLPVGN | 3,785 | 50.9 | DIVTYLMALIPNPSTQ | 409 | 595.0 | ||
| MKSWVTAVAKIMSDYP | 4,417 | 54.8 | SSSGVFTPGIKAGVRL | 849 | 600.3 | ||
| YSKFLLKAEPLALIVS | 1,929 | 58.4 | FSRNYQISKSASLPMF | 633 | 634.6 | ||
| GSLKSNVPKASKAIYD | 4,033 | 62.9 | NVYSNLYNWSASYTGG | 1,361 | 656.3 | ||
| IKIPLRFSTPEFTLLN | 2,633 | 90.9 | RMGLAFESTKSTSSPK | 305 | 670.2 | ||
| FHFSLAPFTLGIDTHT | 1,897 | 92.6 | RTMEQVMPALKSSVLS | 497 | 759.9 |
Affinity IC50 is <1 µM.
Tse et al. (44).
Several previous studies have suggested that Tregs may be protective in atherosclerosis, but antigen specificity was not investigated. Adoptive transfer of polyclonal CD25+ Tregs induced a significant decrease in Th1 responses revealed by reduced IFN-γ production, increased IL-10 production, and significant reduction in atherosclerotic lesion size (30). Adoptive transfer of polyclonal CD4+CD25+ natural Tregs into Apoe−/−Rag2−/− mice ameliorated atherosclerosis in the aortic root (1). A previous study using a human ApoB peptide (p210) showed that Tregs were expanded in spleens of immunized Apoe−/− mice that showed reduced plaque (50). In that study, MHC-II binding of p210 was not tested. p210 is an xenoantigen (human ApoB peptide, not conserved in mouse ApoB). Here, we show that immunization with an MHC-II-binding autoantigenic mouse ApoB peptide induces Tregs and is atheroprotective. Although FoxP3 is a master regulator of Treg development and function, it is thought that other types of Treg cells are induced from naive T cells in the periphery (39). We also found some IL-10+FoxP3– CD4+ T cells, consistent with the phenotype of Tr1 cells that are produced by antigenic stimulation of naive T cells in the presence of IL-10 (9, 14). Our data suggest that both Tregs and Tr1 cells are involved in vaccination-induced atheroprotection.
Several studies support the suppressive role of IL-10 in inflammatory immune responses. It has been reported that systemic IL-10 deficiency increases proinflammatory cytokines including IFN-γ (35) and IL-6 (22) and that IL-10−/− mice develop spontaneous inflammatory bowel disease (23). In IL-10-deficient Apoe−/− mice, Th1 responses and atherosclerotic lesion were significantly increased compared with Apoe−/− control mice (8). Conversely, overexpression of IL-10 in T cell reduces atherosclerosis in Ldlr−/− mice (36). Transfer of dendritic cells loaded with ApoB protein and IL-10 reduces atherosclerosis (17), consistent with our observation that the protective immune response against ApoB is associated with IL-10 production.
We found elevated antibody titers against each specific peptide as shown in Fig. 2. This shows that immunization with peptide induces a specific humoral immune response. However, the role of anti-ApoB IgG antibodies is unclear. In most autoimmune diseases, antibody production is believed to be associated with disease development. By contrast, most epidemiologic studies suggest that autoantibodies are negatively correlated with atherosclerotic plaque burden and clinical events. This is well investigated for autoantibodies to (modified) LDL (3, 20, 46). In humans, IgM and IgG antibodies to oxLDL (3) negatively correlate with lesion burden (37, 46). Some studies suggest that the protective capacity of antibody was found in the IgM compartment (25). However, opposite data were also reported, which show positive correlation between atherosclerosis and autoantibodies to modified LDL (47). One study suggested that autoantibodies against a peptide derived from ApoB are inversely related to atherosclerosis (43). Other studies reported weak positive correlations between autoantibodies to modified LDL and cardiovascular disease (12). Some studies suggest that antibodies to (modified) LDL can be protective in mice (6, 7, 11, 47). Importantly, a positive correlation between antibodies and atherosclerosis does not necessarily mean that the antibodies are proatherogenic. Although recombinant antibodies to modified LDL were shown to induce regression of atherosclerosis in mouse models (40, 41), the results need to be carefully interpreted because human, not mouse, antibodies were infused in these studies. In our study, there is no obvious correlation between the reduction of atherosclerotic plaque and antibody titer (Figs. 1C and 2, F and G); thus the therapeutic effect of increased IgG remains unclear.
We show that vaccination with ApoB peptides reduces atherosclerotic lesion size, which could be due to increased peritoneal IL-10-producing CD4+ T cells. We show that some of the cells with the expected phenotype are found in atherosclerotic plaque. These findings do not exclude the possibility that other cell types also could produce IL-10 such as regulatory B cells (51) and myeloid cells (32). This remains to be investigated in more detail, but a recent study suggests that IL-10 derived from B cells does not affect the development of atherosclerosis in mice (38).
The role of CCR5 in atherosclerosis has attracted attention in terms of immune cell migration. CCR5-deficient Apoe−/− mice were protected from diet-induced atherosclerosis (52). However, systemic deficiency in CCR5 is not sufficient to understand T cell-mediated immune responses because CCR5 is expressed on various cell types including monocytes and macrophages (27). Administration of a CCR5 antagonist reduced T cell infiltration in plaque (48). In addition, a recent study has reported that adoptive transfer of T cells from CCR5 deficient mice showed reduced homing of CD4+ T cells to aorta, which supports the concept that CCR5 may play an important role in the recruitment of T cells in atherosclerotic lesion (28). Another study has shown that some CCR5+ T cells produce IL-10 and mediate recruitment of Tregs into infarcted myocardium, followed by suppression of inflammation (10). We show increased CCR5 expression on Tregs induced by vaccination with ApoB peptides as well as an increase in the number of Tregs, which may allow these cells to home to sites of atherosclerosis. This is supported by recent data showing that CCR5 is important for CD4+ T cell homing to atherosclerotic arteries (28) and our present data showing increased CCR5+CD4+ T cell infiltration in aortic plaque (Fig. 3).
We show that vaccination with ApoB peptides induces IL-10 production by peritoneal CD4+ T cells including Tregs, some of which express CCR5 and can be found in plaque. These findings are consistent with an ApoB-specific effect mediated by IL-10-producing Tregs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-121697 (to K. Ley). T. Kimura was funded by a research fellowship from Uehara Memorial Foundation and the Japan Society for the Promotion of Science.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.K., K.T., J.S., A.S., and K.L. conceived and designed research; T.K., K.T., S.M., T.G., J.M., and J.S. performed experiments; T.K., K.T., S.M., T.G., J.M., Z.M., J.S., and D.W. analyzed data; T.K., D.W., and K.L. interpreted results of experiments; T.K. prepared figures; T.K. drafted manuscript; T.K. and K.L. edited and revised manuscript; T.K., K.T., S.M., T.G., J.M., Z.M., J.S., A.S., D.W., and K.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Margaret Chadwell for technical help in histology.
REFERENCES
- 1.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 12: 178–180, 2006. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 2.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest 114: 427–437, 2004. doi: 10.1172/JCI200420479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder CJ, Shaw PX, Chang MK, Boullier A, Hartvigsen K, Hörkkö S, Miller YI, Woelkers DA, Corr M, Witztum JL. The role of natural antibodies in atherogenesis. J Lipid Res 46: 1353–1363, 2005. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol 23: 454–460, 2003. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 5.Butcher MJ, Filipowicz AR, Waseem TC, McGary CM, Crow KJ, Magilnick N, Boldin M, Lundberg PS, Galkina EV. Atherosclerosis-driven treg plasticity results in formation of a dysfunctional subset of plastic IFNγ+ Th1/Tregs. Circ Res 119: 1190–1203, 2016. doi: 10.1161/CIRCRESAHA.116.309764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, Kohler HV, Kaveri SV, Nicoletti A. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol 50: 540–546, 2007. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 7.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest 109: 745–753, 2002. doi: 10.1172/JCI7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med 9: 10–17, 2003. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265: 1237–1240, 1994. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 10.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol 176: 2177–2187, 2010. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, Skaflen MD, Cutchins A, Lee DJ, Glover DK, Kelly KA, Galkina EV, Ley K, Witztum JL, Tsimikas S, Bender TP, McNamara CA. B-cell aortic homing and atheroprotection depend on Id3. Circ Res 110: e1–e12, 2012. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dotevall A, Hulthe J, Rosengren A, Wiklund O, Wilhelmsen L. Autoantibodies against oxidized low-density lipoprotein and C-reactive protein are associated with diabetes and myocardial infarction in women. Clin Sci (Lond) 101: 523–531, 2001. doi: 10.1042/cs1010523. [DOI] [PubMed] [Google Scholar]
- 13.Freigang S, Hörkkö S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol 18: 1972–1982, 1998. doi: 10.1161/01.ATV.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 14.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MGA. A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis. Nature 389: 737–742, 1997. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 15.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 12: 204–212, 2011. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 16.Herbin O, Ait-Oufella H, Yu W, Fredrikson GN, Aubier B, Perez N, Barateau V, Nilsson J, Tedgui A, Mallat Z. Regulatory T cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol 32: 605–612, 2012. doi: 10.1161/ATVBAHA.111.242800. [DOI] [PubMed] [Google Scholar]
- 17.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation 123: 1083–1091, 2011. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 18.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med 207: 1081–1093, 2010. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564, 2012. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karvonen J, Päivänsalo M, Kesäniemi YA, Hörkkö S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 108: 2107–2112, 2003. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 21.Kimura T, Tse K, Sette A, Ley K. Vaccination to modulate atherosclerosis. Autoimmunity 48: 152–160, 2015. doi: 10.3109/08916934.2014.1003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, Johnson RW. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. Am J Physiol Regul Integr Comp Physiol 295: R1109–R1114, 2008. doi: 10.1152/ajpregu.90302.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274, 1993. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- 24.Laurat E, Poirier B, Tupin E, Caligiuri G, Hansson GK, Bariéty J, Nicoletti A. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 104: 197–202, 2001. doi: 10.1161/01.CIR.104.2.197. [DOI] [PubMed] [Google Scholar]
- 25.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 120: 417–426, 2009. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol 31: 1506–1516, 2011. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Ley K. Lymphocyte migration into atherosclerotic plaque. Arterioscler Thromb Vasc Biol 35: 40–49, 2015. doi: 10.1161/ATVBAHA.114.303227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, McArdle S, Gholami A, Kimura T, Wolf D, Gerhardt T, Miller J, Weber C, Ley K. CCR5+T-bet+FoxP3+ effector CD4 T cells drive atherosclerosis. Circ Res 118: 1540–1552, 2016. doi: 10.1161/CIRCRESAHA.116.308648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest 123: 27–36, 2013. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation 108: 1232–1237, 2003. doi: 10.1161/01.CIR.0000089083.61317.A1. [DOI] [PubMed] [Google Scholar]
- 31.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 208: 1279–1289, 2011. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 10: 1178–1184, 2009. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osborne BA, Smith SW, Liu ZG, McLaughlin KA, Grimm L, Schwartz LM. Identification of genes induced during apoptosis in T lymphocytes. Immunol Rev 142: 301–320, 1994. doi: 10.1111/j.1600-065X.1994.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 34.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA 92: 821–825, 1995. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pils MC, Pisano F, Fasnacht N, Heinrich JM, Groebe L, Schippers A, Rozell B, Jack RS, Müller W. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. Eur J Immunol 40: 443–448, 2010. doi: 10.1002/eji.200939592. [DOI] [PubMed] [Google Scholar]
- 36.Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, Curtiss LK, Berliner JA, Boisvert WA. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ Res 90: 1064–1071, 2002. doi: 10.1161/01.RES.0000018941.10726.FA. [DOI] [PubMed] [Google Scholar]
- 37.Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Tsimikas S. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J Lipid Res 52: 1829–1836, 2011. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sage AP, Nus M, Baker LL, Finigan AJ, Masters LM, Mallat Z. Regulatory B cell-specific interleukin-10 is dispensable for atherosclerosis development in mice. Arterioscler Thromb Vasc Biol 35: 1770–1773, 2015. doi: 10.1161/ATVBAHA.115.305568. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 133: 775–787, 2008. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Schiopu A, Bengtsson J, Söderberg I, Janciauskiene S, Lindgren S, Ares MP, Shah PK, Carlsson R, Nilsson J, Fredrikson GN. Recombinant human antibodies against aldehyde-modified apolipoprotein B-100 peptide sequences inhibit atherosclerosis. Circulation 110: 2047–2052, 2004. doi: 10.1161/01.CIR.0000143162.56057.B5. [DOI] [PubMed] [Google Scholar]
- 41.Schiopu A, Frendéus B, Jansson B, Söderberg I, Ljungcrantz I, Araya Z, Shah PK, Carlsson R, Nilsson J, Fredrikson GN. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1(-/-)/low-density lipoprotein receptor(-/-) mice. J Am Coll Cardiol 50: 2313–2318, 2007. doi: 10.1016/j.jacc.2007.07.081. [DOI] [PubMed] [Google Scholar]
- 42.Sidney J, Southwood S, Moore C, Oseroff C, Pinilla C, Grey HM, Sette A. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. Curr Protoc Immunol 18: 18.3, 2013. doi: 10.1002/0471142735.im1803s100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sjögren P, Fredrikson GN, Samnegard A, Ericsson CG, Ohrvik J, Fisher RM, Nilsson J, Hamsten A. High plasma concentrations of autoantibodies against native peptide 210 of apoB-100 are related to less coronary atherosclerosis and lower risk of myocardial infarction. Eur Heart J 29: 2218–2226, 2008. doi: 10.1093/eurheartj/ehn336. [DOI] [PubMed] [Google Scholar]
- 44.Tse K, Gonen A, Sidney J, Ouyang H, Witztum JL, Sette A, Tse H, Ley K. Atheroprotective vaccination with MHC-II restricted peptides from ApoB-100. Front Immunol 4: 493, 2013. doi: 10.3389/fimmu.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol 25: 615–622, 2013. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res 48: 425–433, 2007. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 21: 95–100, 2001. doi: 10.1161/01.ATV.21.1.95. [DOI] [PubMed] [Google Scholar]
- 48.van Wanrooij EJ, Happé H, Hauer AD, de Vos P, Imanishi T, Fujiwara H, van Berkel TJ, Kuiper J. HIV entry inhibitor TAK-779 attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 25: 2642–2647, 2005. doi: 10.1161/01.ATV.0000192018.90021.c0. [DOI] [PubMed] [Google Scholar]
- 49.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Iinterferon Cytokine Res 22: 661–670, 2002. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 50.Wigren M, Kolbus D, Dunér P, Ljungcrantz I, Söderberg I, Björkbacka H, Fredrikson GN, Nilsson J. Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J Intern Med 269: 546–556, 2011. doi: 10.1111/j.1365-2796.2010.02311.x. [DOI] [PubMed] [Google Scholar]
- 51.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev 223: 284–299, 2008. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 52.Zernecke A, Liehn EA, Gao JL, Kuziel WA, Murphy PM, Weber C. Deficiency in CCR5 but not CCR1 protects against neointima formation in atherosclerosis-prone mice: involvement of IL-10. Blood 107: 4240–4243, 2006. doi: 10.1182/blood-2005-09-3922. [DOI] [PubMed] [Google Scholar]
- 53.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 102: 2919–2922, 2000. doi: 10.1161/01.CIR.102.24.2919. [DOI] [PubMed] [Google Scholar]


