Estrogen-related receptor-β (ESRRβ) is highly expressed in the heart and cardiac-specific deletion results in the development of a dilated cardiomyopathy (DCM). ESRRβ is mislocalized in human myocardium samples with DCM, suggesting a possible role for ESRRβ in the pathogenesis of DCM in humans.
Keywords: estrogen-related receptor, dilated cardiomyopathy, calcium handling
Abstract
Mechanisms underlying the development of idiopathic dilated cardiomyopathy (DCM) remain poorly understood. Using transcription factor expression profiling, we identified estrogen-related receptor-β (ESRRβ), a member of the nuclear receptor family of transcription factors, as highly expressed in murine hearts and other highly oxidative striated muscle beds. Mice bearing cardiac-specific deletion of ESRRβ (MHC-ERRB KO) develop DCM and sudden death at ~10 mo of age. Isolated adult cardiomyocytes from the MHC-ERRB KO mice showed an increase in calcium sensitivity and impaired cardiomyocyte contractility, which preceded echocardiographic cardiac remodeling and dysfunction by several months. Histological analyses of myocardial biopsies from patients with various cardiomyopathies revealed that ESRRβ protein is absent from the nucleus of cardiomyocytes from patients with DCM but not other forms of cardiomyopathy (ischemic, hypertrophic, and arrhythmogenic right ventricular cardiomyopathy). Taken together these observations suggest that ESRRβ is a critical component in the onset of DCM by affecting contractility and calcium balance.
NEW & NOTEWORTHY Estrogen-related receptor-β (ESRRβ) is highly expressed in the heart and cardiac-specific deletion results in the development of a dilated cardiomyopathy (DCM). ESRRβ is mislocalized in human myocardium samples with DCM, suggesting a possible role for ESRRβ in the pathogenesis of DCM in humans.
heart failure is a major health concern and a leading cause of death in the developing world. Idiopathic dilated cardiomyopathy (DCM) occurs in the absence of epicardial or other known cardiac diseases. Its etiology is often genetic, most frequently involving mutations in TTN, the gene encoding for the large sarcomeric protein titin (19, 20). However, the molecular triggers that lead to the development of DCM, often later in adult life, remain poorly understood. The heart is a highly metabolic organ, one-third of which is composed of mitochondria, and consuming up to 20% of systemic oxygen consumption at rest. Mutations in various components of mitochondrial function lead to DCM (21, 36), underscoring the importance of oxidative metabolism in the heart. Some pathways that negatively affect metabolism have also been suggested as being contributors to the development of more common forms of cardiomyopathy (21, 32), and human late-stage heart failure reveals defects in oxidative metabolism (11, 24), and is often considered to be “energy-starved” (22, 31, 33).
To identify novel transcriptional regulators of cardiac and oxidative metabolism, we performed a gene expression screen in various oxidative striated muscles beds and cells. To accomplish this, we took advantage of the proliferator-activated receptor-γ coactivator-1α (PGC-1α) transcriptional coactivator as a potent regulator of oxidative metabolism. PGC-1α was first identified in a yeast two-hybrid screen looking for factors that interact with the peroxisome proliferator-activated receptor-γ (PPARγ) transcription factor (TF) in brown adipocyte cells (37) and has since emerged as a critical regulator of oxidative metabolism and mitochondrial function (27, 40, 44). Ectopic overexpression of PGC-1α in cardiomyocytes in both cell culture and in vivo markedly increases oxygen consumption capacity and fatty acid oxidation with a concomitant decrease in glucose oxidation (25, 47). Conversely PGC-1α null mice exhibit impaired bioenergetics in cardiomyocytes, associated with decreased ATP generation (3, 26). Moreover, under conditions of stress such as aortic constriction, PGC-1α null hearts are more susceptible to heart failure (4). PGC-1α does not bind to DNA directly, and requires the induction and subsequent activation of TFs to mediate its effect on gene transcription.
Here we identify estrogen-related receptor-β (ESRRβ) as being highly expressed in conditions of high oxidative metabolic capacity. We show that mice lacking cardiac ESRRβ develop DCM in mid-life, preceded by pronounced defects in calcium handling and cellular contractility. Lastly, we show that ESRRβ nuclear expression is lost in human DCM suggesting a role in the pathogenesis of human DCM.
MATERIALS AND METHODS
Human studies.
All procedures were approved by the University of Pennsylvania and Beth Israel Deaconess Medical Center Institutional Review Boards (IRB).
Animal studies.
All animal experiments were performed according to procedures approved by the Beth Israel Deaconess Medical Center’s Institutional Animal Care and Use Committee. Mice floxed for exon 2 of ESRRβ (ESRRBflox/flox) as previously described (9), and mice expressing Cre recombinase under the control of the α-myosin heavy chain promoter (α-MHC-Cre+/−) as previously described (1, 4, 35), were obtained from (Jackson Laboratories Stock No. 007674 and 018972, respectively) and were crossed to generate a cardiac-specific ESRRβ KO (MHC-ERRB KO) (ESRRBflox/flox/α-MHC-Cre+/−) animals. Muscle-specific PGC-1α transgenic mice were previously described (29). All animals were maintained on C57BL/6 background. Mice were maintained on standard rodent chow with a 12-h light-dark cycles. Echocardiogrpahy was performed on nonanesthetized mice using a Vivid FiVe ECHO system (GE Medical Systems), and M-mode recordings at the mid-ventricle region of the heart were taken. Unless otherwise stated animals were taken at 4, 6, and 9 mo.
Gene expression studies.
Total RNA was isolated from tissues and cells using Trizol (Invitrogen) following manufacturer’s instructions and subjected to reverse transcription using High Capacity Reverse Transcription (Invitrogen). Quantitative real-time PCR was performed on cDNA using the intercalating fluorescent dye SYBR green (Bio-Rad) with gene-specific primers using a CFX 384 Touch real-time PCR machine (Bio-Rad). Relative expression was determined using the Comparative cycle threshold method (2−ΔΔCt) with 36B4, HPRT, and TBP used as housekeeping genes. For human samples used for gene expression studies, whole human hearts were procured from two separate patient groups: patients with end-stage heart failure who received heart transplants and hearts from brain dead organ donors. The failing hearts (dilated CM n = 13 and ischemic CM n = 13) came from patients undergoing transplants at the University of Pennsylvania. Hearts from brain-dead organ donors were made available through the Gift of Life Donor Program (Philadelphia, PA) and the selected cases had no history of heart failure or evidence of significant myocardial pathology (nonfailing controls n = 13). Tissue from age-matched individuals with no pathological or clinical evidence of heart disease were subjected to the same protocol and used as controls.
Complex IV and citrate synthase enzymatic activity.
Heart samples were snap frozen with liquid nitrogen until ready to process. Complex IV activity was measured by oxidation of cytochrome c as previously described (6, 43). Citrate synthase activity was measured as previously described (42).
Cardiomyocyte calcium imaging and contractility.
Adult murine ventricular cardiomyocytes from wild-type and transgenic mice were isolated as previously described (16) and loaded with 0.25 μg fura 2-AM for subsequent calcium transient analysis using the MMSYS IonOptix imaging system. Isolated cardiomyocytes were stimulated with 15 V at a frequency of 5 Hz to induce uniform cell contraction at 37°C. Contractility measurements were calculated using sarcomere shortening distances from real-time phase-contrast images. Intracellular calcium concentrations were calculated from the ratio of bound to unbound fura 2-AM These measurements were used to determine contractile and calcium transients depicting the cell’s overall contractility or the flux of calcium across the cell membrane. Both contractility measurements and calcium transients were recorded for 5–10 min, at which point each curve was averaged to generate a representative contractile or calcium transient plot for each cell recorded. These plots were then analyzed using a monotransient data analysis algorithm to generate surrogate parameters for systolic and diastolic function for that cell.
Immunofluorescence staining of human heart samples.
De-identified samples came from native hearts of patients who had undergone cardiac transplantation due to end-stage heart failure. Transmural sections of right and left ventricles from patients with hypertrophic (n = 3), with dilated (n = 4) and with ischemic (n = 3) cardiomyopathies were analyzed. The second set of samples came from patients who had died suddenly and diagnosed with arrhythmogenic right ventricular (n = 3) cardiomyopathy at postmortem examination. Tissue from age-matched individuals with no pathological or clinical evidence of heart disease were subjected to the same protocol and used as controls (n = 3). Samples were processed in formalin and subjected to paraffin embedding. In preparation for immunofluorescence microscopy, deparaffinized, rehydrated slide-mounted sections were heated in citrate buffer (10 mmol/l, pH 6.0) to enhance specific immunostaining. After being cooled to room temperature, the tissue sections were simultaneously permeabilized and blocked by incubating them in phosphate-buffered saline (PBS) containing 1% Triton X-100, 3% normal goat serum and 1% bovine serum albumin. The sections were then incubated first with a primary antibody and then with indocarbocyanine-conjugated goat anti-rabbit IgG. Primary antibodies included polyclonal rabbit anti-ERR1 (Thermo Fisher), polyclonal rabbit anti-ERRB (Thermo Fisher) and polyclonal rabbit N-Cadherin (Sigma). Immunostained preparations were analyzed by confocal microscopy (Sarastro Model 2000; Molecular Dynamics) as previously described (41).
Statistical analysis.
The data are presented as means ± SE. Statistical analysis was performed with Student’s t-test for in vitro experiments and ANOVAs for all in vivo experiments. Univariate statistics were performed on Contraction and calcium variables to assess distribution of values. For each time point, the effect of ERRB KO status on each response variable was modeled using generalized estimating equations, accounting for the distribution of each response variable as well as nesting of cell samples within the same mouse. For comparisons of presence or absence of nuclear ESRRB on human biopsies, the nonparametric Chi-Square test of association was used. P < 0.05 were considered statistically significant.
RESULTS
Identification of ESRRβ in a screen for TFs highly expressed in oxidative muscle.
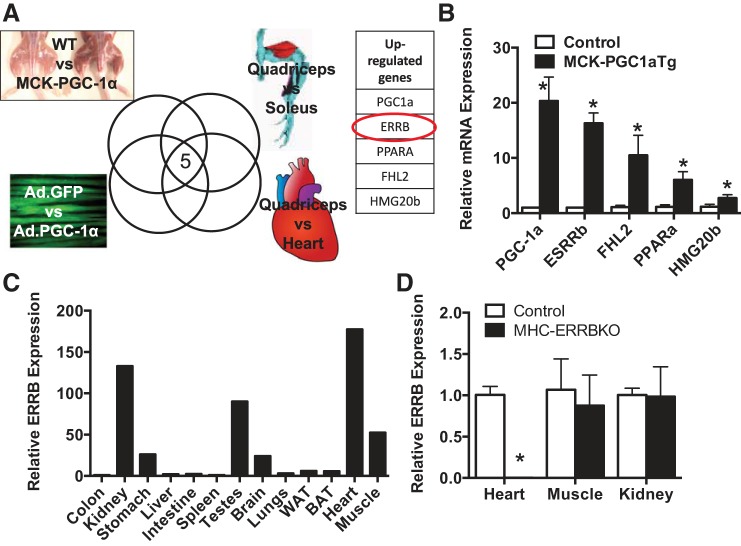
We sought to identify TFs that were differentially expressed in striated muscle beds of high vs. low oxidative capacity. We utilized a high throughput screen in which the expression of ~2,000 known or putative TFs are screened by qPCR (17, 18, 38). We compared the expression of the TFs in murine muscle or cells in four contexts: 1) slow twitch soleus muscle vs. fast twitch quadriceps muscle, 2) quadriceps vs cardiac muscle, 3) PGC-1α overexpression in cultured myotubes vs. GFP-only expression, and 4) transgenic PGC-1α overexpression in quadriceps vs. littermate control. As shown in Fig. 1A, five TFs were found in all four cases to be more highly expressed in the more oxidative tissue or cells. The five TFs included PGC-1α itself, as well as PPARα, a nuclear receptor well known to control programs of fatty acid oxidation in these contexts (8, 28). The most highly differentially expressed TF after PGC-1α was ESRRβ (Fig. 1B). ESRRβ is a member of the three-member ESRR family of TFs, named for their primary homology to the estrogen receptor (ESR), although importantly estrogen likely does not bind to members of the ESRR family. ESRRα and ESRRγ have been studied extensively as drivers of oxidative metabolism in muscle and other tissues (15, 46), but little is known of the role of ESRRβ in muscle tissues. qPCR analysis of RNA isolated from various tissues revealed that ESRRβ is most highly expressed in the heart, followed by muscle, kidney, testes, brain, and stomach, all of which are highly oxidative tissues (Fig. 1C).
Fig. 1.
Identification of estrogen-related receptor-β (ESRRβ) in cardiac metabolism. A: schematic of high-throughput qPCR screen. B: fold expression of upregulated genes in skeletal muscle of MCK-PGC-1Tg compared with control littermates. C: relative ESRRβ mRNA expression in various tissues. D: relative ESRRβ mRNA expression in heart, skeletal muscle, and kidney of MHC-ERRBKO and control mice at 2 mo. Data are presented as means ± SE; n = 4–6 per group; *P < 0.05 compared with control animals.
Cardiac-specific deletion of ESRRβ leads to profound adult-onset DCM.
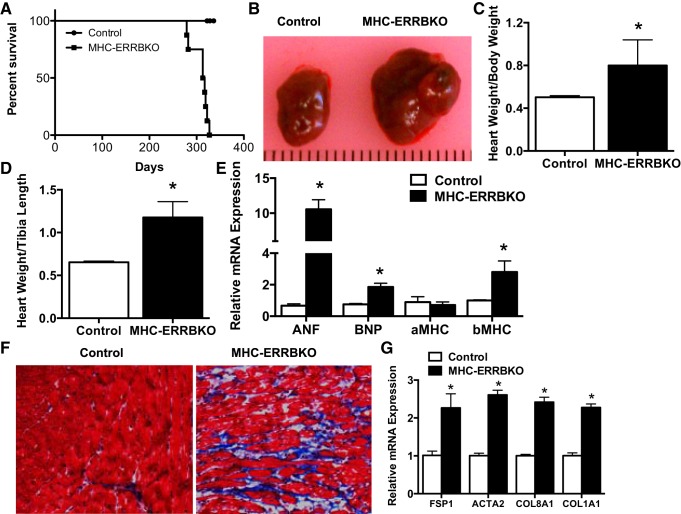
ESRRβ is most highly expressed in the heart, yet its role in cardiac physiology is unknown. To determine the role of ESRRβ in the heart we generated a cardiac-specific knockout of ESRRβ using the α-MHC promoter driving the expression of Cre recombinase and ESRRβ floxed mice to obtain MHC-ERRB KO animals. The MHC-ERRB KO animals had a 95% reduction in ESRRβ in the heart, with no loss of ESRRβ expression in skeletal muscle and kidney (Fig. 1D). The significant deletion of ESRRβ with the α-MHC promoter suggests that in the heart ESRRβ is primarily expressed within cardiomyocytes and not other cells. The MHC-ERRB KO mice were born at Mendelian ratios with no overt phenotype at birth. However, the MHC-ERR β KO had poor survival rates after 9 to 10 mo of age as quantified by Kaplan-Meier survival curve analysis (Fig. 2A). Gross analysis of the hearts from these animals at 9 mo revealed a significantly enlarged heart (Fig. 2B). Gravimetric analysis of heart parameters revealed a significant increase in heart weight to body weight (Fig. 2C) as well as heart weight to tibial length (Fig. 2D). qPCR analysis of ventricular RNA revealed an increase in the expression of ANF, BNP, and β-MHC, and a trend toward a decrease in α-MHC (Fig. 2E). These data indicate a heart failure gene signature in the hearts of the MHC-ERRB KO. Masson’s trichrome staining of the left ventricle revealed a significant increase in fibrosis (Fig. 2F). This increase in fibrosis was confirmed by qPCR analysis revealing a significant increase in collagen and other fibrotic markers (Fig. 2G).
Fig. 2.
Cardiac phenotype of MHC-ERRB KO mice. A: Kaplan-Meier survival curve of MHC-ERRBKO animals. B: gross heart anatomy of the MHC-ERRBKO animals compared with control animals. C: heart weight normalized to body weight (HW/BW). D: heart weight normalized to tibial length (HW/TL). E: mRNA expression of heart failure markers. F: Masson trichrome staining. G: qPCR expression of markers of fibrosis of MHC-ERRBKO and control animals at 9–10 mo of age. Data are presented as means ± SE; n = 4–6 per group; *P < 0.05 compared with control animals.
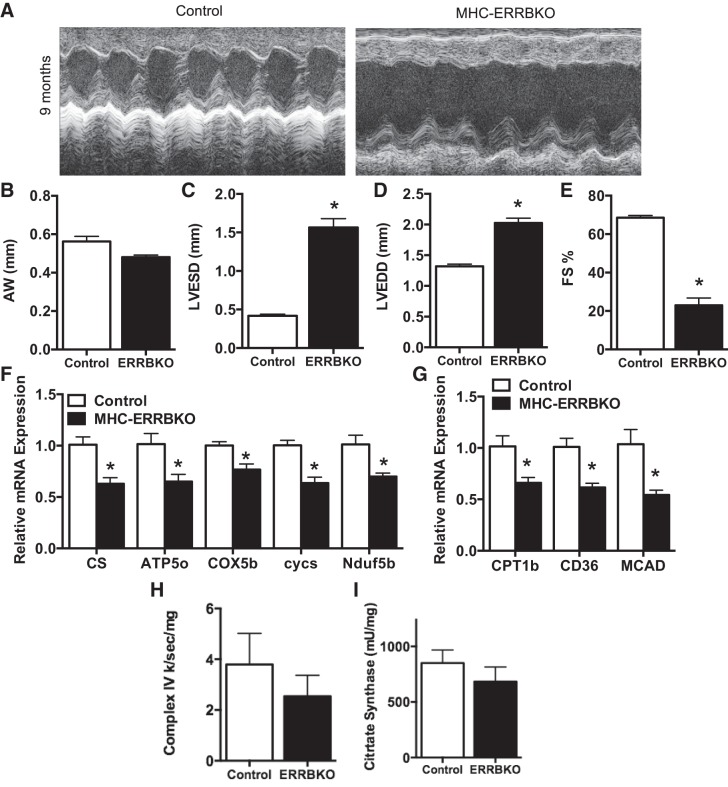
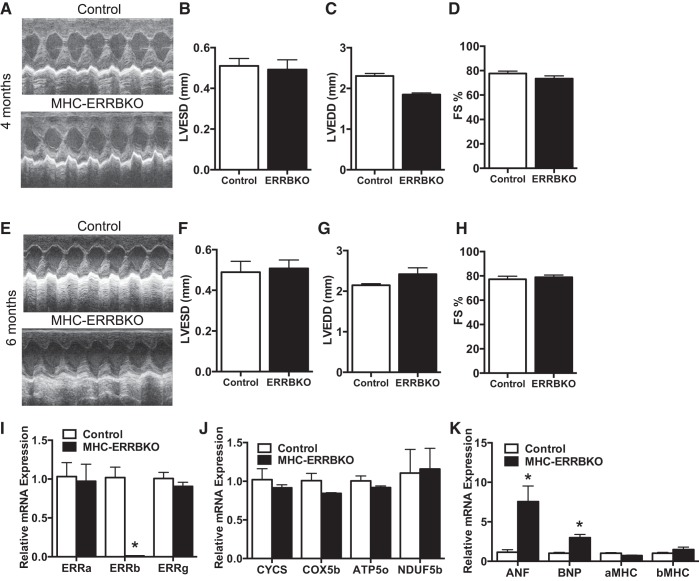
We next sought to evaluate cardiac function in the MHC-ERRB KO by noninvasive echocardiography (ECHO). Two-dimensional M-mode analysis of the ECHOs revealed a significant DCM (Fig. 3A) associated with a decrease in anterior wall thickness (AW) (Fig. 3B), significantly increased left ventricular end systolic diameter (LVESD) (Fig. 3C) and left ventricular end diastolic diameter (LVEDD) (Fig. 3D), and a 60% decrease in fractional shortening (FS) (Fig. 3E). This decrease in cardiac function is associated with a decrease in OXPHOS genes and FAO genes as revealed by qPCR analysis (Fig. 3, F and G), and a small but not statistically significant decline in complex IV activity and citrate synthase activity (Fig. 3, H and I). Interestingly, no evidence of cardiac dysfunction on echocardiography was seen at earlier time points in life, including 4 and 6 mo (Fig. 4, A–H). We did not observe any changes in expression of the other ESRR family members (Fig. 4I) or genes involved in OXPHOS (Fig. 4J), although at 6 mo the expression of ANF and BNP did start to increase (Fig. 4K). Taken together these data demonstrate that loss of ESRRβ result in the development of pronounced DCM during mid-to-late murine life.
Fig. 3.
Noninvasive ECHOs of MHC-ERRBKO. A: sample M-mode echocardiograms from the left ventricle (LV). B: anterior wall thickness (AW). C: left ventricular end systolic diameter (LVESD). D: left ventricular end diastolic diameter (LVEDD). E: percent fractional shortening (%FS). F: qPCR expression of OXPHOS genes. G: qPCR expression of FAO genes. H: complex IV enzymatic activity. I: citrate synthase activity of MHC-ERRBKO and control animals. Data are presented as means ± SE; n = 4–6 per group; *P < 0.05 compared with control animals.
Fig. 4.
Normal function in younger MHC-ERRBKO animal. A: sample M-mode echocardiograms from the left ventricle (LV). B: left ventricular end systolic diameter (LVESD). C: left ventricular end diastolic diameter (LVEDD). D: percent fractional shortening (%FS) at 4 mo. E: sample M-mode echocardiograms from the left ventricle (LV). F: left ventricular end systolic diameter (LVESD). G: left ventricular end diastolic diameter (LVEDD). H: percent fractional shortening (%FS) at 6 mo. I: qPCR expression of ESRR isoforms. J: qPCR expression of OXPHOS genes. K: mRNA expression of heart failure markers. Data are presented as means ± SE; n = 4–6 per group; *P < 0.05 compared with control animals.
Cardiomyocytes from MHC-ERRB KO mice exhibit impaired contractility and calcium handling.
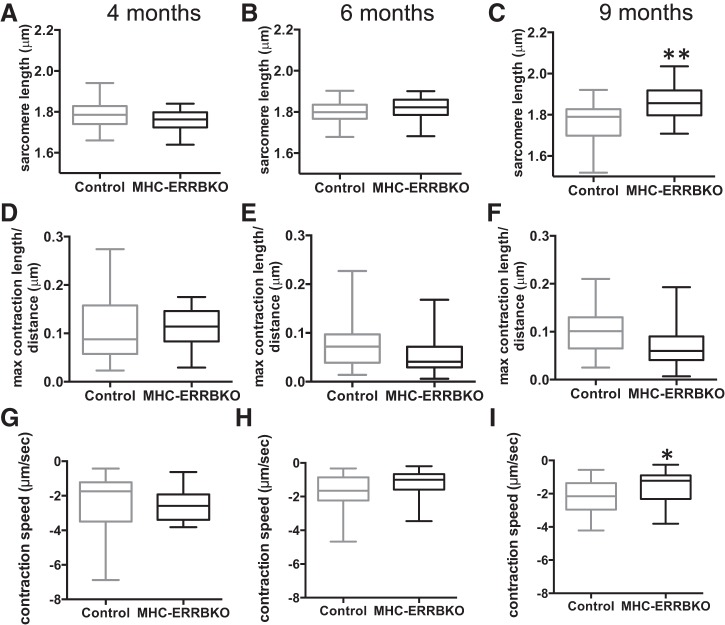
We next sought to determine whether the loss of ESRRβ had a cell autonomous effect on cardiomyocyte function. Ventricular cardiomyocytes were isolated from MHC ERRB KO mice at 4, 6, and 9 mo of age and compared with wild type, age-matched littermates (Fig. 5, A–C). Length measurements of the isolated cardiomyocytes revealed a significant increase in sarcomere length only after 9 mo (Fig. 5C), consistent with the timing of echocardiographic phenotype of left ventricular dilation (Fig. 3). However, this structural defect was preceded at 6 mo by contractile dysfunction evidenced by a decrease in both contractile distance (sarcomere shortening, Fig. 5, D–F) and speed of contraction (Fig. 5, G–I), both of which persisted at the 9-mo time point. Evidence of cell-autonomous contractile defects is thus apparent in MHC-ERRB KO mice as early as 6 mo (Fig. 5, E and H).
Fig. 5.
Decreased contractility in ERRB deleted cardiomyocytes. A–C: sarcomere length (micrometers). D–F: maximum contraction length/distance (micrometers). G–I: contraction speed (micrometers/second) of MHC-ERRBKO and control mice at 4, 6 and 9 mo. Data are presented as whisker plots with medians and min/max values (N = 3–4 animals per group; n = 12 – 20 cells per animals); *P < 0.05 and **P < 0.001 compared with control animals.
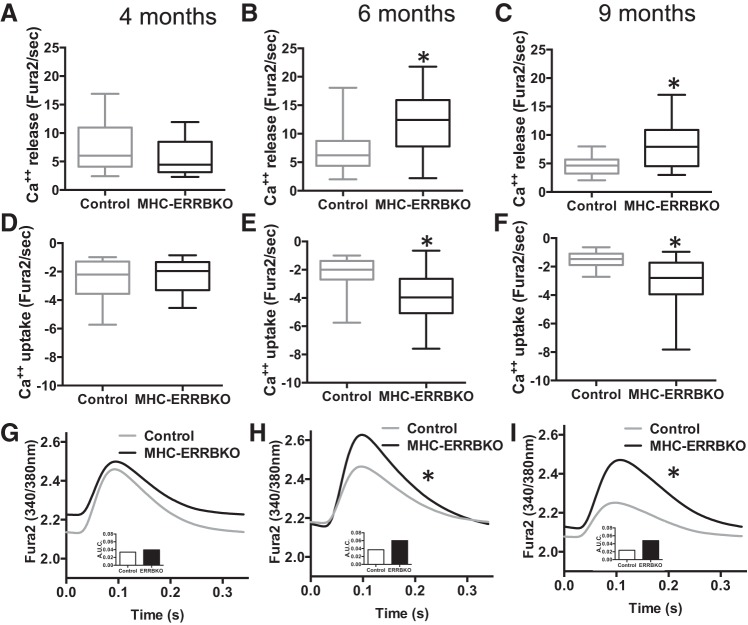
To evaluate calcium handling in these same cardiomyocytes, we measured calcium release and uptake using fura 2-AM fluorescent probes (Fig. 6, A–F). We found that dysfunction in calcium handling began as early as 4 mo, with Ca2+ release being significantly decreased (Fig. 6A), with a coincident trend in decrease in Ca2+ uptake (Fig. 6D). Curiously, the observed decreases in calcium release and uptake at 4 mo (Fig. 6, A and D) is reversed and becomes a markedly increased calcium release and uptake at 6 mo of age (Fig. 6, B and E), which persists at 9 mo of age (Fig. 6, C and F). This increase in calcium release was associated with a time dependent increase in calcium transients at 6 and 9 mo (Fig. 6, G–I). Taken together, these data indicate that deregulation of the excitation-contraction coupling apparatus appear in MHC-ERRB KO mice as early as four months of age, significantly preceding both cellular contractile defects and echocardiographic abnormalities.
Fig. 6.
Impaired cardiomyocytes calcium homeostasis with ESRRβ deletion. A–C: calcium release (Fura2/s). D–F: calcium uptake (Fura2/s). G–I: calcium transients traces (340/380 nm) of MHC-ERRBKO and control mice at 4, 6 and 9 mo. Data are presented as whisker plots with medians and min/max values (N = 3–4 animals per group; n = 12–20 cells per animals); *P < 0.05 and **P < 0.001 compared with control animals.
ESRRβ protein localization in human heart failure sections.
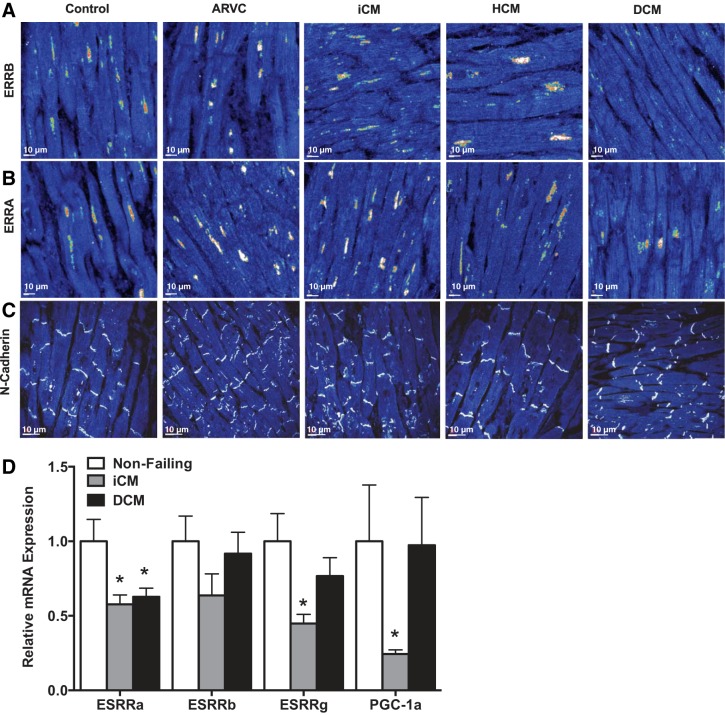
We next sought to determine if ESRRβ localization was affected in human heart failure. Samples were obtained from native hearts at the time of transplant from patients with diagnoses of ischemic cardiomyopathy (iCM), hypertrophic cardiomyopathy (HCM), idiopathic DCM, or postmortem samples of patients, who died suddenly with a diagnosis of arrhythmogenic right ventricular cardiomyopathy (ARVC), and compared with control donor hearts. Transmural sections immunostained for ESRRβ revealed the presence of ESRRβ in the nucleus in all sections with the exception of the DCM samples (Fig. 7A). The pattern of absent ESRRβ staining was seen in four of four DCM samples, but in zero of 3 control and other cardiomyopathy samples (P < 0.01). Moreover, this localization of ESRRβ was specific, as neither ESRRα (nuclear) (Fig. 7B) nor N-cadherin (membrane-bound) revealed any differences in protein distribution (Fig. 7C). Interestingly, in contrast to ESRRα, we did not observe any significant difference in expression of ESRRβ mRNA in cardiac samples from patients with DCM or iCM, compared with donor hearts (Fig. 7D), indicating that ESRRβ protein expression, stability, or localization is affected posttranscriptionally. Taken together the data indicate that ESRRβ protein is undetectable or mislocalized in human DCM.
Fig. 7.
ESRRβ localization and expression in human heart samples. A: immunostaining for ESRRβ (A), ESRRα (B), and N-Cadherin (C) in human transmural sections from arrhythmogenic right ventricular cardiomyopathy (ARVC) (n = 3), ischemic cardiomyopathy (iCM) (n = 3), hypertrophic cardiomyopathy (HCM) (n = 3), dilated cardiomyopathy (DCM) (n = 4), and control donor (n = 3) staining of human hearts. D: mRNA expression of ESRR isoforms and PGC-1 in nonfailing, ischemic cardiomyopathy (iCM) (n = 13) and dilated cardiomyopathy (DCM) (n = 13). *P < 0.05.
DISCUSSION
The PGC-1s and many of their downstream transcriptional regulators have been shown to be important in maintaining cardiac metabolic function (5, 39, 40). Members of the estrogen-related receptor (ESRR) family of orphan receptors have emerged as being important regulators of cardiac function (46). While bearing significantly homology to the estrogen receptor, estrogen-related receptors do not bind estrogen and consist of three members α, β, and γ (15). ESRRα has been studied extensively in the heart and other metabolic tissues (15) and has been shown to be a key component of the gene regulatory machinery that regulates mitochondrial biogenesis and function in these tissue. ESRRγ has been studied much less extensively, but most data indicate a significant overlap in function between ESRRα and ESRRγ (12). Moreover, germline deletion of ESRRγ results in postnatal lethality presumably due to impaired cardiac function (2). ESRRβ appears to have very different role compared with the other family members, despite their homology. Germline deletion of ESRRβ is embryonic lethal due to abnormal placental formation during early embryogenesis (30). However, the role of ESRRβ in cardiac function was unknown.
Here, we identify ESRRβ as a key regulator in the development of the DCM. The precise mechanism by which loss of ESRRβ leads to CM remains uncertain. Our data indicate that impaired calcium homeostasis precedes evidence of defects in contractility both in cell culture and in intact animals, suggesting that ESRRβ may regulate calcium handling. The calcium handling phenotype suggested an early defect in Ca release and uptake, which may account for the alterations in contractility noted. The later changes that suggested an increase in Ca release and uptake may be secondary or adaptive, although the mechanisms are still to be elucidated. However, we did not find that expression of known calcium-handling genes, such as RYR, SERCA2, or PL, was altered in hearts lacking ESRRβ at the time that calcium-handling defects appear. It is possible that ESRRβ affects calcium homeostasis via mechanisms other than regulating gene expression. Such nongenomic mechanisms have been noted for various nuclear receptors, including estrogen receptor (7, 10, 34). ESRRβ is also well recognized as a pluripotency factor, able to substitute for classical Yamanaka factors in certain contexts (13, 14, 23). However, it seems unlikely that cardiomyopathy in the ESRRβ KO hearts stems from loss of pluripotency, because cardiomyocytes in adult hearts replicate very little.
Our results suggest that loss of ESRRβ may contribute to the development of DCM. Nuclear receptors, including ESRRβ, are unique TFs in that they are typically ligand-activated. Nuclear receptors are therefore more easily drugged targets, providing a potentially amenable translational avenue. An endogenous ligand for ESRRβ is not known, but various synthetic ligands can activate it (45, 48). It will therefore be of great interest to test these ligands in models of DCM.
It is interesting that the absence of nuclear ESRRβ was noted in idiopathic DCM samples, but not in other causes of DCM, suggesting that 1) dilation of the heart per se does not cause loss of nuclear ESRRβ, and 2) loss of nuclear ESRRβ may uniquely contribute to the pathogenesis of idiopathic DCM. As noted, at least a quarter of idiopathic DCM cases are caused by truncating mutations in TTN, the gene encoding the sarcomeric protein titin. How mutations in a sarcomeric protein should affect the nuclear localization of a transcription factor is unclear. Evaluation of ESRRβ location and function in mouse models bearing mutations in ttn, once available, will therefore be of interest. Therefore, targeting ESRRβ with specific agonist could provide a new powerful intervention in the treatment of DCM.
GRANTS
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-062128 (to G. C. Rowe) and National Heart, Lung, and Blood Institute Grants HL-094499 and HL-126797 (to Z. Arany) and the American Heart Association (to Z. Arany).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.C.R., A.A., E.L.G., and K.B.M. performed experiments; G.C.R., E.L.G., K.D.M., and K.B.M. analyzed data; G.C.R., A.A., K.D.M., S.D., J.E.S., and Z.A. interpreted results of experiments; G.C.R. prepared figures; G.C.R. and Z.A. drafted manuscript; G.C.R., A.A., E.L.G., S.D., J.E.S., and Z.A. edited and revised manuscript; G.C.R. and Z.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the University of Alabama at Birmingham Diabetes Research Center Bio-Analytical Redox Biology Core, supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-079626, for help with electron transport complex activity assays.
REFERENCES
- 1.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100: 169–179, 1997. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguère V, Evans RM. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6: 13–24, 2007. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1: 259–271, 2005. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-γ coactivator 1α. Proc Natl Acad Sci USA 103: 10086–10091, 2006. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert G, Vega RB, Kelly DP. Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochim Biophys Acta 1833: 840–847, 2013. doi: 10.1016/j.bbamcr.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballmann C, Tang Y, Bush Z, Rowe GC. Adult expression of PGC-1α and -1β in skeletal muscle is not required for endurance exercise-induced enhancement of exercise capacity. Am J Physiol Endocrinol Metab 311: E928–E938, 2016. doi: 10.1152/ajpendo.00209.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19: 833–842, 2005. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 8.Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117: 3930–3939, 2007 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Nathans J. Estrogen-related receptor beta/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell 13: 325–337, 2007. doi: 10.1016/j.devcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol 213: 610–617, 2007. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- 11.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113: 709–724, 2013. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguère V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab 5: 345–356, 2007. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Feng B, Jiang J, Kraus P, Ng JH, Heng JC, Chan YS, Yaw LP, Zhang W, Loh YH, Han J, Vega VB, Cacheux-Rataboul V, Lim B, Lufkin T, Ng HH. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat Cell Biol 11: 197–203, 2009. doi: 10.1038/ncb1827. [DOI] [PubMed] [Google Scholar]
- 14.Festuccia N, Osorno R, Halbritter F, Karwacki-Neisius V, Navarro P, Colby D, Wong F, Yates A, Tomlinson SR, Chambers I. Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell Stem Cell 11: 477–490, 2012. doi: 10.1016/j.stem.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev 29: 677–696, 2008. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 16.Graham EL, Balla C, Franchino H, Melman Y, del Monte F, Das S. Isolation, culture, and functional characterization of adult mouse cardiomyocytes. J Vis Exp 2013: e50289, 2013. doi: 10.3791/50289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science 306: 2255–2257, 2004. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- 18.Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature 464: 619–623, 2010. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJ, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med 366: 619–628, 2012. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, Haghighi A, Homsy J, Hubner N, Church G, Cook SA, Linke WA, Chen CS, Seidman JG, Seidman CE. Heart disease. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349: 982–986, 2015. doi: 10.1126/science.aaa5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 115: 547–555, 2005. doi: 10.1172/JCI24405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingwall JS. On the control of metabolic remodeling in mitochondria of the failing heart. Circ Heart Fail 2: 275–277, 2009. doi: 10.1161/CIRCHEARTFAILURE.109.885301. [DOI] [PubMed] [Google Scholar]
- 23.Iseki H, Nakachi Y, Hishida T, Yamashita-Sugahara Y, Hirasaki M, Ueda A, Tanimoto Y, Iijima S, Sugiyama F, Yagami K, Takahashi S, Okuda A, Okazaki Y. Combined Overexpression of JARID2, PRDM14, ESRRB, and SALL4A Dramatically Improves Efficiency and Kinetics of Reprogramming to Induced Pluripotent Stem Cells. Stem Cells 34: 322–333, 2016. doi: 10.1002/stem.2243. [DOI] [PubMed] [Google Scholar]
- 24.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res 106: 1541–1548, 2010. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED, Kelly DP. The transcriptional coactivator PGC-1α is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol 295: H185–H196, 2008. doi: 10.1152/ajpheart.00081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leone TC, Kelly DP. Transcriptional control of cardiac fuel metabolism and mitochondrial function. Cold Spring Harb Symp Quant Biol 76: 175–182, 2011. doi: 10.1101/sqb.2011.76.011965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA 96: 7473–7478, 1999. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 30.Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguère V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature 388: 778–782, 1997. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- 31.Lygate CA, Schneider JE, Neubauer S. Investigating cardiac energetics in heart failure. Exp Physiol 98: 601–605, 2013. doi: 10.1113/expphysiol.2012.064709. [DOI] [PubMed] [Google Scholar]
- 32.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L’Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 293: H3270–H3278, 2007. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- 33.Neubauer S. The failing heart–an engine out of fuel. N Engl J Med 356: 1140–1151, 2007. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 34.Ordóñez-Morán P, Muñoz A. Nuclear receptors: genomic and non-genomic effects converge. Cell Cycle 8: 1675–1680, 2009. doi: 10.4161/cc.8.11.8579. [DOI] [PubMed] [Google Scholar]
- 35.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del Monte F, Hilfiker-Kleiner D, Karumanchi SA, Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485: 333–338, 2012. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planavila A, Dominguez E, Navarro M, Vinciguerra M, Iglesias R, Giralt M, Lope-Piedrafita S, Ruberte J, Villarroya F. Dilated cardiomyopathy and mitochondrial dysfunction in Sirt1-deficient mice: a role for Sirt1-Mef2 in adult heart. J Mol Cell Cardiol 53: 521–531, 2012. doi: 10.1016/j.yjmcc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 38.Rasbach KA, Gupta RK, Ruas JL, Wu J, Naseri E, Estall JL, Spiegelman BM. PGC-1α regulates a HIF2α-dependent switch in skeletal muscle fiber types. Proc Natl Acad Sci USA 107: 21866–21871, 2010. doi: 10.1073/pnas.1016089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riehle C, Abel ED. PGC-1 proteins and heart failure. Trends Cardiovasc Med 22: 98–105, 2012. doi: 10.1016/j.tcm.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res 107: 825–838, 2010. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saffitz JE, Green KG, Kraft WJ, Schechtman KB, Yamada KA. Effects of diminished expression of connexin43 on gap junction number and size in ventricular myocardium. Am J Physiol Heart Circ Physiol 278: H1662–H1670, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Shepherd D, Garland PB. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J 114: 597–610, 1969. doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264: 484–509, 1996. doi: 10.1016/S0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 44.Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J 282: 647–672, 2015. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 45.Wang SC, Myers S, Dooms C, Capon R, Muscat GE. An ERRbeta/gamma agonist modulates GRalpha expression, and glucocorticoid responsive gene expression in skeletal muscle cells. Mol Cell Endocrinol 315: 146–152, 2010. doi: 10.1016/j.mce.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, McDonald C, Petrenko NB, Leblanc M, Wang T, Giguere V, Evans RM, Patel VV, Pei L. Estrogen-related receptor α (ERRα) and ERRγ are essential coordinators of cardiac metabolism and function. Mol Cell Biol 35: 1281–1298, 2015. doi: 10.1128/MCB.01156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wende AR, Huss JM, Schaeffer PJ, Giguère V, Kelly DP. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 25: 10684–10694, 2005. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu DD, Forman BM. Identification of an agonist ligand for estrogen-related receptors ERRbeta/gamma. Bioorg Med Chem Lett 15: 1311–1313, 2005. doi: 10.1016/j.bmcl.2005.01.025. [DOI] [PubMed] [Google Scholar]