This is the first study to establish, using innovative optogenetic approaches in a transgenic rat model, that there are robust heterogeneous projections from orexin neurons to paraventricular spinally projecting neurons, including excitatory glutamatergic and inhibitory GABAergic neurotransmission. Endogenous orexin release modulates glutamatergic, but not GABAergic, neurotransmission in these pathways.
Keywords: optogenetic, electrophysiology, orexin neurons, sympathetic, cardiovascular regulation
Abstract
Orexin neurons, and activation of orexin receptors, are generally thought to be sympathoexcitatory; however, the functional connectivity between orexin neurons and a likely sympathetic target, the hypothalamic spinally projecting neurons (SPNs) in the paraventricular nucleus of the hypothalamus (PVN) has not been established. To test the hypothesis that orexin neurons project directly to SPNs in the PVN, channelrhodopsin-2 (ChR2) was selectively expressed in orexin neurons to enable photoactivation of ChR2-expressing fibers while examining evoked postsynaptic currents in SPNs in rat hypothalamic slices. Selective photoactivation of orexin fibers elicited short-latency postsynaptic currents in all SPNs tested (n = 34). These light-triggered responses were heterogeneous, with a majority being excitatory glutamatergic responses (59%) and a minority of inhibitory GABAergic (35%) and mixed glutamatergic and GABAergic currents (6%). Both glutamatergic and GABAergic responses were present in the presence of tetrodotoxin and 4-aminopyridine, suggesting a monosynaptic connection between orexin neurons and SPNs. In addition to generating postsynaptic responses, photostimulation facilitated action potential firing in SPNs (current clamp configuration). Glutamatergic, but not GABAergic, postsynaptic currents were diminished by application of the orexin receptor antagonist almorexant, indicating orexin release facilitates glutamatergic neurotransmission in this pathway. This work identifies a neuronal circuit by which orexin neurons likely exert sympathoexcitatory control of cardiovascular function.
NEW & NOTEWORTHY This is the first study to establish, using innovative optogenetic approaches in a transgenic rat model, that there are robust heterogeneous projections from orexin neurons to paraventricular spinally projecting neurons, including excitatory glutamatergic and inhibitory GABAergic neurotransmission. Endogenous orexin release modulates glutamatergic, but not GABAergic, neurotransmission in these pathways.
spinally projecting neurons (SPNs) in the paraventricular nucleus of the hypothalamus (PVN) play an important role in sympathetic regulation, contributing to increase in sympathetic nerve discharge, heart rate, blood pressure, and breathing (21, 25, 40, 45). Whereas magnocellular PVN cells project to the posterior pituitary, parvocellular neurons in the PVN modulate the autonomic nervous system via direct projections to the intermediolateral cell column of the thoracic spinal cord and collateral fibers to the rostral ventrolateral medulla, another brain region involved in sympathetic regulation of autonomic function (25, 26, 49).
In close proximity to spinally projecting cells in the PVN are orexin-containing neurons in the lateral, dorsomedial, and perifornical regions of the hypothalamus. Like SPNs, orexin neurons are involved in modulation of autonomic function in addition to controlling arousal, sleep/wake states, feeding, ventilatory chemosensitivity, and breathing (5, 6, 10, 12, 13, 34, 56, 60–62). Orexin neurons release neuropeptides orexin-A and orexin-B and project to multiple neuronal systems in the brain and spinal cord, including dense innervation in the PVN (8, 9, 38). Intracerebroventricular, intracisternal, and intrathecal administration of orexin has been shown to increase heart rate and blood pressure (1, 7, 32, 43, 50), suggesting orexin receptor activation increases sympathetic nervous system activity. Since high c-fos expression was found in the PVN following intracerebroventricular injection of orexin-A (14), this nucleus was proposed to be a possible site of action for orexin (15). Supporting this hypothesis, both magnocellular and parvocellular neurons in the PVN were depolarized in response to orexin application (15, 49). Taking together, the results from previous studies point to sympathoexcitatory effects of orexin in the PVN; however, the functional connectivity between orexin neurons and SPNs in the PVN has not been established.
This study was undertaken to identify and characterize the synaptic pathway from orexin neurons to SPNs in the PVN. To accomplish this goal, we used a transgenic strain of orexin-Cre rats to express light-activated channelrhodopsin-2 (ChR2) in orexin cells and combined this control with whole cell recording from fluorescently identified SPNs in the PVN in an in vitro slice preparation.
MATERIALS AND METHODS
Animals and ethnical approval.
Experiments were conducted by using both sexes of transgenic rats in which Cre recombinase is exclusively expressed in a majority of orexin neurons. To generate orexin-enhanced green fluorescent protein (EGFP)-2A-Cre rats, the transgene was constructed on the plasmid (pCR-BluntII TOPO). A 3.2-kb fragment of the 5 upstream region of the human prepro-orexin gene was used as a promoter, which drives the expression of the Cre recombinase connected with EGFP via viral 2A peptide (EGFP-2A-Cre). The transgene was excised by restriction enzyme reaction to remove plasmid backbone sequence. Transgene was microinjected into pronuclei of fertilized rat eggs (Wistar) to generate transgenic founders. Founders were bred with Wistar rats to produce stable orexin-EGFP-2A-Cre transgenic lines. Homozygous orexin-EGFP-2A-Cre rats were received from Nagoya University (Nagoya, Japan) and housed in the George Washington University animal care facility under standard environmental conditions. For electrophysiological experiments we used homozygous orexin-EGFP-2A-Cre animals. All animal procedures were performed in compliance with the institutional guidelines at George Washington University and are in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the George Washington University Institutional Animal Care and Use Committee.
SPN labeling and viral injections into the lateral hypothalamus.
SPNs were labeled as previously described (13, 21, 59). Orexin-Cre rats (postnatal days 2–3) were anesthetized with hypothermia. The upper thoracic spinal cord was exposed, and the retrograde tracer cholera toxin subunit B conjugated with Alexa Fluor 555 (1%, Invitrogen, Carisban, CA) was injected bilaterally into T2-T4 spinal segments (1–2 deposits per side, total volume, 50 nl). Whereas the injection sites were not restricted to the intermediolateral cell column because of the small size of the spinal cord (19), neurons in the sympathoexcitatory regions that were retrogradely labeled from the thoracic spinal cord have been shown to selectively innervate the intermediolateral cell column with no projections to thoracic dorsal or ventral horns (42). After surgery, buprenorphine was administered, and animals were monitored for 30 min and every 20 min thereafter until ambulatory.
ChR2 fused to enhanced yellow fluorescent protein (EYFP) was targeted to the plasma membrane of orexin neurons and axons. Adeno-associated viral vector with “FLEX-switch” ChR2 construct [AAV1.EF1a.DIO.hChR2(H134R)-EYFP.WPRE.hGH, Catalog No. AV-1–20298P Penn Vector Core, Philadelphia, PA] was injected into the lateral hypothalamus of orexin-Cre rats. Injections were made while the animals were anesthetized with hypothermia and mounted in a stereotactic apparatus with a neonatal adapter (Stoelting, Wood Dale, IL). The skull was exposed, and a small burr hole was made to position a pulled calibrated pipette (VWR, Radnor, PA) containing viral vector at the following coordinates: 1.7–1.9 mm posterior (depending on the distance between bregma and lambda) and 0.4 mm lateral relative to bregma. The pipette tip was lowered 5.2 mm from the dorsal surface of the brain and 60 nl of viral vector was slowly injected. The pipette was left in place for 5 min, before retraction. In addition to orexin-Cre animals, identical viral injections into the lateral hypothalamus were performed in wild-type Wistar rats. After surgery, buprenorphine was administered, and animals were monitored for 30 min and every 20 min thereafter until ambulatory.
Slice preparation and electrophysiology.
On the day of experiment, animals (21–24 days old) were anesthetized with isoflurane, and glycerol-based artificial cerebrospinal fluid (aCSF) was perfused transcardially before death. Glycerol-based aCSF (4°C) contained (in mM): 252 glycerol, 1.6 KCl, 1.2 NaH2PO4, 1.2 MgCl, 2.4 CaCl2, 26 NaHCO3, and 11 glucose. The brain was carefully removed, and 300-μm-thick coronal slices of the hypothalamus were obtained with a vibratome. The slices were transferred to a solution of the following composition (in mM) 110 N-methyl-d-glucamine (NMDG), 2.5 KCl, 1.2 NaH2PO4, 25 NaHCO3, 25 glucose, 0.5 CaCl2, and 10 mM MgSO4 equilibrated with 95% O2-5% CO2 (pH 7.4) at 34°C for 15 min. This brief protective recovery step using NMDG-based aCSF reduces neuronal swelling during rewarming. The slices were then transferred from NMDG-based aCSF to a recording chamber, which allowed perfusion (5–10 ml/min) of aCSF at room temperature (25°C) containing (in mM) 125 NaCl, 3 KCl, 2 CaCl2, 26 NaHCO3, 5 glucose, and 5 HEPES equilibrated with 95% O2-5% CO2 (pH 7.4) for at least 30 min before experiments were conducted.
Individual SPNs were identified in the PVN by the presence of the fluorescent tracer. These identified cells were then imaged with differential interference contrast optics, infrared illumination, and infrared-sensitive video detection cameras to gain better spatial resolution. To examine light-triggered postsynaptic currents in SPNs, patch pipettes (2.5–3.5 MΩ) were filled with a solution consisting of (in mM) 150 KCl, 2 MgCl2, 2 EGTA, 10 HEPES, and 2 Mg-ATP, pH 7.3, and guided to the surface of individual SPNs. Voltage clamp whole cell recordings were made at a holding potential of −80 mV with an Axopatch 200 B and pClamp 8 software (Axon Instruments, Union City, CA). Spontaneous glutamatergic miniature postsynaptic currents (mEPSCs) as well as action potential firing in SPNs were recorded with the patched pipets filled with a solution consisting of 135 mM K-gluconic acid, 10 mM HEPES, 10 mM EGTA, 1 mM CaCl2, and 1 mM MgCl2, pH 7.3. The recording of mEPSCs was performed in the presence of tetrodotoxin (TTX, 1 µM), gabazine (25 µM), and glycine (1 µM). At the end of experiments, AP-5 (50 µM) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (50 µM) were added to block glutamatergic neurotransmission. All drugs used in this electrophysiological study, except almorexant and orexin, were purchased from Sigma-Aldrich Chemical (St. Louis, MO). Almorexant, a dual orexin receptor antagonist, and orexin were purchased from Selleckchem (Houston, TX).
Selective photostimulation of ChR2-expressing fibers surrounding SPNs was performed by using a 473-nm blue laser light (CrystaLaser, Reno, NV). The laser was attached to the microscope with a dual housing adapter (Nikon). The numeric aperture of the ×40 water immersion objective was 0.8 and the working distance 2.0 mm. A series of 60 consecutive single stimulations (3-ms duration, at 1 Hz) were applied to each neuron studied in voltage clamp configuration. In another set of experiments, train stimulation (4 pulses, 3-ms duration, at frequency 10 Hz) was applied to each neuron while recording voltage in current clamp configuration. Laser light intensity was kept constant across all experiments at an output of 10 mW.
Immunohistochemistry and imaging.
To determine the specificity of EGFP-ChR2 expression in orexin neurons, immunostaining was used. Three weeks after AAV1-ChR2-EYFP viral injections, forebrain slices that included the lateral hypothalamus (100 µm) were prepared and soaked 3 h in 10% formalin. The slices were then incubated with 0.2% Triton X-100 and processed for orexin immunoreactivity by using rabbit anti-orexin-A as a primary antibody (1:15,000 dilution, overnight incubation, Phoenix Pharmaceuticals, Burlingame, CA) and anti-rabbit Alexa Fluor 633 as a secondary antibody (1:200, 4 h, Life Technologies, Carlsbad, CA). For analysis of colocalization of orexin immunoreactivity and ChR2-EYFP expression, the Zeiss 710 confocal imaging system was used.
Data analysis and statistics.
Synaptic latency of the evoked responses was measured as the time between the onset of the stimulation and the onset of synaptic current. Light-triggered postsynaptic currents were measured by using the pClamp 8 software (Molecular Devices, Sunnyvale, CA). The responses to a series of 60 consecutive photostimulations in each neuron were averaged in all series of experiments. The mean value from each neuron in the population was averaged for the population of neurons to create a summary of results for each condition. A series of 60 consecutive single stimulations were applied 1 min before and 4 min after almorexant application, and also at 9 min of almorexant washout. Analysis of firing activity and spontaneous glutamatergic mEPSCs was performed by using Mini Analysis (version 5.6.12; Synaptosoft, Decatur, GA). Firing activity was analyzed 3 s before and 3 s after photostimulation. Glutamatergic mEPSCs were analyzed with threshold set at root-mean-square noise, multiplied by 5. Postsynaptic neurotransmission was analyzed during the last 2 min of the control period, the last 2 min of the 4-min orexin application period, as well as during 8–10 min postorexin application.
Results are presented as means ± SE and statistically compared by using Student’s paired t-test, Kolmogorov-Smirnov (K-S) test, or ANOVA with repeated-measures and Dunnett’s posttest, as appropriate. Significant difference was set at P < 0.05.
RESULTS
Orexin immunoreactivity was detectable in 85 ± 2% of ChR2-EYFP neurons, and 70 ± 3% of orexin-containing neurons were labeled with ChR2-EYFP (n = 9 slices from 3 rats, see Fig. 1). In addition to ChR2-EYFP expression in orexin neurons and axons in the lateral hypothalamus, a dense network of ChR2-EYFP-expressing fibers was detected in the PVN (Fig. 2A). Fluorescently identified SPNs in the PVN were surrounded by ChR2-EYFP-expressing terminals (Fig. 2B). No fluorescent EYFP expression was found in the PVN 3 wk postinjections of AAV1-ChR2-EYFP viral vector into the lateral hypothalamus of wild-type Wistar rats (4 animals, data not shown).
Fig. 1.
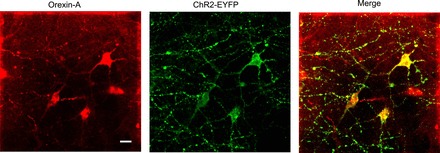
Representative example of colocalization of orexin-A immunoreactivity (red) with ChR2 enhanced yellow fluorescent protein (EYFP) (green) driven by orexin-Cre expression in the lateral hypothalamus. Scale bar, 20 µm.
Fig. 2.
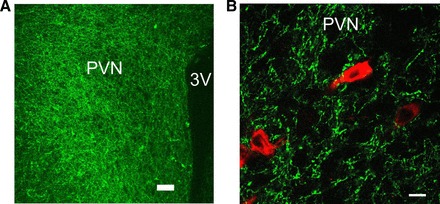
A: dense ChR2-EYFP orexinergic fiber expression (green) in the paraventricular nucleus (PVN) (scale bar, 100 µm). B: typical network of ChR2-EYFP-expressing fibers (green) that surround spinally projecting neurons (SPNs) (red) in the PVN (scale bar, 10 µm).
Selective photoactivation of orexin neurons.
Each optogenetic photostimulation (3 ms, 1 Hz) of fluorescently identified orexin neurons in the lateral hypothalamus generated a large rapid inward current (596 ± 132 pA, n = 11, Fig. 3B, in voltage clamp configuration) and an action potential firing (n = 11 cells, Fig. 3A, in current clamp mode). This robust and reliable excitation on photoactivation indicates there is a strong expression of ChR2-EYFP (46) in orexin positive neurons. No electrophysiological responses in orexin neurons in the lateral hypothalamus were observed in orexin-Cre rats that did not receive AAV1-ChR2-EYFP viral injections (n = 4 cells, data not shown), indicating that the responses were not due to nonspecific cellular activation by blue light pulses.
Fig. 3.
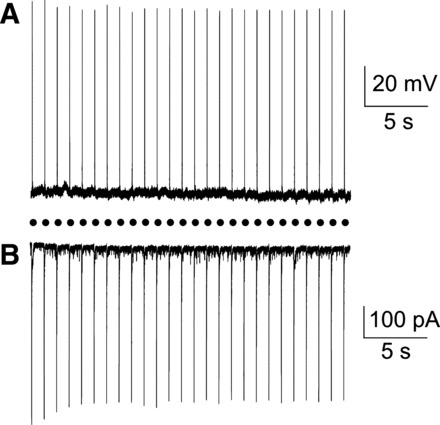
Optogenetic stimulation of orexin cell bodies in the lateral hypothalamus evoked reliable action potentials (A) as well as large inward whole cell currents (B) in all orexin neurons tested (n = 11). Black circles represent light pulses (3 ms, 1 Hz).
Projections from orexin-containing neurons to SPNs in the PVN.
To identify and characterize the functional projection from orexin neurons to SPNs in the PVN, postsynaptic responses in SPNs were obtained on optogenetic stimulation of ChR2-containing orexin fibers. Photostimulation of orexin ChR2-EYFP fibers in the PVN with brief light pulses (3 ms) generated fast postsynaptic responses in SPNs studied (n = 34, in voltage clamp mode). The majority of postsynaptic currents (n = 20 neurons, 59%) were blocked by application of the glutamate receptor antagonist CNQX (50 µM, peak amplitude diminished from 52.6 ± 5.4 pA to 2.7 ± 0.2 pA, P < 0.001, Student’s paired t-test, Fig. 4A), indicating that this synaptic neurotransmission was mediated via postsynaptic glutamatergic AMPA receptors. These excitatory postsynaptic currents (EPSCs) occurred with a short latency of 4.5 ± 0.2 ms (ranging from 2.1 to 6.4 ms, n = 20 cells).
Fig. 4.
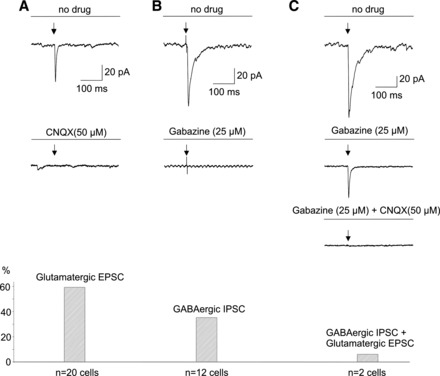
Optogenetic stimulation (indicated by the arrows in this and all subsequent figures) of ChR2-EYFP fibers in the PVN resulted in postsynaptic currents in all SPNs tested (n = 34), including glutamatergic, GABAergic, and mixed glutamatergic and GABAergic responses. Representative traces are shown at top, whereas the summary data are illustrated at bottom of A, B, and C.
In addition to glutamatergic EPSCs, inhibitory postsynaptic currents (IPSCs) were detected in a minority of SPNs. Twelve photoactivated postsynaptic responses out of 34 studied (35%, Fig. 4B) were abolished by application of gabazine (25 µM, peak amplitude diminished from 99.8. ± 26.6 pA to 3.0 ± 0.5 pA, P < 0.005, Student’s paired t-test). The GABAergic IPSCs had latency of 4.4 ± 0.3 ms (ranging from 2.1 to 5.3 ms).
A very small minority of ChR2 photoactivated postsynaptic currents in SPNs (n = 2, 6%, see Fig. 4C) were abolished only by application of both gabazine (25 µM) and CNQX (25 µM), suggesting that in a very small minority of PSNs GABAergic and glutamatergic neurotransmitters were likely coreleased on optogenetic stimulation of orexin fibers.
Glutamatergic and GABAergic inputs from orexin neurons to SPNs are monosynaptic.
We next assessed in voltage clamp mode whether projections from orexin neurons to SPNs were mono- or polysynaptic. Application of the sodium channel blocker TTX (1 µM) eliminated light-triggered glutamatergic current, but a ChR-2-dependent glutamatergic response was reinstated by coapplication of 4-aminopyridine (4-AP, 200 µM) and blocked by CNQX (50 µM) at the end of the experiment (see Fig. 5A). We observed this reinstatement of glutamatergic EPSC in all SPNs studied (n = 5). Similarly, GABAergic postsynaptic current evoked by photostimulation of orexin fibers was eliminated by TTX (1 µM), reinstated by coapplication of 4-AP (200 µM), and blocked by gabazine (n = 5 neurons, Fig. 5B). This approach using coapplication TTX and 4-AP has been used in previous studies to isolate monosynaptic release of neurotransmitter during photostimulation (11, 37, 44, 51). Since postsynaptic responses in SPNs occurred in the presence of TTX and 4-AP, it is likely these glutamatergic and GABAergic responses represent a monosynaptic connection between orexin-containing neurons and SPNs.
Fig. 5.
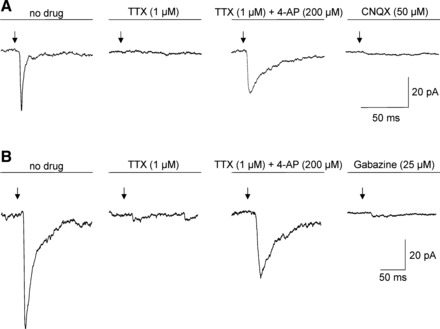
A: in a typical experiment, tetrodotoxin (TTX) eliminated glutamatergic response evoked by photostimulation. A slower glutamatergic response was reinstated by coapplication of 4-AP and blocked by CNQX. B: a typical experiment demonstrates GABAergic postsynaptic current evoked by photostimulation of orexin fibers. This response was eliminated by TTX, reinstated by coapplication of 4-AP, and blocked by gabazine.
Photostimulation of orexin fibers affects action potential firing in SPNs.
To determine if the light-triggered neurotransmission was strong enough to evoke changes in action potential firing in SPNs, we performed recording from SPNs neurons in current clamp configuration. SPNs studied in a current clamp mode fired spontaneously at a frequency of 3.0 ± 0.8 Hz and had a resting membrane potential of −69.8 ± 3.8 mV (n = 9 neurons). Train stimulation (4 light pulses, 3 ms, at 10 Hz) elicited increase in the frequency discharge in seven out of nine neurons studied (K-S test, see Fig. 6A). This frequency increase was abolished by application of CNQX (50 µM, P > 0.05, K-S test, Fig. 6B), suggesting that light-triggered increased firing of SPNs occurred via postsynaptic glutamatergic AMPA receptor activation. No significant changes were detected in two remaining SPNs (P > 0.05, K-S test, see Fig. 6B). However, a light-triggered increase in the frequency of firing occurred in these two initially unresponsive SPNs after application of the GABAergic receptor antagonist gabazine (25 µM, K-S test, Fig. 6B), suggesting GABAergic neurotransmission can sufficiently inhibit the firing activity in a minority of SPNs.
Fig. 6.
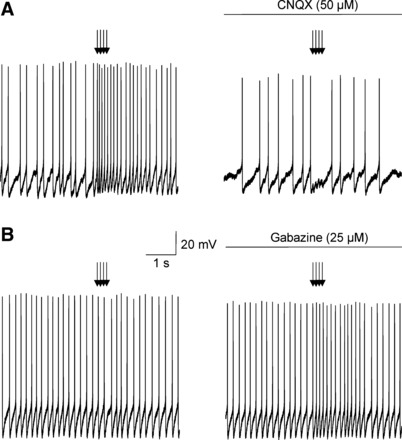
A: representative example in panel at left demonstrates a facilitation of action potential firing (P < 0.01, K-S test) in individual SPNs in response to train stimulation (4 light pulses, 3 ms, at 10 Hz) of orexin ChR2-expressing fibers. As shown in panel at right this facilitation was abolished by application CNQX at a concentration of 50 µM (P > 0.05, K-S test). B: representative example in panel at left demonstrates no significant changes in the action potential firing in another individual SPN occur on photostimulation (P > 0.05, K-S test). In panel at right, however, a light-triggered increase in firing activity occurred in these initially unresponsive SPNs after application of gabazine at a concentration of 25 µM (P < 0.05, K-S test).
Orexin is involved in the generation of EPSCs in presympathetic neurons.
We also studied in voltage clamp mode whether application of an orexin receptor antagonist affects the light-triggered postsynaptic response in SPNs in the PVN. Bath application of almorexant (1 µM), a dual orexin receptor antagonist, did not change the peak amplitude of photoactivated GABAergic IPSCs in SPNs (104.7 ± 26 pA, control vs. 106.2 ± 27 pA, almorexant, P > 0.05, 1-way repeated-measures ANOVA and Dunnett’s posttest, n = 6, Fig. 7). However, almorexant (1 µM) significantly and reversibly diminished the peak amplitude of glutamatergic EPSCs in SPNs in the PVN (from 62.0 ± 6.5 pA, control to 34.8 ± 6.6 pA, almorexant, P < 0.01, 1-way repeated-measures ANOVA and Dunnett’s posttest, n = 5, Fig. 8).
Fig. 7.
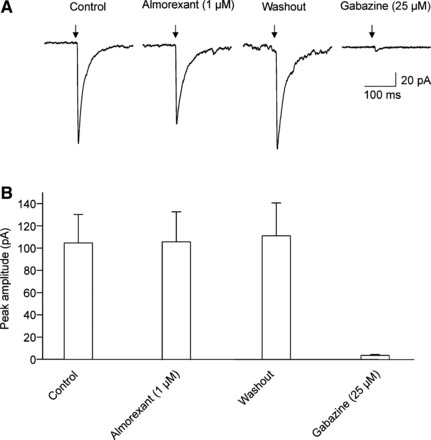
As shown in a typical experiment (A) and in the summary data (B), application of the dual orexin receptor almorexant did not alter the peak amplitude of the GABAergic inhibitory postsynaptic currents (IPSCs) in SPNs in the PVN (P > 0.05, 1-way repeated-measures ANOVA and Dunnett’s posttest, n = 6).
Fig. 8.
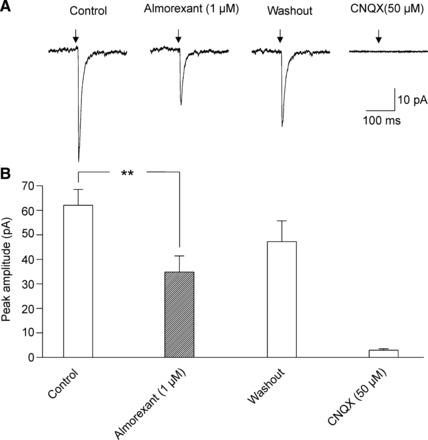
In contrast to GABAergic IPSCs, almorexant significantly and reversibly diminished the peak amplitude of glutamatergic excitatory postsynaptic currents (EPSCs) in PSNs in the PVN (P < 0.01, 1-way repeated-measures ANOVA and Dunnett’s posttest, n = 5). A typical experiment is shown in A, whereas the summary data are illustrated in B. **P < 0.01.
We next performed experiments to determine a possible site of orexin action involved in facilitating glutamatergic neurotransmission. Orexin (0.1 µM) bath applied in the presence of TTX (1 µM) reversibly increased the frequency of spontaneous glutamatergic mEPSCs in SPNs (from 0.6 ± 0.1 Hz to 1.1 ± 0.2 Hz, P < 0.01, one-way ANOVA and Dunnett’s posttest, n = 7, Fig. 9). In contrast, the amplitude of mEPSCs in SPNs was unchanged on orexin application (10.6 pA ± 0.7, control vs. 11.2 ± 0.9 pA, P < 0.05, one-way ANOVA and Dunnett’s posttest, n = 7, Fig. 9). This increase in the mEPSC frequency suggests that orexin likely acts at presynaptic terminals to facilitate glutamatergic neurotransmission to SPNs in the PVN.
Fig. 9.
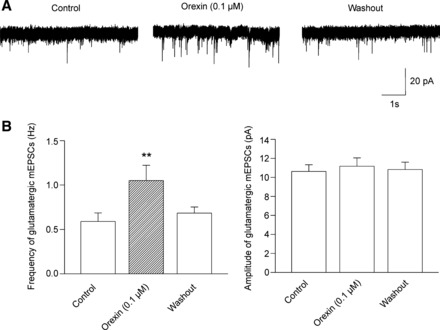
In the presence of TTX (1 µM), orexin (0.1 µM) elicited reversible increase in the frequency of spontaneous glutamatergic miniature postsynaptic currents (mEPSCs) in SPNs (P < 0.01, 1-way ANOVA and Dunnett’s posttest, n = 7). However, orexin (0.1 µM) did not change the amplitude of mEPSCs in SPNs (1-way ANOVA and Dunnett’s posttest, n = 7). A representative experiment is shown in A, whereas the summary data are illustrated in B. **P < 0.01.
DISCUSSION
Major findings.
To the best of our knowledge, this is the first report identifying and characterizing functional projections from orexin neurons to SPNs in the PVN. There are five major findings in this study. 1) Optogenetic stimulation of ChR2-EYFP-expressing orexin neurons in the lateral hypothalamus results in reliable excitation of these orexin neurons, observed both by action potential firing as well as large inward currents. 2) Optogenetic stimulation of ChR2-EYFP-expressing orexin fibers evokes short-latency postsynaptic responses in SPNs in the PVN. There are three types of neurotransmission in the connection from orexin neurons to SPNs, including glutamatergic (59%), GABAergic (35%), and mixed glutamatergic and GABAergic (6%) postsynaptic currents. 3) Both light-triggered EPSC and IPSC are preserved with TTX and 4-AP, suggesting this neurotransmission is a monosynaptic release of glutamate and GABA. 4) In addition to postsynaptic currents, optogenetic stimulation of orexin fibers elicits an AMPA receptor-mediated increase in the frequency of action potential firing in a majority of SPNs. 5) Almorexant, a dual orexin receptor antagonist, does not alter GABAergic IPSCs but does diminish glutamatergic EPSCs in SPNs.
Excitatory and inhibitory projections from orexin-containing neurons to PSNs.
Both SPNs in the PVN and orexin neurons in the lateral hypothalamus are known to play an important role in autonomic cardiovascular regulation (12, 22, 24, 25, 48, 49). Previous studies have found that the PVN is densely innervated by orexin-containing fibers (9, 38, 39), and bath-applied orexin depolarizes neurons in this nucleus (49); however, the endogenous release of neurotransmitters and postsynaptic receptor activation in the pathway from orexin neurons to identified SPNs in the PVN has not previously been established.
Selective optogenetic stimulation of orexin neurons elicits postsynaptic currents in all SPNs tested (100%, n = 34). This indicates a robust connectivity between orexin cells and PSNs. The light-triggered responses include glutamatergic, GABAergic, and mixed glutamatergic and GABAergic postsynaptic currents. The latencies of the responses range from 2.1 to 6.4 ms, suggesting a monosynaptic connection (36). In addition to the short latencies, the present study provides pharmacological evidence of monosynaptic input from orexin-containing neurons to SPNs. Both glutamatergic and GABAergic neurotransmission to SPNs could still be evoked by photostimulation when sodium channels are blocked by TTX in the presence of the potassium blocker 4-AP. This combination of Na and K channel antagonists is commonly used to distinguish between mono- and polysynaptic connections, as in previously published studies (11, 37, 44).
The majority of the responses in PSNs (59%) are mediated by glutamate release and postsynaptic AMPA receptor activation, as these postsynaptic responses are completely blocked by application of the AMPA receptor antagonist CNQX (50 µM). Light-triggered glutamate release is large enough to evoke changes in action potential firing. Indeed, photostimulation of ChR2-containing fibers results in increase in the frequency of action potential firing in a majority of SPNs, and this excitation of SPNs is eliminated by AMPA receptor blocker CNQX (50 µM). These results provide for the first time evidence that orexin neurons excite, via glutamatergic neurotransmission, SPNs in the PVN. Our data are similar to a previously published report that optogenetic stimulation of ChR2-containing fibers from orexin neurons elicits glutamatergic postsynaptic currents in histaminergic tuberomammillary neurons (47). In addition, our data advance previous work from immunohistochemical studies, indicating that orexin neurons contain glutamate (20, 54).
We propose that this glutamatergic excitatory pathway from orexin to SPNs in the PVN is an important component of the mechanism by with orexin-containing cells mediate cardiovascular function. Orexin is well known to evoke sympathetic activation in different regions of the central nervous system. Intracerebroventricular, intracisternal, or intrathecal administration of orexin elicits pressor effects (1, 7, 32, 43, 50). Microinjection of orexin into the rostral ventrolateral (7, 29) or the nucleus tractus solitarius also increases arterial pressure and heart rate (52). The results from this study suggest that in addition to acting on orexin receptors, orexin-containing neurons may modulate cardiovascular sympathoactivation via direct release of the fast neurotransmitter glutamate on SPNs in the PVN.
The orexin receptor blocker almorexant (1 µM) diminishes the amplitude of light-triggered glutamatergic EPSCs, indicating that orexin signaling facilitates this excitatory neurotransmission in a subpopulation of SPNs. Almorexant has been characterized as balanced orexin receptor 1/orexin receptor 2 antagonist, which acts as a competitive and fast equilibrating antagonist at orexin 1 receptors and a noncompetitive and slowly equilibrating antagonist at orexin 2 receptors (30, 31). The results from previous in vitro studies demonstrate that almorexant completely antagonizes the effects of orexin on neurons in the ventral tegmental area (30, 53). In this study, almorexant most likely acts on both orexin 1 and orexin 2 receptors. Although the site of action of orexin involved in facilitating light-triggered glutamatergic neurotransmission is unknown, our data, indicating that orexin application increases the frequency of spontaneous glutamatergic mEPSCs, suggest a likely presynaptic site of action for orexin.
In addition to excitatory glutamatergic inputs, there are also, albeit a minority of responses that are inhibitory, GABAergic projections from orexin neurons to SPNs in the PVN. Thirty-five percent of PSN light-triggered postsynaptic currents were insensitive to CNQX but were abolished by application GABAergic blocker gabazine (25 µM). The GABAergic responses in the presence of TTX and 4-AP, as well as the short latencies of the responses (from 2.1 to 5.3 ms), suggest a monosynaptic inhibitory connection between orexin cells and SPNs. These electrophysiological results are consistent with the possibility that a subpopulation of orexin neurons may corelease GABA. However, neurochemical results from other studies concerning GABA release from orexin neurons are not consistent or uniform. Henny and coauthors (20) found that orexin varicosities contain the presynaptic markers for glutamate, but no immunohistochemical evidence of GABA release from orexin terminals was found within the locus coeruleus. No colocalization of orexin immunoreactivity and GAD67 mRNA has been found in the hypothalamus by using the in situ hybridization method (41). In contrast, the results from another recent study that used real-time PCR methods demonstrate the presence both glutamate and GABA among different orexin cells (18). GABA immunoreactivity was observed within orexin-labeled varicosities in the ventral tegmental area (3). The possibility that orexin neurons may release GABA is also supported by GABA immunoreactivity present in 10–25% of orexin neurons as well as electrophysiological data showing that photostimulation of orexin cells produces short-latency (2–10 ms) GABAergic IPSC in a subpopulation of melanin-concentrating hormone neurons (2).
Interestingly, in 2 out of 34 cells tested in this study, postsynaptic currents were only blocked by application of both CNQX and gabazine, indicating there may be corelease of glutamate and GABA from the same axon terminal. Supporting this hypothesis, vesicular glutamate transporter 1/2 and vesicular GABA transporter have been found in the same nerve terminals in the cerebral cortex, hippocampus, ventral tegmental area, preoptic area, and retina (16, 23, 35, 55, 63). Alternately, excitatory and inhibitory inputs to PSNs may originate from different orexin neurons.
Tonic GABAergic inputs to the PVN have been shown to play a key role in the direct modulation of excitatory outflow from the PVN (25). Indeed, the majority of SPNs in the PVN are subject to a tonic GABAergic inhibition, and GABAergic activity dominates inhibitory-excitatory balance in the PVN under basal conditions (25, 28, 58). The results from our study suggest inhibitory GABAergic projections from orexin-containing neurons may contribute to the endogenous tonically active GABAergic system influencing the outflow activity of SPNs in the PVN.
Physiological relevance and clinical implications.
This study is the first report demonstrating heterogeneous synaptic neurotransmission from orexin neurons to identified SPNs. There are different subpopulations of orexin neurons, including those with excitatory and inhibitory inputs to SPNs in the PVN. These subpopulations of orexin-containing cells are likely differently influenced by various environmental stimuli and/or internal factors and may therefore play differential roles in sympathetic regulation of cardiovascular function and/or cardiovascular dysregulation. For example, glutamatergic/orexinergic neurotransmission from orexin neurons to SPNs may contribute to sympathetic activation of cardiovascular function under normal conditions, whereas dysfunction of GABAergic pathway from orexin neurons to SPNs may play a role in cardiovascular abnormalities associated with the orexin deficiency disorder narcolepsy. Increased sympathetic activity to the heart and blood vessels was reported in individuals with narcolepsy with cataplexy (17, 57). The results from animal studies indicate that chronic lack of orexin signaling results in reduction of sleep-related decrease in blood pressure (4). The present view linking orexin signaling to sympathoactivation only (7, 27, 33, 43) cannot explain evidence of elevated sympathetic tone in orexin-deficient animals and humans. However, the findings from our study suggest that dysfunction of GABAergic transmission from orexin neurons to SPNs in the PVN may lead to disinhibition of SPNs and sympathoactivation in patients with narcolepsy. Further work is needed to understand the differences between subpopulations of orexin neurons and their pathways to SPNs in the PVN and how they may serve disparate roles in cardiovascular regulation and cardiovascular abnormalities associated with disorders.
Conclusions.
This report is the first demonstration of heterogeneous projections from orexin neurons to SPNs in the PVN including monosynaptic glutamatergic and GABAergic neurotransmission. This study elucidates network mechanisms by which orexin neurons contribute to sympathetic control of cardiovascular function and provides insight into possible pathophysiological mechanisms of cardiovascular abnormalities associated with orexin deficiency disorders such as narcolepsy.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-72006 (to D. Mendelowitz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
O.D., A.Y., and D.M. conceived and designed research; O.D. performed experiments; O.D. analyzed data; O.D., A.Y., A.R.S., V.Y.P., and D.M. interpreted results of experiments; O.D. prepared figures; O.D. drafted manuscript; O.D., A.Y., A.R.S., V.Y.P., and D.M. edited and revised manuscript; O.D., A.Y., A.R.S., V.Y.P., and D.M. approved final version of manuscript.
REFERENCES
- 1.Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 281: R1801–R1807, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Apergis-Schoute J, Iordanidou P, Faure C, Jego S, Schöne C, Aitta-Aho T, Adamantidis A, Burdakov D. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. J Neurosci 35: 5435–5441, 2015. doi: 10.1523/JNEUROSCI.5269-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol 503: 668–684, 2007. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- 4.Bastianini S, Silvani A, Berteotti C, Elghozi JL, Franzini C, Lenzi P, Lo Martire V, Zoccoli G. Sleep related changes in blood pressure in hypocretin-deficient narcoleptic mice. Sleep 34: 213–218, 2011. doi: 10.1093/sleep/34.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branch AF, Navidi W, Tabuchi S, Terao A, Yamanaka A, Scammell TE, Behn CD. Progressive loss of the orexin neurons reveals dual effects on wakefulness. Sleep 39: 369−377, 2016. doi: 10.5665/sleep.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium-calcium exchanger. J Neurosci 23: 4951–4957, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol 278: R692–R697, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Ciriello J, de Oliveira CV. Cardiac effects of hypocretin-1 in nucleus ambiguus. Am J Physiol Regul Integr Comp Physiol 284: R1611–R1620, 2003. doi: 10.1152/ajpregu.00719.2002. [DOI] [PubMed] [Google Scholar]
- 9.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96: 748–753, 1999. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol 103: 1772–1779, 2007. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- 11.DePuy SD, Stornetta RL, Bochorishvili G, Deisseroth K, Witten I, Coates M, Guyenet PG. Glutamatergic neurotransmission between the C1 neurons and the parasympathetic preganglionic neurons of the dorsal motor nucleus of the vagus. J Neurosci 33: 1486–1497, 2013. doi: 10.1523/JNEUROSCI.4269-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dergacheva O. Chronic intermittent hypoxia alters neurotransmission from lateral paragigantocellular nucleus to parasympathetic cardiac neurons in the brain stem. J Neurophysiol 113: 380–389, 2015. doi: 10.1152/jn.00302.2014. [DOI] [PubMed] [Google Scholar]
- 13.Dergacheva O, Boychuk CR, Mendelowitz D. Developmental changes in GABAergic neurotransmission to presympathetic and cardiac parasympathetic neurons in the brainstem. J Neurophysiol 110: 672–679, 2013. doi: 10.1152/jn.01054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol 160: R7–R12, 1999. doi: 10.1677/joe.0.160R007. [DOI] [PubMed] [Google Scholar]
- 15.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol 545: 855–867, 2002. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez-Lira G, Lamas M, Romo-Parra H, Gutiérrez R. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci 25: 6939–6946, 2005. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimaldi D, Calandra-Buonaura G, Provini F, Agati P, Pierangeli G, Franceschini C, Barletta G, Plazzi G, Montagna P, Cortelli P. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep 35: 519–528, 2012. doi: 10.5665/sleep.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harthoorn LF, Sañé A, Nethe M, Van Heerikhuize JJ. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol Neurobiol 25: 1209–1223, 2005. doi: 10.1007/s10571-005-8184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayar A, Guyenet PG. Alpha2-adrenoceptor-mediated presynaptic inhibition in bulbospinal neurons of rostral ventrolateral medulla. Am J Physiol Heart Circ Physiol 277: H1069–H1080, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 169: 1150–1157, 2010. doi: 10.1016/j.neuroscience.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosoya Y, Sugiura Y, Okado N, Loewy AD, Kohno K. Descending input from the hypothalamic paraventricular nucleus to sympathetic preganglionic neurons in the rat. Exp Brain Res 85: 10–20, 1991. doi: 10.1007/BF00229982. [DOI] [PubMed] [Google Scholar]
- 22.Kannan H, Shirasaka T, Watanabe S, Yu NS, Kuitake T, Takasaki M [Central action of orexins on sympathetic outflow and cardiovascular function with a focus on the paraventricular nucleus of the hypothalamus]. Masui 56: 30–39, 2007. [PubMed] [Google Scholar]
- 23.Kao YH, Lassová L, Bar-Yehuda T, Edwards RH, Sterling P, Vardi N. Evidence that certain retinal bipolar cells use both glutamate and GABA. J Comp Neurol 478: 207–218, 2004. doi: 10.1002/cne.20221. [DOI] [PubMed] [Google Scholar]
- 24.Kc P, Balan KV, Tjoe SS, Martin RJ, Lamanna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol 588: 725–740, 2010. doi: 10.1113/jphysiol.2009.184580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kc P, Dick TE. Modulation of cardiorespiratory function mediated by the paraventricular nucleus. Respir Physiol Neurobiol 174: 55–64, 2010. doi: 10.1016/j.resp.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73: 553–566, 2012. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Li A, Nattie E. Orexin, cardio-respiratory function, and hypertension. Front Neurosci 8: 22, 2014. doi: 10.3389/fnins.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res 454: 123–130, 1988. doi: 10.1016/0006-8993(88)90810-4. [DOI] [PubMed] [Google Scholar]
- 29.Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept 104: 75–81, 2002. doi: 10.1016/S0167-0115(01)00351-2. [DOI] [PubMed] [Google Scholar]
- 30.Malherbe P, Borroni E, Pinard E, Wettstein JG, Knoflach F. Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol 76: 618–631, 2009. doi: 10.1124/mol.109.055152. [DOI] [PubMed] [Google Scholar]
- 31.Mang GM, Dürst T, Bürki H, Imobersteg S, Abramowski D, Schuepbach E, Hoyer D, Fendt M, Gee CE. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep 35: 1625–1635, 2012. doi: 10.5665/sleep.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumura K, Tsuchihashi T, Abe I. Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension 37: 1382–1387, 2001. doi: 10.1161/01.HYP.37.6.1382. [DOI] [PubMed] [Google Scholar]
- 33.Murakami M, Ohba T, Kushikata T, Niwa H, Kurose A, Imaizumi T, Watanabe H, Yanagisawa T, Nakaji S, Ono K, Hirota K. Involvement of the orexin system in sympathetic nerve regulation. Biochem Biophys Res Commun 460: 1076–1081, 2015. doi: 10.1016/j.bbrc.2015.03.157. [DOI] [PubMed] [Google Scholar]
- 34.Nattie E, Li A. Respiration and autonomic regulation and orexin. Prog Brain Res 198: 25–46, 2012. doi: 10.1016/B978-0-444-59489-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci 24: 8097–8105, 2004. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10: 663–668, 2007. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 37.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature 457: 1142–1145, 2009. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plaza-Zabala A, Flores Á, Maldonado R, Berrendero F. Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol Psychiatry 71: 214–223, 2012. doi: 10.1016/j.biopsych.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 40.Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res 120: 164–172, 1998. doi: 10.1007/s002210050390. [DOI] [PubMed] [Google Scholar]
- 41.Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol 465: 593–603, 2003. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- 42.Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol 228: 168–185, 1984. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- 43.Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res 831: 248–253, 1999. doi: 10.1016/S0006-8993(99)01457-2. [DOI] [PubMed] [Google Scholar]
- 44.Saunders A, Granger AJ, Sabatini BL. Corelease of acetylcholine and GABA from cholinergic forebrain neurons. eLife 4: e06412, 2015. doi: 10.7554/eLife.06412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205: 260–272, 1982. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 46.Schoenenberger P, Schärer YP, Oertner TG. Channelrhodopsin as a tool to investigate synaptic transmission and plasticity. Exp Physiol 96: 34–39, 2011. doi: 10.1113/expphysiol.2009.051219. [DOI] [PubMed] [Google Scholar]
- 47.Schöne C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci 32: 12437–12443, 2012. doi: 10.1523/JNEUROSCI.0706-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharpe AL, Calderon AS, Andrade MA, Cunningham JT, Mifflin SW, Toney GM. Chronic intermittent hypoxia increases sympathetic control of blood pressure: role of neuronal activity in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol 305: H1772–H1780, 2013. doi: 10.1152/ajpheart.00592.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H. Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol Regul Integr Comp Physiol 281: R1114–R1118, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol Regul Integr Comp Physiol 277: R1780–R1785, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci USA 104: 11453–11458, 2007. doi: 10.1073/pnas.0702041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res 950: 261–267, 2002. doi: 10.1016/S0006-8993(02)03048-2. [DOI] [PubMed] [Google Scholar]
- 53.Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, Hopf FW, Bonci A, Bartlett SE. The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS One 7: e44726, 2012. doi: 10.1371/journal.pone.0044726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119: 1033–1044, 2003. doi: 10.1016/S0306-4522(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 55.Uchida N. Bilingual neurons release glutamate and GABA. Nat Neurosci 17: 1432–1434, 2014. doi: 10.1038/nn.3840. [DOI] [PubMed] [Google Scholar]
- 56.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci 18: 7962–7971, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Meijden WP, Fronczek R, Reijntjes RH, Corssmit EP, Biermasz NR, Lammers GJ, van Dijk JG, Thijs RD. Time- and state-dependent analysis of autonomic control in narcolepsy: higher heart rate with normal heart rate variability independent of sleep fragmentation. J Sleep Res 24: 206−214, 2015. doi: 10.1111/jsr.12253. [DOI] [PubMed] [Google Scholar]
- 58.Watkins ND, Cork SC, Pyner S. An immunohistochemical investigation of the relationship between neuronal nitric oxide synthase, GABA and presympathetic paraventricular neurons in the hypothalamus. Neuroscience 159: 1079–1088, 2009. doi: 10.1016/j.neuroscience.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Womack MD, Pyner S, Barrett-Jolley R. Inhibition by alpha-tetrahydrodeoxycorticosterone (THDOC) of pre-sympathetic parvocellular neurones in the paraventricular nucleus of rat hypothalamus. Br J Pharmacol 149: 600–607, 2006. doi: 10.1038/sj.bjp.0706911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu M, Zhang Z, Leranth C, Xu C, van den Pol AN, Alreja M. Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J Neurosci 22: 7754–7765, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamanaka A. [Controlling sleep/wakefulness using optogenetics]. Nihon Shinkei Seishin Yakurigaku Zasshi 35: 97–102, 2015. [PubMed] [Google Scholar]
- 62.Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol 98: 1387–1395, 2005. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]
- 63.Zander JF, Münster-Wandowski A, Brunk I, Pahner I, Gómez-Lira G, Heinemann U, Gutiérrez R, Laube G, Ahnert-Hilger G. Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses. J Neurosci 30: 7634–7645, 2010. doi: 10.1523/JNEUROSCI.0141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


