Oxidative injury to endothelial cells by oxidized LDL reduced cystathionine γ-lyase (CSEγ) expression and H2S production, leading to endothelial dysfunction, which was prevented by histone deacetylase 6 (HDAC6) inhibition. Our data suggest HDAC6 as a novel therapeutic target to prevent the development of atherosclerosis.
Keywords: endothelial function, OxLDL, HDAC6, transcriptional regulation, CSEγ, H2S, atherosclerosis
Abstract
Endothelial cystathionine γ-lyase (CSEγ) contributes to cardiovascular homeostasis, mainly through production of H2S. However, the molecular mechanisms that control CSEγ gene expression in the endothelium during cardiovascular diseases are unclear. The aim of the current study is to determine the role of specific histone deacetylases (HDACs) in the regulation of endothelial CSEγ. Reduced CSEγ mRNA expression and protein abundance were observed in human aortic endothelial cells (HAEC) exposed to oxidized LDL (OxLDL) and in aortas from atherogenic apolipoprotein E knockout (ApoE−/−) mice fed a high-fat diet compared with controls. Intact murine aortic rings exposed to OxLDL (50 μg/ml) for 24 h exhibited impaired endothelium-dependent vasorelaxation that was blocked by CSEγ overexpression or the H2S donor NaHS. CSEγ expression was upregulated by pan-HDAC inhibitors and by class II-specific HDAC inhibitors, but not by other class-specific inhibitors. The HDAC6 selective inhibitor tubacin and HDAC6-specific siRNA increased CSEγ expression and blocked OxLDL-mediated reductions in endothelial CSEγ expression and CSEγ promoter activity, indicating that HDAC6 is a specific regulator of CSEγ expression. Consistent with this finding, HDAC6 mRNA, protein expression, and activity were upregulated in OxLDL-exposed HAEC, but not in human aortic smooth muscle cells. HDAC6 protein levels in aortas from high-fat diet-fed ApoE−/− mice were comparable to those in controls, whereas HDAC6 activity was robustly upregulated. Together, our findings indicate that HDAC6 is upregulated by atherogenic stimuli via posttranslational modifications and is a critical regulator of CSEγ expression in vascular endothelium. Inhibition of HDAC6 activity may improve endothelial function and prevent or reverse the development of atherosclerosis.
NEW & NOTEWORTHY Oxidative injury to endothelial cells by oxidized LDL reduced cystathionine γ-lyase (CSEγ) expression and H2S production, leading to endothelial dysfunction, which was prevented by histone deacetylase 6 (HDAC6) inhibition. Our data suggest HDAC6 as a novel therapeutic target to prevent the development of atherosclerosis.
dyslipidemia is one of several underlying etiologies of atherosclerosis and its associated cardio- and cerebrovascular complications, the leading causes of morbidity and mortality in developed countries (10). The initial injury and downstream complications of atherosclerosis are known to originate in the endothelium. Inflammation marks the early stages of atherosclerotic disease and, ultimately, leads to plaque development, as evidenced by induction of adhesion and migration of leukocytes into the intima and maturation of monocytes into macrophages and, later, evolution into lipid-laden foam cells. Endothelial dysfunction is considered to be an early and critical event in the development and progression of atherosclerosis (20). Accumulating evidence suggests that elevated oxidized low-density lipoprotein (OxLDL) is a proinflammatory trigger that initiates and promotes the atherosclerotic cascade.
Endothelial function is regulated by a plethora of vasoactive transmitters, including the gasotransmitters nitric oxide (NO), carbon monoxide, and hydrogen sulfide (H2S) (27). The most recently discovered gasotransmitter H2S and its depletion have been linked to hypertension and impaired vascular relaxation to acetylcholine (ACh) (33). H2S is synthesized by vascular cells, including endothelial cells, through the action of cystathionine γ-lyase (CSEγ) from substrates including cysteine, cystine, and homocysteine (36). Gasotransmitters are highly reactive and therefore they are not locally stored, but rather produced upon cellular demand. The cellular redox state is known to regulate CSEγ expression in rat mesangial cells (9). Additionally, NADPH oxidase 4 (Nox4), a major source of reactive oxygen species in endothelial cells, has been shown to regulate transcription of CSEγ (25). OxLDL is known to modulate the redox state of endothelial cells through the lectin-like OxLDL receptor-1 (LOX-1). Moreover, OxLDL-LOX-1 signaling leads to initiation of the inflammatory cascade and promotes endothelial dysfunction (18).
A critically important group of transcriptional and posttranscriptional cellular regulators are the histone deacetylases (HDACs). The 18 different HDACs that have been identified are further subcategorized into 4 groups: class I (HDAC1, -2, -3, and -8), class IIa (HDAC4, -5, -7, and -9), class IIb (HDAC6 and -10), class III (SIRT1–7), and class IV (HDAC11). Recent studies describe the role of HDACs in transcription of genes important for endothelial function (5, 29). In addition to their critical role in gene transcription in the nucleus, HDACs modulate posttranslational modifications of structural and contractile proteins outside the nucleus that are important in the development and progression of atherosclerotic lesions (3, 7).
Inhibition of HDACs is known to downregulate Nox4 transcription in endothelial cells and alter the vascular redox state (32). HDAC6, a class IIb HDAC, is an important regulator of myocardial and vascular contractile function and deacetylates the substrates α-tubulin and cortactin (40). Furthermore, pharmacological inhibition of HDAC6 in macrophages has been shown to protect against lipopolysaccharide-induced inflammation by reducing deacetylation of microtubules (35). A recent review by Zheng et al. summarizes current knowledge of the roles of HDACs in atherosclerosis and raises an important question regarding whether HDAC6 is an important negative regulator of vasculature contractile function that leads to the progression of atherosclerosis (41).
The aim of this study was to investigate the effect of OxLDL on endothelial CSEγ expression and describe the specific HDAC(s) that are responsible for mediating these changes.
METHODS
Animals and reagents.
All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine. C57BL/6 and apolipoprotein E (ApoE) knockout (ApoE−/−) mice (30) were purchased from Jackson Laboratory (Bar Harbor, ME). OxLDL, which was prepared by the reaction of LDL and CuSO4, was purchased from Alfa Aesar (Haverhill, MA). All HDAC inhibitors except trichostatin A were purchased from Selleckchem (Houston, TX); trichostatin A was purchased from Cell Signaling Technology (Danvers, MA). The following primary antibodies were used: CSEγ (1:500 dilution; catalog no. 60234-1, Proteintech, Rosemont, IL), HDAC6 (1:1,000 dilution; catalog no. 2144S, Cell Signaling Technology), HDAC2 (1:1,000 dilution; catalog no. AP9762c, Abgent, San Diego, CA), endothelial NO synthase (eNOS, 1:1,000 dilution; catalog no. sc-136977, Santa Cruz Biotechnology, Dallas, TX), phosphorylated (Ser1177) eNOS (1:1,000 dilution; catalog no. 9571, Cell Signaling Technology), Akt (1:1,000 dilution, catalog no. 9272, Cell Signaling Technology), phosphorylated (Thr308) Akt (1:1,000 dilution; catalog no. 4056, Cell Signaling Technology), green fluorescent protein (1:1,000 dilution; catalog no. sc-9996, Santa Cruz Biotechnology), VE-cadherin (1:1,000 dilution; catalog no. 160840, Cayman Chemical, Ann Arbor, MI), GAPDH (1:2,000 dilution; catalog no. ab14247, Abcam, Cambridge, MA), β-tubulin (1:2,000 dilution; catalog no. ab6046, Abcam), α-tubulin (1:1,000 dilution; catalog no. 2144S, Cell Signaling Technology), and acetylated α-tubulin (1:1,000, catalog no. T7461, Sigma, St. Louis, MO).
Cell culture.
Human aortic endothelial cells (HAEC) were maintained in ECM culture medium (Science Cell Research Laboratories, Carlsbad, CA) according to the supplier’s protocol. Lipofectamine 2000 (ThermoFisher Scientific, Waltham, MA) was used for transfection of HEK-293 cells according to the manufacturer’s protocol, and the Amaxa transfection system (Lonza, Allendale, NJ) was used for transfection of HAEC.
RT-PCR and real-time PCR.
TRIzol and PureLink (ThermoFisher Scientific) were used to extract total RNA from mouse aortic segments and HAEC. The RNA was reverse-transcribed with oligo(dT) primers to obtain cDNA. Quantitative real-time PCR (ThermoFisher Scientific) was performed with SYBR Green Supermix (ThermoFisher Scientific), whereas semiquantitative RT-PCR was performed with a conventional PCR machine (Bio-Rad, Hercules, CA) and the following primer sets: 5′-CCCCACAAACCCCACCCAGAA-3′ (forward) and 5′-GACACCAGGCCCATTAC-3′ (reverse) for human CSEγ, 5′-AAGTAGGCAGAACCCCCAGT-3′ (forward) and 5′-GTGCTTCAGCCTCAAGGTTC-3′ (reverse) for human HDAC6, and 5′-CGTTCAGCCACCCGAGATT-3′ (forward) and 5′-GACCCGCACTTACTGGGAATT-3′ (reverse) for human 18s.
DNA constructs and adenoviruses.
We created a CSEγ mammalian expression construct by PCR using an existing CSEγ construct as a template with the following primers: 5′-CACCATGCAGGAAAAAGAC-3′ (forward) and 5′-GCTGTGACTTCCACTTGGAGG-3′ (reverse). The PCR product was cloned into pcDNA3.1 by directional TOPO cloning (ThermoFisher Scientific). We constructed adenoviruses encoding CSEγ expression by subcloning CSEγ into the pENTR1A entry vector using EcoRI restriction enzymes followed by LR recombination with the destination vector pDEST. The CSEγ-pDEST DNA was digested with PacI, ethanol-precipitated, and transfected into HEK-293 cells. After cytopathic effect, adenoviruses were collected and purified with three freeze-thaw cycles and an adenovirus purification kit (Millipore, Temecula, CA).
Force tension myography.
Mouse aorta was isolated and cleaned in ice-cold Krebs-Ringer bicarbonate solution containing (in mM) 118.3 NaCl, 4.7 KCl, 1.6 CaCl2, 1.2 KH2PO4, 25 NaHCO3, 1.2 MgSO4, and 11.1 dextrose. The aorta was immersed in a bath filled with constantly oxygenated Krebs buffer at 37°C. Equal-sized (2-mm) thoracic aortic rings were mounted under microscopy to ensure no damage to the smooth muscle or endothelium. One end of the aortic rings was connected to a transducer and the other to a micromanipulator. The rings were passively stretched to an optimal resting tension with the micromanipulator, 60 mM KCl was administered, and passive stretch was repeated after a wash with Krebs buffer. Thereafter, all vessels were allowed to equilibrate for 20–30 min in the presence of indomethacin (3 μM). Phenylephrine (1 μM) was administered to induce vasoconstriction. We then carried out dose-response testing (1 nM–10 μM) with the muscarinic agonist ACh or the NO donor sodium nitroprusside (SNP), as necessary. The responses were repeated in the presence of inhibitors. Relaxation responses were calculated as a percentage of tension after preconstriction. Sigmoidal dose-response curves were fitted to data with the minimum constrained to 0. Two to four rings were isolated from each animal, and the number of animals in each group (n) was six.
Human CSEγ promoter assay.
Chromosomal DNA was prepared from HAEC with TRIzol reagent according to the manufacturer’s protocol (ThermoFisher Scientific). HAEC were transfected with the plasmids by using FuGENE 6 reagent (Roche, Indianapolis, IN), and luciferase activity was measured by the dual-luciferase reporter assay system (Promega, Madison, WI). We reported luciferase activity as relative luciferase units by dividing firefly luciferase activity by Renilla luciferase activity. We amplified the CSEγ promoter fragment 1 kb upstream of the transcription start site by PCR using specific primer sets as follows: 5′-TTGCAACAACAGAAATATCCCC-3′ (forward) and 5′-CGGCACACAGCGATTGTTGGGG-3′ (reverse). Furthermore, we cloned these into the restriction sites for MluI and HindIII on the pGL3-enhancer vector (Promega) using the following primer sets: 5′-GGGCCCACGCGTTTGCAACAACAGAAATATCCCC-3′ (forward) and 5′-GGGCCCAAGCTTCGGCACACAGCGATTGTTGGGG-3′ (reverse). To create an adenoviral reagent for these constructs, we cloned the complete promoter followed by luciferase cassette into the restriction sites for EcorI and NotI on the Gateway entry vector pENTR1A using the following primer set: 5′-GGGCCCGAATTCGGTACCGAGCTCTTACGC-3′ (forward) and 5′-GGGCCCGCGGCCGCGCATCGGTCGACGGATC-3′ (reverse). The pDEST-CSEγ promoter-luciferase construct DNA was digested with PacI, ethanol-precipitated, and transfected into HEK-293 cells. After cytopathic effect, adenoviruses were collected and purified with three freeze-thaw cycles and an adenovirus purification kit (Millipore).
Western blotting.
After 48 h of HAEC transfection, cells were lysed in ice-cold RIPA lysis buffer consisting of 20 mM Tris·HCl at pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP40, 1% sodium deoxycholate, 1 mM Na3VO4, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 μg/ml leupeptin, and 1:1,000-diluted protease inhibitor cocktail (Sigma).
Measurements of NO production.
We determined NO levels in the cell culture medium as described previously by measuring nitrite levels with a Siever’s NO analyzer (GE Instruments, Boulder, CO) (28).
In vitro amperometric H2S measurement.
We measured cellular H2S production by amperometry using the Apollo 4000 free radical analyzer detector (World Precision Instruments, Sarasota, FL) and a 3-mm H2S-selective electrode (World Precision Instruments). Reactions were performed as previously described (34). Briefly, cell lysates were incubated in 1.6 mM pyridoxal-5′-phosphate (Sigma), 0.4 mM dithiothreitol (New England Biolabs, Ipswich, MA), and 50 mM l-cysteine (Sigma) for 120 min. Five milliliters of headspace gas were withdrawn from the gas-tight vial and injected into a scintillation vial containing 10 ml of 10× PBS (ThermoFisher Scientific) in which the amperometric probe was equilibrating. The amount of H2S produced by the reaction was measured by the probe in units of picoamperes. Data were recorded 2 min after the headspace gas was injected.
Statistics.
All statistical analyses were carried out with Prism 7 for Macintosh (GraphPad Software, La Jolla, CA). Results are expressed as means ± SE. Student’s t-test, one-way ANOVA, and Bonferroni’s post hoc test for multiple comparison were used to compare all experimental data sets and pairs of data sets, respectively. P < 0.05 was considered statistically significant.
RESULTS
OxLDL triggers endothelial dysfunction by decreasing CSEγ expression.
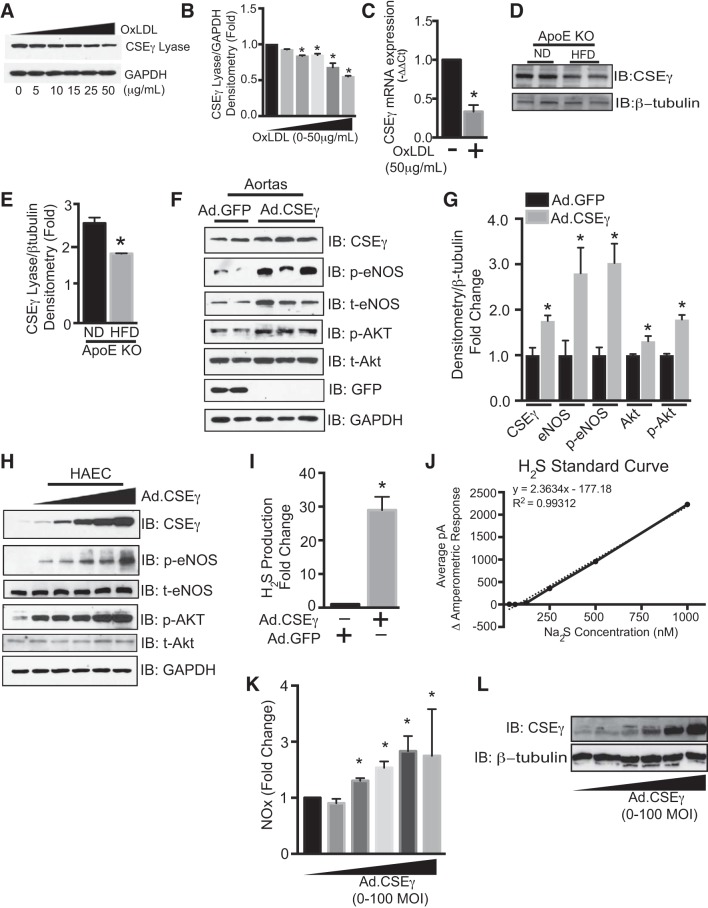
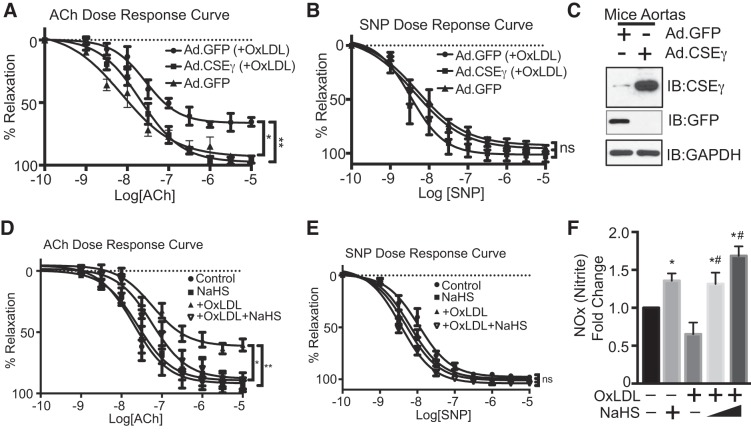
We examined the effects of atherogenic stimuli on endothelial CSEγ expression in two settings: HAEC exposed to OxLDL and ApoE−/− mice fed a high-fat diet (HFD). Levels of CSEγ protein were lower in HAEC exposed to 50 μg/ml OxLDL for 24 h (Fig. 1, A–C) and freshly isolated aortas from ApoE−/− mice fed a HFD for 12 wk (Fig. 1, D and E) than controls. Additionally, CSEγ mRNA levels were reduced by these same conditions in HAEC (Fig. 1C). A previous study showed that H2S and NO have interrelated and synergistic impact on the regulation of vascular function (2). To determine whether increased expression of CSEγ can activate the eNOS signaling pathway, we measured phosphorylation of eNOS at Ser1177 and Akt at Thr308, well-described signaling events for eNOS activation and increased NO production (4). Indeed, overexpression of CSEγ in aortas (Fig. 1, F and G) and HAEC (Fig. 1H) significantly increased phosphorylated eNOS and phosphorylated Akt levels. Interestingly, aortas that overexpressed CSEγ also exhibited increased total eNOS and total Akt levels (Fig. 1, F and G). H2S measurements demonstrated the effectiveness of transduction with the adenoviral CSEγ construct in augmenting H2S production (Fig. 1, I and J). To determine whether increased eNOS phosphorylation led to increased NO production, we measured a by-product of NO metabolism, nitrite, in HAEC that had been transduced with increasing amounts of adenoviruses encoding CSEγ. We observed a dose-dependent increase in NO production with increased expression of CSEγ, as shown in Fig. 1, K and L. Furthermore, we investigated the functional consequences of CSEγ expression in vascular relaxation (Fig. 2, A and B). Isolated murine aortic rings incubated with OxLDL (50 μg/ml) for 24 h showed marked impairment of endothelium-dependent relaxation as determined by ACh dose response. However, aortic rings that overexpressed CSEγ via adenoviral delivery displayed no significant changes in endothelium-dependent relaxation after exposure to OxLDL. The endothelium-independent relaxation, as assessed by NO donor SNP response, did not differ significantly between groups. Western blots of lysates from green fluorescent protein- and CSEγ-transduced aortas reveal the levels of overexpression achieved by the CSEγ adenoviral construct (Fig. 2C). We next determined whether the protective effect of CSEγ against OxLDL-mediated impairment of relaxation was mediated by the production of H2S. Indeed, a 15-min preincubation of phenylephrine-constricted and stabilized murine aortic rings in a low concentration of the H2S donor NaHS (30 µM; a concentration that does not cause either vascular constriction or relaxation) prevented the negative impact of OxLDL on ACh-mediated relaxation (Fig. 2D). In contrast, the SNP dose response (nonendothelium-dependent relaxation) did not differ between groups (Fig. 2E).
Fig. 1.
A and B: human aortic endothelial cells (HAEC) exposed to oxidized LDL (OxLDL, 0, 5, 10, 15, 25, and 50 µg/ml) for 24 h were subjected to Western blotting with cystathionine γ-lyase (CSEγ) and GAPDH antibodies. C: mRNA levels of CSEγ and 18s (housekeeping gene) were assessed by real-time PCR in HAEC exposed to OxLDL (50 µg/ml) for 24 h and presented as 2–ΔΔCt (cycle threshold). D and E: isolated aortas from apolipoprotein E (ApoE) knockout (KO) mice fed a normal diet (ND) or a high-fat diet (HFD) for 12 wk were subjected to Western blotting with CSEγ and β-tubulin antibodies. IB, immunoblot. F–H: mouse aortas (F and G) and HAEC (H) were transduced with CSEγ adenoviruses (Ad) for 24 h, and cell lysates were subjected to Western blotting with total (t) endothelial nitric oxide (NO) synthase (eNOS), total Akt, phosphorylated (Ser1177) eNOS (p-eNOS), phosphorylated (Thr308) Akt (p-Akt), and GAPDH antibodies. I: electrochemical probes were used to assay cell lysates from HAEC transduced with CSEγ adenoviral construct [30 multiplicity of infection (MOI)] for H2S content. J: standard curve for H2S measurements by electrochemical probes. K: NO [nitrite (NOx)] release from HAEC transduced with increasing amounts of adenoviruses encoding CSEγ (0–100 MOI) measured using Siever’s NO analyzer. L: cell lysates from HAEC transduced with increasing amounts of adenoviruses encoding CSEγ (0–100 MOI) were immunoblotted with anti-CSEγ antibodies. *P < 0.05 vs. respective controls. Western blots are representative of 3 immunoblots.
Fig. 2.
A and B: wire myography measurement of acetylcholine (ACh)- and sodium nitroprusside (SNP)-mediated vascular relaxation in mouse aortic rings incubated with or without OxLDL (50 µg/ml) for 24 h in the presence or absence of adenoviral CSEγ overexpression. Adenoviruses encoding green fluorescent protein (GFP) were used as a transfection control. C: Western blots show CSEγ and GFP protein levels in CSEγ- and GFP-transduced aortas after 24 h of infection using CSEγ and GFP antibodies. D and E: phenylephrine-constricted and stabilized aortic rings were incubated in the presence or absence of the H2S donor NaHS (50 µM) for 15 min, and ACh- and SNP-mediated vascular relaxation in mouse aortic rings incubated in medium with or without OxLDL (50 µg/ml) for 24 h was measured by wire myography. F: nitrite levels in HAEC medium incubated in the presence and absence of OxLDL (50 µg/ml) for 24 h with or without 10–100 µM NaHS for 1 h. *, #P < 0.05 vs. respective controls. **P < 0.05, −OxLDL vs. +OxLDL. Values are means ± SE; n = 6. Western blots are representative of 3 immunoblots. ns, Not significant.
We next determined the effect of increased H2S on NO production. As shown in Fig. 2F, HAEC exposed to NaHS (10–100 µM for 1 h) exhibited upregulation of NO production, and NaHS blocked the inhibitory effects of OxLDL on NO production in a dose-dependent fashion. These results highlight the likelihood that endothelial CSEγ is very important for normal vascular function and protection of the vascular endothelium from atherogenic stimuli. We therefore pursued mechanisms that might regulate CSEγ in vascular endothelium. HDACs are important endothelial transcriptional regulators. Recently, our group showed the importance of HDAC2 for control of endothelial arginase 2 expression and downstream vascular function (29). In the current work we screened for specific HDACs that might serve as transcriptional regulators of endothelial CSEγ expression.
CSEγ expression in endothelium is regulated by the class II HDAC, HDAC6.
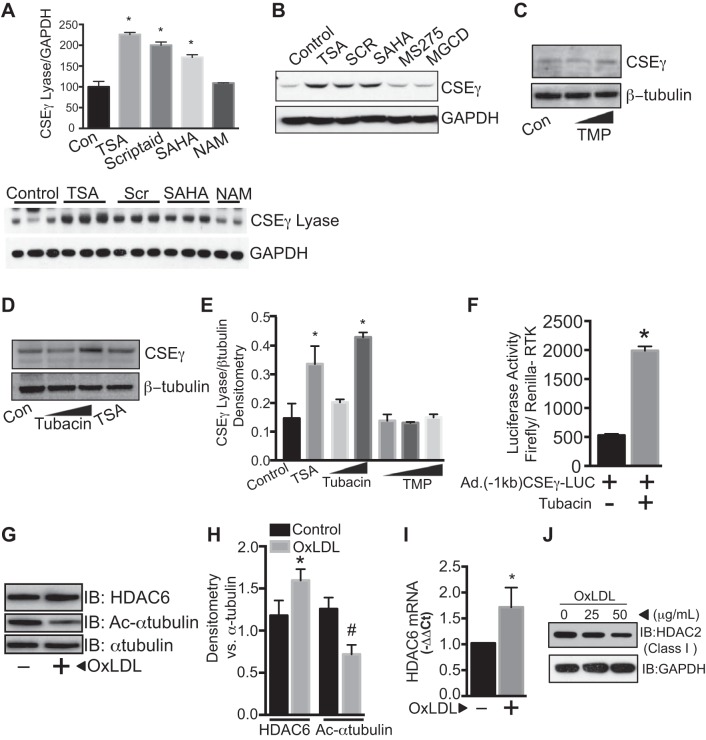
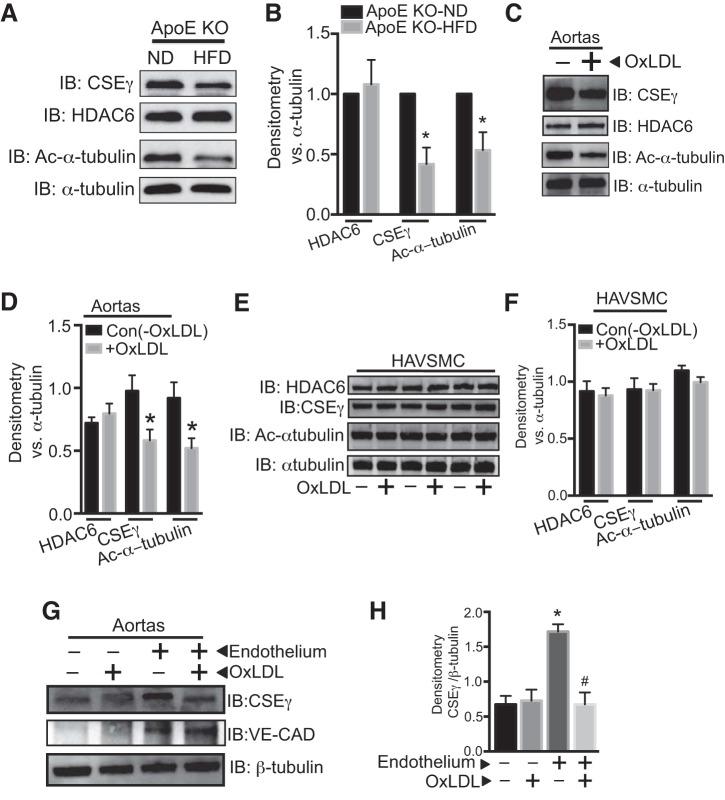
To identify the specific HDACs that are important for CSEγ regulation in HAEC, we first evaluated the effect of the broad-spectrum HDAC inhibitors trichostatin A (0.5 μM), Scriptaid (0.5 μM), and suberoylanilide hydroxamic acid (0.5 μM), as well as the class-specific and narrower-spectrum inhibitors nicotinamide (class III, 5 μM), MGCD0103 and MS275 (class I, 1 μM), MC1568 (class II, 1 μM), TMP269 (class IIa, 0.5 μM), and tubacin (HDAC6, 1, 3, and 10 μM) on endothelial CSEγ expression. Nonspecific HDAC inhibitors, MC1568 and tubacin, significantly upregulated CSEγ expression, whereas nicotinamide, MGCD0103, and MS275 had no effect on CSEγ expression. These results collectively suggest that class II HDAC6 is the regulator of endothelial CSEγ expression (Fig. 3, A–E). Furthermore, utilizing a luciferase reporter assay in HAEC transduced with adenoviruses encoding the firefly luciferase gene under the control of a CSEγ promoter (1 kb upstream of the transcription start site), we demonstrated that HDAC6 inhibition by tubacin can significantly activate the CSEγ promoter (Fig. 3F). We next investigated the impact of OxLDL on HDAC6 expression and activity in HAEC. HDAC6 activity was assessed by measuring levels of acetylated α-tubulin, one of the critical functions of HDAC6 (12). We found increased HDAC6 protein expression and mRNA with a concomitant reduction in acetylated levels of α-tubulin in HAEC exposed to OxLDL (50 μg/ml) for 24 h (Fig. 3, G–I). In contrast, class I HDACs, such as HDAC2, were downregulated in OxLDL-exposed HAEC (Fig. 3J). We further evaluated HDAC6 expression/activity in isolated aortas from ApoE−/− mice on a HFD regimen for 12 wk and in isolated aortic rings exposed to OxLDL (50 μg/ml) for 24 h. Interestingly, HDAC6 protein expression in aortas from HFD-fed ApoE−/− mice was not different from that in ApoE−/− mice fed a normal diet. However, acetylated levels of α-tubulin (a reciprocal index of HDAC6 activity) were lower in the HFD group (Fig. 4, A and B). Isolated aortas that were incubated in OxLDL for 24 h exhibited significant increases in HDAC6 activity (reduced acetylated levels of α-tubulin) without any changes in expression, as shown in Fig. 4, C and D. We also examined whether OxLDL directly affected HDAC6 and CSEγ in human vascular smooth muscle cells or in the medial layer of the murine aortas and found that they did not (Fig. 4, E–H). These data strongly suggest that HDAC6 is a critical component of endothelial injury signaling that is elicited by OxLDL and results in reduction of CSEγ levels and H2S production.
Fig. 3.
A–D: HAEC were incubated under control conditions (Con) or with the broad-spectrum HDAC inhibitors trichostatin A (TSA, 0.5 μM), Scriptaid (SCR, 0.5 μM), and suberoylanilide hydroxamic acid (SAHA, 0.5 μM) or the class III-specific HDAC inhibitor nicotinamide (NAM, 5 μM); MS-275 (1 μM, an inhibitor of class I and III HDACs) or MGCD0103 (MGCD, 1 μM, an inhibitor of class I HDACs); increasing doses of TMP269 (TMP, 3 and 10 µM); or the HDAC6-specific inhibitor tubacin (1 and 10 μM). Cell lysates were subjected to Western blotting using CSEγ and GAPDH antibodies. E: densitometric analyses of data from replicate blots from conditions in C and D. F: CSEγ promoter activity for the fragment 1 kb upstream of the transcription start site was measured using a luciferase reporter assay in HAEC transduced with adenoviruses encoding firefly luciferase controlled by the 1-kb CSEγ promoter in the presence or absence of tubacin (10 μM). Adenoviruses encoding Renilla luciferase controlled by a ubiquitous receptor tyrosine kinase (RTK) promoter were used for normalization. G and H: HAEC incubated with or without OxLDL (50 μg/ml) were subjected to Western blotting using HDAC6, acetylated (Ac) α-tubulin, and total α-tubulin antibodies. I: real-time PCR using HDAC6- and 18s-specific primers. J: HAEC were cultured in the absence or presence of OxLDL (25 and 50 μg/ml) for 24 h, and cell lysates were immunoblotted with HDAC2 or GAPDH antibodies. *, #P < 0.05 vs. respective controls. Values are means ± SE. Western blots are representative of 3 immunoblots.
Fig. 4.
A and B: isolated aortas from ApoE KO mice fed HFD or ND for 12 wk were subjected to Western blotting using CSEγ, acetylated α-tubulin, total α-tubulin, HDAC6, and β-tubulin antibodies. C and D: isolated aortic segments were incubated in medium with or without OxLDL (50 µg/ml) for 24 h, and cell lysates were subjected to Western blotting using CSEγ, acetylated α-tubulin, total α-tubulin, and HDAC6 antibodies. E and F: human aortic vascular smooth muscle cells (HAVSMC) were incubated in medium with or without OxLDL (50 µg/ml) for 24 h, and cell lysates were subjected to Western blotting using CSEγ, acetylated α-tubulin, total α-tubulin, and HDAC6 antibodies. G and H: CSEγ and VE-cadherin (CAD) protein expression were determined by Western blotting in control and deendothelialized mouse aortas in the presence or absence of OxLDL (50 µg/ml) for 24 h. *P < 0.05 vs. respective controls. #P < 0.05 vs. endothelium-intact group not treated with OxLDL. Western blots are representative of 3 immunoblots.
Pharmacological and genetic suppression of HDAC6 attenuates OxLDL-induced vascular dysfunction.
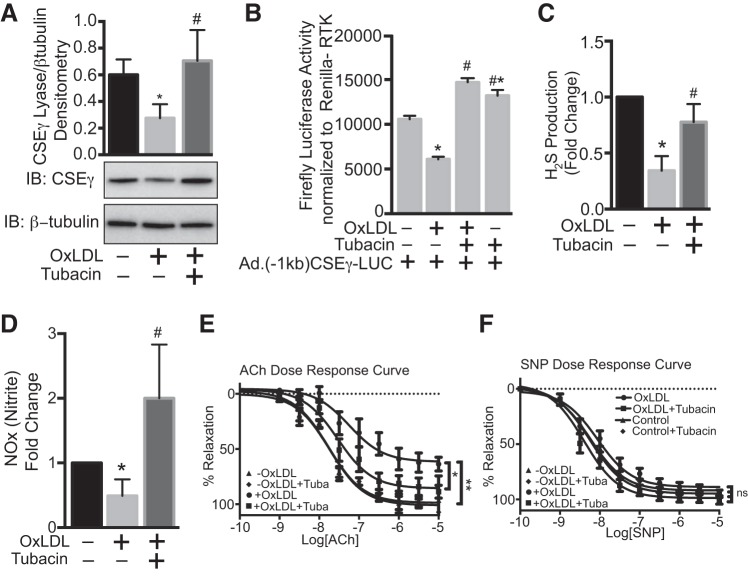
To confirm whether the OxLDL-mediated decrease in endothelial CSEγ is indeed due to upregulation of HDAC6, we used both pharmacological and siRNA approaches to inhibit HDAC6 function in HAEC and examined the effect on CSEγ expression. As shown in Fig. 5A, OxLDL (50 μg/ml) exposure for 24 h significantly downregulated endothelial CSEγ expression, but this effect was prevented in HAEC preincubated with the HDAC6-specific inhibitor tubacin (10 μM). We next investigated whether OxLDL directly modulates CSEγ promoter activity and found that this was, indeed, the case (Fig. 5B). These changes in CSEγ promoter activity were abolished by preincubation of HAEC with tubacin before OxLDL exposure. We next determined if changes in endothelial CSEγ expression translate into changes in H2S production. As shown in Fig. 5C, OxLDL significantly reduced H2S production, but pretreatment with tubacin abolished this effect of OxLDL. Previous studies have shown that CSE-derived H2S can activate eNOS and augments NO production (15, 17). Consistent with this finding, the changes in H2S with OxLDL with or without tubacin reflected changes in NO production in HAEC (Fig. 5D). To determine whether the cellular findings reported here translate into a physiological response, we measured vascular reactivity in isolated mouse aortic rings that were incubated in the presence or absence of OxLDL (50 μg/ml) for 24 h with or without tubacin (10 μM for 1 h). The marked impairment of endothelium-dependent relaxation caused by OxLDL was substantially rescued by tubacin pretreatment (Fig. 5E). Endothelium-independent relaxation, as assessed by SNP response, did not differ significantly in any of the groups (Fig. 5F).
Fig. 5.
A: HAEC pretreated with DMSO (vehicle) or the HDAC6-specific inhibitor tubacin (10 μM) were incubated in medium with or without OxLDL (50 µg/ml) for 24 h, and cell lysates were immunoblotted for CSEγ and β-tubulin. B: HAEC expressing firefly and Renilla luciferase under the control of the 1-kb CSEγ and RTK promoters, respectively, were pretreated with or without tubacin before incubation in medium with or without OxLDL (50 µg/ml) for 24 h, and cell lysates were analyzed using a luciferase activity assay (Promega Dual Glo). C and D: HAEC pretreated with DMSO (vehicle) or tubacin (10 μM) were incubated in medium with or without OxLDL (50 µg/ml) for 24 h, and cell lysates were analyzed quantitatively for H2S using H2S-specific electrochemical probes and for nitrite (NOx) using Siever’s NO analyzer. E and F: mouse aortic rings incubated for 24 h with or without OxLDL (50 µg/ml) in the presence or absence of tubacin (10 μM) were subjected to wire myography to determine the dose-response effect of ACh and SNP on vascular relaxation. Values are means ± SE; n = 6. Western blots are representative of 3 immunoblots. *, # P < 0.05 vs. respective controls. **P < 0.05, −OxLDL vs. +OxLDL.
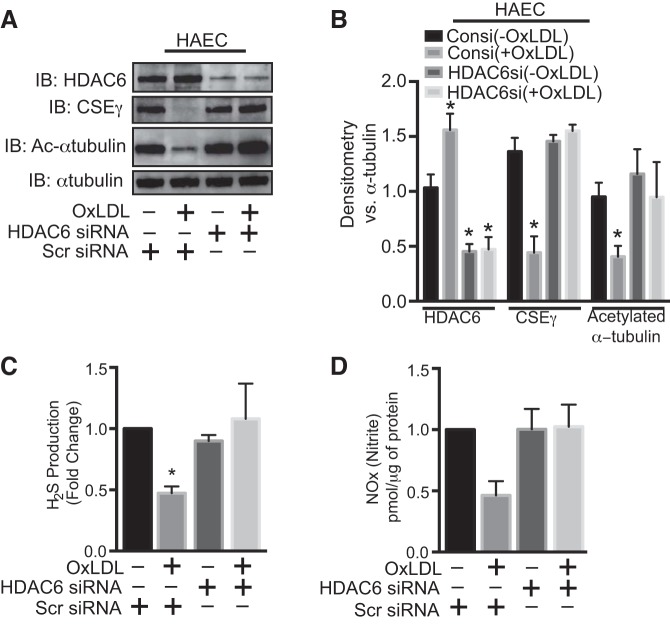
It is well known that pharmacological inhibitors can have off-target effects. We therefore complemented inhibitor studies using HDAC6-specific siRNA. Effectiveness of HDAC6 siRNA was demonstrated by decreased HDAC6 expression in HAEC and increased acetylated levels of α-tubulin. As shown in Fig. 6, this HDAC6 knockdown abolished OxLDL-mediated reduction of CSEγ expression and prevented decreased production of both H2S and NO. These findings clearly establish HDAC6 as a critical effector molecule for OxLDL-mediated changes in vasoactive gases in HAEC.
Fig. 6.
A and B: after 48 h of transfection with nontargeted siRNA (Consi) or HDAC6-specific siRNA (HDAC6si) using RNAimax (Invitrogen), HAEC were incubated in medium with or without OxLDL for 24 h, and cell lysates were subjected to Western blotting with CSEγ, acetylated α-tubulin, total α-tubulin, and acetylated α-tubulin and HDAC6 antibodies. C and D: H2S in cell lysates and nitrite levels in culture medium. *P < 0.05 vs. respective controls. Western blots are representative of 3 immunoblots. Values are means ± SE; n = 6.
DISCUSSION
We have demonstrated that OxLDL significantly impaired endothelium-dependent vascular relaxation; however, relaxation was significantly protected by increased expression of CSEγ. OxLDL concomitantly downregulated CSEγ expression and activity while upregulating HDAC6 expression and activity in endothelial cells. Notably, inhibition of HDAC6 by its small-molecule inhibitor tubacin or by siRNA blocked the inhibitory effects of OxLDL on CSEγ expression/activity and, interestingly, on eNOS activity and, thus, endothelium-dependent vasorelaxation.
H2S, one of the three major gasotransmitters in vascular endothelium, is a volatile and labile endogenously produced mediator with a variety of important functions in the cardiovascular system (33). Although there are three known cellular sources for the production of H2S, cystathionine β-synthase, CSEγ, and 3-mercaptoypyruvate sulfurtransferase, CSEγ is known to be the predominant source of H2S production in some blood vessels (21), although coronary arteries may be an exception (19). The major functions of CSEγ-derived H2S in the vasculature include regulation of vasodilatation and stimulation of angiogenesis (13, 16, 26). Moreover, the importance of the CSEγ-H2S axis has been investigated in CSEγ-deficient mice, which exhibit marked hypertension, endothelial dysfunction, and accelerated atherosclerosis (24, 39). Although extensive literature describes the protective effect of CSEγ-derived H2S on vascular function, no studies have reported how CSEγ expression is regulated in vascular cells. We found that not only does adenoviral-mediated CSEγ overexpression in murine aortas and HAEC protect against OxLDL, it also activates eNOS and the prosurvival serine/threonine kinase Akt. Interestingly, we observed increased eNOS and Akt expression in intact aortas that were overexpressed with CSEγ, which could be due to the differential effect of H2S on epigenetics programming in endothelium vs. smooth muscle cell compartments of aortas (6, 14, 31, 37, 38).
We and others have described the importance of HDACs in the transcriptional and posttranslational regulation of key genes important for endothelial function (29). In the present study we show that the expression of CSEγ and downstream H2S, as well as NO, production are regulated by a class IIb HDAC, HDAC6. Furthermore, the adverse effects of the atherogenic stimulus OxLDL on endothelial function were reversed by pharmacological inhibition of HDAC6 with tubacin, a specific small-molecule inhibitor of HDAC6 tubulin deacetylase activity (8), and by siRNA-mediated HDAC6 knockdown.
HDAC6 regulates many important cytoplasmic and nuclear processes by forming complexes with its partner proteins (22). Because of its nuclear export signal and SE14 motif (1), HDAC6 is found predominantly in the cytoplasm, where it regulates cell migration and degradation of misfolded proteins. However, Liu et al. showed that acetylation of HDAC6 causes nuclear shuttling and interaction of HDAC6 with a number of nuclear factors (23). Therefore, it can also exhibit transcriptional control. We found that OxLDL concomitantly increased HDAC6 mRNA and protein levels and downregulated CSEγ mRNA and protein levels and that each of these effects was dose-dependent. Pharmacological inhibition of HDAC6 and siRNA-mediated HDAC6 knockdown reversed the OxLDL-induced changes in CSEγ protein expression, suggesting that HDAC6 is critical for CSEγ expression. As we found no difference in CSEγ ubiquitination with global HDAC inhibition, the effect of HDAC6 on CSEγ expression most likely occurs at a transcriptional level.
Class I HDACs, mainly HDAC2 and -3, have recently been implicated in endothelial function, although the inhibition of these HDACs was found to be damaging to the endothelium. In the current study we introduce a novel strategy to improve endothelial function by blocking HDAC6. It is quite intriguing that the effect of OxLDL on class I HDAC2 was the opposite of that on class II HDAC6. This finding further highlights the need to use specific HDAC inhibitors to execute unique function and achieve the desired outcome (29).
Targeted drug development for atherogenesis has included both preventive and remedial strategies and has largely focused on decreasing plaque burden. Our data indicate that transcriptional regulators such as HDAC6 may offer a safer and more effective way forward, provided that specific agents can be developed (11). A strategy of using small-molecule inhibitors of HDAC6 to protect against stimuli that are injurious to vasoprotective CSEγ expression offers a promising therapy for atherosclerotic cardiovascular diseases.
GRANTS
This work was supported by American Heart Association Scientist Development Grant 16SDG27340010 to D. Pandey, a 2017 StAAR Award from the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine to D. Pandey, and National Heart, Lung, and Blood Institute Training Grant T32 HL-007227-40 to T. Leucker.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.M.L., Y.N., J.H.K., A.B., V.W., D.E.B., L.R., and D.P. conceived and designed research; T.M.L., Y.N., J.H.K., A.B., V.W., A.W., S.S.J., L.S., and D.P. performed experiments; T.M.L., Y.N., J.H.K., A.B., V.W., A.W., S.S.J., L.S., D.E.B., L.R., and D.P. analyzed data; T.M.L., Y.N., J.H.K., A.B., V.W., A.W., S.S.J., L.S., D.E.B., L.R., and D.P. interpreted results of experiments; T.M.L., Y.N., J.H.K., A.B., V.W., A.W., S.S.J., L.S., and D.P. prepared figures; T.M.L., J.H.K., and D.P. drafted manuscript; T.M.L., Y.N., J.H.K., D.E.B., L.R., and D.P. edited and revised manuscript; T.M.L., Y.N., J.H.K., A.B., V.W., A.W., S.S.J., L.S., D.E.B., L.R., and D.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gary Gerstenblith (Division of Cardiology) for critical analysis and comments on the manuscript. We also thank Claire Levine (Department of Anesthesiology and Critical Care Medicine) for editorial assistance.
REFERENCES
- 1.Bertos NR, Gilquin B, Chan GK, Yen TJ, Khochbin S, Yang XJ. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J Biol Chem 279: 48246–48254, 2004. doi: 10.1074/jbc.M408583200. [DOI] [PubMed] [Google Scholar]
- 2.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA 109: 9161–9166, 2012. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demos-Davies KM, Ferguson BS, Cavasin MA, Mahaffey JH, Williams SM, Spiltoir JI, Schuetze KB, Horn TR, Chen B, Ferrara C, Scellini B, Piroddi N, Tesi C, Poggesi C, Jeong MY, McKinsey TA. HDAC6 contributes to pathological responses of heart and skeletal muscle to chronic angiotensin-II signaling. Am J Physiol Heart Circ Physiol 307: H252–H258, 2014. doi: 10.1152/ajpheart.00149.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 5.Dje N’Guessan P, Riediger F, Vardarova K, Scharf S, Eitel J, Opitz B, Slevogt H, Weichert W, Hocke AC, Schmeck B, Suttorp N, Hippenstiel S. Statins control oxidized LDL-mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler Thromb Vasc Biol 29: 380–386, 2009. doi: 10.1161/ATVBAHA.108.178319. [DOI] [PubMed] [Google Scholar]
- 6.Fish JE, Matouk CC, Rachlis A, Lin S, Tai SC, D’Abreo C, Marsden PA. The expression of endothelial nitric-oxide synthase is controlled by a cell-specific histone code. J Biol Chem 280: 24824–24838, 2005. doi: 10.1074/jbc.M502115200. [DOI] [PubMed] [Google Scholar]
- 7.Gupta MP, Samant SA, Smith SH, Shroff SG. HDAC4 and PCAF bind to cardiac sarcomeres and play a role in regulating myofilament contractile activity. J Biol Chem 283: 10135–10146, 2008. doi: 10.1074/jbc.M710277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA 100: 4389–4394, 2003. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan MI, Boosen M, Schaefer L, Kozlowska J, Eisel F, von Knethen A, Beck M, Hemeida RA, El-Moselhy MA, Hamada FM, Beck KF, Pfeilschifter J. Platelet-derived growth factor-BB induces cystathionine γ-lyase expression in rat mesangial cells via a redox-dependent mechanism. Br J Pharmacol 166: 2231–2242, 2012. doi: 10.1111/j.1476-5381.2012.01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegele RA, Gidding SS, Ginsberg HN, McPherson R, Raal FJ, Rader DJ, Robinson JG, Welty FK. Nonstatin low-density lipoprotein-lowering therapy and cardiovascular risk reduction–statement from ATVB Council. Arterioscler Thromb Vasc Biol 35: 2269–2280, 2015. doi: 10.1161/ATVBAHA.115.306442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helin K, Dhanak D. Chromatin proteins and modifications as drug targets. Nature 502: 480–488, 2013. doi: 10.1038/nature12751. [DOI] [PubMed] [Google Scholar]
- 12.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458, 2002. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 13.Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. Am J Physiol Heart Circ Physiol 304: H1446–H1454, 2013. doi: 10.1152/ajpheart.00506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamat PK, Kalani A, Tyagi SC, Tyagi N. Hydrogen sulfide epigenetically attenuates homocysteine-induced mitochondrial toxicity mediated through NMDA receptor in mouse brain endothelial (bEnd3) cells. J Cell Physiol 230: 378–394, 2015. doi: 10.1002/jcp.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA 111: 3182–3187, 2014. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolluru GK, Bir SC, Yuan S, Shen X, Pardue S, Wang R, Kevil CG. Cystathionine γ-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc Res 107: 590–600, 2015. doi: 10.1093/cvr/cvv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolluru GK, Yuan S, Shen X, Kevil CG. H2S regulation of nitric oxide metabolism. Methods Enzymol 554: 271–297, 2015. doi: 10.1016/bs.mie.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kugiyama K, Kerns SA, Morrisett JD, Roberts R, Henry PD. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature 344: 160–162, 1990. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- 19.Kuo MM, Kim DH, Jandu S, Bergman Y, Tan S, Wang H, Pandey DR, Abraham TP, Shoukas AA, Berkowitz DE, Santhanam L. MPST but not CSE is the primary regulator of hydrogen sulfide production and function in the coronary artery. Am J Physiol Heart Circ Physiol 310: H71–H79, 2016. doi: 10.1152/ajpheart.00574.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Förstermann U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr Pharm Des 15: 3133–3145, 2009. doi: 10.2174/138161209789058002. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Moore PK. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol Sci 29: 84–90, 2008. doi: 10.1016/j.tips.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Shin D, Kwon SH. Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J 280: 775–793, 2013. doi: 10.1111/febs.12079. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Peng L, Seto E, Huang S, Qiu Y. Modulation of histone deacetylase 6 (HDAC6) nuclear import and tubulin deacetylase activity through acetylation. J Biol Chem 287: 29168–29174, 2012. doi: 10.1074/jbc.M112.371120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhoták Š, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 127: 2523–2534, 2013. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 25.Mistry RK, Murray TV, Prysyazhna O, Martin D, Burgoyne JR, Santos C, Eaton P, Shah AM, Brewer AC. Transcriptional regulation of cystathionine-γ-lyase in endothelial cells by NADPH oxidase 4-dependent signaling. J Biol Chem 291: 1774–1788, 2016. doi: 10.1074/jbc.M115.685578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naik JS, Osmond JM, Walker BR, Kanagy NL. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Am J Physiol Heart Circ Physiol 311: H1437–H1444, 2016. doi: 10.1152/ajpheart.00465.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey D, Bhunia A, Oh YJ, Chang F, Bergman Y, Kim JH, Serbo J, Boronina TN, Cole RN, Van Eyk J, Remaley AT, Berkowitz DE, Romer LH. OxLDL triggers retrograde translocation of arginase2 in aortic endothelial cells via ROCK and mitochondrial processing peptidase. Circ Res 115: 450–459, 2014. doi: 10.1161/CIRCRESAHA.115.304262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey D, Sikka G, Bergman Y, Kim JH, Ryoo S, Romer L, Berkowitz D. Transcriptional regulation of endothelial arginase 2 by histone deacetylase 2. Arterioscler Thromb Vasc Biol 34: 1556–1566, 2014. doi: 10.1161/ATVBAHA.114.303685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA 89: 4471–4475, 1992. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhakar NR. Sensing hypoxia: physiology, genetics and epigenetics. J Physiol 591: 2245–2257, 2013. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siuda D, Zechner U, El Hajj N, Prawitt D, Langer D, Xia N, Horke S, Pautz A, Kleinert H, Förstermann U, Li H. Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res Cardiol 107: 283, 2012. doi: 10.1007/s00395-012-0283-3. [DOI] [PubMed] [Google Scholar]
- 33.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 34.Tanizawa K. Production of H2S by 3-mercaptopyruvate sulphurtransferase. J Biochem 149: 357–359, 2011. doi: 10.1093/jb/mvr018. [DOI] [PubMed] [Google Scholar]
- 35.Wang B, Rao YH, Inoue M, Hao R, Lai CH, Chen D, McDonald SL, Choi MC, Wang Q, Shinohara ML, Yao TP. Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nat Commun 5: 3479, 2014. doi: 10.1038/ncomms4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 37.Weber GJ, Pushpakumar S, Tyagi SC, Sen U. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacol Res 113, Pt A: 300–312, 2016. doi: 10.1016/j.phrs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang G, Wang R. H2S and blood vessels: an overview. Handb Exp Pharmacol 230: 85–110, 2015. doi: 10.1007/978-3-319-18144-8_4. [DOI] [PubMed] [Google Scholar]
- 39.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322: 587–590, 2008. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D, Wu CT, Qi X, Meijering RA, Hoogstra-Berends F, Tadevosyan A, Cubukcuoglu Deniz G, Durdu S, Akar AR, Sibon OC, Nattel S, Henning RH, Brundel BJ. Activation of histone deacetylase-6 induces contractile dysfunction through derailment of α-tubulin proteostasis in experimental and human atrial fibrillation. Circulation 129: 346–358, 2014. doi: 10.1161/CIRCULATIONAHA.113.005300. [DOI] [PubMed] [Google Scholar]
- 41.Zheng XX, Zhou T, Wang XA, Tong XH, Ding JW. Histone deacetylases and atherosclerosis. Atherosclerosis 240: 355–366, 2015. doi: 10.1016/j.atherosclerosis.2014.12.048. [DOI] [PubMed] [Google Scholar]