Silica particles are used as novel nanotechnology-based vehicles for diagnostics and therapeutics for the heart. However, their potential hazardous effects remain unknown. Here, the cardiotoxicity of silica nanoparticles in rat myocytes has been described for the first time, showing an impairment of mitochondrial function that interfered directly with Ca2+ handling.
Keywords: cardiomyocyte, silicon dioxide, nanoparticle, toxicity, Ca2+
Abstract
Recent evidence has shown that nanoparticles that have been used to improve or create new functional properties for common products may pose potential risks to human health. Silicon dioxide (SiO2) has emerged as a promising therapy vector for the heart. However, its potential toxicity and mechanisms of damage remain poorly understood. This study provides the first exploration of SiO2-induced toxicity in cultured cardiomyocytes exposed to 7- or 670-nm SiO2 particles. We evaluated the mechanism of cell death in isolated adult cardiomyocytes exposed to 24-h incubation. The SiO2 cell membrane association and internalization were analyzed. SiO2 showed a dose-dependent cytotoxic effect with a half-maximal inhibitory concentration for the 7 nm (99.5 ± 12.4 µg/ml) and 670 nm (>1,500 µg/ml) particles, which indicates size-dependent toxicity. We evaluated cardiomyocyte shortening and intracellular Ca2+ handling, which showed impaired contractility and intracellular Ca2+ transient amplitude during β-adrenergic stimulation in SiO2 treatment. The time to 50% Ca2+ decay increased 39%, and the Ca2+ spark frequency and amplitude decreased by 35 and 21%, respectively, which suggest a reduction in sarcoplasmic reticulum Ca2+-ATPase (SERCA) activity. Moreover, SiO2 treatment depolarized the mitochondrial membrane potential and decreased ATP production by 55%. Notable glutathione depletion and H2O2 generation were also observed. These data indicate that SiO2 increases oxidative stress, which leads to mitochondrial dysfunction and low energy status; these underlie reduced SERCA activity, shortened Ca2+ release, and reduced cell shortening. This mechanism of SiO2 cardiotoxicity potentially plays an important role in the pathophysiology mechanism of heart failure, arrhythmias, and sudden death.
NEW & NOTEWORTHY Silica particles are used as novel nanotechnology-based vehicles for diagnostics and therapeutics for the heart. However, their potential hazardous effects remain unknown. Here, the cardiotoxicity of silica nanoparticles in rat myocytes has been described for the first time, showing an impairment of mitochondrial function that interfered directly with Ca2+ handling.
the technological advances of the 20th century have been the most powerful and fastest phenomena in human history. From the largest to the smallest artifacts, newly developed micro/nanomaterials have provided the main base for creating better and more versatile items in almost every area of knowledge. In this regard, nanotechnology, as we know it in the 21st century, is gaining enormous acceptance in the electronic, manufacturing, agriculture, consumer-product, and food additive industries as well as, more recently, the biotechnological and biomedical fields. Nanomaterials, such as titania (titanium dioxide) and silica (silicon dioxide, SiO2), are used to improve and create new functional properties for common products (51). While nanomaterials based on biomaterials like polymers, such as poly (lactic-co-glycolic acid) (PGLA) or hydrogels, are considered of low toxicity, recent evidence has shown that some nanoparticles, such as metal, metal oxides (e.g., silica and titania), and carbon nanotubes, may pose potential risks to the environment, the food chain, and human health (35). Several studies have shown that nanoparticles can penetrate the body by different routes, including the skin, respiratory system, and gastrointestinal tract. In normal conditions, the inhalation of SiO2 is likely the major route by which nanoparticles enter the body, and defective particle clearance in the airways increases the chance of particles translocating to different organs, including the heart, lungs, and kidneys (1, 45). Moreover, novel clinical diagnostic protocols and therapeutic drug treatments allow biological systems to undergo major and extended nano- and microparticle exposure, which can lead to an inflammatory response, fibrosis, oxidative stress, and cell death (15). Nano- and micro-SiO2 particles have drawn a lot of attention in the biomedical field. Currently, different sizes of SiO2 particles are being used in the clinical setting (23). Silica’s usefulness as a biomedical tool has led to focused use in relation to human diagnostic, imaging, and labeling and in new targeting systems, which allows these nano- and micromaterials to interact directly with the body, leading to increased concentrations in the bloodstream and higher organ accumulation. In recent years, SiO2 nano- and microparticles have been used extensively because of their chemical properties and the capacity to manipulate their surface for different biomedical purposes, including for imaging and for therapeutic vehicles (5, 42, 50). However, while silicon-based particles have been considered to be of low toxicity and safe for use as a therapeutic vehicle (40), recent studies have shown evidence of toxicity, though this toxicity depends on a variety of factors, including the composition, differential toxicity among cell types, and—remarkably—particle size (13, 34, 38, 45). In particular, nanoscale particles (<100 nm) are a controversial topic of debate, and the related research conclusions lack consistency. For instance, in vitro and in vivo effects exerted by SiO2 particles have recently been studied at the cardiovascular level, which showed cytotoxicity related to induced oxidative stress (10). Acute in vivo SiO2 exposure also leads to an inflammatory response and endothelial dysfunction, which are strongly related to oxidative stress production and to impairments in myocardial antioxidant enzymes (8). Chronic exposure to SiO2 particles has also been associated with alterations to different molecular mechanisms related to angiogenesis, heart formation and development, and pericardial edema and bradycardia, which can lead to cardiovascular diseases (10). More recently, microarray analysis showed that SiO2 cardiotoxicity in a zebrafish model was related to oxidative stress and neutrophil-mediated cardiac inflammation (9). However, the mechanisms involved in SiO2-induced toxicity in mammalian heart cells are still unknown.
In this study, we explored, for the first time, SiO2-induced toxicity and impaired contractility and calcium handling in isolated adult cardiomyocytes exposed to 24 h incubation with two different sizes of SiO2 particles: 7-nm AEROSIL 380 Fumed Silica, which will be referred to henceforth as nano-SiO2, and 670-nm particles synthesized by our group, which will be referred to henceforth as micro-SiO2. We explored the mechanism of cell death caused by nano- and micro-SiO2 at their half-maximal inhibitory concentration (IC50) and related these effects to the phenomenon of SiO2 particle association with membrane and cellular uptake. Using confocal microscopy, we evaluated cardiomyocyte shortening and intracellular Ca2+ handling. In addition, we measured the mitochondrial membrane potential, oxidative stress, and ATP content to determine whether SiO2 particles induced mitochondrial dysfunction and whether impairment of bioenergetics contributed to SiO2-induced cardiac dysfunction.
MATERIALS AND METHODS
Chemicals.
All reagents were obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO) unless otherwise stated.
Synthesis of micro-SiO2.
The 670-nm SiO2 particles were synthesized in accordance with the Stöber method (49). Briefly, a mix of 85% of ethanol, 3.6% of concentrated ammonium hydroxide solution, and 11.5% (vol/vol) of ultrapure water was stirred. Separately, a solution was prepared with 30.2% of tetraethyl orthosilicate, 13.2% of (3-aminopropyl) triethoxysilane, and 56.6% (vol/vol) of ethanol. This second solution was added slowly to the first one, and the mixture was magnetically stirred for 12 h. The resulting colloidal suspension was centrifuged, and the precipitate was rinsed with pure ethanol at least three times. The solid was then dried in a muffle at 70°C for 24 h. For the preparation of fluorescent silica spheres (F-micro-SiO2), dried micro-SiO2 spheres obtained of the aforementioned procedure were subsequently calcined at 400°C for 2 h. These calcined particles were then washed three times with ethanol in an ultrasonic bath. The fluorescence is attributed to the introduction of carbon and oxygen defects in the silica network after the calcination of (3-aminopropyl) triethoxysilane (20).
Nano-SiO2 and micro-SiO2 characterization.
Nano-SiO2 (Degussa, Parsippany, NJ), micro-SiO2, and F-micro-SiO2 were characterized by a field emission gun–scanning electron microscope (FEG-SEM) (Model 200 Nova NanoSEM FEI Company). The measurements were performed under low vacuum conditions with a Helix detector and a 10–18 kV electron beam. Zeta potential and size measurements using dynamic light scattering (DLS) were performed on a Malvern Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK). Briefly, nano- and micro-SiO2 were suspended in ultrapure water, Tyrode, M-199, and M-199 + bovine serum albumin (BSA) (5%) solutions and were irradiated with a red laser (HeNe laser, wavelength λ = 632.8 nm), and the intensity fluctuations of the scattered light (detected at a backscattering angle of 173°) were analyzed to obtain an autocorrelation function. The software (DTS v5.03) provided both the mean hydrodynamic diameter and polydispersity index by using the method of cumulants (according to the international standard ISO 13321:19963) and a size distribution by using a regularization scheme by intensity, volume, and number. Crystalline structure was determined by X-ray diffraction (XRD) on a Panalytical Empyrean diffractometer (PANalytical, Westborough, MA).
Cardiomyocytes isolation.
All the studies were performed in accordance with the animal care guidelines of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). All procedures were approved by the Institutional Animal Use and Care Committee (protocol number 2011-Re-017). Rat ventricular myocytes were isolated by collagenase II digestion of perfused hearts (14). Briefly, male Wistar rats weighing 250–300 g were used to isolate cardiac cells. Animals were heparinized and anesthetized with pentobarbital sodium (1,000 U/kg and 100 mg/kg, ip, respectively) before removal and hanging the heart. Hearts were mounted on a Langendorff apparatus and then perfused with Tyrode medium (TM) (in mM): 128 NaCl, 0.4 NaH2PO4, 6 glucose, 5.4 KCl, 0.5 MgCl-6H2O, 5 creatinine, 5 taurine, and 25 HEPES, pH 7.4 at 37°C for 5 min and digested by 0.1% collagenase type II (Worthington Biochemical, Lakewood, NJ), dissolved in TM for 15 min. Ventricles were dissected and cells mechanically disaggregated. Cardiomyocytes were washed in crescent concentrations of calcium (0.25, 0.5, 1, and 1.5 mM) plus 0.1% albumin contained in the TM. Intact cardiomyocytes were cultured in M-199 medium supplemented with (in mM) 5 taurine, 5 creatine, 2 l-carnitine, 2.5 sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin on laminin-coated culture dishes at a density of 1 × 104 viable cells/cm2. Cells were incubated at 37°C in 95% air-5% CO2 for 2 h before all experiments.
Determination of nano-SiO2 and micro-SiO2 cytotoxicity and cell death mechanisms.
To determine SiO2 cytotoxicity, varying concentrations of nano- and micro-SiO2 were added to culture media, and the incubation was continued for 24 h. At the end of the incubation period, cytotoxicity of SiO2 was determined by the Alamar blue viability test (Life Technologies, Carslbad, CA). The IC50 was calculated with Origin software (Northampton, MA). Necrosis and apoptosis were measured with Ghost Dye Red 780 and Annexin V-PE-Cy7-stained cells by using a BD FACSCantoII flow cytometer (BD Biosciences, Heidelberg, Germany). Cells were stained according to the manufacturer’s instructions. Briefly, 1 μl of Ghost Dye (Tonbo Bioscience, San Diego, CA) solution was added in 1 ml of TM and incubated for 30 min, washed, and resuspended in 300 μl with 5 μl of fluorochrome-conjugated Annexin-V-PE-Cy7 (eBioscience, San Diego, CA) for 10 min at room temperature. Finally, cells were washed and fixed using 4% paraformaldehyde for 20 min. After incubation, cell monolayers were washed twice with TM and detached from the culture plate by scrapping. In total, 10,000 cells were analyzed per measurement in TM solution, and data was analyzed with FlowJo 8.8.6 software (Treestar, Ashland, OR). The release of cytoplasmic lactate dehydrogenase (LDH), as a marker of cell integrity and necrosis, was determined enzymatically following the interconversion of pyruvate and lactate and with concomitant oxidation of NADH to NAD+, followed by using spectrophotometry at 340 nm. Values were adjusted to an LDH calibration curve. The activity of caspases-3 and -7, to assess SiO2-induced apoptosis, was measured in cell pellets by using the Apo-ONE fluorescent substrate (Promega, Madison, WI).
Characterization of nano-SiO2 and micro-SiO2 in the cardiomyocyte.
To assess the interaction between cardiomyocytes membrane and SiO2 particles, a 24-h cell incubation with nano- and micro-SiO2 was analyzed by FEG-SEM. Samples were prepared by seeding cardiomyocytes on to 5- × 7-mm silicon chips specimen supports (Ted Pella, Redding, CA). Samples were fixed with 2.5% glutaraldehyde in PBS buffer (Electron Microscopy Sciences, Hatfield, PA) for 20 min and were then dehydrated in increasing concentrations of ethanol for 10 min. Specimens were mounted on SEM stubs (Ted Pella) with conductive adhesive tape (Ted Pella) and coated with a gold thin film. FEG-SEM surface images were acquired at different magnifications under high vacuum at 15.00 kV, spot size 3.0, with a Nova NanoSEM 200 (FEI, Hillsboro, OR). The energy-dispersive X-ray spectroscopy (EDS) analysis was performed with an Oxford Instruments analyzer at 15 kV. In addition to surface images, transversal cuts were done on random cardiomyocytes by focused ion beam, and EDS was performed to assess the intracellular location of the SiO2 particles using a Zeiss Crossbeam 340, using 30 kV for the focused ion beam and 12 kV for the SEM. Ten individual cells from each treatment were selected randomly for imaging and EDS analysis.
Particle-induced X-ray emission (PIXE) was used to quantify the total amount of silica present in the cardiomyocytes. This technique has been used recently for nanomaterial quantification in complex matrices (25). A 2.5-MeV proton beam was used to irradiate the cells with currents < 1 nA to reduce sample damage. The cardiomyocytes were incubated with nano- and micro-SiO2 at its IC50 with different end time points (0, 1, 12, and 24 h). After each incubation time, cells were 1) washed with M-199 to remove free particles, 2) detached from the culture plate, and 3) centrifuged at 500 rpm through a 250-mM sucrose solution to separate noninternalized and nonadhered silica particles as well as dead cardiomyocytes from the intact cells. The pellet (alive cells) was resuspended in 200 µl of MilliQ H2O, finally transported sequentially into truncated sample holders, and removing the aqueous media was removed as previously reported (26). Data analysis was done with GUPIXWIN software (7).
Intracellular localization of F-micro-SiO2 by confocal microscopy examination.
To determine the intracellular localization of F-micro-SiO2, cardiomyocytes were seeded into laminin-covered glass coverslips. Cells were incubated with F-micro-SiO2 for 24 h, followed by fixation and staining of cellular structures. Briefly, after SiO2 incubation, cells were fixed using 4% paraformaldehyde in PBS buffer for 20 min. Immunofluorescence staining was performed by cell permeabilization with 0.1% Triton X-100 (Thermo Fisher Scientific, Waltham, MA) in PBS for 3 min. Cells were rinsed three times in PBS, and a blocking step with 1% bovine serum in PBS was performed for 10 min. Actin filaments were stained with 0.5 U/μl of Alexa Fluor 568-conjugated phalloidin (Life Technologies, Grand Island, NY) for 20 min at room temperature. Glass coverslips were rinsed with PBS and mounted with Vectashield mounting media (Vector laboratories, Burlingame, CA). Samples were imaged with a Leica TCS SP5 confocal microscope equipped with a D-apochromatic 63X, 1.2 NA, oil objective (Leica Microsystems, Wetzlar, Germany). To assess cellular internalization of F-micro-SiO2, a stack of two-dimensional (2-D) images, with a focal plane thickness of 1 µm, were collected every 1 µm along z-axis using an excitation (ex) and emission (em) of 488 nm and 500–700 nm, respectively. For the representative images to assess cellular internalization of F-micro-SiO2, wavelengths of an ex of 488 and 543 nm and em of 500–600 and 620–720 nm, for F-micro-SiO2 and cytoskeleton were used, respectively. The tridimensional reconstructions were made from z-stacks with a focal plane thickness of 2 µm using the 3-D viewer plugin from ImageJ (National Institutes of Health, Bethesda, MD).
Ca2+ handling and cell shortening in intact cardiomyocytes.
The Ca2+ transients, Ca2+ sparks, and cell shortening parameters were measured as previously described (29, 53). Twenty-four hour cultured cardiomyocytes on laminin-covered glass coverslips, with Nano- and Micro-SiO2 at its IC50, were incubated in TM with Fluo-4 AM or Fluo-3 AM (Life Technologies) to evaluate Ca2+ handling and cell shortening. Afterward, the cells were washed with a fluorophore-free solution. Loaded cells were mounted in a superfusion chamber. All fluorescence measurements were acquired with a Leica TCS SP5 confocal microscope equipped with a D-apochromatic 63X, 1.2 NA, oil objective (Leica Microsystems). Line scan images were recorded along the longitudinal axis of the cell at 400 Hz with an argon laser to excite the fluorophore at 488 nm, and its emission was collected at 500–600 nm. For cell shortening, a pinhole optimized for a 4-µm-thick section in the focal plane was used, while a 1-µm-thick section was used for Ca2+ signaling. Cell shortening was evaluated under field stimulation at 0.5, 1, and 2 Hz (MYP100 MyoPacer Field Stimulator; Ion-Optix, Milton, MA). For Ca2+ transient and Ca2+ sparks, the cells were field stimulated by 3 to 5 electric pulses at 1 Hz to attain steady-state sarcoplasmic reticulum (SR) Ca2+ content. The Ca2+ signaling under β-adrenergic stimulation was evaluated by perfusion of isoproterenol (ISO) at 100 nM, and the records were taken after 10 min of exposition. For SR Ca2+ content, the cells were field stimulated at 1 Hz before a rapid caffeine application at 10 mM. Fluorescence data were normalized as ΔF/F0, where F is fluorescence intensity and F0 is average fluorescence at rest.
Bioenergetic status measurements.
Mitochondrial membrane potential (Δψ) was evaluated in cultured cardiomyocytes by confocal microscopy using a Leica TCS SP5 confocal microscope equipped with a D-apochromatic 63X, 1.2 NA, oil objective (Leica Microsystems). Cardiomyocytes on laminin-coated coverslips were treated with nano-SiO2 for 24 h, washed with TM to remove particles, and loaded with 300 nM tetramethylrhodamine ethyl ester perchlorate (TMRE) (Thermo Fisher Scientific) during 30 min at room temperature and darkness. TMRE accumulates in metabolically active mitochondria producing a fluorescence that is proportional to Δψ (44). Afterward, the cells were rinsed with fluorophore-free TM and placed in a recording chamber. TMRE signal was measured with an ex at 543 nm and an em to 560–700 nm using oil immersion 40X objective. To evaluate the Δψ, records were taken after field stimulation at 1 Hz. The 2-D images were acquired with Leica LAS AF version 2.7 format at 400 Hz with 512 × 512 image size and a focal plane thickness of 1 µm. The total fluorescence from the 2-D images was evaluated and plotted as a percentage of relative fluorescence, the control group being 100%. ATP content was measured in control group (CT) cardiomyocytes as well as those treated with nano-SiO2 at its IC50, after a 24-h exposure using the CellTiter-Glo Luminescent Assay (Promega).
Oxidative stress markers.
A change in the concentration of H2O2 in the medium was detected by fluorescence of the oxidized Amplex Red (Life Technologies) using wavelengths of ex at 550 nm and em at 585 nm. The response of Amplex Red to H2O2 was calibrated by sequential additions of known amounts of H2O2 from 100 to 1,000 pmol/min. Glutathione (GSH) was measured as previously described (39). Briefly, cells were dissolved in medium containing (in mM) the following: 50 KH2PO4, pH 7.5, with the addition of 0.2% Tx-100, 1 PMSF, and 0.6% sulfosalicylic acid for 10 min a 4°C and centrifuged at 8,000 rpm for 10 min. Protein concentration was measured by the Lowry method. Thirty micrograms of protein were incubated in a medium that contained (in mM) the following: 50 KH2PO4, 1 EDTA, pH 7.5 with the addition of GSH reductase 10 U/ml, 0.1 DTNB and 2 NADPH. The reduction of DTNB was followed at 412 nm and quantified with extinction coefficient (13.6 M/cm).
Statistical analyses.
Data were analyzed by ANOVA followed by Dunnett’s multiple comparisons tests using Graph Pad InStat (Graph Pad Software, San Diego, CA) or by Student’s t-test, as indicated. Data were expressed as means ± SE. A P value of <0.05 was considered statistically significant.
RESULTS
Characterization of SiO2 particles.
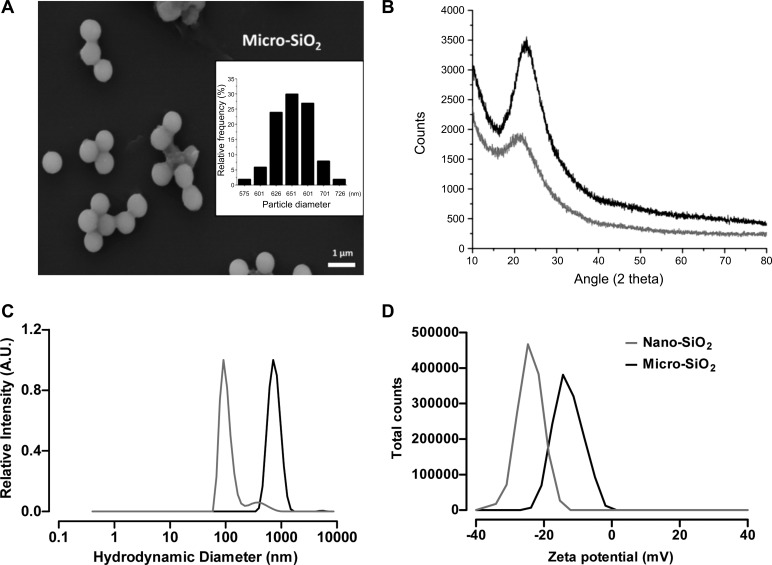
The characterizations of the nano- and micro-SiO2 particles are presented in Fig. 1. The field emission gun-scanning electron microscope (FEG-SEM) images show that the micro-SiO2 had a spherical shape and exhibited dispersibility (Fig. 1A). The size distribution shown by FEG-SEM analysis had an average diameter of 670 ± 32 nm (Fig. 1A, insert), while the average diameter of nano-SiO2 was 7 nm according to the manufacturer’s technical sheet. Analysis of the crystalline structure using XRD showed that the nano- and micro-SiO2 particles presented an amorphous structure (Fig. 1B). The hydrodynamic diameters (Fig. 1C) of the nano- and micro-SiO2 particles in ultrapure water were 91 ± 22 and 712 ± 212 nm, respectively. The ζ-potentials of the nano- and micro-SiO2 particles (Fig. 1D) were −27.1 ± 4.4 and −14.4 ± 5.48 mV, respectively. Hydrodynamic diameters and ζ-potentials were also measured in Tyrode, M-199, and M-199 + BSA solutions (Table 1). In addition, EDS analysis showed that the purity of the silica particles was higher than 95%, and no traces of other metals were detected in the particle suspensions (data not shown).
Fig. 1.
Characterization of silica particles by scanning electron microscope (SEM), X-ray diffraction (XRD), dynamic light scattering (DLS), and ζ-potential. A: representative micro-SiO2 micrograph showing size and morphology. Insert shows size distribution of micro-SiO2 obtained by field emission gun (FEG)-SEM (n = 100 particles). B: XRD analysis with the composition of the talline domains of nano- and micro-SiO2. All samples show the characteristic diffraction pattern of amorphous silica presenting a broad peak (10–30°) with a maximum around 22°. C: the hydrodynamic sizes of nano- and micro-SiO2 particles in ultrapure water. D: the ζ-potentials of nano- and micro-SiO2 particles in ultrapure water. Black and gray curves correspond to micro-SiO2 and nano-SiO2, respectively, in each graphic.
Table 1.
Characterization of silica particles by different techniques
| Particle (SiO2) | Crystallinity (XRD) | Purity (EDS), % | Size Distribution |
Surface Charge (ζ-potential), mV |
|
|---|---|---|---|---|---|
| Feret diameter (FEG-SEM), nm |
Hydrodynamic diameter (DLS), nm | ||||
| Nano-SiO2 | amorphous | >95 | 7* | 91 ± 22 (water) | −24.1 (water) |
| 120 ± 25 (Tyrode) | −22.0 (Tyrode) | ||||
| 220 ± 50 (M-199) | −21.6 (M-199) | ||||
| 220 ± 106 (M-199 + BSA) | −20.8 (M-199 + BSA) | ||||
| Micro-SiO2 | amorphous | >95 | 666 ± 32 | 712 ± 212 (water) | −14.4 (water) |
| 1,106 ± 281 (Tyrode) | −19.5 (Tyrode) | ||||
| 1,190 ± 272 (M-199) | −18.8 (M-199) | ||||
| 1,281 ± 488 (M-199 + BSA) | −12.3 (M-199 + BSA) | ||||
| F-micro-SiO2 | amorphous | >95% | 611 ± 22 | 712 ± 153 (water) | −33.6 (water) |
| 712 ± 152 (Tyrode) | −17.5 (Tyrode) | ||||
| 1,484 ± 294 (M-199) | −13.4 (M-199) | ||||
| 1,484 ± 812 (M-199 + BSA) | −12.9 (M-199 + BSA) | ||||
XRD, X-ray diffraction; EDS, energy-dispersive X-ray spectroscopy; FEG-SEM, field emission gun scanning electron microscope; DLS, dynamic light scattering; ELS, electrophoretic light scattering. BSA, 5 mg/ml
Size as referred by the provider’s data sheet.
Dose- and time-dependent cytotoxicity of nano- and micro-SiO2 particles.
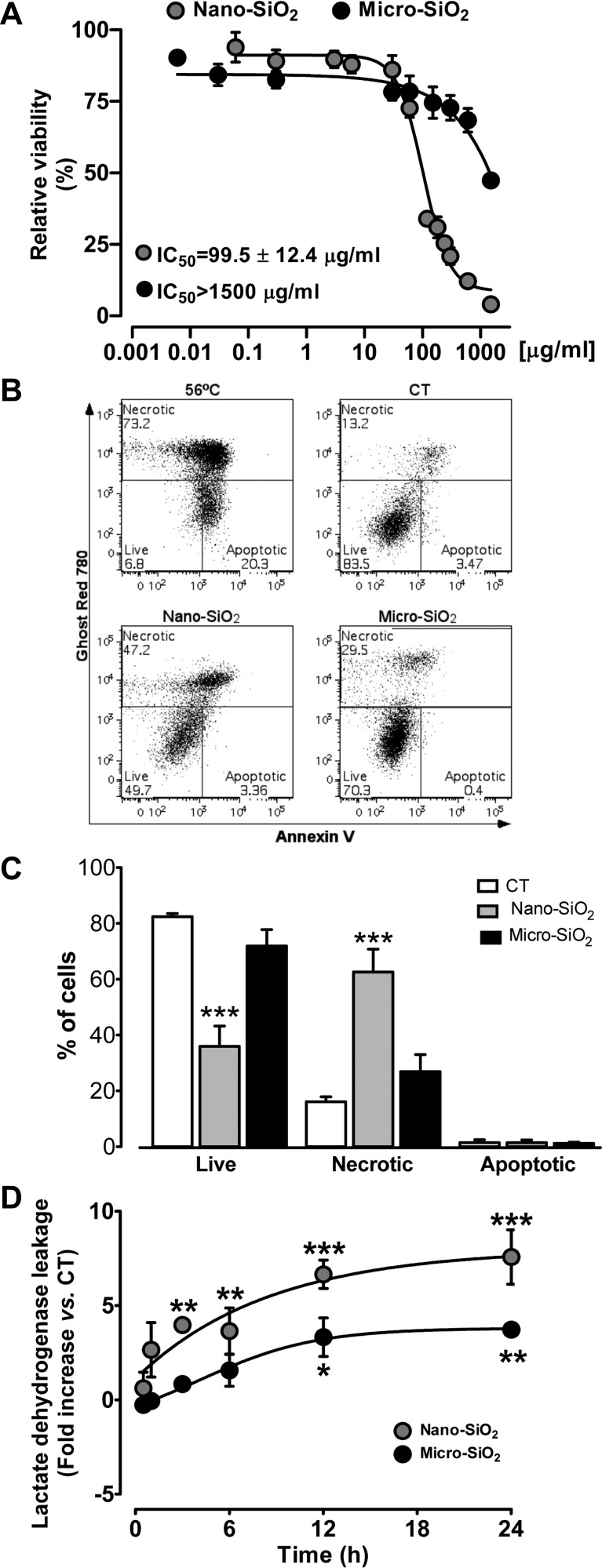
To assess the effects of SiO2 on cardiomyocyte viability, adult rat ventricular myocytes were cultured with different concentrations of nano- and micro-SiO2 particles, and cell death was examined after 24 h of treatment. As shown in Fig. 2A, nano- and micro-SiO2 induced significant cytotoxic effects in a dose-dependent manner, with IC50 values of 99.5 ± 12.4 µg/ml and >1,500 µg/ml, respectively, which indicates that nano-SiO2 has a 15-fold higher toxicity than micro-SiO2. Indeed, when the cardiomyocytes were cultured in the presence of nano- and micro-SiO2 at the IC50 of nano-SiO2, only nano-SiO2-induced cell death occurred as a result of the necrotic pathway. No evidence of apoptosis was observed in any case, as shown by the lack of single positive Annexin-V cells (Fig. 2, B and C) and the lack of caspase-3/7 activity (data not shown). In addition, necrosis induction also occurred faster with nano-SiO2 than with micro-SiO2 as shown in Fig. 2D by the LDH leakage. Nano-SiO2 promoted cardiomyocyte necrosis in the first 3 h of incubation, while micro-SiO2 only led to notable LDH leakage after 12 h at their corresponding IC50 values.
Fig. 2.
Viability of adult cardiomyocytes after 24-h exposure of SiO2 particles and mechanism of cell death. A: nano-SiO2 and micro-SiO2 viability evaluated by Alamar blue viability test. B: representative dot plots from flow cytometry with AnnexinV-PE-Cy7/Ghost Red 780 cell death analysis. A positive control of apoptosis cell death (56°C for 10 min) is shown in comparison to control (CT), nano-SiO2, and micro-SiO2 treated groups at 99.5 µg/ml. C: the percentage of necrotic, live, and apoptotic cells was quantified and is shown in the bar graph. D: time-dependent cytotoxicity by lactate dehydrogenase leakage at 0–24 h, in cultured cardiomyocytes with 24-h incubation of nano-SiO2 and micro-SiO2 at its half-maximal inhibitory concentration (IC50) (99.5 µg/ml for nano-SiO2 and 1,500 µg/ml for micro-SiO2 particles). Values are means ± SE, n = 3–10; *P < 0.05; **P < 0.01; ***P < 0.001 vs. control.
SiO2 particles associate with the cellular membrane and internalize in the cell.
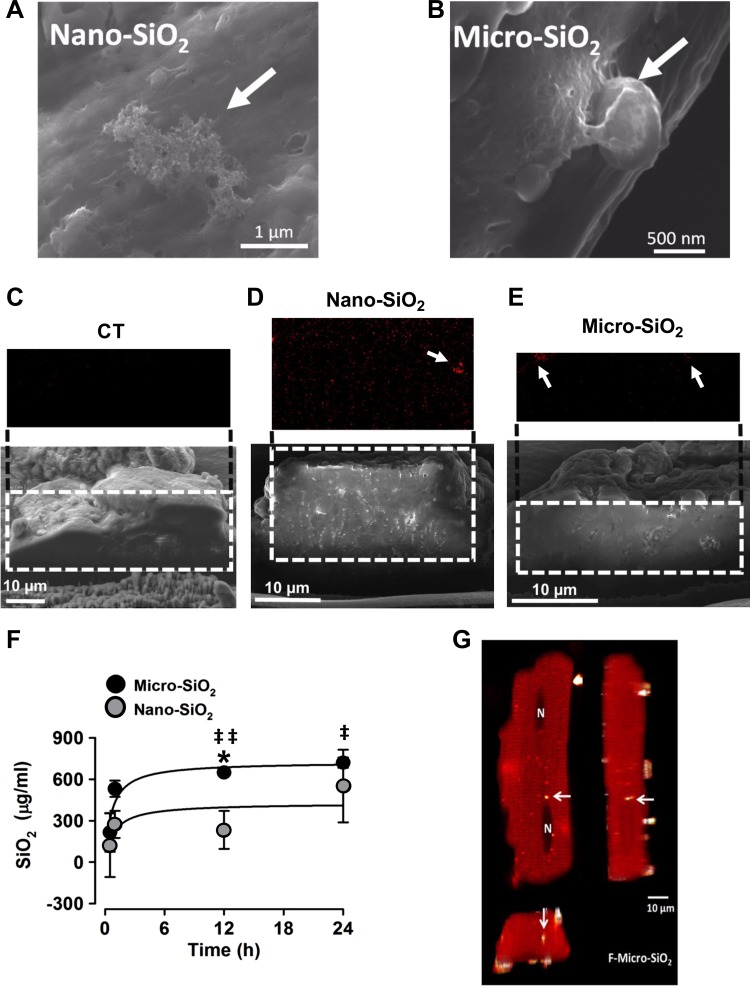
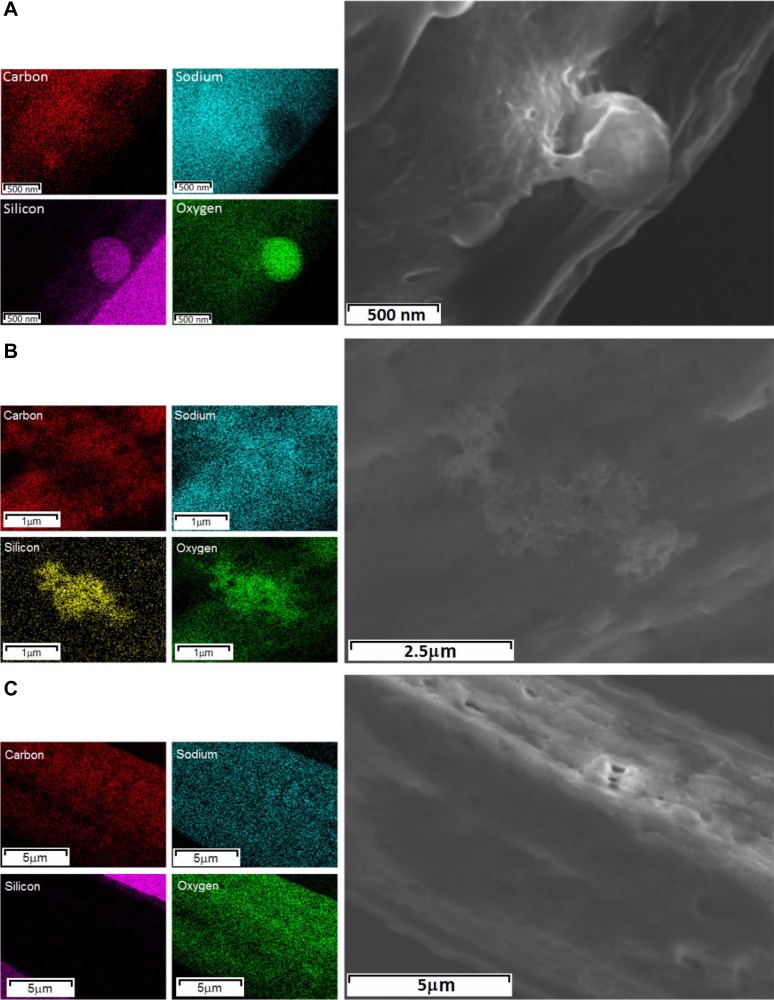
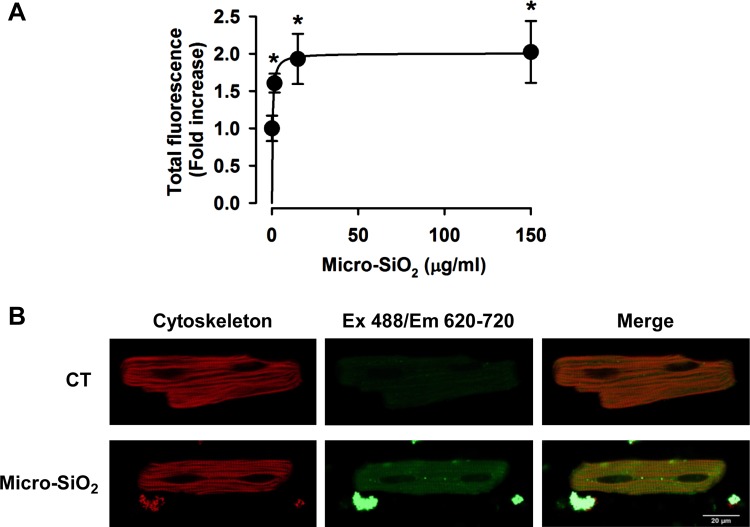
To relate cellular death to particle membrane association or to the potential internalization of the SiO2 particles, we analyzed the association of single or agglomerates/aggregates of nano- and micro-SiO2 to the cardiomyocytes’ cellular membranes, as shown in Fig. 3, A and B. The FEG-SEM images show the association of the SiO2 particles with the cell membrane 24 h after administration in the cultured adult rat cardiomyocytes at 37°C (Fig. 3, A and B). Figure 4 depicts the surface of the cardiomyocytes using EDS, which shows that the nano- and micro-SiO2 particles have individual and agglomerate/aggregate associations with the cellular membrane with respect to the four EDS images of the elemental maps for carbon, sodium, silicon, and oxygen. Transversal cuts in the cardiomyocytes with nano- or micro-SiO2 (Fig. 3, C–E) showed that the particles could internalize in the cardiomyocytes after 24 h of incubation. Figure 3F shows the SiO2 content quantified by PIXE in the cardiomyocytes as a function of time. A time-dependent increase was observed in SiO2 in the cardiomyocytes treated with nano- and micro-SiO2 particles, suggesting an association/internalization of the silica particles in the cardiomyocytes. Furthermore, EDS analysis in the transversal cut in the cardiomyocytes indicates that the SiO2 signal is higher in nano-SiO2 vs. micro-SiO2 particles, which suggests that smaller SiO2 particles are more prone to cellular internalization in cardiomyocytes. As shown in Fig. 3G, we explored, by confocal microscopy, the internalization of micro-SiO2 after 24 h of incubation, and we analyzed the confocal z-stack acquisitions. For this purpose, we synthesized a micro-SiO2 particle with fluorescent properties (F-micro-SiO2) that had a similar size, morphology, dispensability, and ζ-potential as micro-SiO2 (Table 1). Using this approach, we confirmed the presence of multiple single and agglomerate/aggregate F-micro-SiO2 at different subcellular levels by using a tridimensional reconstruction captured from cardiomyocytes, thereby confirming the intracellular localization of SiO2 particles (Fig. 3G and video in Supplemental Material; Supplemental Material for this article is available online at the Journal website). We also visualized the internalization of the F-micro-SiO2 through confocal z-stack analysis, which confirmed the presence of particles (shown as bright spheres) near the nuclei area (shown as dark ovals) (Fig. 3G). Figure 5 shows a semiquantitative analysis wherein the total fluorescence in a z-stack was evaluated to determine the internalization of the F-micro-SiO2 in intact cardiomyocytes exposed to different concentrations of particles during 24 h of incubation. F-micro-SiO2 exhibited dose-dependence at low concentrations (Fig. 5A) and was able to reach a saturating concentration without promoting major cellular death (data not shown), which is similar to micro-SiO2 at the same concentration (Fig. 2A), and we showed that cardiomyocytes treated with 150 µg/ml of F-micro-SiO2 preserved their normal morphology (Fig. 5B). At the maximum evaluated concentration of 150 µg/ml, we observed a twofold increase in fluorescence; the 1 ± 0.17-fold fluorescence presented in the untreated (CT) group increased to 2.02 ± 0.41-fold in the F-micro-SiO2-treated group (Fig. 5B).
Fig. 3.
SiO2 cellular association and internalization in adult rat cardiomyocytes. Representative SEM micrographs showing nano-SiO2 (A) and micro-SiO2 (B) individual and agglomerates/aggregates association to the cellular membrane. Representative SEM–energy-dispersive X-ray spectroscopy (EDS) transversal cut images of untreated (C), Nano-SiO2 (D), and Micro-SiO2 (E) particle internalization after 24 h of incubation (99.5 μg/ml). F: quantification of internalized SiO2 particles by particle-induced X-ray emission, as a function of time (for nano-SiO2 99.5 μg/ml and micro-SiO2 1,500 μg/ml). G: representative confocal microscopy image of the F-micro-SiO2 shown as x, y and z-stacks. The cytoskeleton is shown in red and the brighter dots correspond to F-micro-SiO2 particles. The arrows indicate the SiO2 particles in the cell. The symbol N (in G) marks the nucleus location in the cell. Values are means ± SD; a t-test was used as statistical analysis between nano- and micro-SiO2. Values are means ± SE, n = 3–5. *P < 0.05 vs. nano; ‡P < 0.05 vs. basal (time 0).
Fig. 4.
Cardiomyocytes surface showing silica particles by energy-dispersive X-ray spectroscopy (EDS). Right: FEG-SEM images of micro-SiO2 (A) and nano-SiO2 (B) individual and agglomerates/aggregates association to the cellular membrane and an adult rat cardiomyocyte as a control sample (C). Left: the respective four EDS images of elemental maps for carbon (red), sodium (cyan), silicon (magenta/yellow), and oxygen (green).
Fig. 5.
Micro-SiO2 internalization in adult myocytes. A: semiquantitative increase in fluorescence in a dose-dependent manner of F-micro-SiO2 particles. B: representative images from ventricular myocytes at the control (CT) condition and 150 µg/ml F-micro-SiO2 concentration. The cytoskeleton is shown in red and F-micro-SiO2 in green. Values are means ± SE, n = 4–6. *P < 0.05 vs. CT.
SiO2 treatment induced a reduction in cell shortening, decreased SERCA activity, and impaired β-adrenergic stimulation.
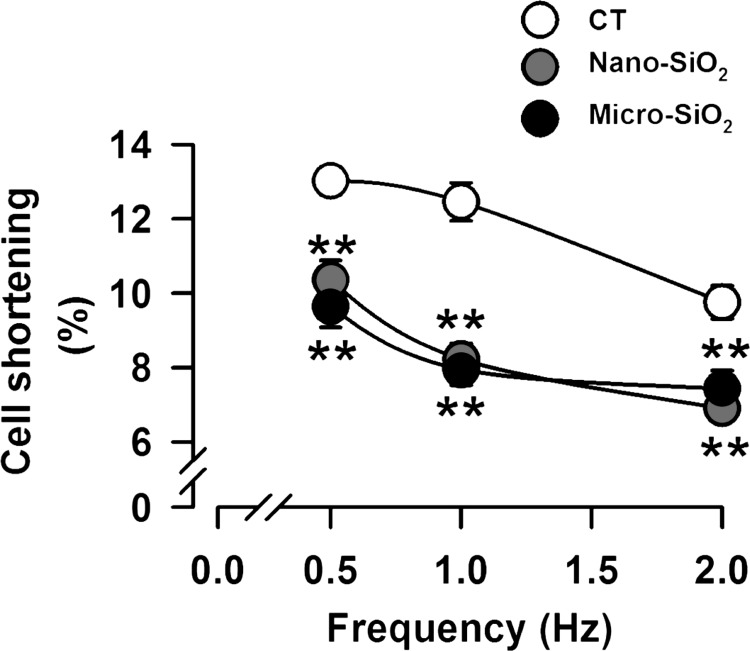
Cardiomyocyte sarcomere shortening (Fig. 6) following nano- and micro-SiO2 treatment reduced by 34 and 36%, respectively, compared with the CT group. This impairment in cell shortening was maintained at 0.5 and 2 Hz. However, no difference was evident between the nano- and micro-SiO2 groups. For the purpose of exploring the mechanism of this impairment, we chose to work with nano-SiO2 particles at 100 µg/ml to match the physiological conditions of airways and to avoid high range concentrations (IC50 values of >1,500 µg/ml for micro-SiO2) that could not be detected in vivo after a short exposure.
Fig. 6.
Cell shortening and Ca2+ handling in cardiomyocytes treated with nano- and micro-SiO2 particles. The percentage of cell shortening before (CT) and after a 24-h treatment of nano-SiO2 and micro-SiO2 at its IC50. Values are means ± SE, n = 15. **P < 0.001 vs. CT.
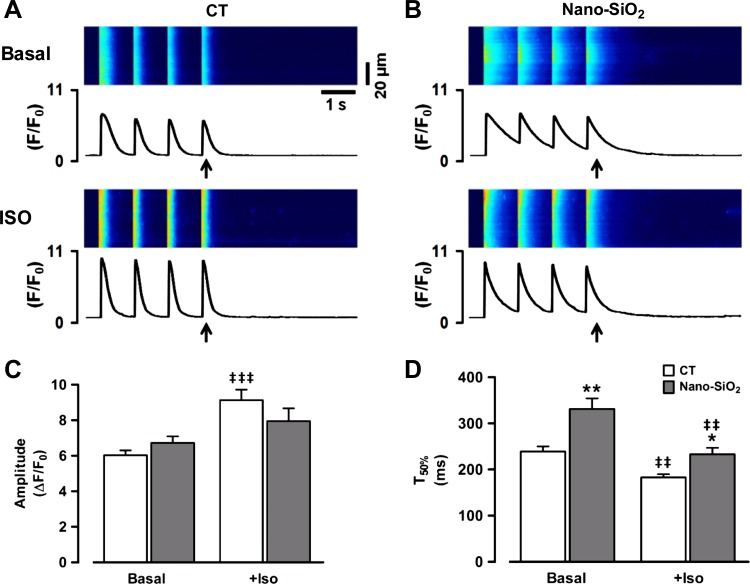
Generally, the changes in cardiomyocyte Ca2+ handling followed the time course of the changes in contractility. In this sense, Fig. 7, A and B, shows a representative recording of Ca2+ transients evoked by field stimulation for the CT and nano-SiO2-treated cells. Figure 7C shows the pooled data of the change in the Ca2+ transient peak amplitude upon physiological β-adrenergic stimulation ISO in both cell treatments. We found that under basal conditions, the peak Ca2+ transient amplitude was similar in the SiO2-treated and CT cells (6.03 ± 0.27 ΔF/F0 CT vs. 6.72 ± 0.37 ΔF/F0 nano-SiO2). However, after 10 min of continuous β-adrenergic stimulation, the Ca2+ transient amplitude increased to only 7.95 ± 0.72 in the nano-SiO2-treated cells, while the CT group increased to 9.13 ± 0.59 (P < 0.05).
Fig. 7.
Calcium transient characterization at basal condition and after β-adrenergic stimulation. A and B: representative images from field-stimulated control and nano-SiO2-treated myocytes under basal conditions and after isoproterenol (ISO) perfusion, the arrow indicates the analyzed transient for semiquantitative results. C: peak Ca2+ transient amplitude. D: time to 50% of decay (T50%). Values are means ± SE. CT: n = 22–33 cells/3 animals; nano-SiO2: n = 19–34 cells/3 animals. *P < 0.05 vs. CT; **P < 0.001 vs. CT; ‡‡P < 0.001 vs. basal; ‡‡‡P < 0.0001 vs. basal.
For relaxation, the released Ca2+ must be removed from the cytosol; SERCA and the sarcolemmal Na+/Ca2+ exchanger perform this activity. In this context, the Ca2+ transient time to 50% decay (T50%) provides an index of the combined activity of the Ca2+ removal systems; however, the SERCA activity underlies more than 90% of Ca2+ removal (4). Figure 7D shows the Ca2+ transient T50% plotted under basal conditions and as a function of time during ISO perfusion. Under basal conditions, the T50% of the nano-SiO2-treated cells was 331 ± 23 ms, while that of the CT cells was 239 ± 11 ms (P < 0.05). In both cases, there was an increase in the cytosolic Ca2+ removal rate after adrenergic stimulation; however, there was still a difference between the CT and nano-SiO2-treated cells (183 ± 7 and 233 ± 14 ms, respectively; P < 0.05). This result suggests impairment in SERCA activity.
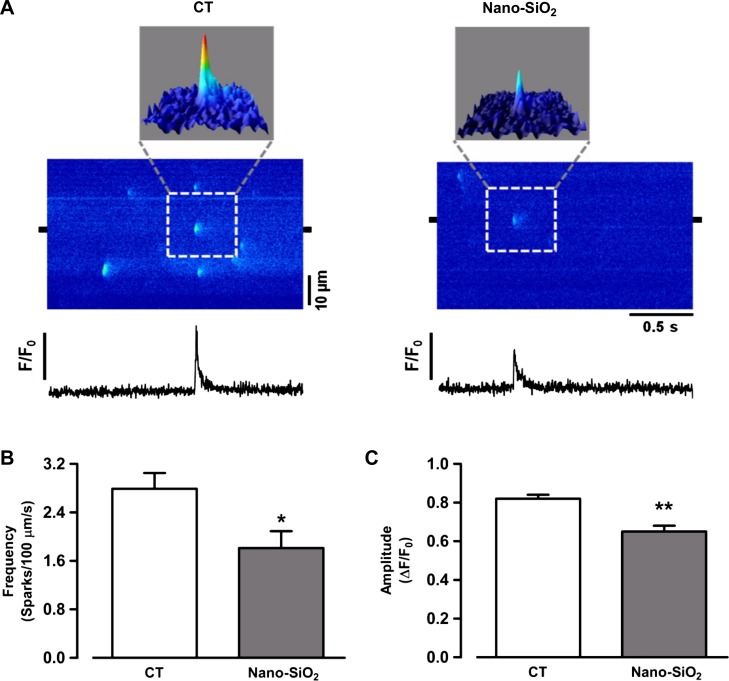
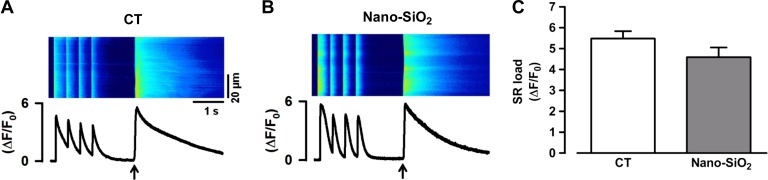
Because alterations in Ca2+ handling in other pathological settings are also reflected at the level of diastolic Ca2+, we measured whether the spontaneous Ca2+ sparks were altered in the SiO2-treated cardiomyocytes. Figure 8A shows the typical Ca2+ spark reconstruction in the CT (left) and nano-SiO2-treated cardiomyocytes (right). Calcium spark analysis revealed a significant decrease in frequency in the nano-SiO2 group (1.81 ± 0.28 sparks·100 µm−1·sec−1 nano-SiO2) compared with the CT group (2.79 ± 0.26 sparks·100 µm−1·sec−1 CT) (P < 0.05) (Fig. 8B) and a significant decrease in amplitude (0.82 ± 0.02 F/F0 CT vs. 0.65 ± 0.03 F/F0 nano-SiO2; P < 0.05) (Fig. 8C), which might indicate reduced SR calcium content. We estimated a steady-state SR Ca2+ content by the cytosolic peak of the caffeine-evoked Ca2+ release in the CT and SiO2-treated cells. Figure 9, A and B, shows representative confocal images of caffeine-evoked SR Ca2+ release in the control and nano-SiO2 treated cells, respectively. Pooled data of the peak caffeine-evoked Ca2+ release is shown in Fig. 9C. The nano-SiO2 cells had a slightly lower SR Ca2+ content; the difference was not statistically significant under basal conditions (5.48 ± 0.35 and 4.59 ± 0.46 for CT and nano-SiO2 treated cells, respectively; P < 0. 12).
Fig. 8.
Calcium sparks characterization in isolated myocytes. A: line scan images from control conditions (left) and after 24-h incubation with Nano-SiO2 (right). Surface plots can be seen above line scan images. Line profiles from selected 2-µm regions (black marks in line scan images) can be seen on images. Pooled data describing spark frequency (B) and amplitude (C). Values are means ± SE, n = 39 cells/3 animals/CT; 33 cells/3 animals/nano-SiO2). *P < 0.05 vs. CT; **P < 0.001 vs. CT.
Fig. 9.
Calcium content in the sarcoplasmic reticulum. Representative images from caffeine-induced Ca2+ transients from control (CT) (A) and nano-SiO2-treated cardiomyocytes (B). Black arrow indicates caffeine application (10 mM). C: pooled data for peak Ca2+ transient amplitude [sarcoplasmic reticulum (SR) load; n = 25 cells/3 animals/CT; 24 cells/3 animals/Nano-SiO2].
SiO2 treatment induces a decrease in mitochondrial membrane potential and ATP content.
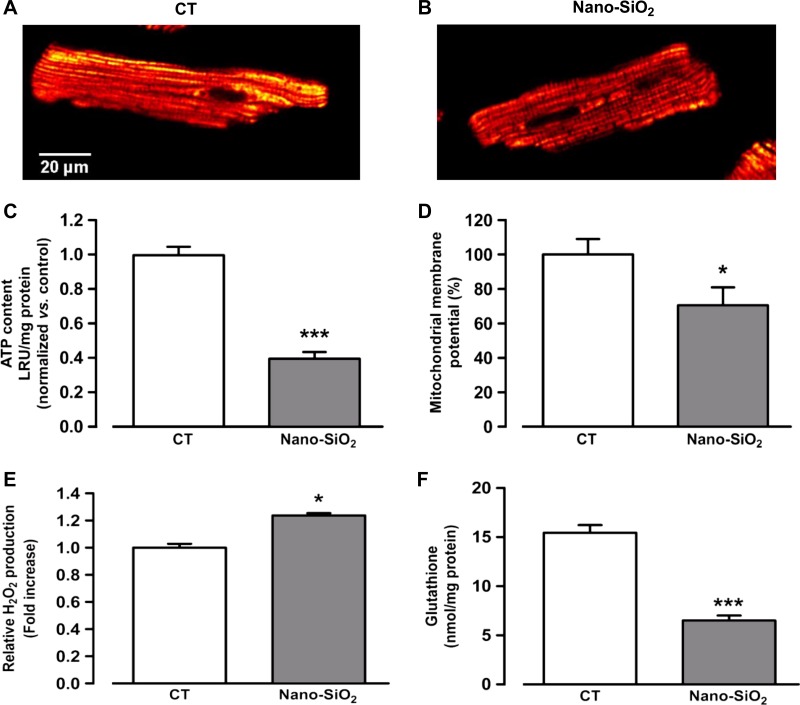
Efficient Ca2+ handling, which is necessary for proper contraction and relaxation, depends strongly on the ATP supply in the vicinity of SERCA (11). In this regard, we explored whether SiO2 treatment affected the bioenergetics status in nano-SiO2-treated cardiomyocytes (Fig. 10, A and B). Figure 10C shows a 29.5% decrease in the mitochondrial membrane potential (Δψ) with nano-SiO2 treatment compared with the CT group after 24 h of incubation. This finding was strongly related to the ATP content in the cardiomyocytes, as nano-SiO2 treatment reduced the ATP content by 60% compared with the CT cells (Fig. 10D). These findings suggest that the SiO2 particles impaired mitochondrial function.
Fig. 10.
Mitochondrial membrane potential, ATP content, reactive oxygen species (ROS) production, and glutathione depletion. Representative images from control (CT) (A) and nano-SiO2-treated cardiomyocytes (B) loaded with TMRE by confocal microscopy. ATP content (luminescence relative units, LRU) (C) and relative fluorescence from mitochondrial membrane potential (%) (D) from CT and nano-SiO2-treated cardiomyocytes. H2O2 production (E) and glutathione content (F) in cultured cardiomyocytes with 24-h incubation of nano-SiO2 at its IC50. Values are means ± SE, n = 10–11 cells (a–D); n = 3 (E and F); *P < 0.05 vs. CT; ***P < 0.0001 vs. CT.
SiO2 induces ROS production and glutathione depletion.
Modification of the redox state by nano- and microparticles has been reported as one of the main mechanisms of cytotoxicity at the cellular level (35). In this regard, we incubated cardiomyocytes with nano-SiO2 for 24 h and then measured H2O2 production and glutathione (GSH) content. Figure 10E shows that the SiO2 particles significantly increased H2O2 production and markedly depleted GSH (Fig. 10F) compared with the CT group. Nano-SiO2 induced a significant reduction in GSH (down to 6.5 nmol/mg) in comparison to the CT group (15.4 nmol/mg), which shows that silica particles exerted a prooxidative status on adult rat cardiomyocytes.
DISCUSSION
The purpose of this study was to evaluate, for the first time, the potential cardiotoxicity of SiO2 particles in adult cardiomyocytes and their effects on excitation-contraction coupling. In addition, recent results have shown that silicon-based microparticles associate and accumulate in a failing myocardium (in contrast to a healthy myocardium) following intravenous administration, and the microparticles reach the cardiomyocytes. This finding points to a novel avenue for developing nanotechnology-based therapeutics and diagnostics for heart failure and other cardiomyopathies that involve endothelial dysfunction and a proinflammatory milieu (40). In addition, there is great interest in using these materials therapeutically as vehicles for carrying genes, siRNA, drugs, or peptides, and there are promising results (5, 42) because of their physicochemical properties, the capacity of biochemical functionality and surface modification to immobilize biomolecules, and the easy preparation of diverse particle sizes (nano and micro) with uniform shapes and structures (37).
SiO2 nanoparticle internalization is associated with cardiotoxicity.
SiO2 particles have displayed various cytotoxic effects at the cellular level, which has led to growing concern about their safety (15, 33, 35). Importantly, the toxicity of SiO2 particles might depend on their crystalline nature and morphology, but this was not considered as a variable in this work because nano- and micro-SiO2 have a spherical shape and the same crystalline nature. However, one important factor for consideration in this work is the particle size (45). It is generally accepted that the smaller the particles, the greater the toxicity exerted in biological systems. In our study, we found differential cardiotoxicity between nano- and micro-SiO2. In agreement with many previous studies (34), nano-SiO2 particles exerted potent cytotoxicity compared with micro-SiO2 particles at the same concentration (e.g., 300 µg/ml). Nano-SiO2 reduced viability nearly 80%, as compared with 30% for micro-SiO2. Our results indicate that nanoparticles are more toxic to adult rat ventricular myocytes than microparticles. Consistent with our results, Duan et al. (9), using 70-nm SiO2 nanoparticles, observed pericardial edema and cardiac toxicity in zebrafish embryos, which affected heart rhythm. These observations should be considered when designing new products for medical applications or for novel vehicles for gene/drug delivery.
We previously mentioned our decision to evaluate SiO2 particle size and stability in ultrapure water and, more importantly, in culture media (Tyrode, M-199, and M-199 + BSA) to know SiO2 particle properties where all experiments have been performed. One of the main characteristics of the DLS technique is that DLS “is a function of the relative refractive indices of the particle or molecule and the dispersant” (24). In our case, when measurements are made in a culture medium, the molecules within it scatter light, which causes interference; thus the data can probably not be gathered with 100% efficiency. However, the changes in the physicochemical properties (particle size distribution and surface charge) of the SiO2 particles in the culture media were determined by using an incubation protocol adapted from Monopoli et al. (32) to assess the formation of the hard protein corona around the particles. The results, as shown in Table 1, effectively indicate that the more complex the media in which the SiO2 particles are exposed, the greater the tendency to increase in size; nevertheless, we showed that their surface charge remained relatively unmodified, as it remained negative for all samples under all tested conditions. The ζ-potential of the nano-SiO2 was −24.1 mV, while for the micro-SiO2 and its fluorescence-modified counterpart it was −14.4 and −33.6 mV, respectively. Overall, these changes are not great enough to expect dramatic changes in their interaction with cells, such as in the internalization effects. It was not until relatively recently that the ζ-potential, which represents the surface charge of a given set of particles, became relevant as a key factor that influences processes such as opsonization and internalization. Examples of such dramatic changes were presented by Arvizo et al. (2), who tested these aforementioned key factors in gold nanoparticle-coated polymers that conferred surface charges that were positive, negative, or neutral. They found that particles with a neutral surface charge (i.e., a ζ-potential < |2| mV) had longer circulation times and consequently higher accumulation in tumors. Other studies with cancer cell lines, such as HeLa (18, 30), and endothelial cells (43) found in vivo that particles with a positive surface charge had higher internalization rates than negative or neutral particles. For cardiac cells specifically, Miragoli et al. (31) found that exposure in cultured neonatal rat myocytes to 50-nm-charged polystyrene particles showed contrasting effects based on the type of surface charge: positively charged particles showed higher cytotoxic effects, while negatively charged particles showed lower cytotoxicity but formed 50–250-nm nanopores that induced proarrhythmic events.
In this work, we observed differences in the cell release levels of LDH after 24-h incubation of SiO2. As shown in Fig. 2C, nano-SiO2 produced a twofold increase in activity compared with micro-SiO2-treated cells. This difference was made more evident by the fact that SiO2-induced necrosis occurred in a time-dependent manner, in accordance with previous reports, and nano-SiO2 caused LDH leakage in endothelial cells at similar times and doses (10). Figure 2D shows cell membrane damage as early as in the first 3 h of incubation with nano-SiO2; this phenomenon is probably due to the size of nano-SiO2, which allows it to penetrate faster through the cell membrane, in turn causing an early permeabilization process that has important cytotoxic effects. This cytotoxic effect was also exerted by the micro-SiO2 as early as the first 2 h, but notable LDH release was shown only after 12 h of incubation. In this scenario, membrane permeabilization and LDH release indicate the beginning of necrosis in SiO2-treated cells. Apoptosis cell death was not observed, potentially because of the reduced energetic metabolites.
As observed, micro-SiO2 exerted a lower cytotoxic effect compared with nano-SiO2. To understand this difference, we developed a hypothesis with two scenarios: 1) micro-SiO2 does not associate properly with cardiomyocyte membranes, and 2) the larger particle size of micro-SiO2 prevents it from penetrating into cardiomyocytes, thus avoiding membrane disruption and cell damage. On the basis of this, we explored the association of SiO2 particles with cellular membranes. The FEG-SEM micrographs show that nano- and micro-SiO2 associated with cells as individuals and agglomerates/aggregates, and there was evidence of an internalization process and a strong interaction between SiO2 and cell membranes. In accordance with this, the quantification by PIXE revealed that there was a concentration of SiO2 in the cardiomyocytes, while no traces of SiO2 were found in the unexposed cells (CT group). The concentration increased 3.3- and 4.5-fold for nano- and micro-SiO2, respectively. These concentrations correspond to particles that were either internalized or that adhered to the cardiomyocytes.
To explore the second hypothesis, we incubated fluorescent micro-SiO2 and analyzed its internalization using confocal microscopy. We confirmed that micro-SiO2 was localized inside the cardiomyocytes, following a dose-dependent manner, with some association near the nucleus, an interesting phenomenon that is widely observed in different phagocytic cellular types (13, 27) but rarely explored in nondividing cells. Adult cardiomyocytes, as a nondividing cell type and with an apparent nonphagocytic capacity, represent an interesting opportunity to study the biological phenomena of nano- and microparticle internalization and intracellular trafficking. Our group recently studied the mechanisms for the internalization, trafficking, and perinuclear particle localization of these apparently not quiescent cardiomyocytes (40).
Calcium mishandling is associated with SiO2 nanoparticle cardiotoxicity.
Aberrant Ca2+ handling is an important contributor to the electrical and contractile dysfunction associated with cell death. Recently, Gilardino et al. (16) found evidence that, in a neuronal cell line, SiO2 nanoparticles induce oscillatory changes in intracellular Ca2+. Although Ca2+ returns to baseline in ~4 h, these authors suggest that several voltage-dependent Ca2+ channels on the plasmatic membrane are responsible for these effects. Our results do not agree with this, as they suggest that extracellular Ca2+ transport remains functional between the L-type Ca2+ channel and SR interaction because the time to peak of Ca2+ release remains intact (data not shown). On the other hand, we observed that the decreased contractility in SiO2-treated cardiomyocytes could also be due to alterations in Ca2+ signaling. In this regard, we found that the electrically evoked Ca2+ transients were blunted with slow decay and were less sensitive to physiological β-adrenergic stimulation during ISO perfusion. The reduced Ca2+ transient amplitude could be due to either decreased SR Ca2+ content or a decrease in the L-type Ca2+ channel current density. The general consensus is that there is a modest change, or no change, in the Ca2+ current, even in the context of pathologies as profound as heart failure. However, there is much evidence associated with a state of SR Ca2+ depletion in pathologies such as doxorubicin-induced cardiomyopathy (36). Decreased SERCA activity and lower intra-SR Ca2+ content in SiO2-treated cardiomyocytes might explain the smaller amplitude of Ca2+ transients and Ca2+ sparks. The lower SR Ca2+ content in SiO2 nanoparticle-treated cells, as well as the slow rate of Ca2+ transient decay, could be explained by different events that can alter the SERCA function, such as low ATP content, as shown in this work (Fig. 10), or a decreased SERCA expression, which was recently observed in microarrays from zebrafish embryos treated with SiO2 nanoparticles (9). However, several studies have shown that decreased SERCA activity is not necessarily accompanied by decreased protein expression (55), and it has been pointed out by our group that Ca2+ signaling alterations may precede the changes in protein expression (24). To explain diminished capacity of SERCA to reload the SR, we should consider the consequences of mitochondrial dysfunction due to the drop in membrane potential and the low ATP content (Fig. 10), particularly during high cardiac workloads. A reduced capacity for energy supply (22) and the ineffective removal of the end products of ATP hydrolysis have been observed in heart failure, which leads to a reduction in the phosphorylation potential, which can in turn affect the ATPases involved in Ca2+ handling (22). SERCA is one of the most energy-demanding, and therefore most sensitive, cardiac enzymes in ATP depletion. On the basis of this, dysfunctional mitochondria and a lower ATP content could undermine cytosolic Ca2+ removal by SERCA, decrease contractile strength, and slow muscle relaxation, which we observed in SiO2-treated cardiomyocytes. However, while the SR Ca2+ content was slightly lower in the SiO2-treated cells, under basal conditions we found a decrease in the spontaneous Ca2+ spark amplitude and frequency. Because a steady-state SR Ca2+ content results from the balance between SERCA activity and SR Ca2+ leakage, the latter reflected spontaneous Ca2+ sparks, and the decrease in SR Ca2+ leakage might explain the lower SR Ca2+ content observed in cells treated with SiO2 nanoparticles; this phenomenon could also be exacerbated and deregulated under continuous β-adrenergic stimulation, as we showed in our electrically evoked Ca2+ transients under ISO stimulation (Fig. 7). Altogether, these findings are in agreement with reports on ventricular dysfunction and ischemic heart disease in which diastolic Ca2+ release is increased (12, 53).
Mitochondrial dysfunction as a trigger of SiO2 toxicity.
As we mentioned before, in cardiomyocytes the excitation-contraction coupling and metabolic adaptations are based on the coordination between the mitochondria and SR, which facilitates the ATP supply for SERCA activity and ensures energy replenishment by Ca2+ and ADP exchange (14, 52). For this reason, mitochondrial function is recognized as an important player in regulatory Ca2+ signaling in the heart (52). However, the impact of SiO2 nanoparticles on energy metabolism and mitochondrial function is largely unclear. Our findings demonstrate that exposing cardiomyocytes directly to SiO2 nanoparticles for 24 h induced mitochondrial membrane depolarization and energy debacle (Fig. 10), and even more membrane depolarization and energy debacle after 10 min of continuous β-adrenergic stimulation under ISO treatment (data not shown).
Xue et al. (54) observed SiO2 nanoparticle-induced suppression of mitochondrial dehydrogenase activity and ATP synthesis in hepatocytes. After in vitro exposure, the internalization of SiO2 nanoparticles correlates with the disturbance of the mitochondrial structure and the release of reactive oxygen species (ROS) in hepatocellular carcinoma cells (47). In this context, cardiac mitochondria allow energy supply-demand matching and regulate the generation of antioxidant defenses, such as nicotinamide adenine dinucleotide phosphate and GSH (28). However, under the Ca2+ handling impairment—as observed during SiO2 nanoparticle interaction—increased electron leakage from the electron transport chain, and the subsequent production of ROS and depletion of antioxidant reserves, has been observed (19). Similar to previous results, it was observed that nano-SiO2 incubation induced a notable amount of H2O2 (a highly toxic type of oxygen-free radical) and a potent depletion in GSH (a first-line antioxidant defense), which indicates SiO2-induced cytotoxicity that is at least partially due to an imbalance in the cellular redox status. In this regard, it is possible that SERCA and/or the ryanodine receptor might be oxidized, as has been described in other works (3), and this could, at least partially, underlie the slow rate of Ca2+ transient decay and the decrease in the amplitude and frequency of sparks. An enhanced diastolic Ca2+ leak may not only contribute to the decreased SR Ca2+ content but also increase the risk of delay after depolarization and Ca2+-triggered arrhythmias, as previously observed by Szebeni et al. (48) with lipid-based nanoparticles in a pig model.
A recent study also showed another possible mechanism of cardiotoxicity with metal-oxide nanoparticles. Savi et al. (41), using nano-TiO2 particles that had a ζ-potential similar to that of our nano-SiO2 particles but a different crystalline nature (a mixture of anatase and rutile, as compared with our amorphous SiO2), observed that nano-TiO2 particles promoted spontaneous contraction in cardiomyocytes (an arrhythmia trigger at the cellular level). This effect was related to the slight depolarization of the resting membrane potential, which consequently reduced the action potential duration. Then, using an in silico model, they suggested that nano-TiO2 particles generate transient nanopores that lead to resting membrane potential instability, possibly due to K+ leakage (41).
In this context, our results with nano-SiO2 particles suggest that there was no change in the action potential because the kinetics of Ca2+ release from the SR were not affected (time to peak; data not shown), and spontaneous contraction was not observed. However, our experiments were performed at 24 h, while the TiO2 particles were analyzed 1 h after particle addition, so further experiments on patch-clamped cardiomyocytes with simultaneous measurement of Ca2+ handling might help to establish a potential connection between these mechanisms of cardiotoxicity. Interestingly, the nano-TiO2 particles induced lipid peroxidation in the heart because of ROS production, which is similar to our findings regarding GSH and H2O2 levels.
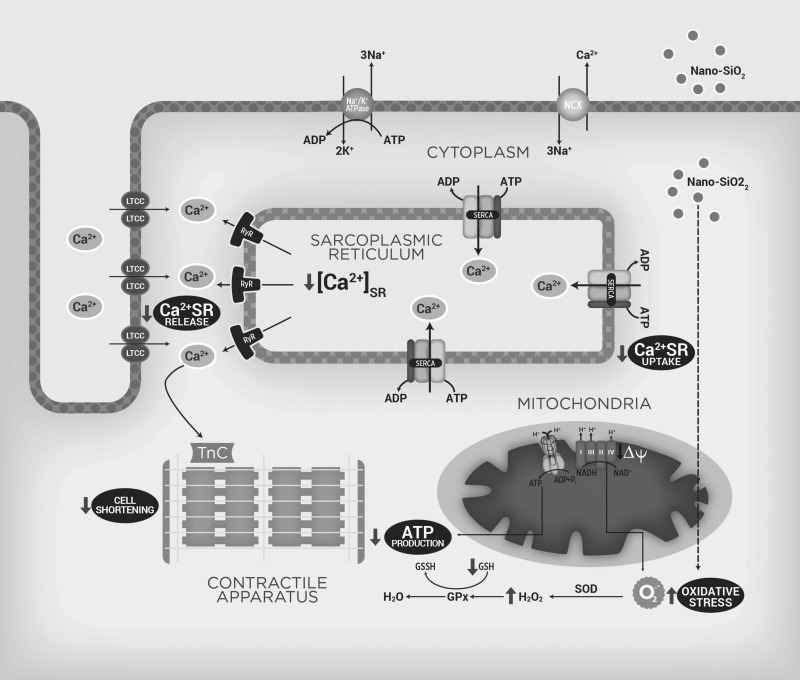
Putting all of this information into context, as summarized in Fig. 11, the Krebs cycle generates the NADH required for oxidative phosphorylation through the electron transport chain (complex I–IV, in blue). This step-by-step transfer of electrons allows protons from the mitochondrial matrix to be transported uphill across the inner mitochondrial membrane, which forms a proton concentration gradient (ΔΨm). Thus free energy released during the oxidation of NADH is stored both as an electric potential and a proton concentration gradient across the inner membrane. The movement of protons back across the inner membrane, driven by this force, is coupled to the synthesis of ATP from ADP and Pi by the ATP synthase. During SiO2 nanoparticle perfusion (indicated by gray arrows), defects in the mitochondrial homeostasis contribute to the energetic mismatch observed (ATP depletion). SiO2 nanoparticles internalize and directly (dotted line) or indirectly produce ROS, such as the superoxide radical (O2·−), which is transformed to H2O2 by superoxide dismutase (SOD) and then converted to H2O using glutathione peroxidase activity (GPx), depleting the (GSH) and increasing oxidative stress. When a cardiomyocyte is depolarized, Ca2+ enters through the L-type calcium channels (LTCC). This Ca2+ triggers a subsequent release of Ca2+ that is stored in the SR; through ryanodine receptors (RyR2), the Ca2+ released by the SR increases the intracellular Ca2+ (lower Ca2+ SR release), and then free Ca2+ binds to troponin C (TnC) and interacts with several proteins, which results in the sarcomere length being shortened (cell shortening decrease). In the relaxation phase, the SR sequesters Ca2+ using an ATP-dependent calcium pump (SERCA2a), which lowers the cytosolic Ca2+ concentration and removes calcium from the TnC, but because of the energy depletion, SERCA reduces its activity, which decreases the Ca2+ SR content.
Fig. 11.
Proposed mechanism of SiO2-induced cardiotoxicity. Scheme of the suggested mechanism by which nano-SiO2 particles induces cardiotoxicity interfering with energetic status and Ca2+ handling in cardiomyocytes. GSH/GSSG, reduced/oxidized glutathione; NCLX, Na+/Ca2+ exchanger; O2·−, superoxide radical; LTCC, L-type calcium channels; SR, sarcoplasmic reticulum; RyR2, ryanodine receptors; TnC, troponin C.
Clinical relevance.
The World Health Organization estimated that there would be ~20 million cardiovascular disease deaths in 2015, accounting for 30% of all deaths worldwide. In this context, the likelihood of cardiovascular diseases being associated with particulate air pollution is well documented. A series of scientific statements from the American Heart Association stressed that exposure to elevated levels of particulate matters is strongly linked with heart diseases, particularly when more than 75% of the total number of particles are nanoparticles (6, 17, 21). However, the correlation between heart disease and nanoscale particles is still being debated, and the related research conclusions lack consistency. Therefore, studying the cardiovascular toxicity of nanoscale particles is necessary and has profound scientific significance, particularly with regard to nanoparticle types, such as metal, metal-oxide, and carbon nanotubes, that have been reported to produce toxicity in other organs (35). To the best of our knowledge, we have demonstrated for the first time that SiO2 nanoparticles impair the bioenergetics status and may consequently impact Ca2+ handling and contraction in rat cardiomyocytes. The mechanism of cardiotoxicity for SiO2 particles mimics the pathological mechanisms of a failing heart. These findings will be helpful in managing risk and providing guidance to reduce the hazardous effects of nanoscale particles. In addition, a better understanding of the mechanism through which nanoparticles produce cardiotoxicity could uncover novel avenues for avoiding the side effects associated with the use of these particles.
In summary, the findings of this study support the notion that SiO2-induced cardiomyocyte toxicity is strongly size dependent. First, we showed differential, dose-dependent toxicity after 24 h of incubation, with differential time-dependent cell death by necrosis (as shown by LDH leakage) and no activation of apoptosis. Second, we visualized, by confocal microscopy, an internalization phenomenon in these nondividing, nonphagocytic primary-culture cell types, thus opening up a new field of study. Third, our results showed the role of oxidative stress and the direct effect of SiO2 particles on cell function: impaired Ca2+ handling and a reduction in cell shortening. These effects were all due to mitochondrial malfunction, which is shown as a drop in the membrane potential and ATP content (Fig. 11). These data enable us to explore and carefully design new medical devices and clinical therapeutic protocols that take into account the advantages and disadvantages of nanoparticles, in particular SiO2.
GRANTS
This work was partially supported by Endowed Chair in Cardiology (Tecnológico de Monterrey, 0020CAT131) as well as the CONACYT Grants 151136, 133591, 269399, and Fronteras de la Ciencia Grant (0682) and Xignus Research Fund. C. Guerrero-Beltrán was supported by Postdoctoral Fellowship CONACYT, 290885. SEM-EDS work was supported by National Institutes of Health Grants 5 G12 RR-013646-12 and G12 MD-007591.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.E.G.-B., J.B.-R., O.L., Y.O.-A., E.C.C., J.R.G., N.G., J.V., A.G.-G., E.O., and N.O.-S. performed experiments; C.E.G.-B., J.B.-R., O.L., Y.O.-A., and E.C.C. analyzed data; C.E.G.-B., J.B.-R., O.L., E.C.C., G.T.-A., and G.G.-R. interpreted results of experiments; C.E.G.-B. and G.G.-R. drafted manuscript; C.E.G.-B., O.L., G.T.-A., and G.G.-R. edited and revised manuscript; J.B.-R. prepared figures; C.E., G.B., and G.G.-R. approved final version of manuscript; C.E.G.-B. and G.G.-R. conceived and designed research.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Tzarara López-Luke and María Christian Álbor Cortés (Centro de Investigaciones en Óptica, CIO, Guanajuato, México), Lilia Magdalena Bautista Carrillo and Nayely Pineda Aguilar (Centro de Investigación en Materiales Avanzados S.C.), and Dr. Flavio Contreras-Torres (Laboratorio de Nanotecnología Ambiental, Tecnológico de Monterrey) for technical support. We acknowledge Valeria Oropeza for the design of Fig. 11.
REFERENCES
- 1.Al-Rasheed NM, Faddah LM, Mohamed AM, Abdel Baky NA, Al-Rasheed NM, Mohammad RA. Potential impact of quercetin and idebenone against immuno-inflammatory and oxidative renal damage induced in rats by titanium dioxide nanoparticles toxicity. J Oleo Sci 62: 961–971, 2013. doi: 10.5650/jos.62.961. [DOI] [PubMed] [Google Scholar]
- 2.Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, Robertson JD, Rotello VM, Reid JM, Mukherjee P. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS One 6: e24374, 2011. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balderas-Villalobos J, Molina-Muñoz T, Mailloux-Salinas P, Bravo G, Carvajal K, Gómez-Viquez NL. Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol 305: H1344–H1353, 2013. doi: 10.1152/ajpheart.00211.2013. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 5.Blanco E, Hsiao A, Ruiz-Esparza GU, Landry MG, Meric-Bernstam F, Ferrari M. Molecular-targeted nanotherapies in cancer: enabling treatment specificity. Mol Oncol 5: 492–503, 2011. doi: 10.1016/j.molonc.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism . Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JL. About GUPIX and GUPIXWIN. Ontario: University of Guelph, 2005. http://pixe.physics.uoguelph.ca/gupix/about/. [22 February 2017]. [Google Scholar]
- 8.Du Z, Zhao D, Jing L, Cui G, Jin M, Li Y, Liu X, Liu Y, Du H, Guo C, Zhou X, Sun Z. Cardiovascular toxicity of different sizes amorphous silica nanoparticles in rats after intratracheal instillation. Cardiovasc Toxicol 13: 194–207, 2013. doi: 10.1007/s12012-013-9198-y. [DOI] [PubMed] [Google Scholar]
- 9.Duan J, Yu Y, Li Y, Li Y, Liu H, Jing L, Yang M, Wang J, Li C, Sun Z. Low-dose exposure of silica nanoparticles induces cardiac dysfunction via neutrophil-mediated inflammation and cardiac contraction in zebrafish embryos. Nanotoxicology 10: 575–585, 2016. doi: 10.3109/17435390.2015.1102981. [DOI] [PubMed] [Google Scholar]
- 10.Duan J, Yu Y, Li Y, Yu Y, Sun Z. Cardiovascular toxicity evaluation of silica nanoparticles in endothelial cells and zebrafish model. Biomaterials 34: 5853–5862, 2013. doi: 10.1016/j.biomaterials.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 11.Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol 206: 2039–2047, 2003. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 12.Endoh M. Cardiac Ca2+ signaling and Ca2+ sensitizers. Circ J 72: 1915–1925, 2008. doi: 10.1253/circj.CJ-08-0838. [DOI] [PubMed] [Google Scholar]
- 13.Farcal LR, Uboldi C, Mehn D, Giudetti G, Nativo P, Ponti J, Gilliland D, Rossi F, Bal-Price A. Mechanisms of toxicity induced by SiO2 nanoparticles of in vitro human alveolar barrier: effects on cytokine production, oxidative stress induction, surfactant proteins a mRNA expression and nanoparticles uptake. Nanotoxicology 7: 1095–1110, 2013. doi: 10.3109/17435390.2012.710658. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Sada E, Silva-Platas C, Villegas CA, Rivero SL, Willis BC, García N, Garza JR, Oropeza-Almazán Y, Valverde CA, Mazzocchi G, Zazueta C, Torre-Amione G, García-Rivas G. Cardiac responses to β-adrenoceptor stimulation is partly dependent on mitochondrial calcium uniporter activity. Br J Pharmacol 171: 4207–4221, 2014. doi: 10.1111/bph.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebel T, Marchan R, Hengstler JG. The nanotoxicology revolution. Arch Toxicol 87: 2057–2062, 2013. doi: 10.1007/s00204-013-1158-6. [DOI] [PubMed] [Google Scholar]
- 16.Gilardino A, Catalano F, Ruffinatti FA, Alberto G, Nilius B, Antoniotti S, Martra G, Lovisolo D. Interaction of SiO2 nanoparticles with neuronal cells: Ionic mechanisms involved in the perturbation of calcium homeostasis. Int J Biochem Cell Biol 66: 101–111, 2015. doi: 10.1016/j.biocel.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Gold DR, Mittleman MA. New insights into pollution and the cardiovascular system: 2010 to 2012. Circulation 127: 1903–1913, 2013. doi: 10.1161/CIRCULATIONAHA.111.064337. [DOI] [PubMed] [Google Scholar]
- 18.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 105: 11613–11618, 2008. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 85: 357–363, 1999. doi: 10.1161/01.RES.85.4.357. [DOI] [PubMed] [Google Scholar]
- 20.Jakob AM, Schmedake TA. A novel approach to monodisperse, luminescent silica spheres. Chem Mater 18: 3173–3175, 2006. doi: 10.1021/cm060664t. [DOI] [Google Scholar]
- 21.Kumar P, Robins A, Vardoulakis S, Britter R. A review of the characteristics of nanoparticles in the urban atmosphere and the prospects for developing regulatory controls. Atmos Environ 44: 5035–5052, 2010. doi: 10.1016/j.atmosenv.2010.08.016. [DOI] [Google Scholar]
- 22.Kuum M, Kaasik A, Joubert F, Ventura-Clapier R, Veksler V. Energetic state is a strong regulator of sarcoplasmic reticulum Ca2+ loss in cardiac muscle: different efficiencies of different energy sources. Cardiovasc Res 83: 89–96, 2009. doi: 10.1093/cvr/cvp125. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev 41: 2590–2605, 2012. doi: 10.1039/c1cs15246g. [DOI] [PubMed] [Google Scholar]
- 24.Lin W, Huang YW, Zhou XD, Ma Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol 217: 252–259, 2006. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Lozano O, Mejia J, Masereel B, Toussaint O, Lison D, Lucas S. Development of a PIXE analysis method for the determination of the biopersistence of SiC and TiC nanoparticles in rat lungs. Nanotoxicology 6: 263–271, 2012. doi: 10.3109/17435390.2011.572301. [DOI] [PubMed] [Google Scholar]
- 26.Lozano O, Mejia J, Piret JP, Saout C, Dogné JM, Toussaint O, Lucas S. How does the deposited dose of oxide nanomaterials evolve in an in vitro assay? J Phys Conf Ser 429: 012013, 2013. doi: 10.1088/1742-6596/429/1/012013. [DOI] [Google Scholar]
- 27.Luo Z, Hu Y, Xin R, Zhang B, Li J, Ding X, Hou Y, Yang L, Cai K. Surface functionalized mesoporous silica nanoparticles with natural proteins for reduced immunotoxicity. J Biomed Mater Res A 102: 3781–3794, 2014. doi: 10.1002/jbm.a.35049. [DOI] [PubMed] [Google Scholar]
- 28.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 99: 172–182, 2006. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonnell SM, García-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, Houser SR. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res 102: e65–e72, 2008. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller CR, Bondurant B, McLean SD, McGovern KA, O’Brien DF. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 37: 12875–12883, 1998. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 31.Miragoli M, Novak P, Ruenraroengsak P, Shevchuk AI, Korchev YE, Lab MJ, Tetley TD, Gorelik J. Functional interaction between charged nanoparticles and cardiac tissue: a new paradigm for cardiac arrhythmia? Nanomedicine (Lond) 8: 725–737, 2013. doi: 10.2217/nnm.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, Dawson KA. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc 133: 2525–2534, 2011. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 33.Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH. The nanosilica hazard: another variable entity. Part Fibre Toxicol 7: 39, 2010. doi: 10.1186/1743-8977-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napierska D, Thomassen LC, Rabolli V, Lison D, Gonzalez L, Kirsch-Volders M, Martens JA, Hoet PH. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small 5: 846–853, 2009. doi: 10.1002/smll.200800461. [DOI] [PubMed] [Google Scholar]
- 35.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science 311: 622–627, 2006. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 36.Olson RD, Gambliel HA, Vestal RE, Shadle SE, Charlier HA Jr, Cusack BJ. Doxorubicin cardiac dysfunction: effects on calcium regulatory proteins, sarcoplasmic reticulum, and triiodothyronine. Cardiovasc Toxicol 5: 269–283, 2005. doi: 10.1385/CT:5:3:269. [DOI] [PubMed] [Google Scholar]
- 37.Qhobosheane M, Santra S, Zhang P, Tan W. Biochemically functionalized silica nanoparticles. Analyst (Lond) 126: 1274–1278, 2001. doi: 10.1039/b101489g. [DOI] [PubMed] [Google Scholar]
- 38.Rabolli V, Thomassen LC, Princen C, Napierska D, Gonzalez L, Kirsch-Volders M, Hoet PH, Huaux F, Kirschhock CE, Martens JA, Lison D. Influence of size, surface area and microporosity on the in vitro cytotoxic activity of amorphous silica nanoparticles in different cell types. Nanotoxicology 4: 307–318, 2010. doi: 10.3109/17435390.2010.482749. [DOI] [PubMed] [Google Scholar]
- 39.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1: 3159–3165, 2006. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz-Esparza GU, Segura-Ibarra V, Cordero-Reyes AM, Youker KA, Serda RE, Cruz-Solbes AS, Amione-Guerra J, Yokoi K, Kirui DK, Cara FE, Paez-Mayorga J, Flores-Arredondo JH, Guerrero-Beltrán CE, Garcia-Rivas G, Ferrari M, Blanco E, Torre-Amione G. A specifically designed nanoconstruct associates, internalizes, traffics in cardiovascular cells, and accumulates in failing myocardium: a new strategy for heart failure diagnostics and therapeutics. Eur J Heart Fail 18: 169–178, 2016. doi: 10.1002/ejhf.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savi M, Rossi S, Bocchi L, Gennaccaro L, Cacciani F, Perotti A, Amidani D, Alinovi R, Goldoni M, Aliatis I, Lottici PP, Bersani D, Campanini M, Pinelli S, Petyx M, Frati C, Gervasi A, Urbanek K, Quaini F, Buschini A, Stilli D, Rivetti C, Macchi E, Mutti A, Miragoli M, Zaniboni M. Titanium dioxide nanoparticles promote arrhythmias via a direct interaction with rat cardiac tissue. Part Fibre Toxicol 11: 63, 2014. doi: 10.1186/s12989-014-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serda RE, Godin B, Blanco E, Chiappini C, Ferrari M. Multi-stage delivery nano-particle systems for therapeutic applications. Biochim Biophys Acta 1810: 317–329, 2011. doi: 10.1016/j.bbagen.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serda RE, Gu J, Bhavane RC, Liu X, Chiappini C, Decuzzi P, Ferrari M. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials 30: 2440–2448, 2009. doi: 10.1016/j.biomaterials.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Silva-Platas C, Guerrero-Beltrán CE, Carrancá M, Castillo EC, Bernal-Ramírez J, Oropeza-Almazán Y, González LN, Rojo R, Martínez LE, Valiente-Banuet J, Ruiz-Azuara L, Bravo-Gómez ME, García N, Carvajal K, García-Rivas G. Antineoplastic copper coordinated complexes (Casiopeinas) uncouple oxidative phosphorylation and induce mitochondrial permeability transition in cardiac mitochondria and cardiomyocytes. J Bioenerg Biomembr 48: 43–54, 2016. doi: 10.1007/s10863-015-9640-x. [DOI] [PubMed] [Google Scholar]
- 45.Sohaebuddin SK, Thevenot PT, Baker D, Eaton JW, Tang L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part Fibre Toxicol 7: 22, 2010. doi: 10.1186/1743-8977-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun L, Li Y, Liu X, Jin M, Zhang L, Du Z, Guo C, Huang P, Sun Z. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicol In Vitro 25: 1619–1629, 2011. doi: 10.1016/j.tiv.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Szebeni J, Alving CR, Rosivall L, Bünger R, Baranyi L, Bedöcs P, Tóth M, Barenholz Y. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J Liposome Res 17: 107–117, 2007. doi: 10.1080/08982100701375118. [DOI] [PubMed] [Google Scholar]
- 49.Tang L, Cheng J. Nonporous silica nanoparticles for nanomedicine application. Nano Today 8: 290–312, 2013. doi: 10.1016/j.nantod.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MM, Decuzzi P, Tour JM, Robertson F, Ferrari M. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol 3: 151–157, 2008. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 11: 313–327, 2015. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Williams GS, Boyman L, Lederer WJ. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 78: 35–45, 2015. doi: 10.1016/j.yjmcc.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willis BC, Salazar-Cantú A, Silva-Platas C, Fernández-Sada E, Villegas CA, Rios-Argaiz E, González-Serrano P, Sánchez LA, Guerrero-Beltrán CE, García N, Torre-Amione G, García-Rivas GJ, Altamirano J. Impaired oxidative metabolism and calcium mishandling underlie cardiac dysfunction in a rat model of post-acute isoproterenol-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 308: H467–H477, 2015. doi: 10.1152/ajpheart.00734.2013. [DOI] [PubMed] [Google Scholar]
- 54.Xue Y, Chen Q, Ding T, Sun J. SiO2 nanoparticle-induced impairment of mitochondrial energy metabolism in hepatocytes directly and through a Kupffer cell-mediated pathway in vitro. Int J Nanomedicine 9: 2891–2903, 2014. doi: 10.2147/IJN.S60661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarain-Herzberg A, García-Rivas G, Estrada-Avilés R. Regulation of SERCA pumps expression in diabetes. Cell Calcium 56: 302–310, 2014. doi: 10.1016/j.ceca.2014.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.