Abstract
Background
To assess the worldwide variation of amyotrophic lateral sclerosis (ALS) incidence, we performed a systematic review and meta-analysis of population-based data published to date.
Methods
We reviewed Medline and Embase up to June 2015 and included all population-based studies of newly diagnosed ALS cases, using multiple sources for case ascertainment. ALS crude and standardized incidence (on age and sex using the US 2010 population) were calculated. Random effect meta-analysis and meta-regression were performed using the subcontinent as the main study level covariate. Sources of heterogeneity related to the characteristics of the study population and the study methodology were investigated.
Results
Among 3216 records, 44 studies were selected, covering 45 geographical areas in 11 sub-continents. A total of 13 146 ALS cases and 825 million person-years of follow-up (PYFU) were co-nsidered. The overall pooled worldwide crude ALS incidence was at 1.75 (1.55–1.96)/100 000 PYFU; 1.68 (1.50–1.85)/100 000 PYFU after standardization. Heterogeneity was identified in ALS standardized incidence between North Europe [1.89 (1.46–2.32)/100 000 PYFU] and East Asia [0.83 (0.42–1.24)/100 000 PYFU, China and Japan P = 0.001] or South Asia [0.73 (0.58–0.89)/100 000/PYFU Iran, P = 0.02]. Conversely, homogeneous rates have been reported in populations from Europe, North America and New Zealand [pooled ALS standardized incidence of 1.81 (1.66-1.97)/100 000 PYFU for those areas].
Conclusion
This review confirms a heterogeneous distribution worldwide of ALS, and sets the scene to sustain a collaborative study involving a wide international consortium to investigate the link between ancestry, environment and ALS incidence.
Keywords: Amyotrophic lateral sclerosis, epidemiology, incidence, ethnic groups
Introduction
Variation in the incidence of amyotrophic lateral sclerosis (ALS) between geographical areas could support the notion that genetic factors, especially populations’ ancestries, along with environmental and lifestyle factors, play a dominant role in the occurrence of the disease.
In 2007, a systematic review argued for a uniform occurrence of ALS across populations of European origin and, by contrast, a lower incidence of ALS among populations of African, Asian and Amerindian origin.1 It was nevertheless difficult to draw firm conclusions, as there was methodological heterogeneity among studies in non-European populations. Also, both the population-based and the clinic-based studies were lumped together, rendering conclusions even more challenging. These results were consistent with those of a subsequent systematic review.2
Since then, numerous new incidence or mortality data have been reported from various parts of the world.3–14 In addition, considering that in some settings ALS mortality rates can be considered as valid proxy for ALS incidence rates,15 lower mortality rates of ALS in a mixed population in Cuba raised the hypothesis that a much wider variety of and different combinations of at-risk alleles lower the overall risk of developing the disease.16 Finally, no formal meta-analyses of ALS incidence have been performed.
An analysis of worldwide incidence rates may provide new clues into the role of ancestry and environment in the occurrence of ALS. We performed a systematic review and meta-analysis of published data concerning the incidence of ALS in relation to subcontinents.
Methods
We applied the guidelines for Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE)17 and followed other specific recommendations for systematic reviews and meta-analyses.18 As this review of literature/meta-analysis did not include ALS patients but only publications on ALS, informed consent of patients was not applicable. Approval of an ethics committee was not applicable.
Definitions
In this study, the disease under consideration was motor neurone disease (MND), which includes ALS and ALS subtypes.19 We included only population-based studies. A population-based study implies using an appropriate methodology, an epidemiological investigation of a sample or the entire population, within defined geographical and time boundaries.20 To investigate the worldwide heterogeneity of ALS incidence, we considered sub-continent as our main study-level covariate. Sub-continent classification was based on the United Nations Statistics Division.21
Search strategy
Medline and Embase were searched. Period of publication (until June 2015) and language were unlimited. We did not use filters as regards species or type of article. Keywords, defined with a medical librarian, are described in eTable 1 (available as Supplementary data at IJE online). We also performed hand-searching (reference lists of articles). All references identified were imported into Endnote X7 and duplicates were deleted.
Table 1.
Population-based studies of ALS incidence included in the review
| Continent | Subcontinent | Country | Area | References (n = 44) | Period | Duration | Number of ALS cases | PYFU | Design | Diagnostic method | Types of sources for case ascertainment |
Duplicates (n = 35) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Hosp | Neuro | Spe | HI | DC | Asso | PCP | ||||||||||||
| Europe | North Europe | Sweden | Varmland | Gunnarson (1984) | 1970-81 | 12 | 89 | 3.41E+06 | R | Neurologist | x | x | x | ||||||
| Vasterbotten, Norbotten, Angermanland | Forsgren (1983) | 1969-80 | 12 | 128 | 7.60E+06 | R | Neurologist | x | x | x | |||||||||
| Finland | Middle Finland | Murros (1983) | 1976-81 | 6 | 36 | 1.45E+06 | R | Neurologist | x | x | |||||||||
| Denmark | North Jutland and Aarhus | Hojer Pedersen (1989) | 1974-86 | 13 | 186 | 1.37E+07 | P | Neurologist | x | x | Christensen (1990) | ||||||||
| Faroe Islands | Countrywide | Joensen (2012) | 1987-2009 | 22 | 28 | 1.04E+06 | P | EEDC | x | x | |||||||||
| Scotland | Countrywide | Forbes (2007) | 1989-98 | 10 | 1226 | 5.13E+07 | P | EEDC | x | x | x | x | Forbes (2004), Chancellor (1993), Chancellor (1993) | ||||||
| England | Devon and Cornwall | Imam (2010) | 2002-07 | 6 | 243 | 9.75E+06 | R | EEDC | x | x | x | ||||||||
| Lancashire and South Cumbria | Logroscino (2010) | 1998-99 | 2 | 54 | 3.25E+06 | P | EEDC | x | x | x | x | ||||||||
| England | South-East England | Abhinav (2007) | 2002-06 | 4.5 | 138 | 1.60E+07 | P | EEDC | x | x | x | x | x | Johnston (2006, sub-region), Scott (2009), Scott (2010), Manjaly (2010), Rojas-Garcia (2012, sub-region) | |||||
| Ireland | Countrywide | O'Toole (2008) | 1995-97/ 2002-04 | 6 | 465 | 2.28E+07 | P | EEDC | x | x | x | x | x | Rooney (2013), Phukan (2011), Donaghy (2010), Donaghy (2009), Traynor (2000), Traynor (1999) | |||||
| Estonia | South Estonia | Gross-Paju (1998) | 1986-95 | 10 | 50 | 3.92E+06 | R | Neurologist | x | x | |||||||||
| West Europe | France | Limousin | Marin (2014) | 2000-11 | 12 | 279 | 8.76E+06 | R | EEDC | x | x | x | |||||||
| The Netherlands | Countrywide | Huisman (2011) | 2006-09 | 4 | 1217 | 6.59E+07 | P | EEDC | x | x | x | x | |||||||
| Germany | Rhineland-Palatinate | Wolf (2014) | 2010-11 | 2 | 146 | 8.01E+06 | P | EEDC | x | x | x | x | x | x | x | ||||
| Swabia | Uenal (2014) | 2008-10 | 2 | 438 | 1.68E+07 | R | EEDC | x | x | x | |||||||||
| South Europe | Italy | Lombardy | Beghi (2007) | 1998-2002 | 5 | 517 | 2.47E+07 | P | EEDC | x | x | x | x | x | Pupillo (2014), Millul (2005) | ||||
| Piemonte and Vallée Aosta | Chio (2009) | 1995-2004 | 10 | 1260 | 4.40E+07 | P | EEDC | x | x | x | x | x | Chio (2011), Chio (2002), Parals (2001), Chio (1999) | ||||||
| Friuli-Venezia Giulia | Drigo (2013) | 2002-09 | 8 | 262 | 9.65E+06 | R | EEDC | x | x | x | x | ||||||||
| Emilia Romagna | Mandrioli (2014) | 2009-11 | 3 | 347 | 1.32E+07 | P | EEDC | x | x | x | x | Mandrioli (2012), Mandrioli (2006), Guidetti (1996), Georgoloupou (2011, sub-region) Bonvicini (2008, sub-region), Mandrioli (2003, sub-region), Govoni (2012, sub-region), Govoni (2003, sub-region), Granieri (1988, sub-region) | |||||||
| Liguria | Bandettini (2013) | 2009-10 | 2 | 104 | 3.23E+06 | P | EEDC | x | x | x | x | ||||||||
| Puglia | Logroscino (2005) | 1998-99 | 2 | 130 | 8.16E+06 | P | EEDC | x | x | x | x | Zoccolella (2008), Zoccolella (2008), Zocolella (2006) | |||||||
| Sardinia (Sassari and Nuoro) | Pugliatti (2013) | 2005-09 | 5 | 89 | 3.55E+06 | R | EEDC | x | x | x | x | x | |||||||
| Sicily (Agrigento, Catania, Caltanissetta, Palermo, Trapani) | Ragonese (2012) | 2005-06 | 2 | 97 | 6.93E+06 | R | EEDC | x | x | x | |||||||||
| Spain | Catalognia | Pradas (2013) | 1999-2001 | 3 | 215 | 1.87E+07 | P | EEDC | x | x | x | x | x | ||||||
| America | North America | Canada | Ontario | Hudson (1986) | 1978-82 | 5 | 139 | 8.54E+06 | R | Neurologist | x | x | |||||||
| Nova Scotia | Bonaparte (2007) | 2003 | 1 | 21 | 9.36E+05 | P | EEDC | x | x | ||||||||||
| USA | Olmsted County | Sorenson (2002) | 1925-98 | 74 | 77 | 5.15E+06 | P | EEDC | x | x | x | x | x | x | Mateen (2010), Mulder (1991), Yoshida (1986) | ||||
| Washington State | McGuire (1996) | 1990-95 | 5 | 235 | 1.28E+07 | P | Neurologist | x | x | x | Del Aguila (2003) | ||||||||
| Harris County | Annegers (1991) | 1985-88 | 4 | 97 | 1.18E+07 | R | Neurologist | x | x | x | x | ||||||||
| Los Angeles County | Valle (2015) | 2009-11 | 3 | 330 | 2.95E+07 | R | EEDC | x | x | x | |||||||||
| San Francisco Bay Area | Valle (2015) | 2009-11 | 3 | 289 | 1.35E+07 | R | EEDC | x | x | x | |||||||||
| New Jersey | Jordan (2014) | 2009-11 | 3 | 493 | 2.64E+07 | R | EEDC | x | x | x | |||||||||
| Florida | Freer (2015) | 2009-11 | 3 | 1021 | 5.64E+07 | R | EEDC | x | x | x | |||||||||
| Baltimore and Philadelphia | Jordan (2015) | 2009-11 | 3 | 142 | 9.72E+06 | R | EEDC | x | x | x | |||||||||
| South America | Argentina | Buenos Aires | Bettini (2013) | 2003-10 | 8 | 63 | 1.01E+06 | R | EEDC | x | x | x | |||||||
| Uruguay | Countrywide | Vazquez (2008) | 2002-03 | 2 | 89 | 6.48E+06 | P | EEDC | x | x | x | x | |||||||
| Hawaii | USA | Hawaii | Matsumoto (1972) | 1952-69 | 18 | 118 | 1.14E+07 | R | Neurologist | x | x | ||||||||
| Latin America and the Caribbean | Caribbean | France | Guadeloupe Islands | Lannuzel (2015) | 1996-2011 | 15 | 32 | 5.90E+06 | R | EEDC | x | x | |||||||
| Africa | North Africa | Libya | Benghazi | Radhakrishnan (1988) | 1980-85 | 5 | 23 | 2.59E+06 | P | Neurologist | x | x | |||||||
| East Asia | Japan | Hokkaido Island | Okumura (2003) | 1980-89 | 10 | 389 | 5.67E+07 | P | Neurologist | x | x | x | |||||||
| China | Taiwan | Lai (2008) | 2000-05 | 5 | 1187 | 1.14E+08 | R | Neurologist | x | x | Lee (2013) | ||||||||
| China | Hong Kong | Fong (2005) | 1997-2002 | 5.1 | 98 | 1.64E+07 | R | EEDC | x | x | Fong (1996) | ||||||||
| Asia | West Asia | Israel | Countrywide | Kahana (1984) | 1959-74 | 16 | 246 | 3.73E+07 | R | Neurologist | x | x | x | Gubbay (1985) | |||||
| South Asia | Iran | Isfahan | Sajjadi (2010) | 2002-06 | 5 | 98 | 2.28E+07 | R | EEDC | x | x | ||||||||
| Oceania | Australia and New Zealand | New Zealand | Canterbury | Murphy (2008) | 1985-2006 | 22 | 215 | 9.21E+06 | R | EEDC | x | x | |||||||
PYFU, person-years of follow-up; Ref, ALS referral centres; Hosp, hospital discharge data; Neuro, neurologist; Spe, other specialist; HI, Health insurance data; DC, death certificates; Asso, patients’ association; PCP, primary care physicians; R, retrospective; P , prospective; Neurologist, diagnostic method based on neurological diagnosis without the use of El Escorial criteria; EEDC, diagnostic method based on neurological diagnosis using El Escorial criteria (original or revised version). For some articles, ‘sub-region’ is mentioned, indicating that those references considered a restricted area included in the main reference selected.
Hawaii and Carribbean included in North American subcontinent for United Nations is presented separately in this table.
Inclusion criteria
Systematic reviews were not included but their references were examined. Proceedings of conferences were not included. We included population-based studies of newly diagnosed ALS cases, using multiple types of sources to ensure the highest level of completeness for case ascertainment: (i) ALS referral centres; (ii) hospital discharge data; (iii) neurologists; (iv) other specialists; (v) health insurance data; (vi) death certificates; (vii) patients’ associations; and (viii) primary care physicians. For each study, the number and type of sources were collected. Appropriate methodology was mandatory in terms of definition of the geographical coverage, study population, investigated period and diagnostic criteria for ALS cases. Only studies with a neurological confirmation of the diagnosis were included.
All population-based studies of newly diagnosed ALS cases were included; nevertheless: (i) when more than one article was available for the same geographical area, the one with the longest follow-up and most person-years was prioritized; (ii) in case of overlap of geographical areas, the article with the widest geographical coverage was prioritized; (iii) and studies from historical ALS foci (Guam ALS-PDC focus and Wakayama prefecture) were not included in order to avoid gathering ALS with variants unrelated to sporadic ALS and potentially implying differing aetiological mechanisms such as toxic exposures, specific nutritional habits and food and water supplies associated with toxins.22 Full texts were examined by one author, who assessed their eligibility. Decision about inclusion was confirmed by a second author.
Data extraction from included studies
During the collection of data from articles included in the review, we did not calculate a ‘quality score’ as its use has been criticized for being inaccurate in some cases.18 Instead we used a checklist, whose components are described below, based on the basic principles of descriptive epidemiology and focused on all aspects of the study design that can influence the quality of case ascertainment and results. The following data were recorded: investigators, year of publication, period, location, design, sources of case ascertainment, diagnosis criteria, number of patients (male, female), population understudy (male, female, age structure) and number of cases by age group and sex. Where not available in the published material, authors (or their collaborators) were contacted in order to obtain more information regarding the variables included in the review. When the attempt to contact the author failed and the article displayed age and sex-specific incidence in a figure, we performed a graphical reading of the figure to obtain numerical rates. For each geographical area, life expectancy at 50 years for men and for women was retrieved from the demographic yearbook published by the United Nations at the mid point of the study period.23 For Libya, we used Egypt life expectancy as proxy due to missing data, and for Hawaii, data were extracted from the United States Census Bureau.24 With the help of a geographer, we accurately identified the geographical area for each study. We collected Global Positioning System (GPS) coordinates of the areas in order to create worldwide maps of crude and standardized incidence.
Data analysis
Crude incidence [number of cases per 100 000 person-years of follow-up (PYFU)] was re-calculated based on number of ALS cases, duration of case ascertainment and study population extracted from each article. Direct standardizations were performed on age and sex on the US 2010 populations.25,26 This reference population was chosen for congruence with usual practices in this field because this is the most frequently used standard population in ALS. To allow homogeneity of reports, we did not exclude any part of the population (when authors considered only subjects older than 15 or 18 years of age, we re-included the youngest subjects in the population under study); 95% confidence intervals (95% CI) were calculated based on the Poisson distribution.
Meta-analysis was conducted and forest plots were obtained.27 Stratification by subcontinents was performed. Pooled incidence rates were calculated. Weights were based on the precision of the incidence estimates for each study, i.e. the inverse of their standard error assuming a Poisson distribution. The I2 value was also calculated.28 Because the heterogeneity was statistically significant, random effects models were used.
Random effects meta-regression with the DerSimonian and Laird method29,30 was used to assess sources of heterogeneity. We used sub-continents as the study level covariate candidate to be the most important source of heterogeneity. The referent sub-continent was the one with the highest number of articles. We considered the following study level covariates as further sources of heterogeneity: (i) characteristics of the study population—life expectancy after 50 years in men and women and sex-ratio (SR) of the study population; and (ii) methods—study design (prospective/retrospective), diagnostic criteria [clinical assessment vs El Escorial original (EEDC)31 or revised (EEDC-R) classification],32 duration of the study period, number of PYFU and period of study. Bubble plots were produced for continuous study-level covariates. R-squared of the models were given. Analyses were done using the statistical software Stata v11.1 (Stata Corporation, College Station, TX, USA).
Results
Included studies
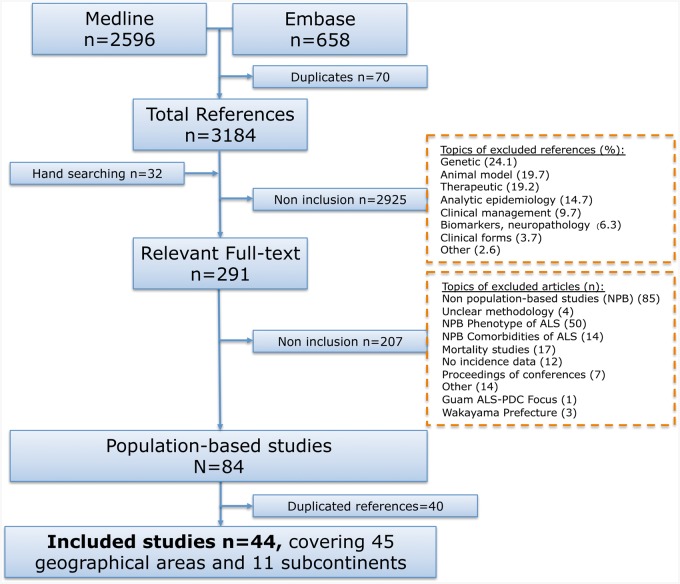
Of 3254 articles identified in the literature search, 70 were duplicates. After screening (title, abstract), 291 full-text articles were considered. After a comprehensive examination of the full texts, 44 articleswere finally included, covering 45 geographical areas and 11 sub-continents3,4,6,8–14,33–36 (Figure 1, Table 1). Types of sources used for case ascertainment are given for each included study; 40 other papers were considered as duplicate material because they described an investigation performed in the same population or in a subset of a population already included.67–106 Reasons for non-inclusion of articles are described in the flowchart (Figure 1).
Figure 1.
Flow chart eligibility criteria of articles on ALS incidence.
Geographical coverage
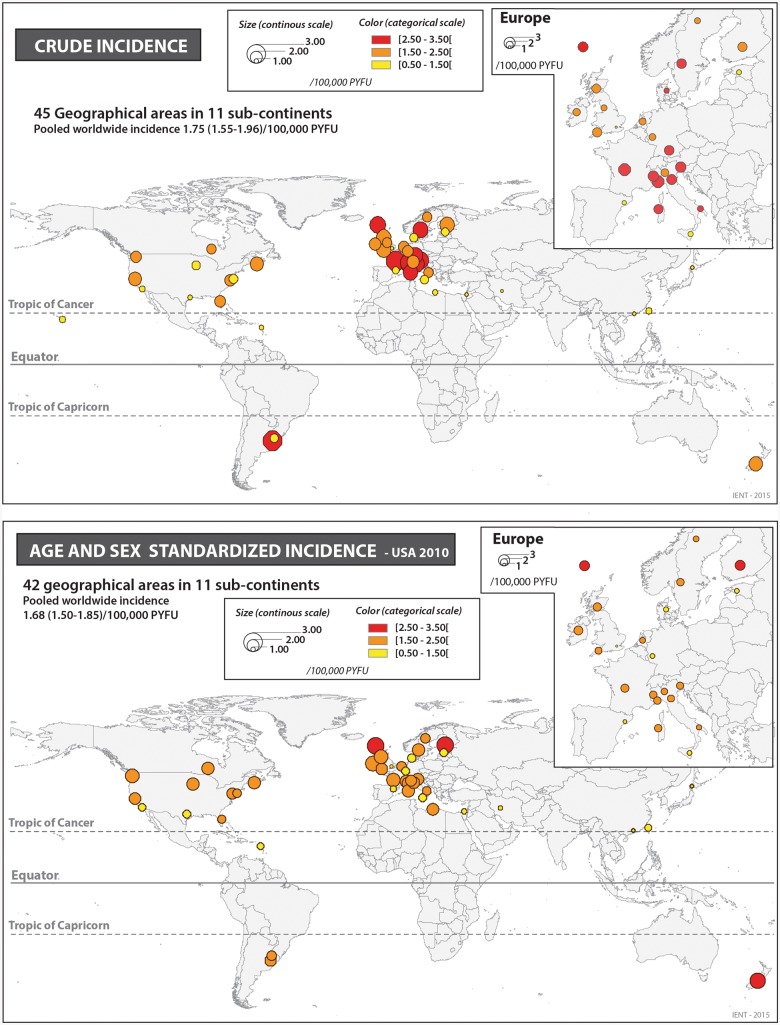
Of the 45 geographical areas investigated (Figure 2), 24 (53.3%) were from Europe: [11 in North Europe, 4 in West Europe, 9 in South Europe (8 from Italy)], and 14 (31.1%) from the American continent: 10 in North America (Canada and the USA), 2 in South America (Uruguay and Argentina), 1 in Hawaii and 1 in the Caribbean (Guadeloupe Island). East Asia was represented by three studies (6.6%). South Asia was represented by only one study (Iran), as was the case for West Asia (Israel), North Africa (Libya) and Oceania (New Zealand).
Figure 2.
Distribution of ALS worldwide: crude incidence and age- and sex-standardized incidence on USA 2010 population.
Crude incidence
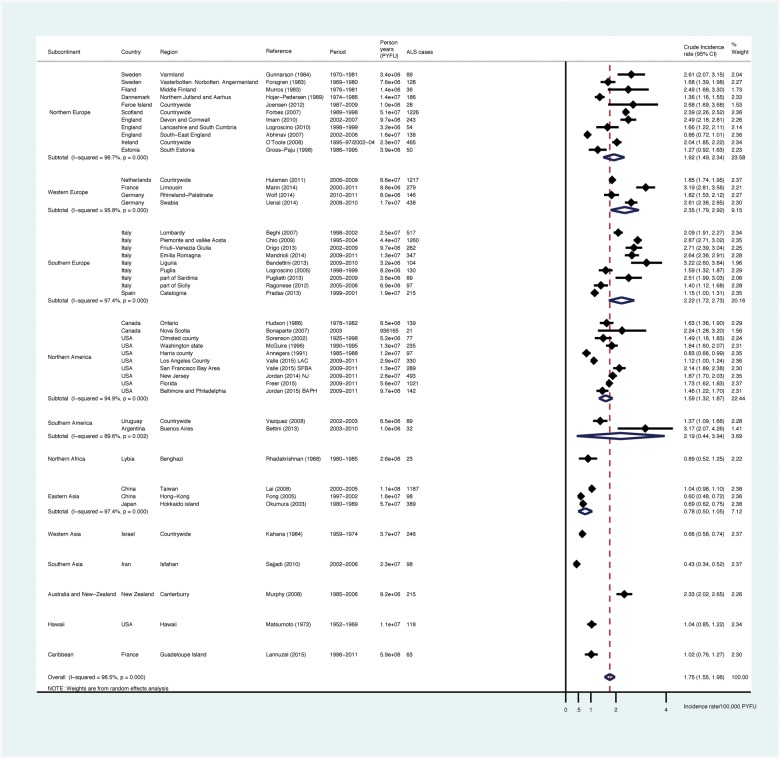
A total of 13 146 ALS cases and 825 million PYFU were considered. The overall pooled worldwide crude incidence of ALS was at 1.75 (1.55-1.96)/100 000 PYFU (Figure 3, Table 2), 2.03 (1.79-2.37) in men and 1.45 (1.25-1.64) in women. Whereas pooled incidence from European sub-continents ranged from 1.92 (1.49-2.34) in North Europe, number of studies = 11) to 2.22 (1.72-2.73) in South Europe (n = 9) and 2.35 (1.79-2.92) in West Europe (n = 4) per 100 000 PYFU, incidence was at 1.59 (1.32-1.87) for North America (n = 10) and 0.78 (0.50-1.05) for East Asia (n = 3). Incidence rates from studies performed in South America varied between 1.37 and 3.17/100 000 PYFU, leading to a pooled estimate of 2.19 (0.44-3.94)/100,000 PYFU. Among studies from the Caribbean (Guadeloupe Island), North Africa (Libya), West Asia (Israel) and South Asia (Iran), ALS incidence appeared remarkably low [1.02 (0.76-1.27), 0.89 (0.52-1.25), 0.66 (0.58-0.74) and 0.43 (0.34-0.52), respectively]. Pooled crude incidence for populations of European origin (Europe, North America and New Zealand) was at 1.96 (1.76-2.17)/100 000. There was heterogeneity in ALS crude incidence (Table 2, first column) in East Asia, South Asia (Iran) and Israel as compared with North Europe (P = 0.006, 0.02 and 0.049, respectively).
Figure 3.
Meta-analysis: forests plots and pooled estimates for ALS crude incidence by sub-continents.
Table 2.
Meta-regression of ALS crude and standardized incidence (2010 USA population)
| Study level covariates | Cruce incidence |
Standardized incidence (2010 USA population) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence rate or intercepta | Coefficientb | P-value | R2 | Incidence rate or intercepta | Coefficientb | P-value | R2 | ||
| Subcontinent | 37.8 | 32.2 | |||||||
| North Europe (Reference, n = 11) | 1.92 | 1.89 | |||||||
| West Europe (n = 4) | 2.35 | 0.21 | 1.71 | 0.68 | |||||
| South Europe (n = 9) | 2.22 | 0.27 | 1.75 | 0.66 | |||||
| North America (n = 10) | 1.59 | 0.27 | 1.79 | 0.84 | |||||
| South America (n = 2) | 2.19 | 0.85 | 1.59 | 0.64 | |||||
| Hawaii (n = 1) | 1.04 | 0.17 | – | – | |||||
| Carribean (n = 1) | 1.02 | 0.16 | 1.15 | 0.16 | |||||
| North Africa (n = 1) | 0.89 | 0.11 | 2.03 | 0.77 | |||||
| East Asia (n = 3) | 0.78 | 0.006 | 0.83 | 0.001 | |||||
| West Asia (n = 1) | 0.66 | 0.049 | 0.94 | 0.057 | |||||
| South Asia (n = 1) | 0.43 | 0.02 | 0.73 | 0.02 | |||||
| Oceania (n = 1) | 2.33 | 0.50 | 2.56 | 0.15 | |||||
| Design | Prospective (n = 26) | 1.81 | 0.40 | 0.0 | 1.75 | 0.43 | 0.0 | ||
| Retrospective (n = 19) | 1.70 | 1.61 | |||||||
| Diagnostic method | El Escorial (n = 32) | 1.93 | 0.018 | 11.4 | 1.71 | 0.51 | 0.9 | ||
| Neurologist only (n = 13) | 1.29 | 1.55 | |||||||
| Mid-point study period | < 1990 (n = 11) | 1.35 | 7.3 | 1.60 | 0.0 | ||||
| 1990-00 (n = 12) | 1.76 | 0.19 | 1.71 | 0.71 | |||||
| > = 2000 (n = 22) | 1.96 | 0.03 | 1.68 | 0.71 | |||||
| Life expectancy after 50 years | In men (/5 years) | −0.71 | 0.45 | 0.057 | 7.8 | 1.74 | −0.01 | 0.95 | 0.0 |
| Life expectancy after 50 years | In women (/5 years) | −2.35 | 0.64 | 0.005 | 18.5 | 1.11 | 0.09 | 0.63 | 0.0 |
| Sex ratio of the underlying population | (/1 unit) | 5.43 | −3.82 | 0.04 | 8.1 | 1.29 | 0.41 | 0.80 | 0.0 |
| Duration of the study | (/5 years) | 1.77 | −0.010 | 0.85 | 0.0 | 1.59 | 0.05 | 0.23 | 1.2 |
| Person-years of follow-up | (/1000000) | 1.89 | −0.007 | 0.17 | 1.4 | 1.80 | −0.006 | 0.09 | 3.5 |
Hawaii included in North American subcontinent for United Nations is presented separately in this table. p values less than 0.05 appear bold.
Incidence rates are calculated for categorical study-levelcovariates, intercept is given for continuous study-level covariate.
Coefficients are calculated for continuous study-level covariates. P-value refers (i) to difference between reference categories and other categories or (ii) to significance of coefficient.
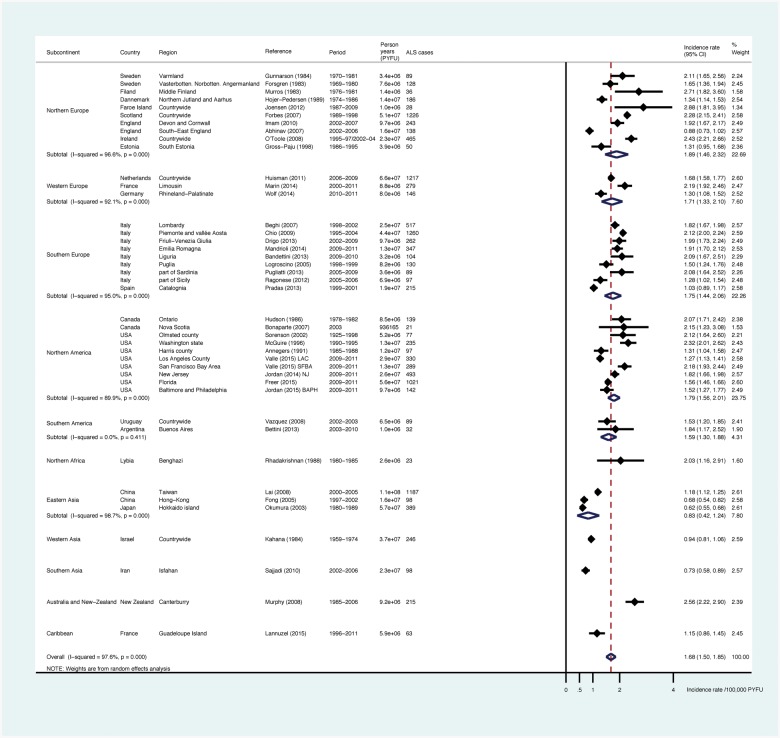
Age- and sex-standardized incidence
We were able to perform a standardization of ALS incidence rates on US 2010 population for 42 geographical areas: for 41 areas on age and sex3,4,6,8–14,22,33–64,66–101,107–111 and, on age only, in one area.65 For the three remaining areas (Swabia, Lancashire, Hawaii) this was not possible; in these latter cases, we did not have access to the number of ALS cases by age and sex groups.9,39,60 Number of cases or incidence rates of ALS by sex and age groups were available (present in the article or in supplementary material or provided on request by authors) for 36 areas.6,8,11–14,35–38,40–51,54–58,61,63,63,66,100,108,112 For the remaining six articles, a graphical reading of the figure of age-specific incidence was performed to obtain rates.4,33,35,52,53,59 The demographic structure of the study population was fully available in 25 cases,6,8,10–14,37,38,40,41,43–50,55,57,58,61,64,65 and in four additional studies the number of subjects for the youngest age groups (0-18) was re-calculated based on national census data.3,42,51,66 For the other studies (n = 12), the structure of the population under study was retrieved from national demographic databases.4,33–36,52–54,56,59,62,63
The pooled worldwide US 2010-standardized incidence of ALS was 1.68 (1.50-1.85)/100 000 PYFU (Figure 4, Table 2), 1.96 (1.75-2.18) for men and 1.39 (1.21-1.56) for women, giving a standardized sex ratio of 1.41. As compared with crude incidence, standardization on the US population reduced the pooled estimates for Europe [1.89 (1.46-2.32), 1.71 (1.33-2.10) and 1.75 (1.44-2.06)/100000 PYFU, respectively in North, South and West Europe]. Conversely, there was an increase in the point estimates and pooled estimates for North America [1.79 (1.56-2.01)], Oceania [New-Zealand: 2.56 (2.22-2.90)], North Africa [Libya: 2.03 (1.16-2.91)], East Asia [0.83 (0.42-1.24)], South Asia [Iran: 0.73 (0.58-0.89)] and the Caribbean [Guadeloupe Island: 1.15 (0.86-1.45)]. The point estimate of standardized incidence in Southern America was at 1.59 (1.30-1.88)/100 000 PYFU and there was no heterogeneity between the two studies published in this area (P = 0.41). We identified heterogeneity in standardized ALS incidence in East Asia and South Asia as compared with North Europe (P-value 0.001 and 0.02, respectively). Pooled ALS standardized incidence for populations of European origin (Europe, North America and New Zealand) was at 1.81 (1.66-1.97)/100 000 PYFU.
Figure 4.
Meta-analysis: forests plots and pooled estimates for ALS age- and sex-standardized incidence on USA 2010 population, by subcontinents.
Robustness analysis
An additional analysis was performed to explore sources of heterogeneity related to (i) the characteristics of the study population and (ii) the study methodology.
Crude incidence.
Using univariable meta-regression, we identified heterogeneity (P = 0.018) in ALS crude incidence between studies that used El Escorial criteria (original or revised version) for case definition [incidence estimate: 1.93 (1.65-2.20)/100 000 PYFU] as compared with the neurologist’s overall judgment [incidence estimate: 1.29 (1.09-1.49)]. Heterogeneity was also identified depending on the time period of the study, with higher crude incidence rates for a more recent period of investigation (Table 2, eFigure 1). An ‘association’ between crude incidence and life expectancy after 50 years in women (P = 0.005) was identified (eFigure 1 presents the bubble plots of these relations, available as Supplementary data at IJE online).
Standardized incidence.
We did not identify any heterogeneity in standardized incidence in relation to (i) characteristics of the study population or (ii) study methodology. Specifically, we did not identify relevant time trends in standardized ALS incidence after controlling for age and sex (eFigure 2, P = 0.95, available as Supplementary data at IJE online).
Discussion
This review is the first to report pooled estimates of ALS crude and USA-standardized incidence of worldwide population-based studies. It emphasizes the heterogeneity between European ALS incidence and incidence from East Asia (China, Japan), South Asia (Iran) and West Asia (Israel). Conversely, there is homogeneity in incidence among populations from Europe, North America and New Zealand.
Input of mixed populations
It is not possible in our study to disentangle the real impact of ancestral origin from lifestyle or environmental factors, because they are highly associated. In this regard, data from mixed populations might give useful information. Such studies allow comparison of incidence rates between different population subgroups that share, at least partially, the same environment. Table 3 presents ALS incidence data estimated in mixed populations (England,104 USA,55,56,113 Hawaii60 Israel,65 ) or estimated with homogeneous methods in different geographical areas (USA, Japan62).
Table 3.
ALS incidence in mixed populations
| Continent | Subcontinent | Reference | Period | Country | Diagnostic method | Incident cases by ethnic group, n (%) | Crude incidence (95% CI) | Standardized incidence (95 CI%) | Reference population for standardization |
|---|---|---|---|---|---|---|---|---|---|
| Europe | North Europe | Rojas-Garcia (2012) | 2002-08 | England | EEDC | 88 | |||
| 74 European origin (84.0) | 1.97 (1.55-2.48) | ||||||||
| 14 African origin (16.0) | 1.35 (0.72-2.30) | ||||||||
| America | North America | McGuire (1996) | 1990-95 | USA | Neurologist | 235 | 2.11 (1.27-2.93) m 1.87 (1.08-2.66) w | USA 1990 | |
| 225 White (95.7) | |||||||||
| 10 non-White (4.3) | 0.74 (0.00-1.96) m 0.53 (0.00-1.91) w | ||||||||
| America | North America | Annegers (1991) | 1885-1998 | USA | Neurologist | 97 | 1.27 (0.95-1.69) m 1.03 (0.75-1.38) w | USA 1970 | |
| White | 1.36 (0.96-1.87) m 1.25 (0.88-1.72) w | ||||||||
| Black | 1.10 (0.48-2.17) m 0.70 (0.28-1.44) w | ||||||||
| Hispanic | 1.27 (0.41-2.96) m 0.10 (0.002-0.46) w | ||||||||
| America | North America | Rechtman (2015) | 2009-11 | USA | EEDC | 3819 | USA 2010 | ||
| 2896 White (75.8) | 1.79 | 1.48 (1.42-1.53) | |||||||
| 319 African American (8.4) | 0.80 | 0.89 (0.79-0.99) | |||||||
| 127 Asian (3.3) | 0.76 | 0.78 (0.64-0.92) | |||||||
| 477 Not reported (12.5) | |||||||||
| 2957 Non-Hispanic (77.4) | 1.65 | 1.36 (1.31-1.41) | |||||||
| 407 Hispanic (10.6) | 0.57 | 0.84 (0.75-0.92) | |||||||
| 455 Not reported (12.6) | |||||||||
| America | North America | Matsumoto (1972) | 1952-69 | Hawaii | Neurologist | 118 | Hawai 1970 | ||
| 23 Caucasian (19.5) | 0.63 | 0.82 | |||||||
| 31 Japanese (26.4) | 0.85 | 0.86 | |||||||
| 42 Filipino (35.6) | 3.38 | 1.56 | |||||||
| 7 Part-Hawaian (5.9) | 0.82 | 1.21 | |||||||
| 5 Hawaian (4.2) | |||||||||
| 7 Chinese (5.9) | |||||||||
| 2 Korean (1.7) | |||||||||
| 1 Samoan (0.8) | |||||||||
| Asia | West Asia | Kahana (1976) | 1960-70 | Israel | Neurologist | 142 | |||
| 96 European (67.0) | 0.72 | USA 1970 | |||||||
| 38 African Asian (26.8) | 0.60 | ||||||||
| 7 Israeli (4.9) | 0.65 | ||||||||
| 1 origin unknown (0.7) | |||||||||
| Okumura (2003) | Neurologist | 401 | |||||||
| America | North America | 1952-91 | USA | 46 USA (Rochester) (11.5) | 2.30 | ||||
| Asia | East Asia | 1980-89 | Japan | 355 Japan (Hokkaido) (88.5) | 0.60 | ||||
M, men; na, not available; w, women; EEDC: El Escorial Diagnosis Criteria; na: non available; 95%CI: 95% confidence interval.
A recent initiative, using a population-based design, investigated the racial variations in ALS incidence in three states (Florida, New Jersey and Texas) and eight metropolitan areas (Atlanta, Baltimore, Chicago, Detroit, Las Vegas, Los Angeles, Philadelphia and San Francisco) of the USA.113,114 Based on 3819 incident cases identified during the study period (2009-11), higher crude and standardized ALS incidences were found in Whites (USA-standardized incidence 1.48/100 000 PYFU) as compared with African Americans (0.89) and with Asians (0.78). Based on ethnic data, a difference was identified between non-Hispanics (1.36) and Hispanics (0.84). These data are in agreement with most previous reports on this topic, which showed in the USA and in the UK a constantly lower point estimate in non-Whites as compared with Whites.55,56,104 The consistency of the findings would exclude under-ascertainment of cases as a possible explanation of the differences. Also, a lower access to health systems for some minorities with lower socioeconomic status in the USA needs to be discussed; Whites have traditionally a better access to health care.115 In this regard in the USA, ALS Asians are less likely to have a federal payer (e.g. 55.2%) as compared with White and African Americans (63.3% and 65.2%, respectively).113 The use of self-pay was also higher in Asians.
Within a given population, a difference in life expectancy between races or ethnic groups might also be implicated in differences of ALS rates. Indeed, a higher number of old people in some groups (the pool of subjects most likely to develop ALS) will lead to higher rates if these are not standardized on race/ethnicity. For example, Chang et al. showed that healthy life expectancy in non-Hispanic Whites was 2.6 years higher than in Hispanics and 7.8 years higher than in non-Hispanic Blacks.116
In our review, it was not possible to accurately assess ALS incidence in Africa due to lack of data. Crude incidence in Libya (North Africa) was low [0.89 (0.52-1.25)/100 000], but after adjustment [2.03 (1.16-2.91)/100 000] it was in the same range as data from Europe or North America. Other data from the African continent are needed to understand more about ALS in this continent.117 Our results comparing incidence data from Europe and from East Asia are in favour of a lower incidence in this latter geographical area. As Eastern Asian studies included in our review are from high-resource areas (Hong Kong, Taiwan, Japan), an under-ascertainment of ALS cases seems unlikely. The report by Okumura et al.,62 comparing incidence between Rochester (USA) and Hokkaido Island (Japan), was also in agreement with ours: i.e. lower incidence in East Asia as compared with Western populations.
Additional clues to disentangle genetic and environmental factors can come from studies on migrants. Chio et al. 199979 raised the issue of ALS incidence among migrants from South Italy who lived in North Italy. Authors identified, in comparison with people born in Piedmont, an increased risk of ALS in migrants from Puglia (South-East Italy) as well as in men migrating from outside Italy. This observation was attributed by the authors to an interaction between environmental and genetic factors or selective migration (people who migrated were of lower socioeconomic status in their native area and most of them were farmers: two characteristics supposed to be associated with an increase in ALS risk).
A clue to a better understanding of ALS incidence variation
The link between the age structure of the population and ALS incidence is well known, as age-specific incidence shows usually a progressive rise with age, with a peak between 65 and 75 years of age followed by a decrease before in men and then in women.118 We evaluated this link through the meta-regression of crude incidence, using life expectancy after 50 years of age as a proxy for the pool of subjects at risk to develop ALS. This link was identified for crude incidence (P-value 0.005 for life expectancy in women). After standardization, this association was not confirmed.
In agreement with others,2 we did not identify a correlation between incidence and duration of study, even when considering the total PYFU. In contrast with others, we did not identify different incident rates according to the modalities of data collection (prospective or retrospective). Previous reviews identified lower incidence in retrospective studies.1,2 However, those reviews considered not only population-based studies with multi-source cases ascertainment but also clinic-based data.
Our data show that, with time, there was an increase in crude incidence but not in standardized incidence. This pattern could be explained, at least partially, by the evolution of the age structure of the populations at risk (which aged especially in the North American continent and in Europe in the past 50 years).
A clue for health care organization
An accurate calculation of synthesized indicators of ALS incidence for various geographical areas allows the estimation of the number of new cases by year with good precision. As the burden of the disease on personal, societal and economic grounds is high, our study can give a better outline of the needs for research and health care organization. Using populations25 and pooled crude incidences estimates, the numbers of incident cases by year are expected to be around 5500 in the North American continent, 9900 in Europe (summing North, South and West Europe) and 12 300 in East Asia.
Strengths
The main strength of this work relies on the material that was used (population-based studies using multiple sources of case ascertainment). The population-based approach has been consistently shown to be the best suited to describe the entire spectrum of the ALS incidence and phenotype.118,119 The literature search was broad, without limitations in terms of dates of publication or language, and exhaustive. The ability to perform systematic standardization of ALS incidence with such a wide panel of data, in line with guidelines on meta-analysis of observational studies17 and recommendations regarding the meta-analysis of incidence studies in neuroepidemiology,18 is without antecedents. We assessed the impact of study methodology and other characteristics (including markers of study quality, i.e diagnostic method, design) and confirmed the robustness of our results.
Limitations
First, as previously mentioned,2 the main body of literature on ALS epidemiology is large but limited geographically. This is especially true for population-based investigations. Most research has been conducted in Europe (53.3% of studies) or more globally in populations of Caucasian origin (77.7%, including Europe, North America, New Zealand).
Second, some methodological requirements chosen during the preparation of the study protocol might have influenced our results. The inclusion in four cases, for homogeneity purposes, of the population aged lower than 15 or 18 years might have diluted the ALS rates.3,42,51,66 The graphical reading of ALS age-specific rates in some cases4,33,34,52,53,59 might have also influenced the results. Nevertheless: (i) the number of cases given in the article and the total number of cases calculated using age-specific rates were highly concordant and (ii) we were able to verify an excellent agreement between our graphical reading and rates that were available in the supplementary web-only material of one study.42
Third, another important limitation is the variability of study designs, populations at risk and settings. We did our best to exclude studies of low quality and we explored sources of heterogeneity related to the design, diagnostic method, time period, population size and life expectancy, and study duration. Nevertheless, we cannot exclude the possibility that some differences are due at least in part to the lack of comparability of the reports included in this review. Differences in health care system organization and access to health care could be also implicated. A differential referral even within the population-based setting cannot be excluded for some categories, i.e. elderly, women and minorities.119,120
Fourth, the criteria for the diagnosis of ALS (clinical assessment versus El Escorial criteria), the evolution with time of El Escorial criteria (original vs revised) and the El Escorial categories included might be implicated in the heterogeneity of ALS incidence. The real impact of these methodological differences is difficult to estimate. For example, studies based on the original EEDC included all categories of ALS (Definite, Probable, Possible, Suspected), n = 12,3,39,40,42–45,48,51,53,66 or excluded cases who, during follow-up, remained suspected ALS (n = 4)4,37,38,54 or suspected/possible ALS (n = 2).6,59 Conversely, most papers that used the revised version of EEDC included all types of ALS (Definite, Probable, Probable laboratory supported, Possible) (n = 13),8–14,46,47,50,57,64 and only one excluded cases with possible ALS during follow-up (n = 1).121 However, when looking at standardized rates (i.e when controlling for age and sex distribution of the underlying population), ALS heterogeneity was not explained by the diagnosis (P = 0.51). This might suggest that the key driver is the modification in populations demographics, which is related to time.
Fifth, ALS rates were standardized on age and sex only, therefore results are susceptible to unmeasured confounding factors which may vary by subcontinent or country.
Sixth, it would have been of major interest to perform subgroup analyses based on ethnic groups. With the exception of recent data from the US National ALS registry initiative,113 accurate information on ethnic groups and, consequently, number of cases by ethnic group among ALS patients were not given in the original articles.
Seventh, we attempted to consider subcontinent as a proxy for ancestral origin of the population, but subcontinent does not represent individual population ancestral origin or ethnicity. Also, within a given subcontinent, the ancestral origin of subjects is not homogeneous. Human population groups and level of admixture vary within a given country.
A clue for future research
This work shows the heterogeneity of ALS worldwide incidence between subcontinents. This heterogeneity could be related to the ancestral origin of the populations. Nevertheless, we cannot exclude that some differences are related to differences in environmental factors in connection with the growing evidence that aetiology relies on multifactorial effects resulting from the combination of environmental and genetic factors.122
As is the case for Alzheimer disease,123,124 ALS phenotype and natural history also vary with geographical area. Marin et al. showed that a major explanatory variable for the variability of ALS phenotype in population-based studies is subcontinent.125 Some markers of ALS phenotype have homogeneous distribution in Western countries (male:female sex ratio, mean age at onset or at time diagnosis) but their distributions in other subcontinents are remarkably different. Other markers (familial ALS, bulbar onset) present variations in European and in other subcontinents. As a consequence, ALS outcome markedly varies, with a median survival time since onset ranging from 24 months (North Europe) to 48 months (South Asia).
Considering this important issue, researchers now need to consider the organization of a wide international consortium to perform with homogeneous methodology an investigation of the link between ancestry, environment and ALS incidence and phenotype. Such an initiative might lead to important advances in the knowledge of the mechanisms of ALS.
Supplementary data
Supplementary data are available at IJE online.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
Supplementary Material
Ackowledgements
We thank the following main authors or co-authors of population-based articles who answered to our solicitation and for the useful material for incidence standardization or phenotype characterization that they were able to provide: Kari Murros, Poul Joensen, Raeburn Forbes, Robert Swingler (the Scottish MND Register is funded by MND Scotland and supported by the Anne Rowling Regenerative Neurology Clinic), Ibrahim Imam, James Rooney, Albert Ludolph, Gabriele Nagel, Marwa Elamin, Orla Hardiman, Mark Huisman, Joachim Wolf, Adriano Chio, Federica Pisa, Jessica Mandrioli, Monica Bandettini, Stefano Zocollela, Maura Pugliatti, Leslie Parish, Paolo Ragonese, Valerie Mc Guire, Will Longstreth, Eric J. Sorenson, Farrah Mateen, James D. Bonaparte, Cristina Vazquez, Carlos Ketzoian, Kurupath Radhakrishnan, Chien-Hsu Lai, Chung Yan G Fong, Hitoshi Okumura, Tameko Kihira, Bruce Taylor and A Lannuzel. We thank Heather Jordan and Jhaqueline Valle for providing data to calculate US incidence. The data came from surveillance projects funded by the Agency for Toxic Substances and Disease Registry's (ATSDR) National ALS Registry [www.cdc.gov/als] (contract #200-2009-32577 and contract #200-2010-F-36614). We also thank Walter Rocca and Brandon R. Grossardt for the detailed data on Olmsted county population with which they provided us, and Hidenao Sasaki, Robert Miller and Eric Denys as contact persons. We thank Vanna Pistotti for her advice during the literature search, as well as Mineko Terao, Lorenzo Moja and Claudio Pelucchi for their help and comments on the manuscript. We thank Limoges teaching hospital for its grant initiative for mobility.
Conflict of interest: Authors have no conflict of interest to disclose.
References
- 1. Cronin S, Hardiman O, Traynor BJ. Ethnic variation in the incidence of ALS: a systematic review. Neurology 2007;68: 1002-07. [DOI] [PubMed] [Google Scholar]
- 2. Chio, Logroscino G, Traynor BJ. et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology 2013;41:118-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joensen P. Incidence of amyotrophic lateral sclerosis in the Faroe Islands. Acta Neurol Scand 2012;126:62-66. [DOI] [PubMed] [Google Scholar]
- 4. Murphy M, Quinn S, Young J, Parkin P, Taylor B.. Increasing incidence of ALS in Canterbury, New Zealand: a 22-year study. Neurology 2008;71:1889-95. [DOI] [PubMed] [Google Scholar]
- 5. Gordon PH, Mehal JM, Holman RC, Rowland LP, Rowland AS, Cheek JE.. Incidence of amyotrophic lateral sclerosis among American Indians and Alaska natives. JAMA Neurol 2013;70:476-80. [DOI] [PubMed] [Google Scholar]
- 6. Sajjadi M, Etemadifar M, Nemati A. et al. Epidemiology of amyotrophic lateral sclerosis in Isfahan, Iran. Eur J Neurol 2010;17:984-89. [DOI] [PubMed] [Google Scholar]
- 7. Bucheli M, Andino A, Montalvo M. et al. Amyotrophic lateral sclerosis: Analysis of ALS cases in a predominantly admixed population of Ecuador. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:106-13. [DOI] [PubMed] [Google Scholar]
- 8. Marin B, Hamidou B, Couratier P. et al. Population-based epidemiology of amyotrophic lateral sclerosis (ALS) in an ageing Europe - the French register of ALS in Limousin (FRALim register). Eur J Neurol 2014;21:1292-300. [DOI] [PubMed] [Google Scholar]
- 9. Uenal H, Rosenbohm A, Kufeldt J. et al. ALS registry study group. Incidence and geographical variation of amyotrophic lateral sclerosis (ALS) in Southern Germany - completeness of the ALS registry Swabia. PLoS One 2014;9:e93932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolf J, Wohrle JC, Palm F. et al. Incidence of amyotrophic lateral sclerosis in Rhineland-Palatinate, Germany. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:269-74. [DOI] [PubMed] [Google Scholar]
- 11. Freer C, Hylton T, Jordan HM, Kaye WE, Singh S, Huang Y.. Results of Florida's Amyotrophic Lateral Sclerosis Surveillance Project, 2009-2011. BMJ Open 2015;5:e007359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jordan H, Rechtman L, Wagner L, Kaye WE.. Amyotrophic lateral sclerosis surveillance in Baltimore and Philadelphia. Muscle Nerve 2015;51:815-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lannuzel A, Mecharles S, Tressieres B. et al. Clinical varieties and epidemiological aspects of amyotrophic lateral sclerosis in the Caribbean island of Guadeloupe: A new focus of ALS associated with Parkinsonism. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:216-23. [DOI] [PubMed] [Google Scholar]
- 14. Valle J,, oberts E, aulukonis S, Collins N, English P, Kaye W. Epidemiology and surveillance of amyotrophic lateral sclerosis in two large metropolitan areas in California. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marin B, Couratier P, Preux PM, Logroscino G.. Can mortality data be used to estimate amyotrophic lateral sclerosis incidence? Neuroepidemiology 2011;36:29-38. [DOI] [PubMed] [Google Scholar]
- 16. Zaldivar T, Gutierrez J, Lara G, Carbonara M, Logroscino G, Hardiman O.. Reduced frequency of ALS in an ethnically mixed population: a population-based mortality study. Neurology 2009;72:1640-45. [DOI] [PubMed] [Google Scholar]
- 17. Stroup DF, Berlin JA, Morton SC. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [DOI] [PubMed] [Google Scholar]
- 18. Fiest KM, Pringsheim T, Patten SB, Svenson LW, Jette N.. The role of systematic reviews and meta-analyses of incidence and prevalence studies in neuroepidemiology. Neuroepidemiology 2014;42:16-24. [DOI] [PubMed] [Google Scholar]
- 19. Mitsumoto H, Chad AD, Pioro EP, Amyotrophic lateral sclerosis. In: Amyotrophic Lateral Sclerosis. Philadelphia, PA: Davis, 1998. [Google Scholar]
- 20. Szklo M. Population-based cohort studies. Epidemiol Rev. 1998;20:81-90. [DOI] [PubMed] [Google Scholar]
- 21. United Nations Statistics Division. Composition of Macro Geographical (Continental) Regions, Geographical Sub-Regions, and Selected Economic and Other Groupings https://unstats.un.org/unsd/methods/m49/m49regin.htm (9 January 2015, date last accessed).
- 22. Plato CC, Garruto RM, Galasko D. et al. Amyotrophic lateral sclerosis and parkinsonism-dementia complex of Guam: changing incidence rates during the past 60 years. Am J Epidemiol 2003;157: 149-57. [DOI] [PubMed] [Google Scholar]
- 23. United Nations. Demographic Yearbook.https://unstats.un.org/unsd/demographic/products/dyb/dyb2.html (25 June 2015, date last accessed).
- 24. United States Census Bureau. National Estimates by Age, Sex, Race: 1900-1979 http://www.census.gov/popest/data/national/asrh/pre-1980/PE-11.html (9 January 2015, date last accessed).
- 25. United States Census Bureau. International Data Base. 2010. http://www.census.gov/population/international/data/idb/informationGateway.php (25 June, 2015, date last accessed).
- 26. United States Census Bureau. 2010 Census Data Products: United States 2010. https://www.census.gov/population/www/cen2010/glance/(9 January 2015, date last accessed).
- 27. Sterne J, Bradburn M, Egger M. Meta-analysis in Stata, in Systematic Reviews in Health Care: Meta-Analysis in Context. BMJ Publishing 2008:347-69. [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson SG, Higgins JP.. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559-73. [DOI] [PubMed] [Google Scholar]
- 30. Egger M, Davey SG, Altman DG. Systematic Reviews in Health Care: Meta-Analysis in Context. Chichester, UK: Wiley, 2008. [Google Scholar]
- 31. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases, and the El Escorial Clinical Limits of Amyotrophic Lateral Sclerosis’ workshop contributors. J Neurol Sci 1994;124(Suppl):96-107. [DOI] [PubMed] [Google Scholar]
- 32. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293-99. [DOI] [PubMed] [Google Scholar]
- 33. Gunnarsson LG, Palm R. Motor neuron disease and heavy manual labor: an epidemiologic survey of Varmland County, Sweden. Neuroepidemiology 1984;3: 195-206. [Google Scholar]
- 34. Forsgren L, Almay BGL, Holmgren G, Wall S.. Epidemiology of motor neuron disease in northern Sweden. Acta Neurol Scand.1983;68: 20-29. [DOI] [PubMed] [Google Scholar]
- 35. Murros K, Fogelholm R.. Amyotrophic lateral sclerosis in Middle-Finland: an epidemiological study. Acta Neurol Scand 1983;67:41-47. [DOI] [PubMed] [Google Scholar]
- 36. Højer-Pedersen E, Christensen PB, Jensen NB.. Incidence and prevalence of motor neuron disease in two Danish counties. Neuroepidemiology 1989;8:151-59. [DOI] [PubMed] [Google Scholar]
- 37. Forbes RB, Colville S, Parratt J, Swingler RJ.. The incidence of motor nueron disease in Scotland. J Neurol 2007;254:866-69. [DOI] [PubMed] [Google Scholar]
- 38. Imam I, Ball S, Wright D, Hanemann CO, Zajicek J.. The epidemiology of motor neurone disease in two counties in the southwest of England. J Neurol 2010;257:977-81. [DOI] [PubMed] [Google Scholar]
- 39. Logroscino G, Traynor BJ, Hardiman O. et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry 2010;81:385-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Toole O, Traynor BJ, Brennan P. et al. Epidemiology and clinical features of amyotrophic lateral sclerosis in Ireland between 1995 and 2004. J Neurol Neurosurg Psychiatry 2008;79:30-32. [DOI] [PubMed] [Google Scholar]
- 41. Gross-Paju K, Oopik M, Luus SM. et al. Motor neurone disease in South Estonia. Diagnosis and incidence rate. Acta Neurol Scand 1998;98:22-28. [DOI] [PubMed] [Google Scholar]
- 42. Huisman MH, de Jong SW, van Doormaal PT. et al. Population based epidemiology of amyotrophic lateral sclerosis using capture-recapture methodology. J Neurol Neurosurg Psychiatry 2011;82:1165-70. [DOI] [PubMed] [Google Scholar]
- 43. Beghi E, illul A, Micheli A, Vitelli E, Logroscino G.. Incidence of ALS in Lombardy, Italy. Neurology 2007;68:141-45. [DOI] [PubMed] [Google Scholar]
- 44. Chio A, Mora G, Calvo A, Mazzini L, Bottacchi E, Mutani R.. Epidemiology of ALS in Italy: a 10-year prospective population-based study. Neurology 2009;72:725-31. [DOI] [PubMed] [Google Scholar]
- 45. Drigo D,, Verriello L, Clagnan E. et al. The incidence of amyotrophic lateral sclerosis in Friuli Venezia Giulia, Italy, from 2002 to 2009: a retrospective population-based study. Neuroepidemiology 2013;41:54-61. [DOI] [PubMed] [Google Scholar]
- 46. Mandrioli J, Biguzzi S, Guidi C. et al. Epidemiology of amyotrophic lateral sclerosis in Emilia Romagna Region (Italy): A population based study. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:262-68. [DOI] [PubMed] [Google Scholar]
- 47. Bandettini di Poggio M, Sormani MP, Truffelli R. et al. Clinical epidemiology of ALS in Liguria, Italy. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:52-57. [DOI] [PubMed] [Google Scholar]
- 48. Logroscino G, Beghi E, Zoccolella S. et al. Incidence of amyotrophic lateral sclerosis in southern Italy: a population based study. J Neurol Neurosurg Psychiatry 2005;76:1094-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pugliatti M, Parish LD, Cossu P. et al. Amyotrophic lateral sclerosis in Sardinia, insular Italy, 1995-2009. J Neurol 2013;260:572-79. [DOI] [PubMed] [Google Scholar]
- 50. Ragonese P, Cellura E, Aridon P. et al. Incidence of amyotrophic lateral sclerosis in Sicily: A population based study. Amyotroph Lateral Scler Frontotemporal Degener 2012;13:284-87. [DOI] [PubMed] [Google Scholar]
- 51. Pradas J,, Puig T, Rojas-Garcia R, Viguera ML, Gich I, Logroscino G: and A.-C. Group. Amyotrophic lateral sclerosis in Catalonia: a population based study. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:278-83. [DOI] [PubMed] [Google Scholar]
- 52. Hudson AJ, Davenport A, Hader WJ.. The incidence of amyotrophic lateral sclerosis in Southwestern Ontario, Canada. Neurology 1986;36:1524-28. [DOI] [PubMed] [Google Scholar]
- 53. Bonaparte JP, Grant IA, Benstead TJ, Murray TJ, Smith M.. ALS incidence in Nova Scotia over a 20-year-period: a prospective study. Can J Neurol Sci 2007;34:69-73. [DOI] [PubMed] [Google Scholar]
- 54. Sorenson EJ, Stalker AP, Kurland LT, Windebank AJ.. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology 2002;59:280-82. [DOI] [PubMed] [Google Scholar]
- 55. McGuire V, Longstreth WT Jr, Koepsell TD, van Belle G.. Incidence of amyotrophic lateral sclerosis in three counties in western Washington state. Neurology 1996;47: 571-73. [DOI] [PubMed] [Google Scholar]
- 56. Annegers JF, Appel S, Lee JRJ, Perkins P.. Incidence and prevalence of amyotrophic lateral sclerosis in Harris County, Texas, 1985-1988. Arch Neurol 1991;48:589-93. [DOI] [PubMed] [Google Scholar]
- 57. Jordan H, Fagliano J, Rechtman L, Lefkowitz D, Kaye W.. Population-based surveillance of amyotrophic lateral sclerosis in New Jersey, 2009-2011. Neuroepidemiology 2014;43: 49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bettini M,, Vicens J,, Giunta DH,, Rugiero M,, Cristiano E.. Incidence and prevalence of amyotrophic lateral sclerosis in an HMO of Buenos Aires, Argentina. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:598-603. [DOI] [PubMed] [Google Scholar]
- 59. Vazquez MC, Ketzoian C, Legnani C. et al. Incidence and prevalence of amyotrophic lateral sclerosis in Uruguay: a population-based study. Neuroepidemiology 2008;30:105-11. [DOI] [PubMed] [Google Scholar]
- 60. Matsumoto N, Worth RM, Kurland LT, Okazaki H.. Epidemiologic study of amyotrophic lateral sclerosis in Hawaii. Identification of high incidence among Filipino men. Neurology 1972;22:934-40. [DOI] [PubMed] [Google Scholar]
- 61. Radhakrishnan K, Ashok PP, Sridharan R, Mousa ME.. Descriptive epidemiology of motor neuron disease in Benghazi, Libya. Neuroepidemiology 1986;5:47-54. [DOI] [PubMed] [Google Scholar]
- 62. Okumura H. Epidemiological and clinical patterns of western pacific amyotrophic lateral sclerosis (ALS) in Guam and sporadic ALS in Rochester, Minnesota, U.S.A. and Hokkaido, Japan: a comparative study. Hokkaido Igaku Zasshi 2003;78: 187-95. [PubMed] [Google Scholar]
- 63. Lai CH, Tseng HF.. Epidemiology and medical expenses of motor neuron diseases in Taiwan. Neuroepidemiology 2008;31: 159-66. [DOI] [PubMed] [Google Scholar]
- 64. Fong G.C, Cheng TS, Lam K. et al. An epidemiological study of motor neuron disease in Hong Kong. Amyotroph Lateral Scler Other Motor Neuron Disord 2005;6:164-68. [DOI] [PubMed] [Google Scholar]
- 65. Kahana E, Zilber N.. Changes in the incidence of amyotrophic lateral sclerosis in Israel. Arch Neurol 1984;41:157-60. [DOI] [PubMed] [Google Scholar]
- 66. Abhinav K, Stanton B, Johnston C. et al. Amyotrophic lateral sclerosis in South-East England: a population-based study. The South-East England register for amyotrophic lateral sclerosis (SEALS Registry). Neuroepidemiology 2007;29:44-48. [DOI] [PubMed] [Google Scholar]
- 67. Christensen PB, Hojer-Pedersen E, Jensen NB.. Survival of patients with amyotrophic lateral sclerosis in 2 Danish counties. Neurology 1990;40:600-04. [DOI] [PubMed] [Google Scholar]
- 68. Forbes RB, Colville S, Cran GW, Swingler RJ.. Unexpected decline in survival from amyotrophic lateral sclerosis/motor neurone disease. J Neurol Neurosurg Psychiatry 2004;75:1753-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chancellor AM, Slattery JM, Fraser H, Swingler RJ, Holloway SM, Warlow CP.. The prognosis of adult-onset motor neuron disease: A prospective study based on the Scottish Motor Neuron Disease Register. J Neurol 1993;240:339-46. [DOI] [PubMed] [Google Scholar]
- 70. Chancellor AM, Swingler RJ, Fraser H, Clarke JA, Warlow CP.. Utility of Scottish morbidity and mortality data for epidemiological studies of motor neuron disease. J Epidemiol Community Health 1993;47:116-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rooney J, Byrne S, Heverin M. et al. Survival Analysis of Irish amyotrophic lateral sclerosis patients diagnosed from 1995-2010. PLoS One 2013;8e74733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Phukan J, Elamin M, Bede P. et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry 2012;83:102-08. [DOI] [PubMed] [Google Scholar]
- 73. Donaghy C, Clarke J, Patterson C, Kee F, Hardiman O, Patterson V.. The epidemiology of motor neuron disease in Northern Ireland using capture-recapture methodology. Amyotroph Lateral Scler Frontotemporal Degener 2010;11: 374-78. [DOI] [PubMed] [Google Scholar]
- 74. Donaghy C, O'Toole O, Sheehan C, Kee F, Hardiman O, Patterson V.. An all-Ireland epidemiological study of MND, 2004-2005. Eur J Neurol 2009;16:148_53. [DOI] [PubMed] [Google Scholar]
- 75. Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O.. Incidence and prevalence of ALS in Ireland, 1995-1997: a population-based study. Neurology 1999;52:504-09. [DOI] [PubMed] [Google Scholar]
- 76. Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: A population-based study. Arch Neurol 2000;57:1171-76. [DOI] [PubMed] [Google Scholar]
- 77. Pupillo E, Messina P, Logroscino G, Beghi E.. Long-term survival in amyotrophic lateral sclerosis: A population-based study. Ann Neurol 2014;75:287-97. [DOI] [PubMed] [Google Scholar]
- 78. Millul A, Beghi E, Logroscino G, Micheli A, Vitelli E, Zardi A.. Survival of patients with amyotrophic lateral sclerosis in a population-based registry. Neuroepidemiology 2005;25: 114-19. [DOI] [PubMed] [Google Scholar]
- 79. Chio A, Cucatto A, Calvo A, Terreni AA, Magnani C, Schiffer D.. Amyotrophic lateral sclerosis among the migrant population to Piemonte, northwestern Italy. J Neurol 1999;246: 175-80. [DOI] [PubMed] [Google Scholar]
- 80. Chio A, Mora G, Leone M. et al. Early symptom progression rate is related to ALS outcome: a prospective population-based study. Neurology 2002;59:99-103. [DOI] [PubMed] [Google Scholar]
- 81. Chio A, Calvo A, Moglia C. et al. Phenotypic heterogeneity of amyotrophic lateral sclerosis: A population based study. J Neurol Neurosurg Psychiatry 2011;82:740-46. [DOI] [PubMed] [Google Scholar]
- 82. Piemonte and Valle d'Aosta Register for Amyotrophic Lateral Sclerosis (PARALS). Incidence of ALS in Italy: evidence for a uniform frequency in Western countries. Neurology 2001; 56:239-44. [DOI] [PubMed] [Google Scholar]
- 83. Mandrioli J, Faglioni P, Merelli E, Sola P.. The epidemiology of ALS in Modena, Italy. Neurology 2003;60:683-89. [DOI] [PubMed] [Google Scholar]
- 84. Mandrioli J, Faglioni P, Nichelli P, Sola P. Amyotrophic lateral sclerosis: Prognostic indicators of survival. Amyotroph Lateral Scler Frontotemporal Degener 2006;7:217-26. [DOI] [PubMed] [Google Scholar]
- 85. Mandrioli J, Salvi F, Sette E. et al. Amyotrophic lateral sclerosis in emilia romagna, Italy: A population based study from 2009 to 2011. the emiliaromagna register for ALS (errals). Amyotroph Lateral Scler Frontotemporal Degener 2012; 13:128. [Google Scholar]
- 86. Govoni V, Granieri E, Capone J, Manconi M, Casetta I.. Incidence of amyotrophic lateral sclerosis in the local health district of Ferrara, Italy, 1964-1998. Neuroepidemiology 2003; 22:229-34. [DOI] [PubMed] [Google Scholar]
- 87. Granieri E, Carreras M, Tola R. et al. , Motor neuron disease in the province of Ferrara, Italy, in 1964-1982. Neurology 1988;38:1604-08. [DOI] [PubMed] [Google Scholar]
- 88. Guidetti D, Bondavalli M, Sabadini R. et al. Epidemiological survey of amyotrophic lateral sclerosis in the province of Reggio Emilia, Italy: influence of environmental exposure to lead. Neuroepidemiology 1996;15:301-12. [DOI] [PubMed] [Google Scholar]
- 89. Georgoulopoulou E, Vinceti M, Bonvicini F. et al. Changing incidence and subtypes of ALS in Modena, Italy: A 10-years prospective study. Amyotroph Lateral Scler Frontotemporal Degener 2011;12:451-57. [DOI] [PubMed] [Google Scholar]
- 90. Bonvicini F, Vinceti M, Marcello N, Rodolfi R, Rinaldi M.. The epidemiology of amyotrophic lateral sclerosis in Reggio Emilia, Italy. Amyotroph Lateral Scler Frontotemporal Degener 2008;9:350-53. [DOI] [PubMed] [Google Scholar]
- 91. Zoccolella S, Beghi E, Palagano G. et al. Signs and symptoms at diagnosis of amyotrophic lateral sclerosis: a population-based study in southern Italy. Eur J Neurol 2006;13:789-92. [DOI] [PubMed] [Google Scholar]
- 92. Zoccolella S, Beghi E, Palagano G. et al. Analysis of survival and prognostic factors in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry 2008;79: 33-37. [DOI] [PubMed] [Google Scholar]
- 93. Zoccolella S, Beghi E, Palagano G. et al. Predictors of long survival in amyotrophic lateral sclerosis: a population-based study. J Neurol Sci 2008;268:28-32. [DOI] [PubMed] [Google Scholar]
- 94. Mateen FJ, Carone M, Sorenson EJ.. Patients who survive 5 years or more with ALS in Olmsted County, 1925-2004. J Neurol Neurosurg Psychiatry 2010;81:1144-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mulder DW, Howard FM Jr.. Patient resistance and prognosis in amyotrophic lateral sclerosis. Mayo Clin Proc 1976;51: 537-41. [PubMed] [Google Scholar]
- 96. Yoshida S, Mulder DW, Kurland LT, Chu CP, Okazaki H.. Follow-up study on amyotrophic lateral sclerosis in Rochester, Minn., 1925 through 1984. Neuroepidemiology 1986; 5: 61-70. [DOI] [PubMed] [Google Scholar]
- 97. del Aguila MA, Longstreth WT Jr, McGuire V, Koepsell TD, van Belle G.. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology 2003;60:813-19. [DOI] [PubMed] [Google Scholar]
- 98. Lee CT, Chiu YW, Wang KC. et al. Riluzole and prognostic factors in amyotrophic lateral sclerosis long-term and short-term survival: a population-based study of 1149 cases in Taiwan. J Epidemiol 2013;23:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fong KY, Yu YL, Chan YW. et al. Motor neuron disease in Hong Kong Chinese: epidemiology and clinical picture. Neuroepidemiology 1996;15:239-45. [DOI] [PubMed] [Google Scholar]
- 100. Gubbay SS, Kahana E, Zilber N, Cooper G, Pintov S, Leibowitz Y.. Amyotrophic lateral sclerosis. A study of its presentation and prognosis. J Neurol 1985;232:295-300. [DOI] [PubMed] [Google Scholar]
- 101. Govoni V, Cesnik E, Casetta I, Tugnoli V, Tola MR, Granieri E. Temporal trend of amyotrophic lateral sclerosis incidence in southern Europe: a population study in the health district of Ferrara, Italy. J Neurol 2012;259:1623-31. [DOI] [PubMed] [Google Scholar]
- 102. Johnston CA, Stanton BR, Turner MR. et al. Amyotrophic lateral sclerosis in an urban setting: a population based study of inner city London. J Neurol 2006;253: 1642-43. [DOI] [PubMed] [Google Scholar]
- 103. Manjaly ZR, Scott KM, Abhinav K. et al. The sex ratio in amyotrophic lateral sclerosis: A population based study. Amyotroph Lateral Scler Frontotemporal Degener 2010;11:439-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rojas-Garcia R, Scott KM, Roche JC. et al. No evidence for a large difference in ALS frequency in populations of African and European origin: a population based study in inner city London. Amyotroph Lateral Scler Frontotemporal Degener 2012;13:66-68. [DOI] [PubMed] [Google Scholar]
- 105. Scott KM, Abhinav K, Stanton BR. et al. Geographical clustering of amyotrophic lateral sclerosis in South-East England: a population study. Neuroepidemiology 2009;32:81-88. [DOI] [PubMed] [Google Scholar]
- 106. Scott KM, Abhinav K, Wijesekera L. et al. The association between ALS and population density: A population based study. Amyotroph Lateral Scler Frontotemporal Degener 2010; 11:435-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Norris F, Shepherd R, Denys E. et al. Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci 1993;118:48-55. [DOI] [PubMed] [Google Scholar]
- 108. Kihira T, Yoshida S, Hironishi M, Miwa H, Okamato K, Kondo T.. Changes in the incidence of amyotrophic lateral sclerosis in Wakayama, Japan. Amyotroph Lateral Scler Other Motor Neuron Disord 2005;6:155-63. [DOI] [PubMed] [Google Scholar]
- 109. Yoshida S, Uebayashi Y, Kihira T. et al. Epidemiology of motor neuron disease in the Kii Peninsula of Japan, 1989-1993: active or disappearing focus? J Neurol Sci 1998;155:146-55. [DOI] [PubMed] [Google Scholar]
- 110. Kihira T, Yoshida S, Kondo T. et al. An increase in ALS incidence on the Kii Peninsula, 1960-2009: a possible link to change in drinking water source. Amyotroph Lateral Scler Frontotemporal Degener 2012;13:347-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wolf J, Safer A, Wohrle JC. et al. Variability and prognostic relevance of different phenotypes in amyotrophic lateral sclerosis - Data from a population-based registry. J Neurol Sci 2014;345:164-67. [DOI] [PubMed] [Google Scholar]
- 112. Okumura H, Moriwaka F, Tashiro K. et al. [Epidemiological study of motor neuron disease in Hokkaido island - its incidence, prevalence and regional distributions - ALS Study Group]. No To Shinkei 1992;44:727-32. [PubMed] [Google Scholar]
- 113. Rechtman L,, Jordan H,, Wagner L,, Horton DK,, Kaye W.. Racial and ethnic differences among amyotrophic lateral sclerosis cases in the United States. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wagner L,, Rechtman L,, Jordan H. et al. State and metropolitan area-based amyotrophic lateral sclerosis (ALS) surveillance. Amyotroph Lateral Scler Frontotemporal Degener 2015; 17:128-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lasser KE, Himmelstein DU, Woolhandler S.. Access to care, health status, and health disparities in the United States and Canada: results of a cross-national population-based survey. Am J Public Health 2006;96:1300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chang MH, Molla MT, Truman BI, Athar H, Moonesinghe R, Yoon PW.. Differences in healthy life expectancy for the US population by sex, race/ethnicity and geographic region: 2008. J Public Health (Oxf) 2015;37:470-79. [DOI] [PubMed] [Google Scholar]
- 117. Marin B,, Kacem I,, Diagana M. et al. Juvenile and adult-onset ALS/MND among Africans: incidence, phenotype, survival: a review. Amyotroph Lateral Scler Frontotemporal Degener. 2012;13:276-83. [DOI] [PubMed] [Google Scholar]
- 118. Logroscino G, Tortelli R, Rizzo G, Marin B, Preux PM, Malaspina A.. Amyotrophic Lateral Sclerosis: An Aging-Related Disease. Curr Geriatr Rep 2015;4:142–53. [Google Scholar]
- 119. Logroscino G, Traynor BJ, Hardiman O. et al. Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry 2008; 79:6-11. [DOI] [PubMed] [Google Scholar]
- 120. Lee JR, Annegers JF, Appel SH.. Prognosis of amyotrophic lateral sclerosis and the effect of referral selection. J Neurol Sci 1995;132:207-15. [DOI] [PubMed] [Google Scholar]
- 121. Bettini M,, Gargiulo-Monachelli GM,, Rodriguez G,, Rey RC,, Peralta LM,, Sica RE.. Epidemiology of amyotrophic lateral sclerosis patients in a centre in Buenos Aires. Arq Neuropsiquiatr 2011;69:867-70. [DOI] [PubMed] [Google Scholar]
- 122. Al-Chalabi A, Hardiman O.. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 2013;9:617-28. [DOI] [PubMed] [Google Scholar]
- 123. Mehta KM, Yaffe K, Perez-Stable EJ. et al. Race/ethnic differences in AD survival in US Alzheimer's Disease Centers. Neurology 2008;70:1163-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y.. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology 2008;71:1489-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Marin B, Logroscino G, Boumediene F. et al. Clinical and demographic factors and outcome of amyotrophic lateral sclerosis in relation to population ancestral origin. Eur J Epidemiol 2016;41:229-45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.