Abstract
Objective
To examine the relative contributions of disease activity and psychological factors to self-reported symptoms and disability in children with Crohn’s Disease.
Study design
Participants (n= 127 children age 8–18 years old) completed questionnaires on symptom severity and disability, as well as psychological measures assessing anxiety, depression, pain beliefs and coping. Disease activity was measured by the Pediatric Crohn’s Disease Activity Index. Structural equation modeling was used to test the effects of disease activity and psychological factors on symptoms and disability.
Results
In the hypothesized model predicting symptoms, psychological factors (β=0.58; p <0.001) were significantly associated with disease symptoms but disease activity was not. The model for disability yielded significant associations for both psychological factors (β=0.75; p<0.001) and disease activity (β =0.61, p<0.05).
Conclusion
Crohn’s disease symptoms in children and adolescents are not only driven by disease activity. Coping, anxiety, depression and illness cognitions are important in the patient-reporting of symptom severity and disability. Physicians need to be aware that symptom self-reporting can be driven by psychological factors and may not always be simply an indicator of disease activity.
Keywords: Crohn’s Disease, Disease activity, Depression, Coping, Anxiety, Catastrophizing
Crohn’s disease is a chronic, relapsing inflammatory bowel disease (IBD). Although symptoms wax and wane with inflammation, a disease marker, the occurrence and severity of symptoms is driven by more than disease severity alone. For example, a recent meta-analysis has shown that 41% of patients with Crohn’s disease in clinical remission continued to report symptoms consistent with irritable bowel syndrome (IBS)1 such as abdominal pain, diarrhea, and bloating. In addition, Crohn’s is a disease that presents multiple challenges to the patient, such as embarrassment over symptoms, invasive procedures and impact on social functioning. These challenges may be of particular importance during adolescence, the primary age at which Crohn’s disease is first diagnosed and a stage at which socializing and fitting in with peers becomes an important developmental milestone.
According to the biopsychosocial model2, 3, symptom presentation and associated disability are likely influenced by psychological and social factors as well as disease mechanisms such as inflammation. There is some evidence for this model in IBD. Coping has been found to influence disability in youth with IBD4, 5. Research reports have been inconsistent regarding the relationship of psychological factors to disease activity in pediatric IBD, with both positive associations as well as no associations reported between anxiety/depression and Crohn’s disease activity6–13. This pattern of findings may partly be explained by methodological differences in measurement of psychological as well as disease factors. For example, studies have used various measures of disease activity such as physician ratings, patient rating, histological/radiological exams, or medication use. Similarly, the measurement of depression/anxiety has ranged from clinician assessment using structured diagnostic criteria to the self-report of symptoms such as negative affect or behavioral functioning. It also may be the case that other measures of stress or coping with the disease may be more important to disease outcomes14 than depression and anxiety. For example, adult patients with IBD have been found to employ less effective coping skills compared with controls, and ineffective coping is associated with worse disease and increased rate of relapse15. Ondersma et al10 observed that subjective symptom reporting was associated with negative affectivity but not with erythrocyte sedimentation rate in adolescents with IBD, raising the intriguing possibility that psychological factors actually may play an important role in patient symptom reporting and disability.
Direct visualization of disease activity is hampered in Crohn’s disease because it requires repeat endoscopies, and therefore the identification of disease activity is more highly dependent on laboratory markers and self-reported symptoms. However, if self-reported symptoms and disability are driven by psychological factors and are not always a sign of inflammation, unnecessary testing and treatments may occur. For example, in adults psychological factors have been found to affect hospitalization rates in IBD16. Recognition of the role of psychological factors associated with patient symptom reporting and disability in pediatric Crohn’s disease is therefore important and needed. The aim of the current study is to examine the contributions of both psychological factors and disease activity to self-reports of symptoms and disability in pediatric Crohn’s disease. We hypothesized that both psychological factors (anxiety, depression and illness cognitions) and disease activity will impact symptom self-reports and disability.
Methods
This study is a secondary data analysis of an existing dataset from a randomized controlled treatment trial for cognitive behavioral therapy versus an education/support control condition for pediatric patients with IBD4, 17, 18 (ClinicalTrials.gov number NCT00679003). Although the trial was registered in May 2008, participants were enrolled beginning in April 2008. Patients enrolled before registration were 3.9% of the final sample, and no data were analyzed in these patients before registration. Before entering the treatment trial, all patients completed baseline measurements, which comprise the data for the current study. The treatment trial included both ulcerative colitis and Crohn’s disease patients; however, only the latter were included in the present study as measures of disease severity were not comparable across groups.
Participants were recruited from pediatric gastroenterology clinics at the Seattle Children’s Hospital and Mary Bridge Children’s Hospital. All parents/caregivers of consecutive patients between 2007 and 2012, meeting inclusion criteria, were contacted by a research coordinator about participating in a randomized controlled trial of a psychosocial intervention for children with IBD and their parents. Patient inclusion criteria were: (1) age 8–18, (2) physician diagnosis of IBD for at least three months, (3) medically approved to engage in typical daily activities, and (4) living with the same primary caregiver for at least the past three months. Patients were excluded if they had a chronic illness other than IBD, major surgery in the past year not related to IBD, and/or developmental disabilities requiring full-time special education or impairing ability to participate in assessment or treatment protocols, or did not speak English. Informed consent for participation was obtained from parents and assent was obtained from children for their participation in the study.
Measures
All children completed a battery of questionnaires over the telephone with an interviewer who was blinded to the treatment allocation of the larger trial from which this data is extracted. All interviewers were trained in use of each questionnaire and conducting interviews with children. The measures were all secondary outcome measure of the original treatment trial. Physician measures were obtained from the physician and medical records. For the current study we used the following baseline (before intervention) assessments.
Crohn’s Disease outcomes
Crohn’s disease symptoms were measured by self-report via the Inflammatory Bowel Disease Symptom Questionnaire (IBDS)19. The IBDS assess 11 common symptoms of IBD including pain, diarrhea, eye disease, bleeding, fever, vomiting, pain/swelling of joints, skin disease, loss of bowel control, bloating/gas and frequent trips to the bathroom rated on a 6-point scale ranging from none (0) to severe (5). All individual items are summed to obtain a total score, with higher scores indicating higher symptom levels. The IBDS has adequate internal consistency and is a good predictor of health status in adult patients with IBD19 but has not yet been psychometrically evaluated in children. In our study we found satisfactory internal consistency (Cronbach alpha = 0.74). We also found significant associations between the IBDS and the Pediatric Crohn’s Disease Activity Index20 (r=0.32, p<0.001), providing preliminary evidence of validity.
Disease related disability was assessed with the Functional Disability Inventory (FDI)21, 22. The FDI assesses children’s self-reported difficulty in physical and psychosocial functioning in the past two weeks due to their physical health. The FDI consists of 15 items concerning perceptions of activity limitations such as “In the last week, would you have any physical trouble or difficulty …. being at school all day”. Responses range from no trouble (0) to impossible (4). Total scores are computed by summing the items. Higher scores indicate greater disability. The FDI has demonstrated reliability and validity21.
Disease activity
Disease activity was assessed with the Pediatric Crohn’s Disease Activity Index (PCDAI)20 completed by the physician for study purposes during the patient recruitment visit. The PCDAI is an 11-item scale completed by the physician and widely used in clinical care to measure disease activity in children and adolescents, with demonstrated reliability and validity. The PCDAI includes physical examination findings, laboratory assessment, data concerning weight gain or loss, as well as growth rates. Scores range from 0–100, with higher scores indicating greater disease activity.
Psychological factors
Depression and Anxiety
Children’s depressive symptoms were measured with the Children’s Depression Inventory (CDI)23, 24. The CDI is a well validated 27-item questionnaire rated on a 3-point scale from 0 to 2. Sample item are: “I am sad” or “I have trouble sleeping”. The item about suicidal ideation was removed. The mean of the remaining 26 Items was multiplied by 27 to get a total mean score. Higher scores indicate more depressive symptoms.
Anxiety was assessed with the Multidimensional Anxiety Scale (MASC)25. The MASC is a well-validated measure of child anxiety with multiple subscales. In this study, we focused on the 12-item Anxiety Disorders Index. Sample items include “I feel tense or uptight” and “I keep my eyes open for danger.” Responses are scored on a 4-point scale ranging from “Never True” to “Often True.” All items are summed to obtain a total score, with higher scores indicative of more anxiety.
Cognitions
Beliefs about the meaning of symptoms, specifically pain which is the most common IBD symptom26, were assessed with the Pain Beliefs Questionnaire (PBQ)27, 28. The PBQ includes a 20-item subscale that measures pain threat by assessing the perceived seriousness, duration, and frequency of pain on a 5-point scale ranging from “Not at all true” to “Mostly true”. These included items such as “My stomach aches go on forever” and “My stomachaches mean I’m really sick”. All scores were summed and averaged to obtain a mean score which indicates more threat with increasing scores.
Catastrophizing
Catastrophizing (a tendency to magnify the seriousness of symptoms in combination with a sense of helplessness to be able to change symptoms) was measured with the Pain Response Inventory (PRI)29 which asks about coping with stomachaches or stomach problems. The PRI contains a 5-item Catastrophizing scale answered on a 5-point scale ranging from “Never” to “Always”. Sample items include “When you have stomach problems, how often do you: …. Think to yourself it is never going to stop” and “Feel like you can’t stand it anymore”. All items are summed and averaged to obtain a mean score. Higher scores indicates more catastrophizing. Catastrophizing has been consistently associated with emotional distress and disability4, 30.
Data analyses
To determine whether psychological factors and disease activity are related to disease outcomes, structural equation modeling was used (IBM® SPSS® AMOS 19.9). Structural equation modelling answers a set of inter-related research questions in a single systematic comprehensive analysis, avoiding the need to run multiple regressions. In addition, structural equation modelling allows for the construction of latent (i.e., unobserved) variables which are constructed of several observed variables.
We ran two separate structural equation models: one with Crohn’s disease symptoms (IBDS) as the dependent variable and another with functional disability (FDI) as the dependent variable. Independent variables were disease activity and psychological factors (PRI, PBQ, MASC, CDI). This part of the model can be recognized as a multiple regression model and the values in Figures 1 and 2 depict standardized regression coefficients. “Psychological factors” was a latent variable in our model constructed of the measured variables catastrophizing (as measured by the PRI), beliefs regarding pain threat (PBQ), depression (CDI) and anxiety (MASC). In order to generate a scale for the latent variable “psychological factors”, the unstandardized regression coefficient between the latent variable and one of the observed variables (in this case catastrophizing) was set at 1.
Figure 1.
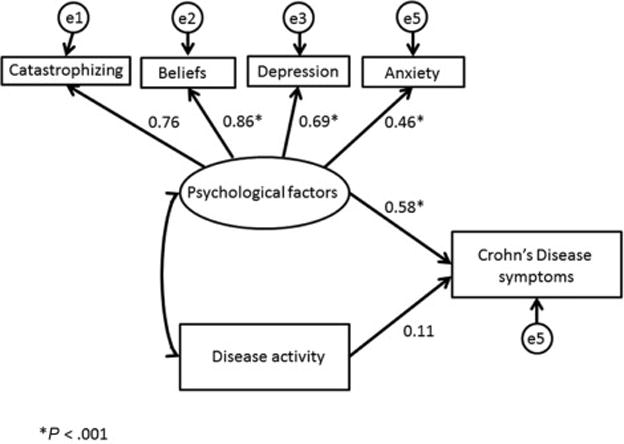
Disease activity and psychological factors predicting Crohn’s disease symptoms (standardized regression weights are depicted)
Figure 2.
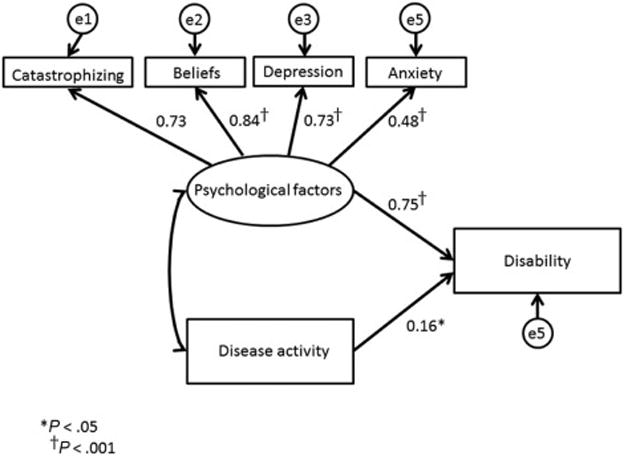
Disease activity and psychological factors predicting disability (standardized regression weights)
Chi-square was used for an overall test of the fit of the model (p > .05 denotes good fit). Goodness of fit was also determined by the Root Mean Square Error of Approximation (RMSEA < .06 indicates good fit31) and Comparative Fit Index (CFI >0.95 indicates good fit31). The contributions of psychological factors and disease activity variables to the outcomes were then evaluated. With five variables in the model, the sample size of 116 was sufficient to test the model, given recommendations that the sample N should be at least 10 to 20 times the number of variables in the model32.
The study was approved by the Institutional Review Boards of Seattle Children’s Hospital and Mary Bridge Children’s Hospital. Written informed consent was obtained from all parents and assent from all children.
Results
Sample
The sample consisted of 127 children and adolescents with Crohn’s disease between the ages of 8 and 18 (mean age 13.8). The majority of participants were male (57.5 %)) and Caucasian (84.3%). Paris classification33, indicating disease phenotype, is provided in Table I. Twenty-seven patients were on corticosteroids for their IBD, and seven patients were prescribed antidepressants. Compared with those not prescribed corticosteroids, these patients did not show increased levels of depression (M=8.4 versus 7.0; p=0.362) or anxiety (M=8.1 vs 8.2; P = 0.964). Table II presents means and standard deviations of all the variables in our model. No univariate or multivariate outliers were found. Missing data were minimal (1 or fewer cases; Table II) and replaced by maximum likelihood estimates34. Pearson correlations between independent variables and dependent variables are presented in Table III (available at www.jpeds.com).
Table 1.
Paris Classification
| N (%) | |
|---|---|
| Age at diagnosis | |
| A1a 0–<10 years | 31 (24.4) |
| A1b 10–< 17 years | 87 (68.5) |
| A2 17–40 years | 3 (2.4) |
| Unknown | 6 (4.7) |
|
| |
| Location | |
| L1 Distal Ileum | 32 (25.2) |
| L2 Colonic | 16 (12.6) |
| L3 Ileocolonic | 53 (41.7) |
| L4 Isolated Upper disease | 14 (11.0) |
| Unknown | 12 (9.4) |
|
| |
| Behavior | |
| B1 Non stricturing / non-penetrating | 95 (74.8) |
| B2 Stricturing | 9 (7.1) |
| B3 Penetrating | 2 (1.6) |
| B2B3 | 5 (3.9) |
| p Perianal Disease modifier | 4 (3.1) |
| B1 + p | 1 (0.8) |
| B3 + p | 1 (0.8) |
| Unknown | 10 (7.9) |
|
| |
| Growth | |
| G0 No evidence of growth delay | 73 (57.5) |
| G1 Growth delay | 45 (35.4) |
| Unknown | 9 (7.1) |
Table 2.
Descriptives for the variables in the models
| Variable | Number of participants | Mean (standard deviation) range | Cut-off scores |
|---|---|---|---|
| PCDAI total score | 126 | 10.04 (11.3) 0–62.5 |
Inactive disease N=66 (52.4%) Mild disease N=54 (42.9%) Moderate/severe disease N=6 (4.8%) |
| Functional Disability (FDI) | 127 | 6.23 (7.2) 0–34 |
|
| Crohn’s Disease symptoms (IBDS) | 127 | 5.01 (5.2) 0–22 |
|
| Catastrophizing (PRI-CAT) | 126 | 1.0 (0.8) 0–3.2 |
|
| Depression (CDI) | 126 | 7.31 (7.0) 0–36.00 |
Possible clinical depression N= 10 (7.9%) |
| Pain beliefs (PBQ) | 126 | 1.73(0.84) 0–3.75 |
|
| Anxiety (MASC) | 126 | 8.15(2.9) 0–17.62 |
Possible clinical anxiety N=9 (7.1%) |
Table 3.
Pearson correlations between variables in the model; online only
| IBD symptoms | Disability | |
|---|---|---|
| Depression | 0.36b | 0.60a |
|
| ||
| Anxiety | 0.24b | 0.42b |
|
| ||
| Beliefs | 0.58b | 0.69a |
|
| ||
| Catastrophizing | 0.44a | 0.54a |
|
| ||
| PCDAI items | ||
| Abdo pain | 0.28b | 0.33b |
| Stool | 0.27b | 0.34b |
| Well-being | 0.32b | 0.39b |
| HCT | 0.17 | 0.19a |
| ESR | 0.12 | 0.13 |
| Albumin | 0.15 | 0.05 |
| Weight | 0.09 | 0.24a |
| Height @ diagnosis | −0.08 | −0.09 |
| Height @ Follow-up | 0.07 | 0.14 |
| Abdo tenderness/mass | 0.21a | 0.30b |
| Perirectal disease | −0.01 | 0.09 |
| Extra-intestinal Symptoms | −0.06 | 0.04 |
p<0.05,
p<0.01
Crohn’s disease symptoms
Figure 1 shows the model predicting Crohn’s disease symptoms reported by patients on the IBDS from psychological factors and disease activity. The model was significant (Chi2 (df=8)= 9.72; p=0.28), and has adequate fit to the data (RMSEA=0.04 (90% CI 0.00-0.117); CFI =0.99). Psychological factors (β=0.58; p<0.001) but not disease activity (β=0.11; p=0.16) were significantly associated with symptoms.
A large proportion of our sample had no clinically active disease (52.4%), based on the PCDAI cut-off score of 11 or higher20. As this could explain the insignificant effect of disease activity on symptom self-reporting, we reran the model including only patients who currently had clinically active disease (n=60). Although underpowered given the low n, this model was significant: Chi2 (df=8)= 14.13; p=0.08). The association between disease activity measures and Crohn’s disease symptoms remained insignificant (p=0.08), while psychological factors still significantly predicted outcomes (β=0.7, p <0.001).
Functional disability
Similar models were run with disability (FDI) as the dependent variable (Figure 2). This model was significant (Chi2 (df=8)=8.4; p=0.40) and had adequate fit to the data (RMSEA =0.02 (90%CI 0.00-0.108); CFI=0.99). Both psychological factors (β=0.75, p <0.001) and disease activity (β=0.16, p=0.01) were associated with disability.
We reran our model including only those patients who had clinically active disease (n=60). Again, this model was significant (Chi2 (df=25)= 29.36; p=0.25). The association between disability and psychological factors remained significant (β=0.8, p <0.001), but no association was found between disability and disease activity (p=.30).
Discussion
The aim of the current study was to examine the relative contribution of psychological factors and disease activity to the self-reporting of symptoms and disability in a pediatric sample. We found that psychological factors were more strongly associated with both Crohn’s disease symptom self-reporting and disability than were validated measures of disease activity. Interestingly, disease activity had no relation to symptoms and only a very small association with disability. This finding is supported by the fact that treatment for depression in adolescents with inflammatory bowel disease, is not linked to changes in disease severity35.
One possible explanation for our findings is that many patients with Crohn’s disease report elevated levels of psychological distress. This disease has an unpredictable course requiring occasional invasive procedures and demanding medical regimens, and there is the constant threat of another flare. Moreover, disease onset often occurs during childhood or adolescence, which is a developmental period when social acceptance is important, yet the sequelae of the disease may be seen as stigmatizing. These factors can lead to elevated symptoms of anxiety and depression. In about 8% of our sample, scores were at or above cutoffs for clinical depression23. The literature has shown that as many as one quarter of pediatric Crohn’s disease patients meet criteria for clinical depression6, 36. Many more children have heightened psychological distress scores even when not meeting these cut offs.
However, there may be a reciprocal relationship between psychological distress and Crohn’s disease symptoms or disability. It has been documented that children who are anxious or depressed are more likely to report gastrointestinal symptoms and disability37, 38. The bidirectional communication between the brain and the gut is the likely explanation of why psychological distress is associated with gastrointestinal symptoms. The gut sends a constant stream of interoceptive information through the spinal dorsal horn, supraspinal sites and finally to cortical areas39. In healthy individuals, these signals are not consciously perceived and mainly are used by the autonomic nervous system to guide homeostasis. However, in some patients these interoceptive signals interact with emotional input and reach consciousness creating symptoms39. Descending emotional pathways via the periaqueductal gray to the dorsal horn can amplify new afferent signals from the gut creating a spiral of more input and more symptoms39. Patients with IBS, who have symptoms not explained by disease activity, show more activity in brain areas related to attention (specifically the Insular Cortex) and emotion (Anterior Cingulate Cortex, hypothalamus, and the amygdala) with gut distension40. In patients with IBD, IBS-like symptoms have been reported during clinical remission and thought to be explained by similar brain processes1. Psychological factors can further modulate these responses. Negative emotions increase brain responsiveness to gut distension41 and removing severe emotional distress reduces brain activation in important areas for pain encoding such as the midcingulate cortex42. Thus, psychological factors can both be a consequence and cause of symptom presentation43.
There are some limitations to our study design that may partly explain why disease activity had little or no measurable association with symptom reporting. First, our sample was largely in a quiescent state of disease and therefore less likely to experience symptoms directly related to disease activity. To address this, we reran our models among a subsample of patients who were currently have clinically active disease and found the same pattern of results. A second limitation is the use of PCDAI as a measure of disease activity. The golden standard for measuring inflammation in CD is an endoscopy, but given the invasive nature of this procedure cannot be performed for study purposes only. The PCDAI is the most widely used measure for Crohn’s disease activity and 91% of experts in pediatric Crohn’s disease reported it has good to very good face validity44. No validated, well-accepted alternatives are available. Fecal calprotectin and fecal lactoferrin45 are good predictors of inflammation (although not disease specific), but were not widely used in clinical care of IBD a few years ago when this sample was collected. We could obtain fecal calprotectin levels from the medical records in only four patients. The correlational design of our study is another limitation, as it does not allow us to determine cause and effect. The data were collected at a single time point and longitudinal data is needed to examine if there are time effects. Do disease activity and psychological factors at time 1 influence symptoms and disability at time 2 or is the order of events reversed? The design also does not allow for examining the lagged effect of psychological distress on disease course in IBD which has been previously observed in adult patients14. This lagged effect may be as long as several months, possibly reflecting changes in cellular function related to distress over time, and that may not have been detectable in our study design. Finally, the study was based on a cohort of patients who participated in a randomized controlled trial, which may limit the generalizability to a larger IBD population. However, it is one of the largest cohorts in the pediatric IBD literature and patients were obtained from multiple centers, including academia as well as private practice.
In conclusion, the current study shows that in this sample of children and adolescents, psychological factors were strongly associated with self-reported Crohn’s disease symptoms and disability. This is an important finding as symptom presentation often plays an important role in treatment decisions, which could lead to unnecessary exposure to tests and treatments, with potential negative side effects. When confronted with a pediatric Crohn’s disease patient who has high levels of psychological distress, independent of their inflammatory status, the clinician should consider incorporating behavioral techniques such as education, reassurance, and cognitive behavior therapy into the management plan.
Acknowledgments
Supported by the National Institutes of Health (R01HD36069 [to R.L.]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Trial registration ClinicalTrials.gov: NCT00679003
Contributor Information
Miranda A.L. van Tilburg, University of North Carolina, Center for Functional GI and Motility, Disorders Chapel Hill, NC.
Robyn Lewis Claar, University of North Carolina, Center for Functional GI and Motility, Disorders Chapel Hill, NC.
Joan M. Romano, University of Washington, Department of Psychiatry and Behavioral Sciences, Seattle, WA.
Shelby L. Langer, University of Washington, School of Social Work Seattle, WA.
Douglas A. Drossman, University of North Carolina, Center for Functional GI and Motility Disorders, Chapel Hill, NC.
William E. Whitehead, University of North Carolina, Center for Functional GI and Motility Disorders, Chapel Hill, NC.
Bisher Abdullah, Prime Health Clinic, Puyallup, WA.
Rona L. Levy, University of Washington, School of Social Work Seattle, WA.
References
- 1.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474–82. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 2.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–36. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA. Presidential address: Gastrointestinal illness and the biopsychosocial model. Psychosom Med. 1998;60:258–67. doi: 10.1097/00006842-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 4.van Tilburg MA, Claar RL, Romano JM, Langer SL, Walker LS, Whitehead WE, et al. The Role of Coping With Symptoms in Depression and Disability: Comparison Between Inflammatory Bowel Disease and Abdominal Pain. J Pediatr Gastroenterol Nutr. 2015;61:431–6. doi: 10.1097/MPG.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wojtowicz AA, Greenley RN, Gumidyala AP, Rosen A, Williams SE. Pain severity and pain catastrophizing predict functional disability in youth with inflammatory bowel disease. J Crohn’s & Col. 2014;8:1118–24. doi: 10.1016/j.crohns.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Szigethy E, Levy-Warren A, Whitton S, Bousvaros A, Gauvreau K, Leichtner AM, et al. Depressive symptoms and inflammatory bowel disease in children and adolescents: a cross-sectional study. J Pediatr Gastroenterol Nutr. 2004;39:395–403. doi: 10.1097/00005176-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Steinhausen HC, Kies H. Comparative studies of ulcerative colitis and Crohn’s disease in children and adolescents. J Child Psychol Psychiatry. 1982;23:33–42. doi: 10.1111/j.1469-7610.1982.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 8.Burke PM, Neigut D, Kocoshis S, Chandra R, Sauer J. Correlates of depression in new onset pediatric inflammatory bowel disease. Child psychiatry Human Dev. 1994;24:275–83. doi: 10.1007/BF02353203. [DOI] [PubMed] [Google Scholar]
- 9.Mackner LM, Crandall WV. Long-term psychosocial outcomes reported by children and adolescents with inflammatory bowel disease. Am J Gastroenterol. 2005;100:1386–92. doi: 10.1111/j.1572-0241.2005.41428.x. [DOI] [PubMed] [Google Scholar]
- 10.Ondersma SJ, Lumley MA, Corlis ME, Tojek TM, Tolia V. Adolescents with inflammatory bowel disease: the roles of negative affectivity and hostility in subjective versus objective health. J Pediatr Psychol. 1997;22:723–38. doi: 10.1093/jpepsy/22.5.723. [DOI] [PubMed] [Google Scholar]
- 11.Reed-Knight B, Lobato D, Hagin S, McQuaid EL, Seifer R, Kopel SJ, et al. Depressive symptoms in youth with inflammatory bowel disease compared with a community sample. Inflamm Bowel Dis. 2014;20:614–21. doi: 10.1097/01.MIB.0000442678.62674.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szigethy EM, Youk AO, Benhayon D, Fairclough DL, Newara MC, Kirshner MA, et al. Depression subtypes in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58:574–81. doi: 10.1097/MPG.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besharat S, Amiriani T, Roshandel G, Besharat M, Semnani S, Kamkar M. Depressive mood and disease activity in inflammatory bowel disease. Arab J Gastroenterol. 2012;13:136–8. doi: 10.1016/j.ajg.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Maunder RG, Levenstein S. The role of stress in the development and clinical course of inflammatory bowel disease: epidemiological evidence. Current molecular medicine. 2008;8:247–52. doi: 10.2174/156652408784533832. [DOI] [PubMed] [Google Scholar]
- 15.McCombie AM, Mulder RT, Gearry RB. How IBD patients cope with IBD: A systematic review. J Crohn’s & Col. 2013;7:89–106. doi: 10.1016/j.crohns.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Drossman DA, Leserman J, Mitchell CM, Li ZM, Zagami EA, Patrick DL. Health status and health care use in persons with inflammatory bowel disease. A national sample. Dig Dis Sci. 1991;36:1746–55. doi: 10.1007/BF01296620. [DOI] [PubMed] [Google Scholar]
- 17.Langer SL, Romano JM, Mancl L, Levy RL. Parental Catastrophizing Partially Mediates the Association between Parent-Reported Child Pain Behavior and Parental Protective Responses. Pain Res Treat. 2014;2014:751097. doi: 10.1155/2014/751097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy RL, van Tilburg MAL, Langer S, Romano J, Walker LS, Mancl L, et al. Effects of a Cognitive Behavioral Therapy Intervention Trial to Improve Disease Outcomes in Children with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:2134–48. doi: 10.1097/MIB.0000000000000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drossman DA, Li Z, Leserman J, Patrick DL. Ulcerative colitis and Crohn’s disease health status scales for research and clinical practice. J Clin Gastroenterol. 1992;15:104–12. doi: 10.1097/00004836-199209000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–47. [PubMed] [Google Scholar]
- 21.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs M. The Children’s Depression Inventory. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- 24.Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica. 1981;46:305–15. [PubMed] [Google Scholar]
- 25.March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–65. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88:995–1000. doi: 10.1136/adc.88.11.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker LS, Baber KF, Garber J, Smith CA. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain. 2008;137:266–75. doi: 10.1016/j.pain.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker LS, Smith CA, Garber J, Claar RL. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychol. 2005;24:364–74. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker LS, Smith CA, Garber J, Van Slyke DA. Development and validation of the Pain Response Inventory for children. Psychological Assessment. 1997;9:392–405. [Google Scholar]
- 30.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 32.Tabachnick BG, Fidell LS. Using multivariate statistics. Fifth. Boston MA: Pearson Education; 2007. [Google Scholar]
- 33.Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–21. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 34.Allison PD. Missing data techniques for structural equation modeling. J Abnorm Psychol. 2003;112(4):545–57. doi: 10.1037/0021-843X.112.4.545. Epub 2003/12/17. [DOI] [PubMed] [Google Scholar]
- 35.Szigethy E, Craig AE, Iobst EA, Grand RJ, Keljo D, DeMaso D, et al. Profile of depression in adolescents with inflammatory bowel disease: implications for treatment. Inflamm Bowel Dis. 2009;15:69–74. doi: 10.1002/ibd.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke P, Meyer V, Kocoshis S, Orenstein DM, Chandra R, Nord DJ, et al. Depression and anxiety in pediatric inflammatory bowel disease and cystic fibrosis. J Am Acad Child Adolesc Psychiatry. 1989;28:948–51. doi: 10.1097/00004583-198911000-00022. [DOI] [PubMed] [Google Scholar]
- 37.Bohman H, Jonsson U, Paaren A, von Knorring L, Olsson G, von Knorring AL. Prognostic significance of functional somatic symptoms in adolescence: a 15-year community-based follow-up study of adolescents with depression compared with healthy peers. BMC psychiatry. 2012;12:90. doi: 10.1186/1471-244X-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Veek SM, Derkx HH, de Haan E, Benninga MA, Boer F. Abdominal pain in Dutch schoolchildren: relations with physical and psychological comorbid complaints in children and their parents. J Pediatr Gastroenterol Nutr. 2010;51:481–7. doi: 10.1097/MPG.0b013e3181d51a59. [DOI] [PubMed] [Google Scholar]
- 39.van Tilburg MAL. Integration of biomedical and psychosocial issues in pediatric functional gastrointestinal and motility disorders. In: Faure C, DiLorenzo C, Thapar N, editors. Pediatric Neurogastroenterology. New York: Springer; 2013. pp. 59–70. [Google Scholar]
- 40.Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. NeurogastroenterolMotil. 2009;21:579–96. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–59. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drossman DA, Ringel Y, Vogt BA, Leserman J, Lin W, Smith JK, et al. Alterations of brain activity associated with resolution of emotional distress and pain in a case of severe irritable bowel syndrome. Gastroenterol. 2003;124:754–61. doi: 10.1053/gast.2003.50103. [DOI] [PubMed] [Google Scholar]
- 43.Drossman DA, Ringel Y. Psychosocial factors in ulcerative colitis and crohn’s disease. In: Sartor RB, Sandborn WJ, editors. Kirsner’s Inflammatory Bowel Disease. 6th. London: W.B. Saunders; 2004. pp. 340–56. [Google Scholar]
- 44.Turner D, Griffiths AM, Walters TD, Seah T, Markowitz J, Pfefferkorn M, et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis. 2012;18:55–62. doi: 10.1002/ibd.21649. [DOI] [PubMed] [Google Scholar]
- 45.Sipponen T, Karkkainen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–9. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]


