SYNOPSIS
Early-onset Alzheimer’s disease (EOAD) with onset <65 years of age, while overshadowed by the more common late-onset AD (LOAD), differs significantly from LOAD. EOAD comprises about 5% of AD and is associated with delays in diagnosis, an aggressive course, and age-related psychosocial needs. One source of confusion is that a substantial percentage of EOAD are phenotypic variants that differ from the usual memory-disordered presentation of typical AD. Patients with EOAD overall have greater parietal atrophy, more white matter abnormalities, and less hippocampal volume loss, compared to those with LOAD. The phenotypic variants also have atrophy and white matter changes corresponding anatomically to the cognitive changes and appear to involve alternate neural networks relative to typical AD. The management of EOAD is similar to that for LOAD but special emphasis should be placed on targeting the specific cognitive areas involved and more age-appropriate psychosocial support and education.
Keywords: Dementia, Alzheimer’s disease, early-onset dementia, young-onset dementia, logopenic variant primary progressive aphasia, progressive cortical atrophy
INTRODUCTION
Alzheimer’s disease (AD) originally meant a disorder of early-onset (EOAD; <65 years of age) and did not include older patients with “senile dementia.” In fact, the first patient reported with the neuropathology of AD, Auguste Deter (1850–1906), appeared to have the onset of symptoms in her late 40’s, before being diagnosed with dementia at age 511. Her symptoms included memory loss, confusion, language impairment, and unpredictable, agitated, aggressive, and paranoid behavior, and, on autopsy, she had what we now recognize as the characteristic neuropathological markers of AD, extracellular amyloid-positive neuritic plaques and intracellular tau-positive neurofibrillary tangles (NFTs). With the observation of similar neuropathology associated with cognitive decline in all age groups, investigators subsequently broadened the diagnosis of AD to include the much more common late-onset AD (LOAD)2. In recent years, the main focus of interest and research has been on LOAD; however, like Auguste Deter, patients with EOAD remain an important and impactful subgroup of patients with this disorder.
EOAD is the most common early-onset neurodegenerative dementia. The few epidemiological studies on EOAD indicate that the vast majority are non-familial, making up about 4–6% of all AD 3, with an annual incidence rate of about 6.3/100,000 4 and a prevalence rate of about 24.2/100,000 in the 45–64 year age group 5, or between 220,000 and 640,000 Americans 6. These incidence and prevalence rates of EOAD rise exponentially as patients approach age 65 7. Unfortunately, EOAD is often atypical and missed, resulting in about a 1.6 year average delay in diagnosis compared to older patients 8. Yet, from 1999 to 2010, mortality reports show that EOAD accounted for a large number of premature deaths among US adults aged 40–64 with many years of potential life lost as well as losses in productivity 9.
EOAD vs. LOAD
EOAD is not just LOAD occurring at an arbitrarily younger age cut-off; EOAD differs from LOAD in many respects (See Table 1). EOAD differs from LOAD in the greater extent of evaluation required for diagnosis 10, the increased impact of dementia risk factors such as lower cardiovascular fitness and cognitive fitness 11, and the potentially increased consequence of traumatic brain injury the lower the age of onset of dementia12. There are psychosocial problems specific to early-onset dementia 13–17, such as the effects of unexpected loss of independence, grief with a sense of an “out-of-step” decline in midlife, difficulty juggling ongoing responsibilities, and relatively preserved insight with associated depression and anxiety. Given that autosomal dominant familial AD tends to be of early onset, there are subgroups of EOAD with higher rates of neurological symptoms than LOAD and a greater risk for the development of AD among relatives 18,19. In contrast, compared to LOAD, EOAD patients have decreased overall comorbidities such as diabetes, obesity, and circulatory disorders 18.
TABLE 1.
Early-Onset Alzheimer’s Disease
| Differences in Comparison to the More Common Late-Onset Disorder |
|
EOAD patients differ, on average, from LOAD patients on a number of clinical, neuropsychological, neuroimaging, and neuropathological variables. Several studies indicate that these early-onset patients have a more aggressive clinical course 20–24. EOAD, compared to LOAD, presents less commonly with memory deficits and more frequently as focal cortical or phenotypic variants (described below)25. Overall, EOAD patients, compared to comparably impaired LOAD patients, have better memory recognition scores and semantic memory 26, but they tend to have worse attention, executive functions, ideomotor praxis, and visuospatial skills25,26. On magnetic resonance imaging (MRI), EOAD shows greater neocortical atrophy, particularly in parietal cortex, with less atrophy in the mesial temporal lobe (MTL) 27,28. MRI shows larger sulcal widths in the temporoparietal cortex among EOAD patients with preserved hippocampal volumes relative to LOAD29. Resting state fluorodeoxy glucose (FDG) positron emission tomography (PET), shows greater parietal hypometabolism, worse on the left in one study 30, in EOAD compared to greater bilateral temporal hypometabolism in LOAD 15. FDG-PET also suggests dysfunction in brain metabolic activity especially in the salience network among EOAD patients with behavioral disturbances 31. Neuropathologically, both EOAD and LOAD have temporoparietal-precuneus atrophy, but EOAD patients have higher burdens of neuritic plaques and NFTs in these regions, and, to a lesser extent, frontal cortex, than LOAD patients 25.
EOAD, regardless of clinical variant, has an early and prominent pattern of WM damage that is more severe in posterior areas32. Diffusion tensor imaging (DTI) measures in EOAD demonstrate more damage to WM pathways in both deep long range limbic and association fibers and superficially located short range association fibers in the frontal, temporal, and parietal lobes associated with fronto-parietal dysfunction33,34. Compared with LOAD, the WM involvement in EOAD patients is particularly greater in posterior WM (posterior cingulate and parietal regions) and main anterior-posterior pathways with less mesial temporal involvement34–36. Moreover, WM damage in EOAD is more widely distributed than would be predicted by the extent of gray matter (GM) atrophy 36. Using graph theory analysis of DTI, EOAD appears to target the nodal connectivity of the brain, mainly affecting the rich club network in the superior frontal regions, precuneus, posterior cingulate and insula with differential disruption of the major central hubs that transfer information between brain regions37.
Variant EOAD Phenotypes (or “Type 2 AD”)
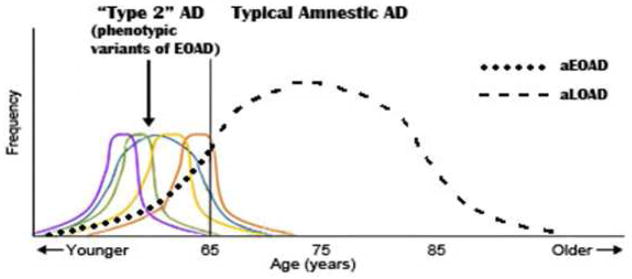
One of the most important aspects of EOAD is its common presentation as a number of non-amnestic, variant phenotypes, potentially justifying their grouping under the label “Type 2” AD. These variants represent the young tail of the normally distributed age of AD onset curve (See Figure 1). About 22–64% of EOAD are non-amnestic variant phenotypes, which differ from typical amnestic AD (either EOAD or LOAD) not only in non-memory presentations 21,38–43, but also in the decreased prevalence of the apolipoprotein E (APOE) ε4 allele23,41, and early posterior cortical NFTs with relative hippocampal sparing 44.
Figure 1.
Type 2 AD (variant phenotypes of EOAD) vs. Typical amnestic AD (aEOAD and aLOAD). Significantly modified and adapted from Van der Flier et al, 2011{van der Flier, 2011 #5}. The non-amnestic variant phenotypes (logopenic variant primary progressive aphasia, posterior cortical atrophy, and other proposed variants) tend to occur in the early-onset age range and are depicted as colored lines.
The variant phenotypes of EOAD constitute a number of syndromes (See Table 2) 38,45,46. The most common may be a language-impaired phenotype known as logopenic progressive aphasia (LPA)35,39,47. Investigators report a “posterior cortical atrophy” (PCA) variant with visuospatial deficits 48,49. Others suggest that a biparietal phenotype with progressive ideomotor apraxia (PIA) and visuospatial and other deficits is a common form of EOAD 45. The literature stresses the occurrence of a behavioral/dysexecutive variant, sometimes referred to as “frontal variant AD50,38. In addition, patients with corticobasal syndrome, characterized by progressive limb apraxia and motor changes, have AD in up to 25% at autopsy51, indicating another manifestation of variant EOAD which greatly overlaps with PIA. These phenotypes are clinical syndromes that appear to overlap with one another, while differing in basic respects from typical amnestic AD 52,53.
Table 2.
Alternative Classifications for Variant Phenotypes of EOAD
| Brain Regions | Koedam et al, 201045 N=87 |
Alladi et al, 200739 N=34 |
Stopford et al, 200838* N=17 |
|---|---|---|---|
| Left Parietal | Apraxia/visuospatial (37.5%) | Corticobasal syndrome (17.5%) | Praxis (23.5%) |
| Left Parietal Left Temporooccipital | Language (28.1%), Aphasia-Apraxia-Agnosia (25%) | Language (56%): [Nonfluent (35%), Semantic (6%), Mixed (15%)] | Language (23.5%) |
| Dorsolateral Frontal | Dysexecutive (6.3%0) | Non-AD: FTD (6%) | Dysexecutive (41.2%) |
| Right Parietal, Right Temporoccipital | Posterior cortical atrophy (3.1%) | Posterior cortical atrophy (20.5%) | Perceptuo-spatial (11.8%) |
Neuroimaging studies indicate differences among the EOAD variants (further discussed below). In general, the typical amnestic EOAD patients have more hippocampal atrophy; whereas, the variant phenotypes of EOAD with language presentations have more left parietal atrophy, and the variant phenotypes of EOAD with visuospatial presentations have more right parietal-occipital changes. Typical amnestic AD has WM damage in the genu and splenium of the corpus callosum and the parahippocampal tract bilaterally36; whereas, the variant phenotypes of EOAD have extensive degeneration of major anterior-posterior connecting fiber bundles and of commissural frontal lobe tracts, implying deafferentation within fronto-parietal cortical networks54.
Functional MRI studies suggest that EOAD is driven by early involvement of fronto-parietal networks (central executive and salience networks; language, working memory, and higher visual networks) rather than the decreased posterior default mode network (DMN) and MTL-hippocampal connectivity of typical amnestic AD 55–68. In typical AD, functional connectivity shows enhanced effective connectivity within frontally-based executive and salience networks, even before the detection of any WM changes 3,69,70. In contrast, fMRI in EOAD demonstrates decreased fronto-parietal connectivity 45,46,71–74.
Logopenic Variant Primary Progressive Aphasia (lvPPA)
A major EOAD phenotypic variant is the progressive decline in language known as lvPPA. Patients with this syndrome present with word-finding difficulty, decreased sentence repetition, and abnormalities in echoic memory, with impairments in their phonological buffer (i.e. limitations in the number of spoken words that they can keep in working memory). Clinicians must distinguish these patients from non-fluent and semantic forms of primary progressive aphasia (PPA) which are typically due to frontotemporal lobar degenerations. The presence of some degree of difficulty in episodic memory and visuospatial skills helps distinguish lvPPA from other PPAs. In addition, a history of dyslexia is common among patients with lvPPA75–77, suggesting a pre-existing vulnerability in language networks. In one study, 25% of lvPPA patients had self or informant reports of delay in spelling or reading 76.
The clinical criteria for lvPPA are as follows (See Table 3) 47: An insidious onset and progression of: 1. Impaired single-word retrieval in spontaneous speech and naming (anomia); 2. Impaired repetition of sentences and phrases; and 3. At least 3 of the following must also be present: a) Speech (phonologic) errors, b) spared single-word comprehension and object knowledge, c) spared motor speech. and/or 4. Absence of frank agrammatism.
TABLE 3.
Characteristics of Logopenic Variant Primary Progressive Aphasia (lvPPA)
|
| Left posterior temporal/inferior parietal dysfunction on neuroimaging |
Although some patients may have frontotemporal lobar degeneration or other pathologies, the clinical syndrome of lvPPA usually results from AD with focal involvement of temporoparietal language areas in the left hemisphere. Neuroimaging shows atrophy, decreased metabolism, and decreased WM in the left temporo-parietal junction78. Patients with lvPPA usually have positive AD biomarkers including amyloid-PET positive scans79 and decreased Aβ42/elevated tau levels in the cerebrospinal fluid (CSF)80. DTI analysis of lvPPA reveals bilateral but predominantly left-sided alterations in frontal origin pathways such as superior and inferior longitudinal fasciculi and the uncinate fasciculus, as well as the parietotemporal junction (See Figure 2) 52,71,81. Compared to typical AD, those with lvPPA have reduced connectivity in the left posterior superior temporal region and temporal language network, the inferior parietal and prefrontal regions and fronto-parietal networks, and the left working memory networks68,73,82, and less involvement of the ventral DMN associated with episodic memory impairment 68.
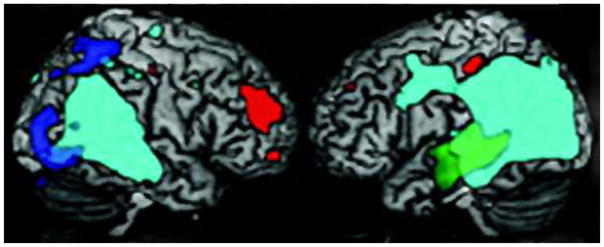
Figure 2. Voxel-based morphometry of parietal overlap of EOAD phenotypes78.
Light green represents overlap of all EOAD variants. Green=Type 2 AD-lvPPA; Blue=Type 2 AD-PCA; Red= Other EOAD.
Source of neuroimage: Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73(19):1571–1578.
There is a pathophysiological explanation for this syndrome’s impairments. In lvPPA, disease in the left inferior parietal lobule and superior and middle temporal gyri disturbs the phonological loop of verbal working memory (phonological short-term memory or store that holds phonological traces for brief periods) 68,83, resulting in deficits in digit, letter, and word span and an absent phonological similarity effect84.
Posterior Cortical Atrophy (PCA)
Many patients with EOAD present with a progressive decline in visuospatial skills, known as PCA or “Benson’s disease” after D. Frank Benson, who described the syndrome in 198885. Patients with this syndrome present with complex visual symptoms including alexia, apperceptive visual agnosia, Balint’s syndrome (simultanagnosia, optic ataxia, oculomotor apraxia) and difficulty with visuospatial localization, Gerstmann’s syndrome, and a possible left visual field deficit with disproportionate impairments on tests of visual constructions (See Table 4). PCA is most commonly a visual variant of AD, but may result from dementia with Lewy bodies, Creutzfeldt-Jakob (Heidenheim variant), or other lesions or disorders involving the posterior visual cortex. PCA patients have better verbal fluency and somewhat less impaired episodic memory than typical AD,86 and they differ from many dementias in having relatively preserved insight into their illness and a tendency to depression. Some investigators suggest that PCA is a focal Alzheimer neurodegeneration of the occipital, occipitoparietal, and occipitotemporal cortex87,88, and that there may be prior learning disabilities and a pre-existing vulnerability in the cortical visual systems77.
Table 4.
Complex Visual Disorders among PCA Patients (approximate order of frequency)
|
Adapted from Mendez et al, 200249
The clinical criteria for PCA are as follows (See Table 5) 48,49: An insidious onset and progression of the following: 1. Visual complaints with intact primary visual functions, except for possible visual field deficits. 2. Evidence of predominant complex visual disorder (oculomotor apraxia, optic ataxia, dressing apraxia, environmental disorientation, abnormal anti-saccades, neglect, constructional difficulty, simultanagnosia, visual agnosia, prosopagnosia). 3. Proportionally less impaired deficits in memory and verbal fluency.
TABLE 5.
Developing Criteria for Posterior Cortical Atrophy{Crutch, 2012 #291}*
| • CLINICAL FEATURES: Insidious onset and gradual progression Prominent early disturbances of visual and/or other posterior cognitive symptoms/signs |
| • COGNITIVE FEATURES: At least 3 of the following must be early or presenting features: Visuospatial difficulty, elements of Balint’s syndrome, visual object agnosia, visuoconstructional difficulty, environmental disorientation, dressing apraxia, alexia, elements of Gerstmann’s syndrome, ideomotor apraxia, apperceptive prosopagnosia, visual field deficit All of following must be evident: Relative sparing of anterograde memory, speech and verbal language, executive functions, and behavior and personality |
| • NEUROIMAGING: Predominant occipito-parietal or occipito-temporal changes |
| • EXCLUSION CRITERIA: Lesions or disorders of the brain that could cause similar symptoms and findings |
Consortium developing criteria under the leadership of Sebastian Crutch, M.D.
Neuroimaging shows predominant areas of atrophy, hypoperfusion, and hypometabolism from primary visual cortex through dorsal visual association cortex and posterior regions of the temporal lobes. On DTI, there may be predominate right-sided WM changes in superior and inferior longitudinal fasciculi, inferior fronto-occipital fasciculus, and right fronto-parietal pathways (See Figure 2) 14,89. These areas and WM tracts impact on mid-level cortical visual processing, resulting in abnormal perceptual integration and organization, and difficulty with figure-ground discrimination and global-local precedence. Many patients have difficulty findings things in their spatial environment, left more than right visual field constriction, and elements of Balint’s syndrome, especially optic ataxia with “magnetic misreaching” towards the point of fixation when reaching for items in their peripheral fields 90.
Other Variants
Other than for lvPPA and PCA, there is no consensus on the number of EOAD variants or on their classification. Two addition EOAD variants are worth noting. One is a progressive ideomotor apraxia (PIA) variant, which overlaps with corticobasal syndrome from AD as well as with lvPPA and PCA. This variant results from focal left parietal neuropathology and manifests as difficulty performing learned limb movements on command and to imitation. It is often accompanied by Gerstmann’s syndrome with acalculia, alexia with agraphia, and problems with visual constructions. Another variant is “behavioral/dysexecutive AD”, also described as “frontal variant AD”50. This variant can present with apathy, and half can meet clinical criteria for behavioral variant frontotemporal dementia (bvFTD). However, persons with behavioral/executive variant EOAD tend to perform worse on memory tests than those with bvFTD and can show marked atrophy in bilateral temporoparietal regions with milder atrophy in frontal cortex50.
The recent literature suggests that the variant phenotypes of EOAD could be related to each other, potentially comprising a “Type 2” AD that differs in its neurocognitive-neural network profile from typical amnestic AD 42,43,52,53. Clinically, they all relatively spare memory, and pathologically, they may all have hippocampal sparing with greater posterior cortical NFTs 44. There is additionally specific involvement of left hemisphere language areas in lvPPA 80,91 and the visual neocortex in PCA 92,93. Neuroimaging data also suggest posterior neocortical rather than mesotemporal cortical overlap of these phenotypes 78,94,95,78,94,95.
Cerebrospinal Fluid (CSF) and Amyloid PET Biomarkers in EOAD
Similar to typical LOAD, amyloid β42 (Aβ) peptide levels are decreased and total tau and phospho-tau levels are increased in CSF in EOAD and its variants96. Some studies suggest phenotypic variations in these CSF biomarkers, particularly lower tau levels in PCA 54,97,98, but this is not confirmed across studies and with neuropathology. Where EOAD differs from LOAD is the better correspondence of lower Aβ levels, rather than increased tau levels, with GM atrophy 96. One possible explanation for this difference is the decreased release of tau into the ventricular space in EOAD in light of the neurodegeneration occurring further from the ventricular surface (e.g. in the neocortex rather than medial temporal lobe lobe).
Amyloid PET is especially useful in the differentiation of EOAD from other dementias of early onset. The prevalence of amyloid positivity decreases in AD from age 50 to 90, particularly among apolipoprotein E (APOE) ε4 noncarriers, while increasing with age in non-AD dementias 99. This suggests an increased utility of amyloid PET among those with dementia onset of less than 65 years of age. Amyloid positivity by PET is almost always associated with low CSF Aβ in symptomatic AD, and amyloid PET positivity is a better predictor of clinical diagnosis 100.
Genetics
Genetic changes are becoming increasing important in the analysis and understanding of EOAD101. There is growing awareness of polymorphisms and genetic mutations that increase susceptibility for EOAD. The identification of determinant AD genes in this population overall, however, is rare. Despite an autosomal dominant history in about 14.2% of persons with EOAD, only 1.6% or so of the total EOAD population carries a presenilin 1 PSEN1), presenilin 2 (PSEN2), or amyloid precursor protein (APP) gene that conveys an autosomal dominant inheritance for AD 102. These three pathogenic mutations, which lead to aberrant cleavage or aggregation of the APP, result in the more typical amnestic AD but can have distinctive features such as spastic paraparesis, early myoclonus, seizures, dysarthria, pseudobulbar affect, more extensive amyloid angiopathy, and atypical amyloid plaque morphology and distribution103{Ringman, 2016 #964}. Nevertheless, there may be a need to screen EOAD patients for these mutations. Investigators report a PSEN1 mutation In an analysis of a specimen from Auguste Deter, Alzheimer’s original patient 104, and some PSEN1 mutations, such as A79V, can be variable and sometimes mild with ages of onset ranging from 53–84105. One study found three PSEN1 and one PSEN2 in 4 (1.5%) of 264 EOAD patients, but no effect of having a positive family history of LOAD 106. Another whole-exome sequencing of 23 German patients with EOAD revealed 3 with potential pathogenic PSEN2 variants107. Finally, on screening 451 sporadic EOAD for known causative mutations of the APP gene, investigators found four heterozygous for A713T, V717I, V717G 108.
There is a polygenic risk for AD from a number of susceptibility genes, but none increases risk as much as does the presence of the APOE ε4 allele. APOE is a regulator of lipoprotein metabolism that binds soluble Aβ and influences its clearance and aggregation. The presence of ε4 alleles accelerate Aβ deposition; one allele increases AD risk three-hold, and two alleles increases AD risk twelve-fold. For typical amnestic AD, the presence of an ε4 allele decreases the age of onset (but, paradoxically, within EOAD it occurs within the older range109); whereas, ε3 alleles tend to be found in variant phenotypes of EOAD, and ε2 alleles decrease the risk or delays AD. Other rare variants that increase risk for EOAD occur in genes including SORL1 (sortilin-related receptor, L(DLR class)), a neuronal APOE receptor that plays a protective role against the secretion of Aβ110; the ABCA7 (ATP binding cassette subfamily A member 7), which was present in 6.6% of EOAD patients compared to only 2.0% of controls111; and coding variants such as PLD3 (phospholipase D Family Member 3), which catalyzes the hydrolysis of membrane phospholipid, and TREM2 (Triggering Receptor Expressed On Myeloid Cells 2), a receptor on microglia that stimulates phagocytosis and suppresses inflammation 101.
Neuropathology
The neuropathology of EOAD resembles that of LOAD in the presence of neuritic plaques and neurofibrillary tangles, but differs in a number of respects. First, there is a greater likelihood of hippocampal sparing and more involvement of neocortex, particularly parietal and occipitoparietal, but also, to a lesser extent, frontal44. Second, despite early Aβ deposition, the clinicopathological manifestations are driven more by tau than by Aβ, with a relatively greater tau burden in EOAD than in LOAD. For example, in lvPPA the regional tau deposition in the left inferior parietal lobule is more closely linked to hypometabolism than amyloid density 112, and in PCA the best correspondence with clinical symptoms is with the tau burden113. Although unclear, it is possible that EOAD variants could result from differences in the “strains” of soluble Aβ or oligomeric Aβ. Third, EOAD variants may also depend on greater WM involvement and selective vulnerability of long, projection neurons which connect higher association cortex76.
Neural Networks
The human brain is organized as separate networks, and there is growing evidence that AD targets and spreads along network pathways with different networks being involved in different clinicopathological forms of AD 97,114,115. 116–118. Progressive changes and disconnection in neural networks are present before symptom onset in AD and before neuronal loss and regional atrophy 119–122. All forms of AD may begin with amyloid-β (Aβ) deposition in the precuneus and related areas years before clinical symptoms, and this amyloid deposition has a permissive effect on tau and NFT spread 123–125. The network degeneration hypothesis postulates that Aβ promotes the spread of pathological forms of tau trans-synaptically along networks, which, in typical amnestic AD follows the “Braak and Braak progression” from the MTL-entorhinal-hippocampus to limbic and then neocortical regions probably along the DMN 126–131. EOAD variant phenotypes reflect differences from typical amnestic AD in probable trans-synaptic spread along alternate fronto-parietal neural networks such as the central executive network 126,132,133. In sum, the literature suggests that Type 2 EOAD proceeds to earlier and more prominent NFTs in posterior neocortex compared to LOAD, and involves alternate, vulnerable neural networks rather than the DMN.27,34,38,73,78,91,134–148.
Management
Management is similar to that for LOAD but with emphasis on targeting the specific cognitive areas involved and more age-appropriate psychosocial support and education. Targeting cognition includes speech therapy for language impairment, interventions for the partially-sighted for PCA, and occupational therapy for ideomotor apraxia. There must be greater psychosocial support for these patients, who are often in a highly productive time of their life, maintaining jobs and careers and supporting families. Clinicians can help these patients and their families by providing information, education, and resources on these frequently poorly understood manifestations of AD. Clinicians must also take care to provide services, such as support groups, that are specifically for those with young-onset dementia, rather than the elderly. Often the best support groups and programs are even more specifically targeted to the EOAD phenotype. For example, groups of lvPPA caregivers may discuss methods to improve communication, and groups of PCA caregivers may discuss methods to improve visual functioning in the home. As for medications, non-memory symptoms may not significantly respond to acetylcholinesterase inhibitors or memantine, but, considering their safety, these interventions are worth trying in these patients. Finally, in the absence of disease-modifying interventions, patients and families usually appreciate the opportunity to participate in clinical drug trials.
CONCLUSIONS
Early-onset Alzheimer’s disease (EOAD) with onset <65 years of age, while overshadowed by the more common late-onset AD (LOAD), differs significantly from LOAD. EOAD comprises about 5% of AD and is associated with delays in diagnosis, distress and confusion over symptoms, an aggressive or problematic course, and age-related psychosocial needs. One source of confusion is that a substantial percentage of EOAD are phenotypic variants (“Type 2 AD”) that differ from the usual memory-disordered presentation of typical AD. These variants include lvPPA, PCA, PIA and corticobasal syndrome from AD, and behavioral/dysexecutive AD. In addition, there is a small percentage (1.5–5%) of persons with EOAD in whom the disease is inherited as an autosomal dominant trait due to identifiable gene mutations.
Patients with EOAD overall have greater parietal atrophy, more white matter disturbances, and less hippocampal volume loss, compared to those with LOAD. The phenotypic variants have atrophy and white matter changes in corresponding cognitive areas of the brain. On neuropathology, patients with EOAD overall have disproportionate regional amyloid and tau accumulation in posterior neocortex. Abnormal tau drives this neocortical pathology with greater posterior cortical NFTs per gray matter atrophy compared to typical AD. The focal neocortical burden of NFTs is greater in left hemisphere language areas in lvPPA and in visual neocortex in PCA. The variants tended to hippocampal sparing compared to typical AD, and, in more advanced stages, the pattern of atrophy converged across the variants149.
Neural network differences characterize EOAD and the different phenotypes. Compared to LOAD, the phenotypic variants of EOAD involve alternate, fronto-parietal and syndrome-specific neural networks rather than the posterior DMN as in typical AD. Language networks are affected in lvPPA, visual networks in PCA, and the posterior cingulate cortex-hippocampal circuit in amnestic EOAD and LOAD. In Type 2 AD there may be primarily spread along alternate neural networks rather than from mesiotemporal entorhinal cortex along the DMN as in more typical amnestic AD.
These scientific advancements in our understanding of EOAD and its variants is only a first step in advancing our management of this disorder, which is particularly devastating because of its onset in middle life. Currently, the management is similar to that for LOAD with the addition of targeting interventions for specific cognitive impairments, the provision of education on the disease, and psychosocial support aimed at the unique patient and caregiver problems due to EOAD. The advancements in our understanding of the neurobiology of EOAD holds great promise for the development of therapeutic interventions specifically targeted to the initiation, spread, and expression of the neuropathology of this disease.
KEY POINTS.
EOAD is not just LOAD at a younger age; there are substantial differences between these two categories of Alzheimer’s disease.
Compared to LOAD, EOAD has greater neocortical pathology, particularly in parietal cortex, greater tau compared to amyloid burden, and less hippocampal disease.
Up to 50% or more of patients with EOAD have non-amnestic, phenotypic variants, including logopenic variant primary progressive aphasia, posterior cortical atrophy, progressive ideomotor apraxia, behavioral/dysexecutive AD, corticobasal syndrome, and others. These may be conceptualized as “Type 2 AD.”
Compared to LOAD, the phenotypic variants of EOAD preferentially involve alternate, fronto-parietal neural networks rather than the posterior default mode network.
The management of EOAD differs from LOAD in the emphasis on targeted cognitive interventions and age-appropriate psychosocial support.
Acknowledgments
Funding Source (author P.I.): NIA R01AG050967; NIA R01 AG034499.
Footnotes
DISCLOSURE STATEMENT
The Author has nothing to disclose. The author has no commercial or financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer’s disease. Lancet. 1997;349(9064):1546–1549. doi: 10.1016/S0140-6736(96)10203-8. [DOI] [PubMed] [Google Scholar]
- 2.Terry RD, Davies P. Dementia of the Alzheimer type. Annu Rev Neurosci. 1980;3:77–95. doi: 10.1146/annurev.ne.03.030180.000453. [DOI] [PubMed] [Google Scholar]
- 3.Zhu XC, Tan L, Wang HF, et al. Rate of early onset Alzheimer’s disease: a systematic review and meta-analysis. Annals of translational medicine. 2015;3(3):38. doi: 10.3978/j.issn.2305-5839.2015.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickel H, Burger K, Hampel H, et al. Presenile dementia in memory clinics--incidence rates and clinical features. Nervenarzt. 2006;77(9):1079–1085. doi: 10.1007/s00115-005-1949-y. [DOI] [PubMed] [Google Scholar]
- 5.Renvoize E, Hanson M, Dale M. Prevalence and causes of young onset dementia in an English health district. Int J Geriatr Psychiatry. 2011;26(1):106–107. doi: 10.1002/gps.2456. [DOI] [PubMed] [Google Scholar]
- 6.Alzheimer’s A. Early-Onset Dementia: A National Challenge, A Future Crisis. Washington, D.C: Alzheimer’s Association; 2006. [Google Scholar]
- 7.Lambert MA, Bickel H, Prince M, et al. Estimating the burden of early onset dementia; systematic review of disease prevalence. Eur J Neurol. 2014;21(4):563–569. doi: 10.1111/ene.12325. [DOI] [PubMed] [Google Scholar]
- 8.van Vliet D, de Vugt ME, Bakker C, et al. Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med. 2013;43(2):423–432. doi: 10.1017/S0033291712001122. [DOI] [PubMed] [Google Scholar]
- 9.Moschetti K, Barragan N, Basurto-Davila R, Cummings PL, Sorvillo F, Kuo T. Mortality and Productivity Losses From Alzheimer Disease Among US Adults Aged 40 to 64 Years, 1999 to 2010. Alzheimer Dis Assoc Disord. 2015;29(2):165–168. doi: 10.1097/WAD.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson H, Fereshtehnejad SM, Falahati F, Farahmand B, Religa D, Eriksdotter M. Differences in routine clinical practice between early and late onset Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2014 doi: 10.3233/JAD-132273. [DOI] [PubMed] [Google Scholar]
- 11.Nyberg J, Aberg MA, Schioler L, et al. Cardiovascular and cognitive fitness at age 18 and risk of early-onset dementia. Brain : a journal of neurology. 2014;137(Pt 5):1514–1523. doi: 10.1093/brain/awu041. [DOI] [PubMed] [Google Scholar]
- 12.Mendez MF, Paholpak P, Lin A, Zhang JY, Teng E. Prevalence of Traumatic Brain Injury in Early Versus Late-Onset Alzheimer’s Disease. J Alzheimers Dis. 2015;47(4):985–993. doi: 10.3233/JAD-143207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemerson G, Walsh S, Isaac C. Towards living well with young onset dementia: An exploration of coping from the perspective of those diagnosed. Dementia (London) 2013 doi: 10.1177/1471301212474149. [DOI] [PubMed] [Google Scholar]
- 14.Migliaccio R, Agosta F, Toba MN, et al. Brain networks in posterior cortical atrophy: a single case tractography study and literature review. Cortex. 2012;48(10):1298–1309. doi: 10.1016/j.cortex.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser NC, Melrose RJ, Liu C, et al. Neuropsychological and neuroimaging markers in early versus late-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2012;27(7):520–529. doi: 10.1177/1533317512459798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducharme F, Kergoat MJ, Antoine P, Pasquier F, Coulombe R. The unique experience of spouses in early-onset dementia. American journal of Alzheimer’s disease and other dementias. 2013;28(6):634–641. doi: 10.1177/1533317513494443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosness TA, Barca ML, Engedal K. Occurrence of depression and its correlates in early onset dementia patients. Int J Geriatr Psychiatry. 2010;25(7):704–711. doi: 10.1002/gps.2411. [DOI] [PubMed] [Google Scholar]
- 18.Gerritsen AA, Bakker C, Verhey FR, de Vugt ME, Melis RJ, Koopmans RT. Prevalence of Comorbidity in Patients With Young-Onset Alzheimer Disease Compared With Late-Onset: A Comparative Cohort Study. Journal of the American Medical Directors Association. 2016;17(4):318–323. doi: 10.1016/j.jamda.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Jarvik L, LaRue A, Blacker D, et al. Children of persons with Alzheimer disease: what does the future hold? Alzheimer disease and associated disorders. 2008;22(1):6–20. doi: 10.1097/WAD.0b013e31816653ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koedam EL, Pijnenburg YA, Deeg DJ, et al. Early-onset dementia is associated with higher mortality. Dement Geriatr Cogn Disord. 2008;26(2):147–152. doi: 10.1159/000149585. [DOI] [PubMed] [Google Scholar]
- 21.Schott JM, Ridha BH, Crutch SJ, et al. Apolipoprotein e genotype modifies the phenotype of Alzheimer disease. Arch Neurol. 2006;63(1):155–156. doi: 10.1001/archneur.63.1.155. [DOI] [PubMed] [Google Scholar]
- 22.Panegyres P, CHY K. Differences between early and late onset Alzheimer’s disease. American Journal of Neurodegenerative Disease. 2013;2(4):6. [PMC free article] [PubMed] [Google Scholar]
- 23.Smits LL, Pijnenburg YA, van der Vlies AE, et al. Early onset APOE E4-negative Alzheimer’s disease patients show faster cognitive decline on non-memory domains. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2015 doi: 10.1016/j.euroneuro.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Stanley K, Walker Z. Do patients with young onset Alzheimer’s disease deteriorate faster than those with late onset Alzheimer’s disease? A review of the literature. International psychogeriatrics / IPA. 2014;26(12):1945–1953. doi: 10.1017/S1041610214001173. [DOI] [PubMed] [Google Scholar]
- 25.Palasi A, Gutierrez-Iglesias B, Alegret M, et al. Differentiated clinical presentation of early and late-onset Alzheimer’s disease: is 65 years of age providing a reliable threshold? J Neurol. 2015;262(5):1238–1246. doi: 10.1007/s00415-015-7698-3. [DOI] [PubMed] [Google Scholar]
- 26.Joubert S, Gour N, Guedj E, et al. Early-onset and late-onset Alzheimer’s disease are associated with distinct patterns of memory impairment. Cortex. 2016;74:217–232. doi: 10.1016/j.cortex.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Cho H, Jeon S, Kang SJ, et al. Longitudinal changes of cortical thickness in early-versus late-onset Alzheimer’s disease. Neurobiology of aging. 2013;34(7):1921 e1929–1921 e1915. doi: 10.1016/j.neurobiolaging.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Migliaccio R, Agosta F, Possin KL, et al. Mapping the Progression of Atrophy in Early- and Late-Onset Alzheimer’s Disease. J Alzheimers Dis. 2015 doi: 10.3233/JAD-142292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamelin L, Bertoux M, Bottlaender M, et al. Sulcal morphology as a new imaging marker for the diagnosis of early onset Alzheimer’s disease. Neurobiol Aging. 2015;36(11):2932–2939. doi: 10.1016/j.neurobiolaging.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Chiaravalloti A, Koch G, Toniolo S, et al. Comparison between Early-Onset and Late-Onset Alzheimer’s Disease Patients with Amnestic Presentation: CSF and (18)F-FDG PET Study. Dementia and geriatric cognitive disorders extra. 2016;6(1):108–119. doi: 10.1159/000441776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballarini T, Iaccarino L, Magnani G, et al. Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer’s disease. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daianu M, Mendez MF, Baboyan VG, et al. An advanced white matter tract analysis in frontotemporal dementia and early-onset Alzheimer’s disease. Brain imaging and behavior. 2015 doi: 10.1007/s11682-015-9458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MJ, Seo SW, Kim ST, Lee JM, Na DL. Diffusion Tensor Changes According to Age at Onset and Apolipoprotein E Genotype in Alzheimer Disease. Alzheimer Dis Assoc Disord. 2016 doi: 10.1097/WAD.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 34.Canu E, Agosta F, Spinelli EG, et al. White matter microstructural damage in Alzheimer’s disease at different ages of onset. Neurobiol Aging. 2013;34(10):2331–2340. doi: 10.1016/j.neurobiolaging.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Migliaccio R, Agosta F, Toba MN, et al. Brain networks in posterior cortical atrophy: A single case tractography study and literature review. Cortex. 2011 doi: 10.1016/j.cortex.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caso F, Agosta F, Mattavelli D, et al. White Matter Degeneration in Atypical Alzheimer Disease. Radiology. 2015;277(1):162–172. doi: 10.1148/radiol.2015142766. [DOI] [PubMed] [Google Scholar]
- 37.Daianu M, Jahanshad N, Mendez MF, Bartzokis G, Jimenez EE, Thompson PM. Communication of brain network core connections altered in behavioral variant frontotemporal dementia but possibly preserved in early-onset Alzheimer’s disease. Proceedings of SPIE--the International Society for Optical Engineering. 2015:9413. doi: 10.1117/12.2082352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stopford CL, Snowden JS, Thompson JC, Neary D. Variability in cognitive presentation of Alzheimer’s disease. Cortex. 2008;44(2):185–195. doi: 10.1016/j.cortex.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130(Pt 10):2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 40.Davidson Y, Gibbons L, Pritchard A, et al. Apolipoprotein E epsilon4 allele frequency and age at onset of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23(1):60–66. doi: 10.1159/000097038. [DOI] [PubMed] [Google Scholar]
- 41.van der Flier WM, Pijnenburg YA, Fox NC, Scheltens P. Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE varepsilon4 allele. Lancet Neurol. 2011;10(3):280–288. doi: 10.1016/S1474-4422(10)70306-9. [DOI] [PubMed] [Google Scholar]
- 42.Palasi A, Gutierrez-Iglesias B, Alegret M, et al. Differentiated clinical presentation of early and late-onset Alzheimer’s disease: is 65 years of age providing a reliable threshold? J Neurol. 2015 doi: 10.1007/s00415-015-7698-3. [DOI] [PubMed] [Google Scholar]
- 43.Park HK, Choi SH, Park SA, et al. Cognitive profiles and neuropsychiatric symptoms in Korean early-onset Alzheimer’s disease patients: a CREDOS study. J Alzheimers Dis. 2015;44(2):661–673. doi: 10.3233/JAD-141011. [DOI] [PubMed] [Google Scholar]
- 44.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis. 2010;19(4):1401–1408. doi: 10.3233/JAD-2010-1337. [DOI] [PubMed] [Google Scholar]
- 46.Smits LL, Pijnenburg YA, Koedam EL, et al. Early Onset Alzheimer’s Disease is Associated with a Distinct Neuropsychological Profile. J Alzheimers Dis. 2012 doi: 10.3233/JAD-2012-111934. [DOI] [PubMed] [Google Scholar]
- 47.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai PH, Teng E, Liu C, Mendez MF. Posterior cortical atrophy: evidence for discrete syndromes of early-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2011;26(5):413–418. doi: 10.1177/1533317511418955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14(1):33–40. doi: 10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- 50.Ossenkoppele R, Pijnenburg YA, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain. 2015;138(Pt 9):2732–2749. doi: 10.1093/brain/awv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70(2):327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magnin E, Sylvestre G, Lenoir F, et al. Logopenic syndrome in posterior cortical atrophy. J Neurol. 2013;260(2):528–533. doi: 10.1007/s00415-012-6671-7. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed S, de Jager CA, Haigh AM, Garrard P. Logopenic aphasia in Alzheimer’s disease: clinical variant or clinical feature? J Neurol Neurosurg Psychiatry. 2012;83(11):1056–1062. doi: 10.1136/jnnp-2012-302798. [DOI] [PubMed] [Google Scholar]
- 54.Cerami C, Crespi C, Della Rosa PA, et al. Brain Changes within the Visuo-Spatial Attentional Network in Posterior Cortical Atrophy. J Alzheimers Dis. 2014 doi: 10.3233/JAD-141275. [DOI] [PubMed] [Google Scholar]
- 55.Gour N, Felician O, Didic M, et al. Functional connectivity changes differ in early and late-onset alzheimer’s disease. Hum Brain Mapp. 2014;35(7):2978–2994. doi: 10.1002/hbm.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laforce R, Jr, Tosun D, Ghosh P, et al. Parallel ICA of FDG-PET and PiB-PET in three conditions with underlying Alzheimer’s pathology. Neuroimage Clin. 2014;4:508–516. doi: 10.1016/j.nicl.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehmann M, Madison CM, Ghosh PM, et al. Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013;110(28):11606–11611. doi: 10.1073/pnas.1221536110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blautzik J, Keeser D, Berman A, et al. Long-term test-retest reliability of resting-state networks in healthy elderly subjects and with amnestic mild cognitive impairment patients. Journal of Alzheimer’s disease : JAD. 2013;34(3):741–754. doi: 10.3233/JAD-111970. [DOI] [PubMed] [Google Scholar]
- 59.Dennis EL, Thompson PM. Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol Rev. 2014;24(1):49–62. doi: 10.1007/s11065-014-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sorg C, Riedl V, Perneczky R, Kurz A, Wohlschlager AM. Impact of Alzheimer’s disease on the functional connectivity of spontaneous brain activity. Curr Alzheimer Res. 2009;6(6):541–553. doi: 10.2174/156720509790147106. [DOI] [PubMed] [Google Scholar]
- 61.Krajcovicova L, Mikl M, Marecek R, Rektorova I. Disturbed Default Mode Network Connectivity Patterns in Alzheimer’s Disease Associated with Visual Processing. Journal of Alzheimer’s disease : JAD. 2014 doi: 10.3233/JAD-131208. [DOI] [PubMed] [Google Scholar]
- 62.Hampel H. Amyloid-beta and cognition in aging and Alzheimer’s disease: molecular and neurophysiological mechanisms. Journal of Alzheimer’s disease : JAD. 2013;33(Suppl 1):S79–86. doi: 10.3233/JAD-2012-129003. [DOI] [PubMed] [Google Scholar]
- 63.Sperling R. Potential of functional MRI as a biomarker in early Alzheimer’s disease. Neurobiology of aging. 2011;32(Suppl 1):S37–43. doi: 10.1016/j.neurobiolaging.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiology of aging. 2012;33(8):1564–1578. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 65.de Haan W, van der Flier WM, Koene T, Smits LL, Scheltens P, Stam CJ. Disrupted modular brain dynamics reflect cognitive dysfunction in Alzheimer’s disease. Neuroimage. 2012;59(4):3085–3093. doi: 10.1016/j.neuroimage.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 66.Das SR, Pluta J, Mancuso L, et al. Increased functional connectivity within medial temporal lobe in mild cognitive impairment. Hippocampus. 2013;23(1):1–6. doi: 10.1002/hipo.22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lehmann M, Madison C, Ghosh PM, et al. Loss of functional connectivity is greater outside the default mode network in nonfamilial early-onset Alzheimer’s disease variants. Neurobiol Aging. 2015;36(10):2678–2686. doi: 10.1016/j.neurobiolaging.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitwell JL, Jones DT, Duffy JR, et al. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer’s dementia. Neurobiol Aging. 2015;36(3):1245–1252. doi: 10.1016/j.neurobiolaging.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neufang S, Akhrif A, Riedl V, et al. Disconnection of frontal and parietal areas contributes to impaired attention in very early Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2011;25(2):309–321. doi: 10.3233/JAD-2011-102154. [DOI] [PubMed] [Google Scholar]
- 70.Balthazar ML, Pereira FR, Lopes TM, et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp. 2014;35(4):1237–1246. doi: 10.1002/hbm.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahoney CJ, Malone IB, Ridgway GR, et al. White matter tract signatures of the progressive aphasias. Neurobiol Aging. 2013;34(6):1687–1699. doi: 10.1016/j.neurobiolaging.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frisoni GB, Pievani M, Testa C, et al. The topography of grey matter involvement in early and late onset Alzheimer’s disease. Brain. 2007;130(Pt 3):720–730. doi: 10.1093/brain/awl377. [DOI] [PubMed] [Google Scholar]
- 74.Kalpouzos G, Eustache F, de la Sayette V, Viader F, Chetelat G, Desgranges B. Working memory and FDG-PET dissociate early and late onset Alzheimer disease patients. Journal of neurology. 2005;252(5):548–558. doi: 10.1007/s00415-005-0685-3. [DOI] [PubMed] [Google Scholar]
- 75.Rogalski E, Johnson N, Weintraub S, Mesulam M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol. 2008;65(2):244–248. doi: 10.1001/archneurol.2007.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller ZA, Mandelli ML, Rankin KP, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013;136(Pt 11):3461–3473. doi: 10.1093/brain/awt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seifan A, Assuras S, Huey ED, Mez J, Tsapanou A, Caccappolo E. Childhood Learning Disabilities and Atypical Dementia: A Retrospective Chart Review. PLoS One. 2015;10(6):e0129919. doi: 10.1371/journal.pone.0129919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73(19):1571–1578. doi: 10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64(4):388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63(6):709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galantucci S, Tartaglia MC, Wilson SM, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134(Pt 10):3011–3029. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leyton CE, Piguet O, Savage S, Burrell J, Hodges JR. The neural basis of logopenic progressive aphasia. J Alzheimers Dis. 2012;32(4):1051–1059. doi: 10.3233/JAD-2012-121042. [DOI] [PubMed] [Google Scholar]
- 83.Baldo JV, Katseff S, Dronkers NF. Brain Regions Underlying Repetition and Auditory-Verbal Short-term Memory Deficits in Aphasia: Evidence from Voxel-based Lesion Symptom Mapping. Aphasiology. 2012;26(3–4):338–354. doi: 10.1080/02687038.2011.602391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer AM, Snider SF, Campbell RE, Friedman RB. Phonological short-term memory in logopenic variant primary progressive aphasia and mild Alzheimer’s disease. Cortex. 2015;71:183–189. doi: 10.1016/j.cortex.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45(7):789–793. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- 86.Ahmed S, Baker I, Husain M, et al. Memory Impairment at Initial Clinical Presentation in Posterior Cortical Atrophy. J Alzheimers Dis. 2016;52(4):1245–1250. doi: 10.3233/JAD-160018. [DOI] [PubMed] [Google Scholar]
- 87.Crutch SJ, Schott JM, Rabinovici GD, et al. Shining a light on posterior cortical atrophy. Alzheimers Dement. 2012 doi: 10.1016/j.jalz.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Tang-Wai DF, Graff-Radford NR. Looking into posterior cortical atrophy: providing insight into Alzheimer disease. Neurology. 2011;76(21):1778–1779. doi: 10.1212/WNL.0b013e31821ccd4f. [DOI] [PubMed] [Google Scholar]
- 89.Migliaccio R, Agosta F, Scola E, et al. Ventral and dorsal visual streams in posterior cortical atrophy: A DT MRI study. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meek BP, Shelton P, Marotta JJ. Posterior cortical atrophy: visuomotor deficits in reaching and grasping. Front Hum Neurosci. 2013;7:294. doi: 10.3389/fnhum.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gefen T, Gasho K, Rademaker A, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012;135(Pt 5):1554–1565. doi: 10.1093/brain/aws076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- 93.Carrasquillo MM, Khan QU, Murray ME, et al. Late-onset Alzheimer disease genetic variants in posterior cortical atrophy and posterior AD. Neurology. 2014;82(16):1455–1462. doi: 10.1212/WNL.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ridgway GR, Lehmann M, Barnes J, et al. Early-onset Alzheimer disease clinical variants: Multivariate analyses of cortical thickness. Neurology. 2012;79(1):80–84. doi: 10.1212/WNL.0b013e31825dce28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lehmann M, Koedam EL, Barnes J, et al. Posterior cerebral atrophy in the absence of medial temporal lobe atrophy in pathologically-confirmed Alzheimer’s disease. Neurobiology of aging. 2012;33(3):627 e621–627 e612. doi: 10.1016/j.neurobiolaging.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ossenkoppele R, Mattsson N, Teunissen CE, et al. Cerebrospinal fluid biomarkers and cerebral atrophy in distinct clinical variants of probable Alzheimer’s disease. Neurobiol Aging. 2015;36(8):2340–2347. doi: 10.1016/j.neurobiolaging.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Teng E, Yamasaki TR, Tran M, Hsiao JJ, Sultzer DL, Mendez MF. Cerebrospinal fluid biomarkers in clinical subtypes of early-onset Alzheimer’s disease. Dement Geriatr Cogn Disord. 2014;37(5–6):307–314. doi: 10.1159/000355555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Molinuevo JL, Blennow K, Dubois B, et al. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 2014;10(6):808–817. doi: 10.1016/j.jalz.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fagan AM. What does it mean to be ‘amyloid-positive’? Brain. 2015;138(Pt 3):514–516. doi: 10.1093/brain/awu387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jarmolowicz AI, Chen HY, Panegyres PK. The patterns of inheritance in early-onset dementia: Alzheimer’s disease and frontotemporal dementia. Am J Alzheimers Dis Other Demen. 2015;30(3):299–306. doi: 10.1177/1533317514545825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Joshi A, Ringman JM, Lee AS, Juarez KO, Mendez MF. Comparison of clinical characteristics between familial and non-familial early onset Alzheimer’s disease. J Neurol. 2012 doi: 10.1007/s00415-012-6481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muller U, Winter P, Graeber MB. A presenilin 1 mutation in the first case of Alzheimer’s disease. Lancet Neurol. 2013;12(2):129–130. doi: 10.1016/S1474-4422(12)70307-1. [DOI] [PubMed] [Google Scholar]
- 105.Ringman JM. Are Late-Onset Autosomal Dominant and Sporadic Alzheimer Disease “Separate but Equal”? JAMA neurology. 2016 doi: 10.1001/jamaneurol.2016.1633. [DOI] [PubMed] [Google Scholar]
- 106.Nicolas G, Wallon D, Charbonnier C, et al. Screening of dementia genes by whole-exome sequencing in early-onset Alzheimer disease: input and lessons. European journal of human genetics : EJHG. 2016;24(5):710–716. doi: 10.1038/ejhg.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blauwendraat C, Wilke C, Jansen IE, et al. Pilot whole-exome sequencing of a German early-onset Alzheimer’s disease cohort reveals a substantial frequency of PSEN2 variants. Neurobiol Aging. 2016;37:208 e211–207. doi: 10.1016/j.neurobiolaging.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 108.Barber IS, Garcia-Cardenas JM, Sakdapanichkul C, et al. Screening exons 16 and 17 of the amyloid precursor protein gene in sporadic early-onset Alzheimer’s disease. Neurobiol Aging. 2016;39:220, e221–227. doi: 10.1016/j.neurobiolaging.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Luca V, Orfei MD, Gaudenzi S, Caltagirone C, Spalletta G. Inverse effect of the APOE epsilon4 allele in late- and early-onset Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 2015 doi: 10.1007/s00406-015-0663-4. [DOI] [PubMed] [Google Scholar]
- 110.Nicolas G, Charbonnier C, Wallon D, et al. SORL1 rare variants: a major risk factor for familial early-onset Alzheimer’s disease. Molecular psychiatry. 2016;21(6):831–836. doi: 10.1038/mp.2015.121. [DOI] [PubMed] [Google Scholar]
- 111.Le Guennec K, Nicolas G, Quenez O, et al. ABCA7 rare variants and Alzheimer disease risk. Neurology. 2016;86(23):2134–2137. doi: 10.1212/WNL.0000000000002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pascual B, Masdeu JC. Tau, amyloid, and hypometabolism in the logopenic variant of primary progressive aphasia. Neurology. 2016;86(5):487–488. doi: 10.1212/WNL.0000000000002340. [DOI] [PubMed] [Google Scholar]
- 113.Ossenkoppele R, Schonhaut DR, Baker SL, et al. Tau, amyloid, and hypometabolism in a patient with posterior cortical atrophy. Ann Neurol. 2015;77(2):338–342. doi: 10.1002/ana.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seppala TT, Nerg O, Koivisto AM, et al. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology. 2012;78(20):1568–1575. doi: 10.1212/WNL.0b013e3182563bd0. [DOI] [PubMed] [Google Scholar]
- 115.Dai Z, He Y. Disrupted structural and functional brain connectomes in mild cognitive impairment and Alzheimer’s disease. Neurosci Bull. 2014;30(2):217–232. doi: 10.1007/s12264-013-1421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bokde AL, Ewers M, Hampel H. Assessing neuronal networks: understanding Alzheimer’s disease. Prog Neurobiol. 2009;89(2):125–133. doi: 10.1016/j.pneurobio.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Sun Y, Yin Q, Fang R, et al. Disrupted functional brain connectivity and its association to structural connectivity in amnestic mild cognitive impairment and Alzheimer’s disease. PLoS One. 2014;9(5):e96505. doi: 10.1371/journal.pone.0096505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pineda-Pardo JA, Garces P, Lopez ME, et al. White matter damage disorganizes brain functional networks in amnestic mild cognitive impairment. Brain Connect. 2014;4(5):312–322. doi: 10.1089/brain.2013.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Savioz A, Leuba G, Vallet PG, Walzer C. Contribution of neural networks to Alzheimer disease’s progression. Brain Res Bull. 2009;80(4–5):309–314. doi: 10.1016/j.brainresbull.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 120.Brier MR, Thomas JB, Fagan AM, et al. Functional connectivity and graph theory in preclinical Alzheimer’s disease. Neurobiology of aging. 2014;35(4):757–768. doi: 10.1016/j.neurobiolaging.2013.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.D’Amelio M, Rossini PM. Brain excitability and connectivity of neuronal assemblies in Alzheimer’s disease: from animal models to human findings. Prog Neurobiol. 2012;99(1):42–60. doi: 10.1016/j.pneurobio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 122.Jacobs HI, Radua J, Luckmann HC, Sack AT. Meta-analysis of functional network alterations in Alzheimer’s disease: toward a network biomarker. Neurosci Biobehav Rev. 2013;37(5):753–765. doi: 10.1016/j.neubiorev.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 123.Mattsson N, Insel PS, Nosheny R, et al. Emerging beta-Amyloid Pathology and Accelerated Cortical Atrophy. JAMA neurology. 2014 doi: 10.1001/jamaneurol.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 127.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129(Pt 11):3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 128.Spreng RN, Turner GR. Structural covariance of the default network in healthy and pathological aging. J Neurosci. 2013;33(38):15226–15234. doi: 10.1523/JNEUROSCI.2261-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82(4):756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jack CR, Jr, Wiste HJ, Knopman DS, et al. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82(18):1605–1612. doi: 10.1212/WNL.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013;80(6):1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de Calignon A, Polydoro M, Suarez-Calvet M, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73(4):685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang-Wai D, Mapstone M. What are we seeing? Is posterior cortical atrophy just Alzheimer disease? Neurology. 2006;66(3):300–301. doi: 10.1212/01.wnl.0000202093.81603.d8. [DOI] [PubMed] [Google Scholar]
- 135.Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122(6):703–713. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 136.Malkani RG, Dickson DW, Simuni T. Hippocampal-sparing Alzheimer’s disease presenting as corticobasal syndrome. Parkinsonism Relat Disord. 2011 doi: 10.1016/j.parkreldis.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 137.Ossenkoppele R, Zwan MD, Tolboom N, et al. Amyloid burden and metabolic function in early-onset Alzheimer’s disease: parietal lobe involvement. Brain. 2012;135(Pt 7):2115–2125. doi: 10.1093/brain/aws113. [DOI] [PubMed] [Google Scholar]
- 138.Shibuya Y, Kawakatsu S, Hayashi H, et al. Comparison of entorhinal cortex atrophy between early-onset and late-onset Alzheimer’s disease using the VSRAD, a specific and sensitive voxel-based morphometry. Int J Geriatr Psychiatry. 2013;28(4):372–376. doi: 10.1002/gps.3834. [DOI] [PubMed] [Google Scholar]
- 139.Ishii K, Kawachi T, Sasaki H, et al. Voxel-based morphometric comparison between early- and late-onset mild Alzheimer’s disease and assessment of diagnostic performance of z score images. AJNR Am J Neuroradiol. 2005;26(2):333–340. [PMC free article] [PubMed] [Google Scholar]
- 140.Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain. 2010;133(Pt 2):512–528. doi: 10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shiino A, Watanabe T, Maeda K, Kotani E, Akiguchi I, Matsuda M. Four subgroups of Alzheimer’s disease based on patterns of atrophy using VBM and a unique pattern for early onset disease. Neuroimage. 2006;33(1):17–26. doi: 10.1016/j.neuroimage.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 142.Kim EJ, Cho SS, Jeong Y, et al. Glucose metabolism in early onset versus late onset Alzheimer’s disease: an SPM analysis of 120 patients. Brain. 2005;128(Pt 8):1790–1801. doi: 10.1093/brain/awh539. [DOI] [PubMed] [Google Scholar]
- 143.Sakamoto S, Ishii K, Sasaki M, et al. Differences in cerebral metabolic impairment between early and late onset types of Alzheimer’s disease. J Neurol Sci. 2002;200(1–2):27–32. doi: 10.1016/s0022-510x(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 144.Mielke R, Herholz K, Grond M, Kessler J, Heiss WD. Differences of regional cerebral glucose metabolism between presenile and senile dementia of Alzheimer type. Neurobiol Aging. 1992;13(1):93–98. doi: 10.1016/0197-4580(92)90015-p. [DOI] [PubMed] [Google Scholar]
- 145.Karas G, Scheltens P, Rombouts S, et al. Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology. 2007;49(12):967–976. doi: 10.1007/s00234-007-0269-2. [DOI] [PubMed] [Google Scholar]
- 146.Whitwell JL, Jack CR, Jr, Przybelski SA, et al. Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol Aging. 2011;32(9):1531–1541. doi: 10.1016/j.neurobiolaging.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moller C, Vrenken H, Jiskoot L, et al. Different patterns of gray matter atrophy in early- and late-onset Alzheimer’s disease. Neurobiology of aging. 2013;34(8):2014–2022. doi: 10.1016/j.neurobiolaging.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 148.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Early-onset Alzheimer’s disease is associated with greater pathologic burden. J Geriatr Psychiatry Neurol. 2007;20(1):29–33. doi: 10.1177/0891988706297086. [DOI] [PubMed] [Google Scholar]
- 149.Ossenkoppele R, Cohn-Sheehy BI, La Joie R, et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp. 2015;36(11):4421–4437. doi: 10.1002/hbm.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mendez MF, Lee AS, Joshi A, Shapira JS. Nonamnestic Presentations of Early-Onset Alzheimer’s Disease. American journal of Alzheimer’s disease and other dementias. 2012 doi: 10.1177/1533317512454711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer’s disease and genetic risk. Cortex. 2007;43(7):835–845. doi: 10.1016/s0010-9452(08)70683-x. [DOI] [PubMed] [Google Scholar]
- 152.Binetti G, Magni E, Padovani A, Cappa SF, Bianchetti A, Trabucchi M. Executive dysfunction in early Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;60(1):91–93. doi: 10.1136/jnnp.60.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56(10):1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 154.Swanberg MM, Tractenberg RE, Mohs R, Thal LJ, Cummings JL. Executive dysfunction in Alzheimer disease. Arch Neurol. 2004;61(4):556–560. doi: 10.1001/archneur.61.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Woodward M, Jacova C, Black SE, Kertesz A, Mackenzie IR, Feldman H. Differentiating the frontal variant of Alzheimer’s disease. Int J Geriatr Psychiatry. 2010;25(7):732–738. doi: 10.1002/gps.2415. [DOI] [PubMed] [Google Scholar]