The new and noteworthy findings are that 1) in rodents, obesity-related deficits in insulin-mediated vasodilation are associated with increased influence of insulin-stimulated ET-1 and depressed influence of insulin-stimulated NOS and 2) a physical activity intervention, initiated after the onset of disease, restores insulin-mediated vasodilation, likely by normalizing insulin-stimulated ET-1 and NOS balance. These data demonstrate that the treatment effects of chronic exercise on insulin-mediated vasodilation extend beyond active skeletal muscle vasculature and include the cerebrovasculature.
Keywords: insulin, cerebrovascular, obesity, physical activity, NO, endothelin
Abstract
This study tested the hypotheses that obesity-induced decrements in insulin-stimulated cerebrovascular vasodilation would be normalized with acute endothelin-1a receptor antagonism and that treatment with a physical activity intervention restores vasoreactivity to insulin through augmented nitric oxide synthase (NOS)-dependent dilation. Otsuka Long-Evans Tokushima Fatty rats were divided into the following groups: 20 wk old food controlled (CON-20); 20 wk old free food access (model of obesity, OB-20); 40 wk old food controlled (CON-40); 40 wk old free food access (OB-40); and 40 wk old free food access+RUN (RUN-40; wheel-running access from 20 to 40 wk). Rats underwent Barnes maze testing and a euglycemic hyperinsulinemic clamp (EHC). In the 40-wk cohort, cerebellum and hippocampus blood flow (BF) were examined (microsphere infusion). Vasomotor responses (pressurized myography) to insulin were assessed in untreated, endothelin-1a receptor antagonism, and NOS inhibition conditions in posterior cerebral arteries. Insulin-stimulated vasodilation was attenuated in the OB vs. CON and RUN groups (P ≤ 0.04). Dilation to insulin was normalized with endothelin-1a receptor antagonism in the OB groups (between groups, P ≥ 0.56), and insulin-stimulated NOS-mediated dilation was greater in the RUN-40 vs. OB-40 group (P < 0.01). At 40 wk of age, cerebellum BF decreased during EHC in the OB-40 group (P = 0.02) but not CON or RUN groups (P ≥ 0.36). Barnes maze testing revealed increased entry errors and latencies in the RUN-40 vs. CON and OB groups (P < 0.01). These findings indicate that obesity-induced impairments in vasoreactivity to insulin involve increased endothelin-1 and decreased nitric oxide signaling. Chronic spontaneous physical activity, initiated after disease onset, reversed impaired vasodilation to insulin and decreased Barnes maze performance, possibly because of increased exploratory behavior.
NEW & NOTEWORTHY The new and noteworthy findings are that 1) in rodents, obesity-related deficits in insulin-mediated vasodilation are associated with increased influence of insulin-stimulated ET-1 and depressed influence of insulin-stimulated NOS and 2) a physical activity intervention, initiated after the onset of disease, restores insulin-mediated vasodilation, likely by normalizing insulin-stimulated ET-1 and NOS balance. These data demonstrate that the treatment effects of chronic exercise on insulin-mediated vasodilation extend beyond active skeletal muscle vasculature and include the cerebrovasculature.
the vasodilatory effects of insulin were documented over 70 years ago (1). Since then it has been established that, after binding to insulin receptors on the vascular endothelium and subsequent insulin receptor substrate 1/2 phosphorylation, insulin stimulates the production of the vasodilator nitric oxide (NO) through phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling. Independently of insulin receptor substrate phosphorylation, insulin also stimulates the production of the vasoconstrictor endothelin-1 (ET-1) through Ras-ERK/mitogen-activated protein kinase (MAPK) signaling (31). Under nonpathological conditions vascular insulin signaling favors endothelium-dependent NO-mediated vasodilation in various vascular beds throughout the body (31, 33), including the middle and posterior cerebral arteries as well as the cortical microvessels (19). However, under obese/insulin-resistant conditions, insulin-induced cerebrovascular vasodilation appears blunted (20). Evidence suggests that attenuated insulin-stimulated vasodilation in cerebral arteries may be explained partially by reduced NO and augmented ET-1 action, indicated by the selective inhibition of insulin-induced phosphorylation of Akt and elevated insulin-induced phosphorylation of ERK-1 and -2 in cerebral arteries from Zucker obese vs. lean rats (20). Whether the increased insulin-induced phosphorylation of ERK/MAPK culminates in depressed insulin-mediated cerebral vasodilation in obesity remains unknown. Altered vascular responses to insulin provide insight into endothelial cell phenotype/pathology (31, 33, 35, 40), and impaired insulin-induced phosphorylation of PI3K/Akt and endothelial nitric oxide synthase (NOS) can impact brain blood flow regulation (20). Importantly, disruption of brain blood flow and metabolism is relevant to the pathogenesis of cognitive decline in obese/insulin-resistant states (19, 20, 22, 35, 36, 44, 53). Although obesity alters cerebrovascular insulin signaling (20), it is uncertain whether this is associated with deficits in cognition.
Chronically increasing physical activity levels appears to be an effective treatment for obesity- and diabetes-induced impairments in insulin-stimulated vasodilation in the skeletal muscle vasculature (10, 27, 29, 35, 38) and in the sciatic nerve vasa nervorum (34), likely through enhanced NOS signaling (35). However, the effects of physical activity on the vascular actions of insulin may be restricted to vascular beds engaged during physical activity (i.e., not a systemic effect) (10). It remains unknown whether physical activity-induced adaptations in insulin signaling extend to the cerebrovasculature. Therefore, the purpose of this study was to examine the contribution of NOS and ET-1a receptor-mediated signaling to insulin-stimulated cerebrovascular vasodilation in lean and obese rats and to determine whether obesity-related impairments in insulin-induced vasodilation can be treated with increases in spontaneous physical activity. First, we hypothesized that obesity-induced decrements in insulin-mediated vasodilation, in isolated arteries, would be reversed with acute ET-1a receptor antagonism. Second, in light of evidence that the posterior circulation (42, 49), including the vascular supply of the cerebellum and hippocampus (11), is engaged during aerobic physical activity, we hypothesized that chronic increases in spontaneous physical activity would improve insulin-mediated posterior cerebrovascular vasodilation through enhanced NOS-mediated dilation. As a result, the development of cerebrovascular insulin resistance would be modified by physical activity, such that accelerated age-related changes in vascular insulin resistance in the setting of obesity would be normalized by a physical activity treatment intervention. Finally, we conducted extended cognitive testing to test the hypotheses that obesity-induced decrements in memory performance may be related to impaired vasoreactivity to insulin in the cerebral circulation and cognitive deficits can be reversed with a physical activity treatment intervention.
METHODS
Experimental design.
Previously, it was demonstrated that at 20 wk of age Otsuka Long-Evans Tokushima Fatty rats (OLETF; model of obesity-induced type 2 diabetes) exhibit obesity as well as overt hyperglycemia and hyperinsulinemia relative to lean control rats. At 40 wk of age, they remain obese and overtly hyperglycemic but display hypoinsulinemia (39). Thus, for the present experiments, these time points were selected to reflect the onset of obesity-induced insulin resistance (20 wk) and the emergence of frank type 2 diabetes (40 wk of age) in mature rats. Four-week-old hyperphagic OLETF rats (n = 48) were obtained from Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). Rats were divided into five groups: 1) 20 wk old food controlled (CON-20; n = 8); 2) 20 wk old obese with free food access (OB-20; n = 8); 3) 40 wk old food controlled (CON-40; n = 8); 4) 40 wk old obese with free food access (OB-40; n = 8); and 5) 40 wk old with free food access + RUN (RUN-40; wheel access from 20 to 40 wk; n = 8). The food-controlled groups (CON-20 and CON-40) were fed ~70% of the free-food access group. All groups were fed standard chow (carbohydrate = 56%, fat = 18%, protein = 26%; Formulab 5008, Purina Mills, St. Louis, MO) from 8 wk of age until euthanasia. Beginning at 20 wk (after the onset of overt insulin resistance in this model), daily running distance and time in the RUN-40 group were recorded by equipping running wheels with a Sigma Sport BC 800 bicycle computer (Sigma Sport USA, St. Charles, IL). This time point was selected to reflect a physical activity treatment intervention, initiated after the onset of obesity-induced insulin resistance (39). To isolate the effects of the spontaneous physical activity treatment intervention on vascular insulin resistance, rats in the RUN-40 group continued to have free food access. Rats were housed individually in cages in a temperature (21°C)-controlled room with a 12:12-h light-dark cycle. Twenty-four hours before metabolic and vascular experiments, running wheels were locked. The experimental protocol was approved by the Animal Care and Use Committee at the University of Missouri.
Cognitive testing was performed with a Barnes maze over 3 days, every 4 wk for 9 mo. At 20 and 40 wk of age, after a 12-h overnight fast rats were anesthetized with 0.1 mg/kg ketamine and underwent body composition assessment and a euglycemic hyperinsulinemic clamp in which carotid artery blood flow was measured. At 40 wk of age, blood flow to the hippocampus and cerebellum was measured with microspheres. Thereafter, rats were decapitated and the posterior cerebral arteries and a branch of the posterior cerebral artery were isolated for in vitro testing of vascular function. The rationale for studying the posterior cerebral artery/circulation was based on evidence that it dilates in response to insulin (19) and that blood flow to the posterior circulation increases during structured exercise, incrementally with exercise intensity, and to a greater extent than the anterior circulation (11, 42, 49). Furthermore, both the hippocampus and cerebellum rely on the posterior circulation, express insulin receptors, and are involved in spatial memory (52). Importantly, visual inspection confirmed that the posterior cerebral artery supplied the hippocampus (8) and revealed that branches of the posterior cerebral artery also supplied the cerebellum.
Body composition.
After sedation, body mass, fat mass, and lean mass were quantified by dual X-ray absorptiometry (Hologic QDR-1000/w calibrated for rats). Thereafter, rats underwent a euglycemic hyperinsulinemic clamp.
Euglycemic hyperinsulinemic clamp and in vivo blood flow measurements.
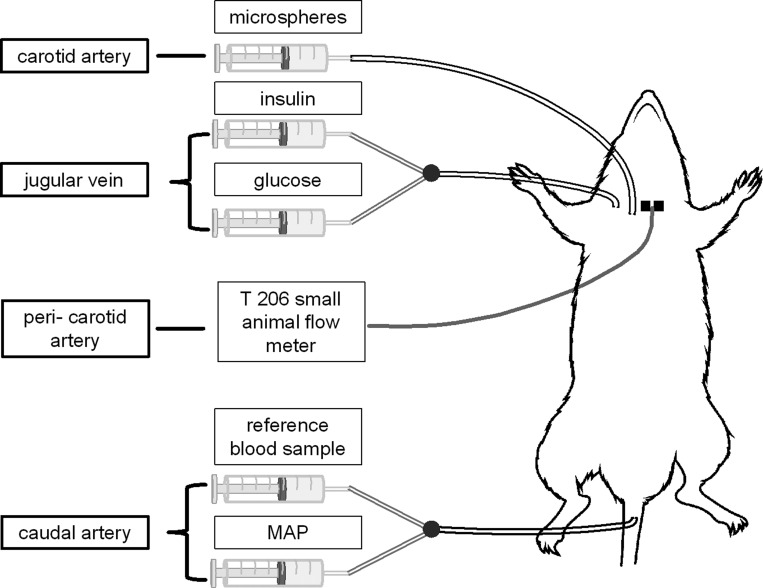
A 60-min insulin clamp was performed in anesthetized rats as previously described (34). Briefly, a catheter (polyethylene-50 tubing) was inserted into the right jugular vein to facilitate constant insulin (10 mU·kg−1·min−1, 0.400 μIU/ml; Novolin R) and variable glucose (0.4 g/ml; EMD Millipore, Darmstadt, Germany) infusions. Glucose infusion rate was adjusted to maintain euglycemia, which was determined by blood glucose measures performed every 5 min for the first 20 min and every 10 min thereafter. Blood samples obtained from the arterial catheter line enabled the measurement of blood glucose (Alpha Trak glucometer; Abbott Group, Chicago, IL) as well as insulin (ELISA kit for rat and human insulin; Crystal Chem, Downers Grove, IL). Carotid artery blood flow was monitored with a perivascular flow probe (T 206 small-animal flowmeter; Transonic Systems, Ithaca, NY) placed around the left carotid artery. To determine blood flow distribution within the brain, a second catheter was inserted into the right carotid artery and advanced to the aorta to facilitate microsphere infusions and a third catheter was inserted in the caudal artery for withdrawal of the microsphere reference sample and for monitoring mean arterial blood pressure (MAP; catheter was connected to a pressure transducer, PX272, Edwards, Lifesciences, Irvine, CA). In total, each rat was instrumented with a jugular vein, caudal artery, and carotid artery catheter as well as a carotid artery flow probe (Fig. 1). Importantly, experimental conditions were the same across groups, and acute unilateral carotid artery ligation does not cause significant changes in cerebral perfusion or the hemispheric uniformity of blood flow distribution (12, 23, 24, 28, 41).
Fig. 1.
Illustration of the experimental setup: jugular vein catheter facilitating constant insulin and variable glucose infusion during the euglycemic hyperinsulinemic clamp, carotid artery catheter positioned in the aorta to enable microsphere infusion, caudal artery catheter to allow for the reference blood sample and continuous monitoring of mean arterial pressure (MAP), and a perivascular flow probe positioned around the carotid artery to monitor carotid artery blood flow continuously throughout the experiment.
Stable isotope-labeled microsphere infusions (samarium and gold, 15-µm diameter; BioPAL, Worcester, MA) were performed at baseline, before insulin infusion, and at the end of the insulin clamp in 40-wk-old anesthetized rats. Briefly, withdrawal of the reference blood sample began at a rate of 0.6 ml/min and was followed (~10 s later) by infusion of a well-mixed suspension of microspheres and a saline flush (performed over ~20 s). Adequate mixing of microspheres was ensured by comparison of flows to the right and left kidneys. Furthermore, blood flow to the cerebellum and hippocampus, both of which rely primarily on the posterior circulation (i.e., not the carotid artery) was determined. Regional brain blood flow was calculated as follows (51):
where RBS is reference blood sample and CPM is counts per minute. Vascular resistance was calculated as the quotient of caudal artery MAP and blood flow (carotid artery blood flow and regional microsphere determinations).
Microsphere data were collected from 20 rats, but data were excluded from 4 rats that did not exhibit adequate mixing or if fewer than 400 microspheres were captured in the reference blood sample or tissue of interest. Data from five CON-40, five OB-40, and six RUN-40 rats are presented.
Isolation of and vascular reactivity of posterior cerebral arteries to insulin.
Sedated rats were decapitated, and their brains were harvested and transferred to a dissecting dish containing ice-cold physiological saline solution (PSS; in mM: 145 NaCl, 4.7 KCl, 2.0 CaCl2, and 1.17 MgSO4 with 10 g/l albumin added) with a pH of 7.4. With the aid of an Olympus microscope, posterior cerebral arteries and the main branch of the posterior cerebral artery were dissected, transferred to a Plexiglas chamber filled with PSS, and cannulated with two glass micropipettes (60–75 µm) filled with PSS and secured with a fine suture. Thereafter, the chambers were transferred to the stage of an inverted microscope (Nikon Diaphot 200) attached to a video camera (Javelin Electronics, Los Angeles, CA), video micrometer (Microcirculation Research Institute, Texas A&M University), and a MacLab data acquisition system. Luminal diameter and pressure were monitored continuously throughout the experiment.
Each vessel was warmed to 37°C and equilibrated at an intraluminal pressure of 60 mmHg for 1 h. With the exception of one vessel (myogenic tone was not studied in this vessel), all vessels studied developed spontaneous tone, indicated by a 20–40% constriction relative to preincubation diameter over the 1-h incubation period. After equilibration, myogenic tone was examined by decreasing intraluminal pressure to 40 mmHg and increasing it in 20-mmHg increments every 5 min (or until a stable diameter was achieved for 2 min) up to an intraluminal pressure of 140 mmHg. Intraluminal pressure was returned to 60 mmHg by the same stepwise procedure. After a washout period, an insulin dose-response curve was conducted (1, 10, 100, 1,000, and 10,000 μIU/ml). This protocol was completed first in untreated and ET-1a receptor antagonism (3 μM BQ123) conditions. After a washout period, untreated arteries were treated with BQ123, and the entire myogenic and insulin-dose response protocols were repeated. Similarly, after a washout period, arteries previously treated with BQ123 (from the beginning) were subsequently treated with the NOS blocker NG-nitro-l-arginine methyl ester (l-NAME; 300 μM), and the insulin dose-response curve was repeated. After the final insulin dose-response curve, vessels were washed twice with Ca2+-free PSS, and the pressure/myogenic curves were repeated. Data were averaged across vessels for myogenic and dose-response curves in the same condition within the same rat. A thromboxane A2 analog, U46691 (0.1 μM), was used to preconstrict a single vessel from one rat in the RUN-40 group. Given the primary focus of our experimental design, multiple cerebral vessels from each rat were cannulated for functional experiments. The time required for this process was such that we were unable to harvest cerebral microvessels in the manner required to confirm functional observations at the gene or protein expression level.
Barnes maze.
The Barnes maze was selected because food intake was being controlled in the CON groups but not the OB or RUN groups (i.e., motivation for food would likely be greater in the chronically food-controlled groups) and this cognitive task does not rely on food motivation. The original description of the Barnes maze can be found in Reference 3, and the design and protocol used in the present study were adapted from Walker and colleagues (48). The maze consisted of a gray circular platform 122 cm in diameter, surrounded by a wall 30.5 cm in height. The maze was elevated 83.8 cm above the floor by a stand. Twenty holes measuring 10.2 cm in diameter were evenly spaced around the perimeter. A rectangular gray escape box (28 cm in length × 12.7 cm wide × 7.6 cm high at the area closest to the maze and tapering to 16.5 cm high) could be placed beneath any hole. The escape box included an entry ramp that provided easy entry access for the rat. A black curtain was hung around the maze to surround the apparatus and ensure that rats would only use the visual cues provided in the maze, instead of distal cues within the testing room. Proximal cues were more likely to remain constant, within subjects and across subjects, during the course of training. Four visual cues consisting of various shapes (triangle, square, circle, cross) were placed at evenly spaced intervals on the inside of the maze walls. Two 86-W, 120-V floodlights producing 1,690 lm hung above the platform served to brightly light the maze in order to create a potentially aversive environment to help motivate the rats to escape from the brightly lit, open surface in favor of the dark environment of the escape box.
Each rat was assigned an escape hole; assigned holes were alternated across rats to eliminate odor cues for consecutively tested rats. The escape box location remained constant for any individual rat across test trials. Behavioral testing consisted of nine training trials (3 trials/day) over a period of 3 days. Testing was conducted at 8, 12, 16, and 20 wk of age in the 20-wk cohort and at 24, 28, 32, 36, and 40 wk of age in the 40-wk cohort.
A training trial began when the rat was placed under the start box positioned in the center of the platform. After 30 s, the box was lifted and the rat had a maximum of 3 min to find and enter the escape box. Latency (time it took for the rat to enter the escape box) and total errors (nose-pokes into nonescape holes) were recorded. If the rat did not enter the escape box within 3 min, it was gently guided there by the experimenter’s hand. After 30 s, the rat was removed from the escape box and returned to its home cage. The platform and escape box were cleaned after every trial with a 20% ethanol solution. Rats were allowed to rest in their home cage in the testing room for 20 min before starting their next daily trial.
Statistical analyses.
Data for body composition, energy intake, fasting blood glucose, plasma insulin, and steady-state glucose infusion rate during the insulin clamp were compared with an unpaired t-test (CON-20 vs. OB-20) and a between-group one-way ANOVA (CON-40, OB-40, RUN-40). Hemodynamic variables (blood flow, MAP, and vascular conductance) examined at baseline and the end of the insulin clamp were compared with a mixed-model ANOVA (group × time) within the 20-wk-old and 40-wk-old animals, respectively. Myogenic tone was expressed as the magnitude of the %myogenic tone at that pressure (i.e., the quotient of Δpassive − active diameter and passive diameter, multiplied by 100). Vasomotor responses during the insulin dose-response curve were expressed as %possible dilation (i.e., the quotient of Δinsulin dose diameter − baseline diameter and Δmaximal diameter − baseline diameter, multiplied by 100). Myogenic tone and %possible dilation during untreated, BQ123-treated, and l-NAME-treated insulin dose-response curves were compared with a mixed-model ANOVA (group × pressure and group × insulin dose) within 20-wk-old and 40-wk-old groups, respectively. To assess a general effect of disease progression on the ET-1a receptor antagonism and NOS inhibition to insulin-induced vasodilation, the difference in %possible dilation between the untreated and BQ123 and l-NAME conditions was averaged across animals at each dose and a cumulative average was obtained. The ΔET-1a receptor antagonism and NOS inhibition data were compared with a one-way ANOVA. Whereas significance testing was used to determine the probability that group differences across a range of insulin doses were the result of chance, Cohen’s d effect size analysis (7, 43) was used to determine the magnitude and directional impact of obesity, age, and chronic physical activity on insulin-induced dilation at 10 µIU/ml insulin (i.e., a physiological insulin concentration). Peak Barnes maze performance (best latency and total entry errors score) was analyzed with a repeated-measures ANOVA (group × time). Significant interactions were explored with a post hoc Student-Newman-Keuls test. All data are presented as means ± SE.
RESULTS
Animal characteristics.
Body mass, percent fat, weekly food intake, and blood glucose and insulin concentrations were greater in OB-20 vs. CON-20 rats (P < 0.01; Table 1). Also, glucose infusion rate during the insulin clamp was lower in OB-20 vs. CON-20 rats (P < 0.01; Table 1). At 40 wk of age, body mass, percent fat, and blood glucose, but not insulin (P = 0.12), were greater and glucose infusion rate during the insulin clamp was lower in the OB-40 groups vs. CON-40 and RUN-40 groups (P ≤ 0.05; Table 1). Food intake was greater in OB-40 and RUN-40 groups vs. the CON-40 group (P < 0.01; Table 1). Despite a greater food intake, blood glucose was lower in the RUN-40 group vs. the CON-40 group (P < 0.01; Table 1). Total running distance and running time for the RUN-40 group are presented in Fig. 2.
Table 1.
Animal characteristics
| CON-20 | OB-20 | CON-40 | OB-40 | RUN-40 | |
|---|---|---|---|---|---|
| Mass, g | 442 ± 6 | 625 ± 7* | 522 ± 5 | 621 ± 27†§ | 525 ± 11 |
| Body fat, % | 12 ± 1 | 28 ± 2* | 18 ± 2 | 24 ± 4†§ | 14 ± 1 |
| Weekly food intake, g | 144 ± 1 | 222 ± 4* | 146 ± 1 | 216 ± 6† | 208 ± 4† |
| Fasting blood glucose, mM | 8.0 ± 0.3 | 11.6 ± 0.8* | 11.8 ± 1.1 | 18.0 ± 0.7†§ | 7.9 ± 0.4† |
| Fasting plasma insulin, µIU/ml | 15.1 ± 1.2 | 26.8 ± 2.0* | 18.3 ± 1.7 | 13.4 ± 1.7 | 17.1 ± 1.0 |
| Plasma insulin EHC, µIU/ml | 332 ± 22 | 307 ± 30 | 285 ± 25 | 302 ± 30 | 297 ± 32 |
| GIR during EHC, mg·kg−1·min−1 | 27 ± 5 | 9 ± 2* | 23 ± 3 | 9 ± 3†§ | 30 ± 4 |
Data are presented as means ± SE. GIR, glucose infusion rate; EHC, euglycemic hyperinsulinemic clamp.
Significantly different from CON-20;
significantly different from CON-40;
significantly different from RUN-40.
Fig. 2.
Average weekly running distance (A) and running time (B) from 21 to 40 wk of age in the RUN-40 group.
Systemic hemodynamics and isolated arterial function—20 wk.
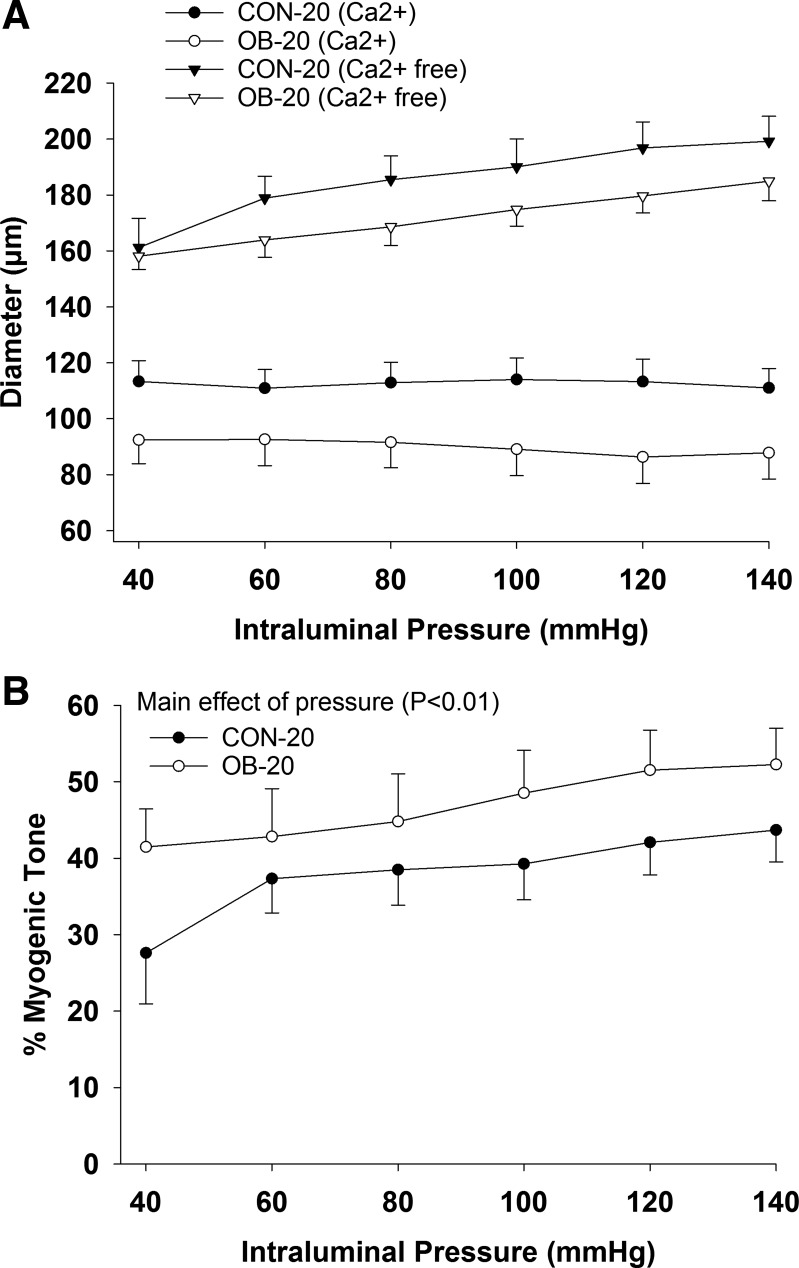
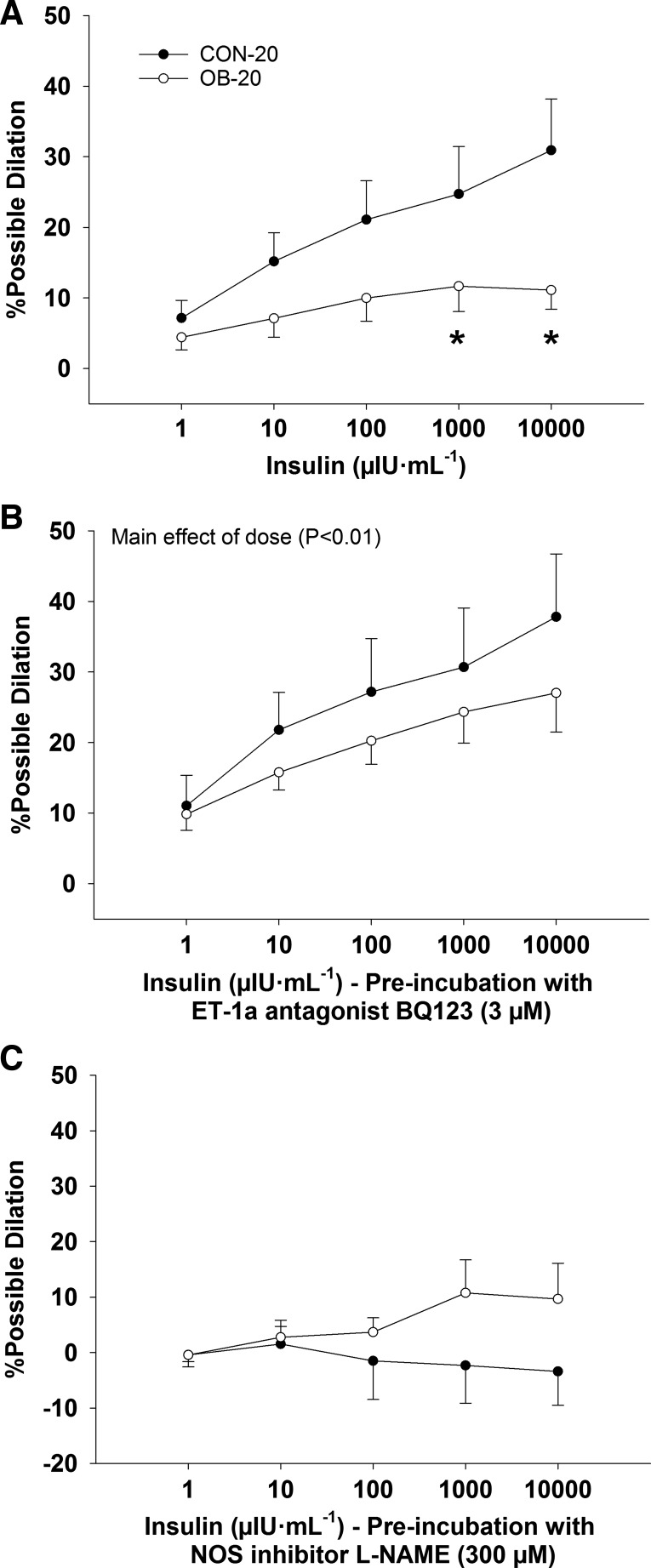
At 20 wk of age, carotid artery blood flow, MAP, and vascular resistance were similar at rest and did not change significantly during the insulin clamp (P ≥ 0.17; data not shown). In isolated cerebral arteries, absolute diameter was similar among groups in PSS and Ca2+-free PSS (P ≥ 0.27; Fig. 3A). Similarly, %myogenic tone was similar among groups (P = 0.29) and increased with intraluminal pressure (main effect of pressure P < 0.01; Fig. 3B). The %myogenic tone was unaffected by BQ123 treatment (P = 0.10; data not shown). Insulin-induced dilation was similar between 1 and 100 µIU/ml (P ≥ 0.09) and was lower in the OB-20 group vs. the CON-20 group at 1,000 and 10,000 µIU/ml (group × dose interaction, P ≤ 0.05; Fig. 4A). However, arterial vasomotor responses were similar among groups (P = 0.62) after treatment with BQ123 (main effect of insulin dose P < 0.01; Fig. 4B) or l-NAME (P ≥ 0.20; Fig. 4C).
Fig. 3.
Arterial diameter during graded increases in pressure in Ca2+ and Ca2+-free buffer (A) and %myogenic tone (B) in CON-20 (n = 7) and OB-20 (n = 7) groups. Data analyzed with a mixed-model ANOVA (group × pressure).
Fig. 4.
Percent possible dilation in response to insulin (A), insulin + BQ123 (ET-1a receptor antagonist; B), and insulin + l-NAME (NOS inhibitor; C) in CON-20 (n = 7) and OB-20 (n = 7) groups. Data analyzed with a mixed-model ANOVA (group × insulin dose). *Significantly less than CON-20.
Systemic hemodynamics and isolated arterial function—40 wk.
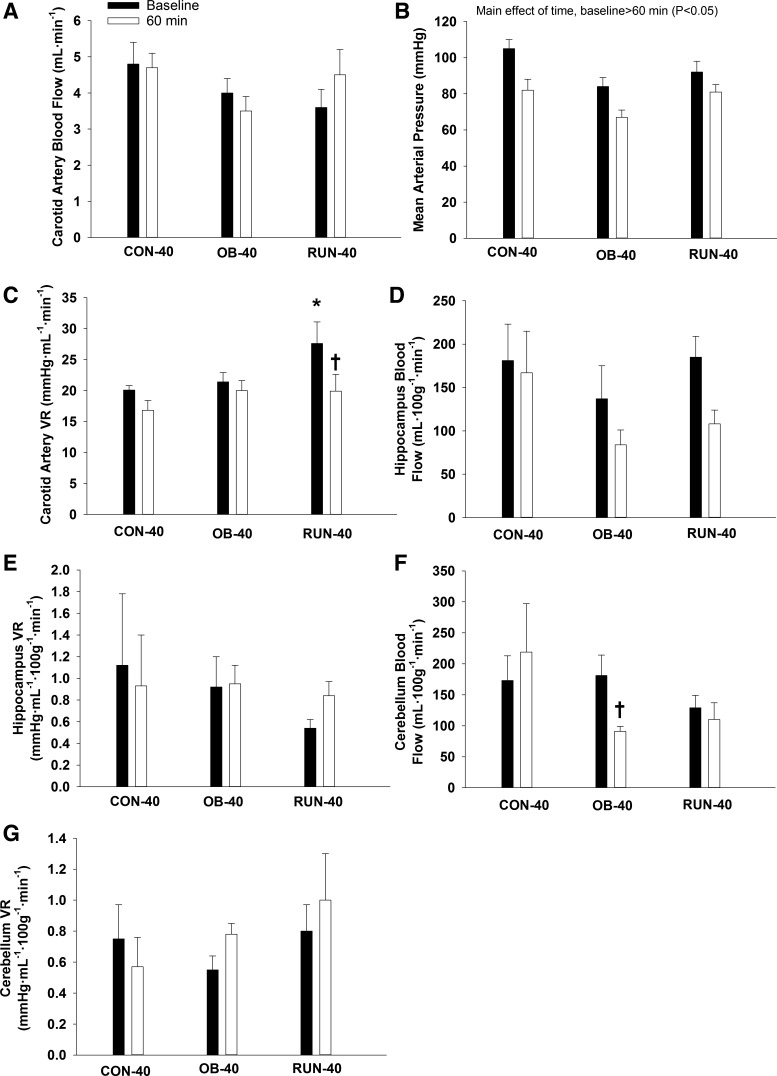
At 40 wk of age, carotid artery blood flow did not change from baseline (P = 0.66; Fig. 5A), but MAP decreased during the insulin clamp (main effect of time P < 0.01; Fig. 5B). Vascular resistance was higher in the RUN-40 group vs. all other groups at baseline and decreased significantly during the insulin clamp (group × time interaction, P ≤ 0.05; Fig. 5C). Hippocampal blood flow and vascular resistance were similar among groups and did not change during the insulin clamp (P ≥ 0.08; Fig. 5, D and E). Cerebellum flow was similar among groups at baseline (P ≥ 0.36), but decreased significantly during the insulin clamp in the OB-40 group (group × time interaction, P = 0.02; Fig. 5F). Cerebellum vascular resistance was similar among groups and did not change during the insulin clamp (P ≥ 0.32; Fig. 5G). Kidney blood flow was similar among groups at baseline (P = 0.44) and decreased during the insulin clamp (main effect of time, P = 0.04, group data: baseline = 624 ± 98 ml/min vs. 60 min = 422 ± 75 ml/min).
Fig. 5.
Carotid artery (CA) blood flow (A), mean arterial pressure (B), CA vascular resistance (VR) (C), hippocampus blood flow (D), hippocampus VR (E), cerebellum blood flow (F), and cerebellum VR (G) at baseline and the end (60 min) of the euglycemic hyperinsulinemic clamp in CON-40 (n = 5–7), OB-40 (n = 5 or 6), and RUN-40 (n = 6 or 7) groups. Data analyzed with a mixed-model ANOVA (group × time point). *Significantly different vs. all other groups; †significantly different from baseline.
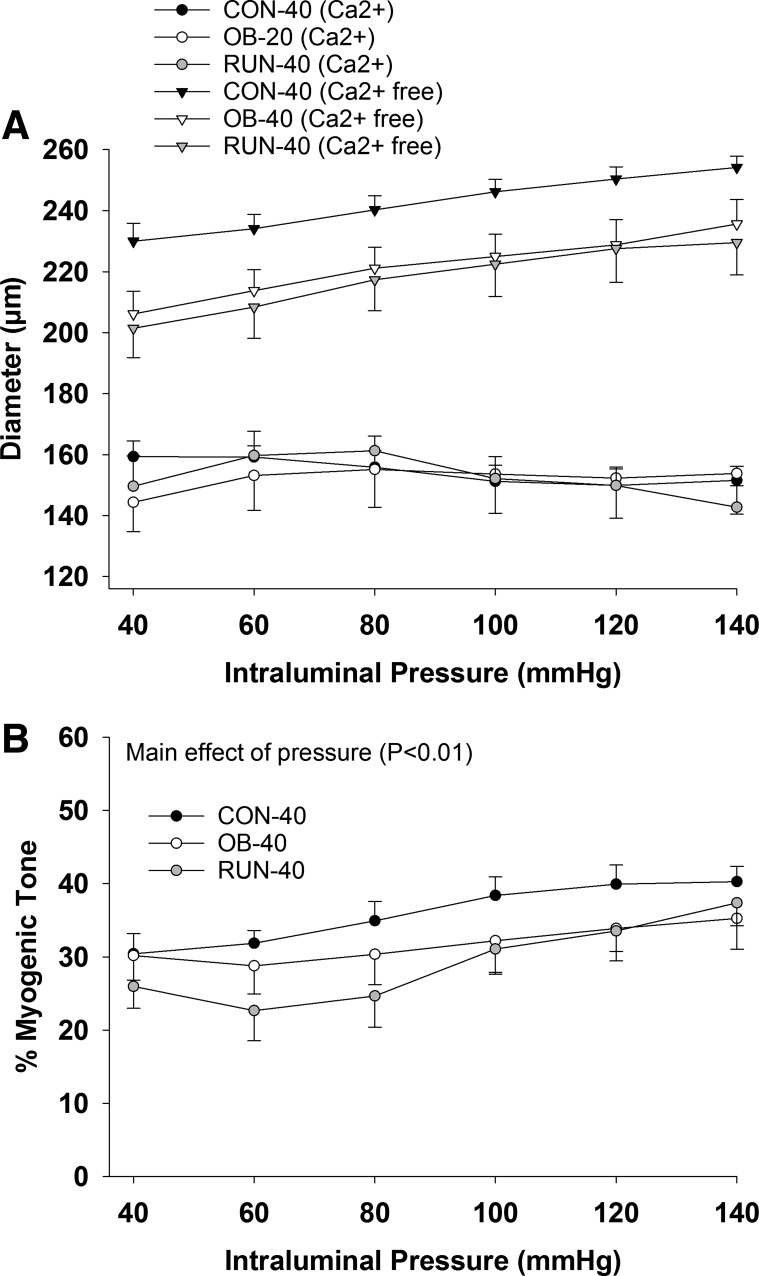
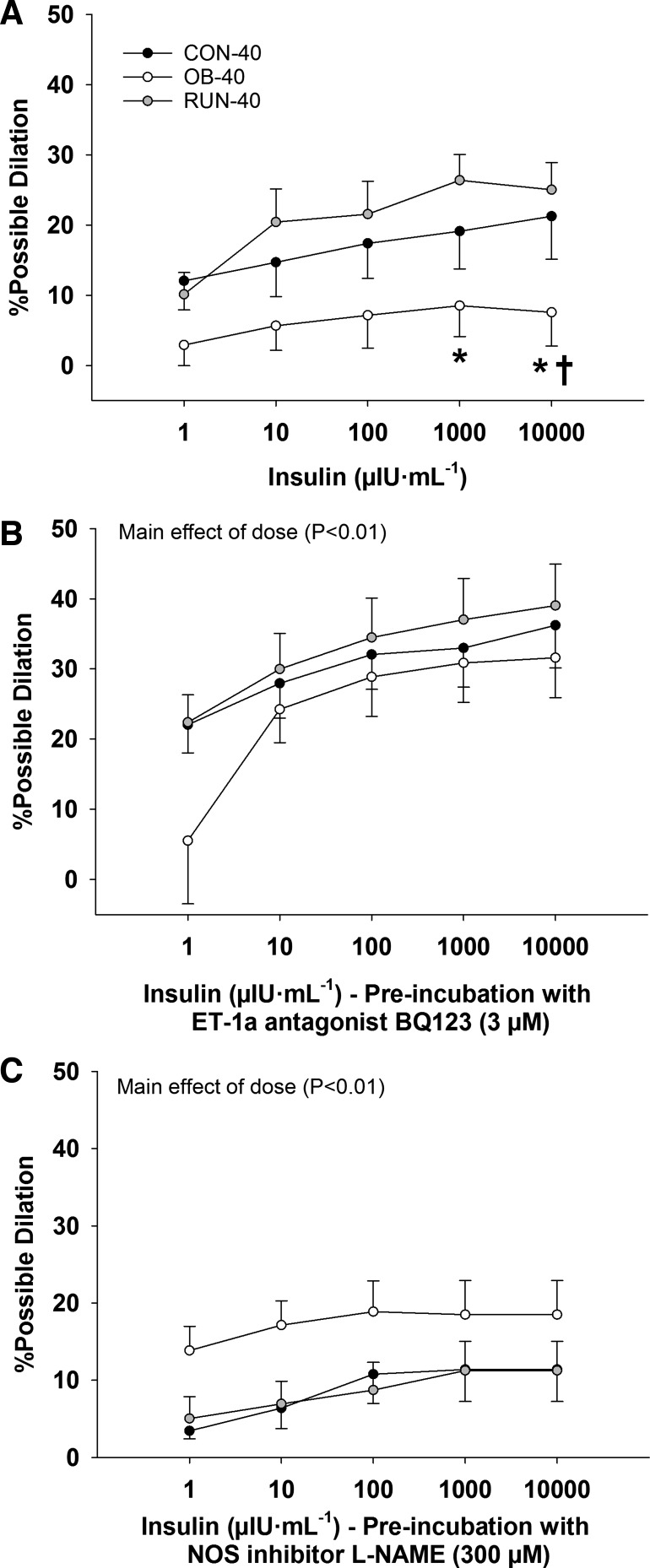
In isolated cerebral arteries, absolute diameter was similar among groups in PSS and Ca2+-free PSS (P ≥ 0.07; Fig. 6A). Similarly, %myogenic tone was similar among groups (P = 0.31) and increased with intraluminal pressure (main effect of pressure P < 0.01; Fig. 6B). The %myogenic tone was unaffected by BQ123 treatment (P = 0.89, data not shown). Insulin-induced dilation was lower in OB-40 vs. CON-40 (at 10,000 µIU/ml) and RUN-40 (at 1,000–10,000 µIU/ml; group × dose interaction, P ≤ 0.04; Fig. 7A) groups. However, arterial dilatory responses to insulin were similar among groups (P ≥ 0.56) during BQ123 (main effect of insulin dose P < 0.01; Fig. 7B) and l-NAME (main effect of insulin dose P < 0.01; Fig. 7C) treatment.
Fig. 6.
Arterial diameter during graded increases in pressure in Ca2+ and Ca2+-free buffer (A) and %myogenic tone (B) in CON-40 (n = 8), OB-40 (n = 8), and RUN-40 (n = 8) groups. Data analyzed with a mixed-model ANOVA (group × pressure).
Fig. 7.
Percent possible dilation in response to insulin (A), insulin + BQ123 (ET-1a receptor antagonist; B), and insulin + l-NAME (NOS inhibitor; C) in CON-40 (n = 8), OB-40 (n = 8), and RUN-40 (n = 8) groups. Data analyzed with a mixed-model ANOVA (group × insulin dose). *Significantly less than RUN-40; †significantly less than CON-40.
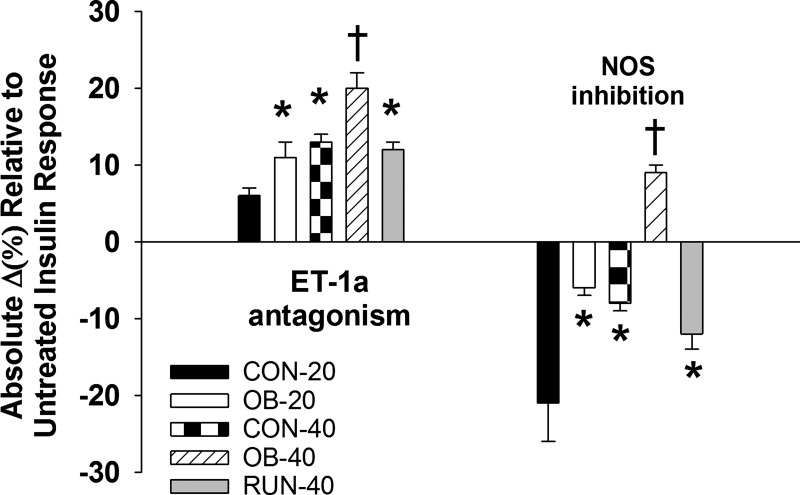
Acute antagonism of ET-1a receptor increased insulin-induced dilation in 40-wk-old vs. 20-wk-old rats (CON-40 > CON-20 and OB-40 > OB-20, P < 0.01; Fig. 8) and in obese vs. lean rats (OB-20 > CON-20 and OB-40 > CON-40, P ≤ 0.02; Fig. 8), indicating that the effect of ET-1 restriction on insulin-induced dilation increases with age and obesity. However, these effects of obesity were reversed with the spontaneous physical activity treatment (OB-40 > RUN-40, P < 0.01; Fig. 8). NOS inhibition reduced insulin-mediated vasodilation less in 40-wk-old vs. 20-wk-old rats (CON-20 < CON-40 and OB-20 < OB-40, P < 0.01; Fig. 8) and less in obese vs. lean rats (CON-20 < OB-20 and CON-40 < OB-40, P < 0.01; Fig. 8), indicating that insulin-induced NO-dependent dilation is reduced with age and obesity. However, these effects of obesity were reversed with spontaneous physical activity treatment (RUN-40 < OB-40, P < 0.01; Fig. 8). In OB-40 rats, insulin-induced dilation was increased during NOS inhibition.
Fig. 8.
Relative restraint exerted by ET-1a receptor on insulin-induced dilation and relative contribution of NOS to insulin-induced dilation in CON-20 (n = 7), OB-20 (n = 7), CON-40 (n = 8), OB-40 (n = 8), and RUN-40 (n = 8) groups. Data analyzed with a 1-way ANOVA.*Significantly different from CON-20; †significantly different from all other groups.
Although the mixed-model ANOVA did not reveal between-group differences in cerebrovascular responses to insulin until 1,000 μIU/ml (i.e., pharmacological dose), Cohen’s d effect size analyses reveal that obesity had a large, negative and a medium, negative effect on posterior cerebral artery dilation to 10 μIU/ml insulin (i.e., physiological dose) at 20 and 40 wk, respectively (Table 2). Age had a very small, negative effect on posterior cerebral artery dilation to 10 μIU/ml insulin in CON and OB groups (Table 2). Consistent with the shift toward decreased ET-1a and increased NOS contribution to total insulin-induced dilation relative to their sedentary OB-40 counterparts, spontaneous physical activity treatment from 20 to 40 wk had a large, positive effect on posterior cerebral artery dilation to 10 μIU/ml insulin (Table 2).
Table 2.
Effect sizes comparing insulin-induced dilation at 10 µIU/ml
| Effect Size | Description | |
|---|---|---|
| Obesity | ||
| CON-20 vs. OB-20 | −0.83 | Large, negative effect of obesity at 20 wk |
| CON-40 vs. OB-40 | −0.72 | Medium, negative effect of obesity at 40 wk |
| Age | ||
| CON-20 vs. CON-40 | −0.04 | Very small, negative effect of age from 20 to 40-wk |
| OB-20 vs. OB-40 | −0.14 | Very small, negative effect of age from 20 to 40-wk |
| Physical activity | ||
| OB-40 vs. RUN-40 | 1.1 | Large, positive effect of physical activity from 20 to 40-wk |
Long-duration cognitive function.
Cognitive performance was similar among CON-20 and OB-20 groups. There were no main or interaction effects for total entry errors or latency from 8 to 20 wk of age (P ≥ 0.61; Table 3). In contrast, cognitive performance was altered by the spontaneous physical activity treatment from 20 to 40 wk of age. Entry errors were greater in the RUN-40 group vs. all other groups (main effect of group P ≤ 0.01; Table 3). Likewise, latency was greater and increased over time in the RUN-40 group vs. all other groups (P ≤ 0.04; Table 3).
Table 3.
Barnes maze
| Weeks of Age | CON | OB | RUN |
|---|---|---|---|
| Entry errors | |||
| 8 | 1.3 ± 0.37 | 0.63 ± 0.38 | |
| 12 | 0.88 ± 0.40 | 1.13 ± 0.30 | |
| 16 | 1.00 ± 0.76 | 0.50 ± 0.19 | |
| 20 | 0.88 ± 0.74 | 1.25 ± 0.62 | |
| 24 | 1.3 ± 0.56 | 2.25 ± 0.66 | 3.25 ± 0.67* |
| 28 | 2.13 ± 1.17 | 0.75 ± 0.44 | 4.00 ± 1.67* |
| 32 | 1.63 ± 0.53 | 0.50 ± 0.35 | 3.88 ± 1.10* |
| 36 | 0.88 ± 0.47 | 0.13 ± 0.13 | 2.38 ± 0.63* |
| 40 | 0.75 ± 0.49 | 0.13 ± 0.13 | 2.00 ± 0.76* |
| Latency, s | |||
| 8 | 18.4 ± 4.1 | 21.6 ± 3.9 | |
| 12 | 16.6 ± 4.9 | 17.8 ± 4.4 | |
| 16 | 15.4 ± 7.45 | 14.3 ± 3.7 | |
| 20 | 15.0 ± 5.4 | 33.9 ± 17.6 | |
| 24 | 20.4 ± 5.9 | 37.3 ± 8.6 | 41.3 ± 8.8 |
| 28 | 36.4 ± 20.7 | 22.1 ± 6.5 | 43.8 ± 12.8 |
| 32 | 21.0 ± 4.5 | 21.1 ± 3.6 | 91.4 ± 25.6†§ |
| 36 | 23.8 ± 7.7 | 14.3 ± 2.7 | 83.4 ± 27.6†§ |
| 40 | 20.6 ± 7.8 | 13.1 ± 3.0 | 80.1 ± 25.3†§ |
Data are presented as means ± SE.
Significant main effect of group, RUN > CON and OB;
significantly greater than CON and OB groups;
significantly greater than 24 and 28 wk.
DISCUSSION
The present study demonstrates that posterior cerebrovascular responses to insulin are impaired in obesity but can be treated effectively with a physical activity treatment intervention that alters both insulin-mediated vasoconstriction and vasodilation pathways, based on the following novel findings: 1) insulin-induced dilation in isolated posterior cerebral arteries was diminished in obese vs. lean rats; 2) the vasoconstrictor influence of insulin-stimulated ET-1 was greater in obese rats and increased with age in lean and obese rats; 3) the vasodilatory influence of insulin-stimulated NOS-generated NO was blunted in obese rats and decreased with age in lean and obese rats; and 4) increases in spontaneous physical activity from 20 to 40 wk of age in obese rats improved insulin-mediated vasodilation. These findings suggest that improved insulin-mediated vasodilation in the posterior cerebral circulation following the physical activity treatment intervention, initiated after the onset of disease, may be the result of normalizing cerebrovascular ET-1 and NO balance in response to insulin stimulation.
Obesity.
At 20 and 40 wk of age, carotid artery blood flow and vascular resistance remained stable in CON and OB groups during the insulin clamp, suggesting that at this stage of disease deficits in insulin-mediated vasodilation are not reflected in total cranial perfusion in vivo. Given that the vasomotor actions of insulin are nonuniform along the arterial tree (18, 27) and can alter microvascular perfusion without changes in bulk flow (6), we also examined regional blood flow responses to insulin in the 40-wk cohort. Unexpectedly, cerebellum flow was decreased at 60 min into the insulin clamp in the OB-40 group. These results suggest that impaired myogenic reactivity to the drop in blood pressure (i.e., impaired autoregulation) or insulin-mediated vasoconstriction of posterior cerebellar arteries may have caused a redistribution of flow within the brain. However, in isolated posterior cerebral arteries, basal arterial diameter and myogenic function were similar among CON and OB groups, suggesting that impaired myogenic reactivity was not responsible for the reduction in cerebellum blood flow during the insulin clamp. In contrast, in isolated posterior cerebral arteries, insulin-mediated vasodilation was depressed in the OB group, owing to increased vasodilatory restraint exerted by ET-1a and depressed insulin-induced NOS-mediated vasodilation. Furthermore, insulin induces sympathetic neural activation (2, 47), which in the absence of a NO-mediated dilatory effect may produce vasoconstriction (47). Thus, in the present study an imbalance in the contribution of ET-1 and NO to the vasoactions of insulin may have contributed to the arteriolar vasoconstriction and reduced cerebellum blood flow during the insulin clamp observed in the OB-40 group.
Spontaneous physical activity.
During the insulin clamp vascular resistance decreased in the RUN-40 group. This was reflected by the nonsignificant combination of a drop in blood pressure and a small (~25%) increase in carotid artery blood flow. In light of the drop in blood pressure, the reduction in vascular resistance may have been the result of decreased myogenic tone to stabilize flow and/or insulin-mediated vasodilation. The available data suggest that myogenic function was similar among groups and point toward improved insulin-mediated vasodilation in the RUN-40 group. However, hippocampus and cerebellum blood flow did not increase during the insulin clamp, despite the fact that posterior cerebral arteries in the RUN-40 group dilated to insulin in vitro. Thus, any cerebral vasodilation in the RUN-40 group under in vivo conditions was not local to the hippocampus or cerebellum, and the dilatory actions of insulin on arteries supplying those structures may have been overcome by elevated ET-1 signaling (4, 18, 27) or other extrinsic mechanisms (i.e., insulin-mediated sympathetic vasoconstriction) (2, 26, 47). Given that the middle cerebral artery also dilates to insulin (19), the reduction in vascular resistance during the insulin clamp may be explained by vasodilation in other brain regions located in the frontal, parietal, or temporal lobes.
In the present study, chronic increases in spontaneous physical activity, initiated after the onset of obesity and cerebrovascular dysfunction, normalized insulin-mediated posterior cerebrovascular vasodilation. This finding builds on previous observations that indicate that chronic physical activity improves whole body insulin sensitivity and insulin-mediated vasodilation in skeletal muscle (10, 27, 29, 35, 38) and the vasa nervorum (34) and demonstrates that the treatment effects of chronic physical activity on insulin-mediated vasodilation extend to the posterior cerebral vasculature. Previously, we demonstrated that when matched for levels of hyperglycemia (34) as well as body weight/metabolic status (9, 29, 30), chronic physical activity improved insulin-mediated vasodilation in the vasa nervorum (34) and skeletal muscle vasculature (9, 29, 30). Furthermore, Dela and colleagues (10) demonstrated that structured exercise training-induced improvements in insulin-mediated vasodilation were restricted to active vs. inactive skeletal muscle. Taken together, these data suggest that the effects of chronic physical activity on insulin-induced vasodilation in a disease setting are not necessarily related to improvements in hyperglycemia or hyperinsulinemia and remain localized to the vascular tissues engaged directly during physical exertion (i.e., vascular beds that undergo physical activity-induced hyperemia). Blood flow through the posterior cerebral circulation (42, 49), including blood flow to the cerebellum and to a lesser extent the hippocampus (11), increases during physical activity; thus, we speculated that the posterior cerebral arteries and the downstream vascular bed would adapt favorably (in the context of insulin-mediated vasodilation) to chronic increases in spontaneous physical activity. Although blood flow to the cerebellum and hippocampus did not increase during the insulin clamp, data from isolated vessels indicate that the posterior cerebral arteries respond predictably to increased physical activity by dilating more in response to insulin stimulation following physical activity treatment (35). Furthermore, the latter observation was associated with decreased ET-1a-mediated restraint and increased NOS-dependent insulin-stimulated vasodilation. These data highlight that in a disease setting chronic increases in spontaneous physical activity may be an effective treatment to normalize or improve selective cerebrovascular insulin resistance (20, 21). It is important to note that posterior cerebral vasomotor responses to insulin were examined 24 h after the cessation of physical activity. Thus, improvements in selective cerebrovascular insulin resistance may have been the result of both acute (i.e., 24 h later) and chronic adaptations, stressing that the present findings apply to chronic, daily physical activity interventions.
Cognition.
Despite impaired cerebrovascular function in the OB rats, Barnes maze performance was similar among CON-20 and OB-20 as well as CON-40 and OB-40 groups (Table 3). These data indicate that, in this model, obesity and cerebrovascular insulin resistance are not associated with overt impairments in memory performance. Recently, Gomez-Smith and colleagues (15) also reported that diet-induced obesity, resulting in hippocampal neuroinflammation, did not impact Barnes maze performance in rats. However, decrements in memory performance have been observed between OLETF rats and their nonhyperphagic, lean counterparts using the Morris water maze (25) and the radial arm maze (32), indicating that cognitive impairment may exist but is task specific. Of note, in the present study the RUN-40 group exhibited the greatest amount of entry errors, and their latency increased over time. Although it is possible that chronic increases in spontaneous physical activity decreased memory performance, the bulk of research contradicts this conclusion, as results indicate that physical activity improves various aspects of cognition including memory (37, 45, 46). Alternatively, the finding that rats exposed to chronic increases in spontaneous physical activity produced longer latencies and more errors may indicate that rats, once they knew the goal location, showed greater exploratory behavior resulting in contact with nongoal locations and increased latency (14, 16). This view is consistent with the observation that latency increased over time in the RUN-40 group. The assumption that all rats, independent of experimental condition, were motivated equally by the bright lights to locate the escape box is an inherent limitation of the Barnes maze. Compared with their lean counterparts OLETF rats display increased anxiety-like behavior (50). Furthermore, spontaneous wheel running increases both resistance to and recovery from external stressors, referred to as “stress robustness” (5, 13, 17). Thus, it is possible that motivation to locate the escape box was increased in the OB group vs. the CON group as well as in both sedentary groups vs. the RUN group. From this perspective, cerebrovascular insulin resistance may be associated with increased anxiety-like behavior/stress vulnerability (reflected by an increased motivation to locate the escape box), which can be reversed and improved by chronic spontaneous physical activity treatment (reflected by decreased motivation to locate the escape box and increased exploratory behavior, which increased over time). Although the Barnes maze historically has been used to evaluate memory performance, the present findings and the emergence of new concepts such as stress robustness provide new insight into the use of the Barnes maze and suggest that the use of this method may extend beyond traditional interpretations.
Conclusions.
This study demonstrated that a progressive and selective imbalance in ET-1 and NO vasoconstrictor and vasodilator actions during insulin stimulation, respectively, underscores obesity-related decrements in insulin-induced posterior cerebrovascular vasodilation. Specifically, obesity was associated with augmented ET-1a-mediated vasoconstriction and depressed NOS-dependent vasodilation during insulin stimulation, and this imbalance worsened with age. However, dysfunctional changes in the cerebrovascular responses to insulin in obese rats were reversed and improved by chronically increasing daily levels of spontaneous physical activity after the onset of disease. These observations should be considered when determining the underlying mechanisms responsible for the adaptations to physical activity and when designing physical activity interventions to improve vascular insulin sensitivity and cerebrovascular function.
GRANTS
This work was supported by a College of Veterinary Medicine Pilot Grant, University of Missouri (principal investigator: T. D. Olver).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.D.O., M.W.M., D.K., R.A.R., J.L.J., T.R.S., and H.T.Y. performed experiments; T.D.O. and D.K. analyzed data; T.D.O., M.W.M., D.K., R.A.R., J.L.J., C.J.M., T.R.S., H.T.Y., C.A.E., and M.H.L. interpreted results of experiments; T.D.O. prepared figures; T.D.O. drafted manuscript; T.D.O., M.W.M., D.K., R.A.R., J.L.J., C.J.M., T.R.S., H.T.Y., C.A.E., and M.H.L. edited and revised manuscript; T.D.O., M.W.M., D.K., R.A.R., J.L.J., C.J.M., T.R.S., H.T.Y., C.A.E., and M.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank C. Juszczyk and K. Clark for their assistance with cognitive testing and P. Thorne for her technical assistance with pressure myography.
REFERENCES
- 1.Abramson DI, Schkloven N, Margolis MN, Mirsky IA. Influence of massive doses of insulin on peripheral blood flow in man. Am J Physiol 128: 124–132, 1939. [Google Scholar]
- 2.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87: 2246–2252, 1991. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93: 74–104, 1979. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 4.Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, Panza JA. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 100: 820–825, 1999. doi: 10.1161/01.CIR.100.8.820. [DOI] [PubMed] [Google Scholar]
- 5.Clark PJ, Amat J, McConnell SO, Ghasem PR, Greenwood BN, Maier SF, Fleshner M. Running reduces uncontrollable stress-evoked serotonin and potentiates stress-evoked dopamine concentrations in the rat dorsal striatum. PLoS One 10: e0141898, 2015. doi: 10.1371/journal.pone.0141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50: 2682–2690, 2001. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
- 8.Coyle P. Vascular patterns of the rat hippocampal formation. Exp Neurol 52: 447–458, 1976. doi: 10.1016/0014-4886(76)90216-8. [DOI] [PubMed] [Google Scholar]
- 9.Crissey JM, Padilla J, Jenkins NT, Martin JS, Rector RS, Thyfault JP, Harold Laughlin M. Metformin does not enhance insulin-stimulated vasodilation in skeletal muscle resistance arteries of the OLETF rat. Microcirculation 20: 764–775, 2013. doi: 10.1111/micc.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dela F, Larsen JJ, Mikines KJ, Ploug T, Petersen LN, Galbo H. Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes 44: 1010–1020, 1995. doi: 10.2337/diab.44.9.1010. [DOI] [PubMed] [Google Scholar]
- 11.Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol 533: 849–859, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K, Newman ED. Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rat. J Pharmacol Methods 11: 1–39, 1984. doi: 10.1016/0160-5402(84)90050-0. [DOI] [PubMed] [Google Scholar]
- 13.Fleshner M, Greenwood BN, Yirmiya R. Neuronal-glial mechanisms of exercise-evoked stress robustness. Curr Top Behav Neurosci 18: 1–12, 2014. doi: 10.1007/7854_2014_277. [DOI] [PubMed] [Google Scholar]
- 14.Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med 25: 78–82, 2004. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Smith M, Karthikeyan S, Jeffers MS, Janik R, Thomason LA, Stefanovic B, Corbett D. A physiological characterization of the Cafeteria diet model of metabolic syndrome in the rat. Physiol Behav 167: 382–391, 2016. doi: 10.1016/j.physbeh.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Goodrick CL. The effects of exercise on longevity and behavior of hybrid mice which differ in coat color. J Gerontol 29: 129–133, 1974. doi: 10.1093/geronj/29.2.129. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev 39: 140–149, 2015. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins NT, Padilla J, Martin JS, Crissey JM, Thyfault JP, Rector RS, Laughlin MH. Differential vasomotor effects of insulin on gastrocnemius and soleus feed arteries in the OLETF rat model: role of endothelin-1. Exp Physiol 99: 262–271, 2014. doi: 10.1113/expphysiol.2013.074047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katakam PV, Domoki F, Lenti L, Gáspár T, Institoris A, Snipes JA, Busija DW. Cerebrovascular responses to insulin in rats. J Cereb Blood Flow Metab 29: 1955–1967, 2009. doi: 10.1038/jcbfm.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katakam PV, Snipes JA, Steed MM, Busija DW. Insulin-induced generation of reactive oxygen species and uncoupling of nitric oxide synthase underlie the cerebrovascular insulin resistance in obese rats. J Cereb Blood Flow Metab 32: 792–804, 2012. doi: 10.1038/jcbfm.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King GL, Park K, Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award lecture. Diabetes 65: 1462–1471, 2016. doi: 10.2337/db16-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev 96: 1169–1209, 2016. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- 23.Laptook AR, Stonestreet BS, Oh W. The effect of carotid artery ligation on brain blood flow in newborn piglets. Brain Res 276: 51–54, 1983. doi: 10.1016/0006-8993(83)90547-4. [DOI] [PubMed] [Google Scholar]
- 24.De Ley G, Nshimyumuremyi JB, Leusen I. Hemispheric blood flow in the rat after unilateral common carotid occlusion: evolution with time. Stroke 16: 69–73, 1985. doi: 10.1161/01.STR.16.1.69. [DOI] [PubMed] [Google Scholar]
- 25.Li XL, Aou S, Hori T, Oomura Y. Spatial memory deficit and emotional abnormality in OLETF rats. Physiol Behav 75: 15–23, 2002. doi: 10.1016/S0031-9384(01)00627-8. [DOI] [PubMed] [Google Scholar]
- 26.Liang C, Doherty JU, Faillace R, Maekawa K, Arnold S, Gavras H, Hood WB Jr. Insulin infusion in conscious dogs. Effects on systemic and coronary hemodynamics, regional blood flows, and plasma catecholamines. J Clin Invest 69: 1321–1336, 1982. doi: 10.1172/JCI110572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin JS, Padilla J, Jenkins NT, Crissey JM, Bender SB, Rector RS, Thyfault JP, Laughlin MH. Functional adaptations in the skeletal muscle microvasculature to endurance and interval sprint training in the type 2 diabetic OLETF rat. J Appl Physiol (1985) 113: 1223–1232, 2012. doi: 10.1152/japplphysiol.00823.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meadow W, Rudinsky B, Raju T, John E, Fornell L, Shankararao R. Correlation of flow probe determinations of common carotid artery blood flow and internal carotid artery blood flow with microsphere determinations of cerebral blood flow in piglets. Pediatr Res 45: 324–330, 1999. doi: 10.1203/00006450-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, Laughlin MH, Thyfault JP. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol (1985) 109: 1203–1210, 2010. doi: 10.1152/japplphysiol.00064.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikus CR, Roseguini BT, Uptergrove GM, Morris EM, Rector RS, Libla JL, Oberlin DJ, Borengasser SJ, Taylor AM, Ibdah JA, Laughlin MH, Thyfault JP. Voluntary wheel running selectively augments insulin-stimulated vasodilation in arterioles from white skeletal muscle of insulin-resistant rats. Microcirculation 19: 729–738, 2012. doi: 10.1111/j.1549-8719.2012.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 32.Nomoto S, Miyake M, Ohta M, Funakoshi A, Miyasaka K. Impaired learning and memory in OLETF rats without cholecystokinin (CCK)-A receptor. Physiol Behav 66: 869–872, 1999. doi: 10.1016/S0031-9384(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 33.Olver TD, Laughlin HM. Endurance, interval sprint, and resistance exercise training: impact on microvascular dysfunction in type 2 diabetes. Am J Physiol Heart Circ Physiol 310: H337–H350, 2016. doi: 10.1152/ajpheart.00440.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olver TD, McDonald MW, Grisé KN, Dey A, Allen MD, Medeiros PJ, Lacefield JC, Jackson DN, Rice CL, Melling CW, Noble EG, Shoemaker JK. Exercise training enhances insulin-stimulated nerve arterial vasodilation in rats with insulin-treated experimental diabetes. Am J Physiol Regul Integr Comp Physiol 306: R941–R950, 2014. doi: 10.1152/ajpregu.00508.2013. [DOI] [PubMed] [Google Scholar]
- 35.Padilla J, Olver TD, Thyfault JP, Fadel PJ. Role of habitual physical activity in modulating vascular actions of insulin. Exp Physiol 100: 759–771, 2015. doi: 10.1113/EP085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16: 59–65, 2005. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical activity and cognitive vitality. Annu Rev Psychol 66: 769–797, 2015. doi: 10.1146/annurev-psych-010814-015249. [DOI] [PubMed] [Google Scholar]
- 38.Rattigan S, Wallis MG, Youd JM, Clark MG. Exercise training improves insulin-mediated capillary recruitment in association with glucose uptake in rat hindlimb. Diabetes 50: 2659–2665, 2001. doi: 10.2337/diabetes.50.12.2659. [DOI] [PubMed] [Google Scholar]
- 39.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev 31: 343–363, 2010. doi: 10.1210/er.2009-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salford LG, Siesjö BK. The influence of arterial hypoxia and unilateral carotid artery occlusion upon regional blood flow and metabolism in the rat brain. Acta Physiol Scand 92: 130–141, 1974. doi: 10.1111/j.1748-1716.1974.tb05729.x. [DOI] [PubMed] [Google Scholar]
- 42.Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 589: 2847–2856, 2011. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawilowsky SS. New effect size rules of thumb. J Mod Appl Stat Methods 8: 597–599, 2009. [Google Scholar]
- 44.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev 24: 855–872, 2000. doi: 10.1016/S0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 45.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med 72: 239–252, 2010. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voelcker-Rehage C, Godde B, Staudinger UM. Physical and motor fitness are both related to cognition in old age. Eur J Neurosci 31: 167–176, 2010. doi: 10.1111/j.1460-9568.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- 47.Vollenweider P, Randin D, Tappy L, Jéquier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest 93: 2365–2371, 1994. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker JM, Fowler SW, Miller DK, Sun AY, Weisman GA, Wood WG, Sun GY, Simonyi A, Schachtman TR. Spatial learning and memory impairment and increased locomotion in a transgenic amyloid precursor protein mouse model of Alzheimer’s disease. Behav Brain Res 222: 169–175, 2011. doi: 10.1016/j.bbr.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 49.Willie CK, Cowan EC, Ainslie PN, Taylor CE, Smith KJ, Sin PY, Tzeng YC. Neurovascular coupling and distribution of cerebral blood flow during exercise. J Neurosci Methods 198: 270–273, 2011. doi: 10.1016/j.jneumeth.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto Y, Akiyoshi J, Kiyota A, Katsuragi S, Tsutsumi T, Isogawa K, Nagayama H. Increased anxiety behavior in OLETF rats without cholecystokinin-A receptor. Brain Res Bull 53: 789–792, 2000. doi: 10.1016/S0361-9230(00)00407-X. [DOI] [PubMed] [Google Scholar]
- 51.Yang HT, Terjung RL. Angiotensin-converting enzyme inhibition increases collateral-dependent muscle blood flow. J Appl Physiol (1985) 75: 452–457, 1993. [DOI] [PubMed] [Google Scholar]
- 52.Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem 274: 34893–34902, 1999. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- 53.Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol 490: 71–81, 2004. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]