Moderate-intensity resistance training causes sex-specific adaptations in skeletal muscle structure and function at the cellular and molecular levels in inactive older adult men and women with knee osteoarthritis. However, these responses were minimal compared with high-intensity resistance training. Thus adjuncts to moderate-intensity training need to be developed to correct underlying cellular and molecular structural and functional deficits that are at the root of impaired physical function in this mobility-limited population.
Keywords: myofilament, sex differences, mitochondria, training
Abstract
High-intensity resistance exercise (REX) training increases physical capacity, in part, by improving muscle cell size and function. Moderate-intensity REX, which is more feasible for many older adults with disease and/or disability, also increases physical function, but the mechanisms underlying such improvements are not understood. Therefore, we measured skeletal muscle structure and function from the molecular to the tissue level in response to 14 wk of moderate-intensity REX in physically inactive older adults with knee osteoarthritis (n = 17; 70 ± 1 yr). Although REX training increased quadriceps muscle cross-sectional area (CSA), average single-fiber CSA was unchanged because of reciprocal changes in myosin heavy chain (MHC) I and IIA fibers. Intermyofibrillar mitochondrial content increased with training because of increases in mitochondrial size in men, but not women, with no changes in subsarcolemmal mitochondria in either sex. REX increased whole muscle contractile performance similarly in men and women. In contrast, adaptations in single-muscle fiber force production per CSA (i.e., tension) and contractile velocity varied between men and women in a fiber type-dependent manner, with adaptations being explained at the molecular level by differential changes in myosin-actin cross-bridge kinetics and mechanics and single-fiber MHC protein expression. Our results are notable compared with studies of high-intensity REX because they show that the effects of moderate-intensity REX in older adults on muscle fiber size/structure and myofilament function are absent or modest. Moreover, our data highlight unique sex-specific adaptations due to differential cellular and subcellular structural and functional changes.
NEW & NOTEWORTHY Moderate-intensity resistance training causes sex-specific adaptations in skeletal muscle structure and function at the cellular and molecular levels in inactive older adult men and women with knee osteoarthritis. However, these responses were minimal compared with high-intensity resistance training. Thus adjuncts to moderate-intensity training need to be developed to correct underlying cellular and molecular structural and functional deficits that are at the root of impaired physical function in this mobility-limited population.
aging and its accompanying acute and chronic diseases hasten the development of physical disability by promoting skeletal muscle atrophy and dysfunction (20). Resistance exercise (REX) is recommended to restore physical function because of its well-recognized effects to increase muscle size and contractility. While much is known regarding the effects of REX in healthy adult populations, our understanding of the effects of REX in populations most in need of the benefits of exercise, such as those with, or at high risk for developing, disability, is limited.
High-intensity REX [e.g., 80% of 1 repetition maximum (1 RM)] incurs the greatest benefits (24, 57), and some older adults can perform high-intensity REX under supervision (19). However, many individuals are unable or unwilling to perform such intense activities, and access to medically monitored REX programs for those with disease or disability is limited. From a practical perspective, moderate-intensity REX programs (e.g., 60% of 1 RM) may be more feasible for these individuals and may produce similar whole body and tissue-level benefits (63, 68). However, it is unclear whether moderate-intensity REX corrects fundamental deficits in muscle size and function that limit physical capacity in older adults.
Knee osteoarthritis (OA) is the leading cause of physical disability in older adults (2). The primary pathological hallmarks of knee OA, articular cartilage loss and progressive bone remodeling, cause knee joint pain and, in turn, reductions in weight-bearing activity (29, 64, 71, 80), which drive muscle atrophy and dysfunction. Accordingly, REX training is a logical intervention to remediate such deficits. However, joint pain from OA limits the intensity at which exercise training can be performed, especially in volunteers with advanced disease (34). In this context, knee OA represents a clinically relevant population in which knowledge of the fundamental structural and functional adaptations to moderate-intensity REX training would provide valuable information that could aid exercise prescription. Extrapolation of data from healthy older adults undergoing high-intensity training protocols suggests that REX might enhance muscle function in older adults with knee OA through fiber hypertrophy (15, 21, 22, 76) and improved intrinsic contractility (16, 21, 75, 76). Although moderate-intensity REX may provide a less robust training stimulus, it yields whole body/muscle functional improvements in older adults that rival high-intensity programs (68). Moreover, in older adults with, or at risk for, disability, who are habituated to a low level of physical activity, moderate-intensity REX may provide a sufficient resistive stimulus to promote myofiber hypertrophy and functional benefits. The effects of moderate-intensity REX training on skeletal muscle size and function in older adults with advanced knee OA, however, have not been defined.
Our overall goal was to examine the effects of a moderate-intensity REX training program on muscle morphology and contractile function at the cellular and molecular levels in inactive older adults at high risk for physical disability. We recruited volunteers with knee OA, but who were nonobese and free from other disease, to ensure that structural and functional adaptations to moderate-intensity REX would not be influenced by any preexisting disease process or its sequelae. This study is an extension of our cross-sectional work in this cohort, which described sex-specific muscle morphological and functional adaptations to knee OA at the cellular, organellar, and molecular levels (9, 10). Considering these sex-specific differences (9, 10, 45), we examined whether the morphological or functional responses to REX training differed between men and women.
METHODS
Subjects
A total of 171 individuals were referred to our study from orthopedic practices or responded to advertisements, of which 26 (13 men, 13 women) met initial eligibility criteria on telephone screening and were invited to an outpatient screening visit. Eight volunteers (5 men, 3 women) did not meet screening eligibility criteria. The remaining 18 (8 men, 10 women; 70 ± 1 yr) volunteers were enrolled, and 17 (7 men, 10 women) completed the entire study. Volunteers selected for study had: radiographic [Kellgren and Lawrence grade 3 or 4 (33)] and symptomatic [Western Ontario and McMaster Universities Osteoarthritis Index (4)] evidence for advanced-stage knee OA in at least one knee. Additionally, volunteers reported being inactive and participating in only light-intensity activities, based on the Stanford Brief Activity Survey, which corresponds to activity levels in the 1.0 to 1.5 metabolic equivalent range (70). That these volunteers were sedentary was confirmed by accelerometry, as compared with moderately active, age-matched control subjects, as previously reported (9), as well as other groups of diseased and sedentary elderly that we have studied in the past (47). To ensure that the functional response to training was not influenced by other chronic diseases or health conditions, volunteers were excluded if they had/have had a history, clinical signs, or symptoms of diabetes, heart failure, pulmonary disease, thyroid disease, peripheral arterial disease, neurological, neuromuscular, or autoimmune disease; a body mass index >30 kg/m2; a current or past (within 10 yr) history of smoking; a current or past (within 10 yr) history of malignancy, excluding nonmelanoma skin cancer; or prior knee replacement in either knee. All volunteers had normal blood counts/chemistry and renal, liver, and thyroid function, based on standard blood tests. None had received an intra-articular injection (hyaluronan or corticosteroid), had participated in any rehabilitation program for 6 mo before testing, or was taking sex steroid replacement therapy, oral or inhaled corticosteroids, or any other medications known to affect muscle function. Four OA volunteers were on stable regimens of HMG CoA reductase inhibitors (statins), although plasma creatine kinase levels were within the normative range in these volunteers and none had symptoms or signs of statin-induced myopathy. We have recently shown that statin use does not affect skeletal muscle structure or function in statin-tolerant older adults (61) and does not impair the response to exercise training (62). Additionally, six participants had hypertension and were on stable antihypertensive therapy, consisting of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (n = 2), diuretics (n = 2), and adrenergic blocking agents (n = 3). Seven volunteers were taking nonsteroidal anti-inflammatory agents but discontinued this medication 5 days before the muscle biopsy to limit its antithrombotic effect. Notably, recent work in humans has shown that nonsteroidal anti-inflammatory agents, such as ibuprofen, enhance REX-induced modifications in muscle size and function, in contrast to inhibitory effects shown in animal studies (77, 78). Nonetheless, the similar frequency distribution of this medication in men and women (3 men, 4 women) suggests that it would not impact the observed sex differences. Written informed consent was obtained from each volunteer before participation, and the protocol was approved by the Committees on Human Research at the University of Vermont. Data from baseline evaluations in a subset of these volunteers (n = 10; 4 men, 6 women) examining whole muscle and single-muscle fiber function, structure, and protein expression have been reported previously in comparison to healthy control subjects (9–11). The present report builds on this work by addressing effects of moderate-intensity REX training on whole muscle, cellular, and molecular muscle function.
Experimental Protocol
Volunteers who met initial eligibility criteria were invited to an outpatient screening visit, at which time medical history, physical examination, blood samples, and bilateral whole muscle strength testing were performed, the latter to determine which leg would be biopsied [weaker leg, as described previously (9)] and to familiarize volunteers with the strength testing procedure. Additionally, knee X-rays were conducted for radiographic confirmation of knee OA. Volunteers who met entry criteria underwent baseline testing during two outpatient visits. During the first visit, leg strength testing was repeated on the study leg. At least 5 days later, muscle tissue was obtained via percutaneous biopsy of the vastus lateralis and body composition was assessed. Shortly after these assessments (1–3 wk), volunteers entered a 14-wk moderate-intensity REX training program. At the completion of the training program, volunteers repeated baseline measurements, with the muscle biopsy performed at least 3 days after the last bout of exercise or strength testing (average 5.4 ± 0.5 days, range 3–10 days; men 5.6 ± 0.9 and women 5.2 ± 0.7 days) to minimize residual effects on muscle structure and function outcomes.
Knee Extensor Muscle Function
We studied the weaker leg, based on isometric knee extensor torque measurements taken at baseline, as described previously (9), using a HUMAC/NORM 770 dynamometer (CSMi, Stoughton, MA). Thereafter, isometric (70°) and isokinetic (60°/s and 180°/s) knee extensor strength testing was performed on the study leg only, as described previously (74), although both legs were trained to prevent asymmetries from developing. Measurements were not conducted on one woman after training because of technical problems with the equipment.
Total and Regional Body Composition
Total and regional fat mass and fat-free mass were measured by dual-energy X-ray absorptiometry (DEXA) (GE Lunar, Madison, WI), including measurement of thigh fat-free tissue mass, as described previously (10). Quadriceps muscle cross-sectional area (CSA) was measured by computerized tomography (CT), as described previously (8). Briefly, the scan location for pre- and posttraining evaluations was standardized as half the distance between the anterior superior iliac crest and the most proximal aspect of the patella with a radiopaque marker. Volunteers were transported to the CT scanner in a wheelchair to limit contraction-induced fluid shifts before the measurement. The time volunteers spent in the supine position was not standardized, but scans were taken shortly after volunteers were positioned in the scanner (~5 min) and changes in muscle volume are not altered dramatically in the thigh (5).
Solutions
Dissection, skinning, and storage solutions for muscle biopsy acquisition and storage were identical to those previously described (9, 10). For mechanical analyses, relaxing, preactivating, and activating solutions were as previously described (9, 10). All solutions used for mechanical experiments were adjusted to an ionic strength of 175 meq with sodium methane sulfate.
Muscle Biopsy and Processing of Tissue Samples
Baseline and posttraining biopsies of the vastus lateralis muscle were performed, as described previously (73), with tissue partitioned for 1) single-fiber mechanical and morphological analysis of chemically skinned fibers, 2) immunohistochemical (IHC) staining, and 3) electron microscopy (EM). For chemically skinned fibers, tissue was placed immediately into cold (4°C) dissecting solution, processed, and stored for single-fiber measurements, as described previously (47). Segments (~2.5 mm) of chemically skinned single fibers were isolated and processed for assessment of morphology or cellular- and molecular-level functional parameters, as described previously (9, 10, 46), within 3 wk of the biopsy procedure. Mechanical assessments were not available on one woman after training because of technical problems. For IHC and EM analyses, muscle tissue was frozen in embedding medium (OCT; Sakura, Torrance, CA) in isopentane cooled with liquid N2 or fixed in glutaraldehyde/paraformaldehyde, respectively, as described previously (10).
Single-Muscle Fiber Cross-Sectional Area
Single-fiber morphology was assessed with two separate techniques. In the first, single-fiber CSA was assessed on segments of manually dissected, chemically skinned single fibers, as described previously (10), followed by placement of the fiber in gel loading buffer for analysis of myosin heavy chain (MHC) expression. Single-muscle fiber CSA was also performed on a subset (n = 8; 6 men, 2 women) of volunteers on frozen sections, as generally described (10), with modifications to assess three isoforms of MHC as detailed by Fry et al. (23). Briefly, 6-µm cross sections were allowed to dry and rehydrated with phosphate-buffered saline (PBS), followed by incubation with antibodies for MHC I [Developmental Studies Hybridoma Bank (DSHB) BA-D5; 1:100], MHC IIA (DSHB SC-71; 1:600), and MHC IIX (DSHB 6H1; 1:50) in PBS containing 1% bovine serum albumin (BSA) and 0.1% Triton X-100 overnight at room temperature in a humidified chamber. After PBS washes, secondary antibodies (all Thermo Fisher: goat anti-mouse Alexa Fluor 646 A-21242; goat anti-mouse Alexa Fluor 488 A-21121; goat anti-mouse Alexa Fluor 568 A-21043; all 1:500) in PBS-1% BSA-0.1% Triton X-100 were applied for 1 h, followed by postfixing with 100% methanol. Image acquisition was performed with an Olympus BX51 microscope (Olympus America, Center Valley, PA) at ×20, with analysis using ImageJ.
Myonuclear Number
Myonuclear numbers of MHC I and II subtypes were determined as described previously (10). Although this estimate may include satellite cells, these are a small fraction (<3%) of nuclei under the laminin border (69). At each time point an average of 96 ± 7 fibers were studied per volunteer, with a total of 1,805 fibers studied overall.
Electron Microscopy
Myofilament ultrastructure was assessed by EM, as described previously (47), including myofilament fractional content and A-band length. Mitochondrial content and morphology were assessed in both the subsarcolemmal (SS) and intermyofibrillar (IMF) (see Fig. 2, A–D) regions, as described previously (8).
Fig. 2.
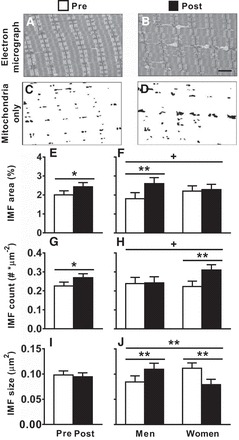
A–D: representative images showing electron micrographs (A and B) and highlighted mitochondria only (C and D) for an older male volunteer pre- and posttraining. E–J: training-induced adaptations in intermyofibrillar mitochondria (IMF) in the entire cohort (E, G, and I) and in men and women separately (F, H, and J), as assessed by electron microscopy. Data represent means ± SE. Lines extending across all sexes and times reflect training × sex interaction effects, while lines extending across pre- and posttraining assessments within each sex denote differences from post hoc, pairwise comparisons. *P < 0.05, **P < 0.01, +trends (P = 0.07 and 0.08 for IMF count and IMF area, respectively) for training or training × sex interaction effects. Note that differences in fiber CSA between chemically skinned single-muscle fiber CSA and IHC CSA are related to the swelling that occurs upon chemical skinning, as we have demonstrated previously (10).
Cellular-Level Contractile Function Measurements
Cellular-level contractility was assessed, as described in detail elsewhere (9), to assess isometric tension (defined as force per CSA) and maximum shortening velocity (Vmax) and maximum power production (Pmax) with isotonic load clamps. Measurements were conducted at 15°C to maintain stability of the preparation throughout the experimental protocol. If fiber force decreased by >10% from the first to the last activation or if sarcomere patterns were skewed or disrupted, results for that fiber were not included in the final analyses. At each time point an average of 13 ± 4 fibers were studied per volunteer, with a total of 351 fibers studied overall. Approximately 9% of fibers that were dissected and studied were discarded on the basis of these criteria and were not included in final analyses because of noticeable tears in the fiber, disruption of sarcomere patterns, or diminished tension.
Molecular-Level Contractile Function Measurements
The experimental apparatus and procedures used to chemically fix fiber attachment points were described previously (46). Sinusoidal analysis was conducted under maximal Ca2+-activated conditions (pCa 4.5) to estimate myosin-actin cross-bridge mechanics and kinetics, as detailed previously (46). Measurements were conducted at 25°C, as lower temperatures did not allow resolution of certain parameters of cross-bridge mechanics/kinetics represented by viscoelastic properties of the fiber under low-frequency sinusoidal length perturbations. This analysis yields three characteristic processes, A, B, and C, which relate to various mechanical (A, B, C, and k) and kinetic [2πb and (2πc)−1] properties of the cross-bridge cycle, as previously described in detail (46). Briefly, regarding the frequency portion of the B and C processes, respectively, 2πb is interpreted as the apparent rate of myosin force production or the rate of myosin transition between the weakly and strongly bound states and (2πc)−1 as the average myosin attachment time (ton) to actin (51). The magnitudes of the B and C processes (parameters B and C) are proportional to the number of myosin heads strongly bound to actin and cross-bridge stiffness. Finally, the A process has no kinetic or enzymatic dependence (48) and, under Ca2+-activated conditions, reflects the viscoelastic properties of the nonenzymatic, passive elements in the myofilaments, reflecting the underlying stiffness of the lattice structure and the attached myosin heads in series under Ca2+-activated conditions. The parameter A is separated into its real [A cos(kπ/2)] and imaginary [A sin(kπ/2)] parts, as previously described (45), to characterize the elastic and viscous properties of the nonenzymatic, passive structural elements in myofilaments, respectively. Finally, the parameter k represents the relationship of the viscous to elastic modulus of the A process (k = 0 purely elastic vs. k = 1 purely viscous). At each time point (pre- and posttraining), an average of 18 ± 2 fibers were studied per volunteer, with a total of 566 fibers used in the final analyses. Approximately 12% of fibers that were dissected and studied were discarded and not included in final analyses because of noticeable tears in the fiber, disruption of sarcomere patterns, or diminished tension.
Single-Fiber MHC Isoform Expression
After all morphological and mechanical assessments, chemically skinned fibers were placed in gel loading buffer for determination of MHC isoform composition by SDS-PAGE to identify fiber type, as described previously (47), with the relative proportion of IIA and IIX isoforms in MHC IIAX fibers determined by densitometry, as described previously (72).
Resistance Exercise Training Program
Before beginning the 12-wk training program, all volunteers underwent a 2-wk run-in training period in which they visited the training center 3 times/week to be instructed on the proper form for each exercise and to undergo 1 RM testing, as described previously (1). Exercises included 1) leg press, 2) leg extension, 3) leg curls, 4) calf raises, 5) hip extension, and 6) hip flexion, utilizing both machines and free weights, with both concentric and eccentric phases of contraction lasting 3–4 s. Each training session started with a 5-min warm-up on a cycle ergometer and light stretching. The purpose of this run-in period was to reduce subsequent dropouts by identifying volunteers who might have trouble completing the training program because of physical/pain limitations. Volunteers completed one set of eight repetitions of all exercises at 30% of 1 RM, with emphasis on proper technique and full range of motion. During this run-in period and throughout the remainder of the training program both legs were trained (3 times/week), but each was trained separately (i.e., unilateral performance of each exercise) to account for any asymmetries between legs that might cause volunteers to favor their stronger leg when performing exercises on equipment that permits bilateral effort against the resistance.
After the 2-wk run-in period, volunteers underwent 12 additional weeks of resistance exercise training. The target REX training intensity was set at 60% of 1 RM. This intensity was chosen because volunteers with knee OA will have problems completing higher-intensity training because of physical and pain limitations (60, 79). This type of training regimen has been shown to yield functional benefits in knee OA (58). Volunteers completed each exercise at 40% of 1 RM during the first week and 50% of 1 RM during the second week and reached the target of 60% of 1 RM by the third week. A second set of eight repetitions was added at week 4 and remained at this frequency for the duration of the program. We restrained the volume of training during each session in lieu of knee joint pain in advanced-stage knee OA so that the demands of the training did not adversely affect adherence. 1 RM of each leg was reassessed every 3 wk throughout the training program, and the exercise prescriptions were adjusted accordingly. The same trainer (P. D. Savage) oversaw all training and 1 RM testing.
Statistics
Changes in physical characteristics and whole muscle strength were determined via repeated-measures analysis of variance, with training (pre- vs. posttraining) as a within-subject factor (SPSS version 22; IBM SPSS Statistics, Armonk, NY) and sex as a between-subject factor. For variables in which multiple observations were performed within the same individual (e.g., single-fiber mechanical/morphological parameters), a linear mixed model (SAS version 9.3; SAS Institute, Cary, NC) was used because a general linear model makes the assumption that each measurement is independent, which is invalid when multiple fibers are evaluated within each volunteer. To account for clustering of observations within individuals, we included a repeated effect in the model, as described previously (73). By accounting for this variance within each volunteer at each time point, this model can be considered more conservative than the simple process of taking an average value for each volunteer before and after training, which yields a single value with no variance estimate. Sex was included in the mixed model to evaluate whether the response to training differed between men and women. When a training × sex interaction effect was found, pairwise comparisons were made to identify the location of differences. Relationships between variables were determined with Pearson’s r values, with normality confirmed by the Shapiro-Wilk test. Partial correlation analysis was used to examine the relationship between variables after statistical removal of covariates. All data are reported as means ± SE.
RESULTS
Subjects
The majority of the OA cohort had a Kellgren and Lawrence grade of 4 (n = 13; 7 men, 6 women), indicating end-stage knee OA, with the remaining volunteers being grade 3 (n = 5; 1 man, 4 women), reflecting advanced-stage knee OA, with an average Western Ontario and McMaster Universities Osteoarthritis Index score of 66 ± 3. One male volunteer dropped out for personal reasons before starting the training program. There were no adverse events from participation in the study or the REX program, and all volunteers who began exercise training reached the target intensity and completed the program. Compliance with the training program was excellent (91.7 ± 1.5% of sessions completed), with no difference in compliance between men and women (91.2 ± 2.4% vs. 92.1 ± 2.0% of sessions completed, respectively).
Structural Adaptations
Whole body composition and quadriceps CSA.
Body size and total and regional fat and fat-free tissue mass from dual-energy X-ray absorptiometry were unchanged with training (Table 1), with no training × sex interaction effects, although training increased quadriceps muscle CSA measured by CT (7%, P < 0.05; Fig. 1A) similarly in men and women (P < 0.05 for each sex; Fig. 1B). There was no change in CT muscle tissue attenuation with training [study leg: 49.1 ± 1.0 vs. 49.1 ± 1.1 HU; nonstudy leg: 50.7 ± 0.9 vs. 51.7 ± 0.9 HU) and no training × sex interaction.
Table 1.
Age and body composition
| Male |
Female |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age, yr | 69.4 ± 1.6 | 70.0 ± 1.7 | ||
| Body mass index, kg/m2 | 26.9 ± 1.6 | 27.1 ± 1.6 | 27.6 ± 1.3 | 27.6 ± 1.3 |
| Body mass, kg | 80.4 ± 4.2 | 80.9 ± 4.3 | 67.7 ± 3.5 | 67.5 ± 3.6 |
| Fat mass, kg | 22.0 ± 2.7 | 22.3 ± 2.8 | 28.9 ± 2.3 | 28.3 ± 2.3 |
| Fat-free mass, kg | 56.1 ± 2.1 | 56.0 ± 2.0 | 36.8 ± 1.8 | 36.9 ± 1.7 |
| SL thigh fat-free mass, kg | 3.2 ± 0.2 | 3.2 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.1 |
| NSL thigh fat-free mass, kg | 3.3 ± 0.2 | 3.3 ± 0.2 | 2.1 ± 0.2 | 2.1 ± 0.1 |
Data are means ± SE for n = 17 volunteers (7 men, 10 women). SL, studied leg; NSL, nonstudied leg. Total and regional body composition were measured by dual-energy X-ray absorptiometry. No training or training × sex interaction effects were noted for any variables.
Fig. 1.
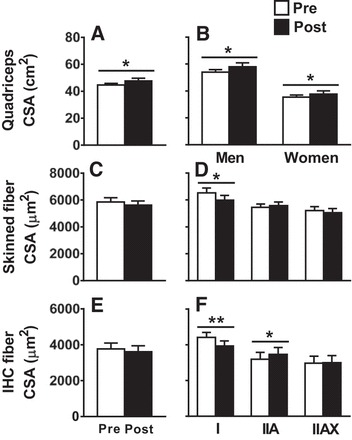
Training-induced adaptations in quadriceps (A and B) and single-fiber (C–F) cross-sectional area (CSA). IHC, immunohistochemistry. Data represent means ± SE *P ≤ 0.05, **P < 0.01 for training effect. Note that there were no training × sex interaction effects for any comparisons.
Single-muscle fiber CSA.
The increase in quadriceps muscle CSA was not accompanied by an overall increase in single-muscle fiber CSA by two separate techniques (Fig. 1, C and E). In manually dissected, chemically skinned fibers, further evaluation of specific fiber types showed a reduction in MHC I CSA (P < 0.05; Fig. 1D), with no significant changes in MHC IIA or IIAX CSA and no training × sex interactions. At each time point, an average of 20 ± 0.2 fibers were analyzed per volunteer (n = 601 total). Because measurements were taken every 250 µm along the length of these fibers (2,982 ± 12 µm), there were ~10 CSA measurements per fiber, which yields ~200 CSA measurements per volunteer per time point. Note that data for pre- and posttraining values for MHC I/IIA, MHC IIX, and MHC I/IIA/IIX fibers for both morphological and mechanical variables are not presented for these fibers or IHC fiber CSA because they were too few to permit comparisons (<3% for each). Analysis of single-fiber CSA by IHC showed no change in average muscle fiber CSA, although there were reciprocal changes in MHC I and IIA fiber CSAs, with a reduction (P < 0.05) in MHC I and an increase (P < 0.05) in MHC IIA fibers (Fig. 1F), and no training × sex interaction effects. At each time point, an average of 107 ± 10 fibers were analyzed for each volunteer (n = 1,713). Finally, EM data showed no changes in myofilament fractional area (Pre 87.6 ± 1.2% vs. Post 86.1 ± 1.2%) or A-band length (Pre 1.51 ± 0.02 µm vs. Post 1.51 ± 0.02 µm) and no training × sex effects (not shown in figure), arguing for no effects of training at the myofilament ultrastructural level.
Tissue MHC isoform expression.
In tissue homogenates (n = 16, as tissue was limited on 1 woman before training), we observed a shift in MHC protein isoform expression, as evidenced by a reduction in expression of MHC I (36.7 ± 1.0% to 34.3 ± 0.8%; P < 0.05) and an increase in MHC IIX (24.9 ± 0.8% to 26.6 ± 0.9%; P < 0.05), with no change in MHC IIA (38.5 ± 1.0% to 39.0 ± 0.7%) or training × sex interaction effects.
Myonuclear content.
Training did not affect myonuclear number per fiber in MHC I (1.95 ± 0.13 vs. 2.05 ± 0.13 nuclei/fiber) or II (2.10 ± 0.12 vs. 2.00 ± 0.12 nuclei/fiber) fibers. At each time point, an average of 96 ± 2 fibers were evaluated for each volunteer (n = 1,806 total).
Mitochondrial content/structure.
Training increased IMF mitochondrial fractional content (Fig. 2E; P < 0.05) via increases in mitochondrial number (Fig. 2G; P < 0.05), with no change in average mitochondrial size (Fig. 2I). Trends (P = 0.08 and 0.07) toward training × sex interaction effects for IMF mitochondrial fractional content and count (Fig. 2, F and H, respectively) and a significant training × sex interaction effect for IMF mitochondria average size (Fig. 2J; P < 0.01) prompted us to examine pairwise sex differences. Men showed an increase in mitochondrial fractional content (P < 0.01), whereas women exhibited no change (Fig. 2F). The increase in content in men was due to increased mitochondrial size (P < 0.01; Fig. 2J), with no change in number (Fig. 2H). Despite the lack of change in fractional content, mitochondria underwent remodeling in women, with increased number (P < 0.01; Fig. 2H) and reduced size (P < 0.01; Fig. 2J). In contrast, there were no training or training × sex interaction effects for SS mitochondrial content, number, or size (data not shown).
Functional Adaptations: Whole Muscle Function
Training improved 1 RM in the studied leg for all lower extremity resistance exercises (range: 54–69%; all P < 0.001; Table 2), with similar relative improvements in the nonstudied leg (43–71%; all P < 0.001; data not shown). Peak isometric knee extensor torque increased (+9%; P < 0.01), although peak isokinetic torque at either speed (60 or 180°/s) was unchanged. There were no training × sex interaction effects for 1 RM or peak isometric or isokinetic knee extensor torque responses to training.
Table 2.
One repetition maximum strength and isometric and isokinetic knee extensor torque
| Male |
Female |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Leg press, kg | 29.8 ± 3.1 | 49.2 ± 5.1 | 19.5 ± 2.6 | 32.0 ± 4.2** |
| Leg curl, kg | 8.7 ± 0.6 | 14.3 ± 0.9 | 5.1 ± 0.5 | 8.4 ± 0.8** |
| Leg extension, kg | 18.8 ± 2.1 | 27.9 ± 3.2 | 10.3 ± 1.7 | 16.7 ± 2.7** |
| Hip extension, kg | 4.9 ± 0.3 | 8.2 ± 0.3 | 4.9 ± 0.2 | 8.4 ± 0.2** |
| Hip flexion, kg | 4.9 ± 0.3 | 8.2 ± 0.3 | 5.1 ± 0.3 | 8.4 ± 0.2** |
| 70° isometric torque, Nm | 144.2 ± 12.8 | 153.4 ± 12.3 | 81.3 ± 11.3 | 93.1 ± 10.8* |
| 60°/s isokinetic torque, Nm | 103.7 ± 8.1 | 109.3 ± 7.5 | 66.0 ± 7.1 | 67.3 ± 6.6 |
| 180°/s isokinetic torque, Nm | 70.4 ± 5.1 | 69.0 ± 4.8 | 40.8 ± 4.5 | 41.8 ± 4.2 |
Data are means ± SE for n = 17 volunteers (7 men, 10 women) for 1 repetition maximum (1 RM) data and n = 16 volunteers (7 men, 9 women) for isometric and isokinetic knee extensor peak torque measures. No sex × training interaction effects were noted for any variables.
Training effect, P < 0.01;
training effect, P < 0.001.
Cellular and Molecular Functional Adaptations
MHC I fibers.
No training effects were found for maximal tension (Tmax; Pre 86.6 ± 4.8 vs. Post 86.9 ± 5.1 mN/mm2), Vmax [0.392 ± 0.030 vs. 0.366 ± 0.032 muscle lengths (ML)/s], Pmax (1.29 ± 0.11 vs. 1.25 ± 0.12 mN/mm2 × ML/s), velocity at maximum power output (Vopt; 0.075 ± 0.004 vs. 0.070 ± 0.005 ML/s), or tension at maximum power production (Topt; 17.2 ± 0.8 vs. 18.0 ± 0.8 mN/mm2) in MHC I fibers [n = 97 (43 men, 54 women) pretraining fibers and n = 96 (24 men, 72 women) posttraining fibers]. Training × sex interaction effects were noted for Tmax in MHC I fibers at 15°C (P = 0.05; Fig. 3A) and at 25°C [P < 0.05; Fig. 3B; n = 188 (77 men, 111 women) pretraining fibers and n = 159 (55 men, 104 women) posttraining fibers], with the interaction at 25°C being driven by a reduction (P < 0.01) in women. There was also a training × sex interaction effect for Topt (P < 0.05; Fig. 3C), due to increased Topt (P < 0.05) in men. Changes in Topt and Tmax with training in men and women are illustrated graphically in Fig. 3D. Training × sex effects were not found for any other variables. Parenthetically, because no training or training × sex interaction effects were found for myofilament fractional content (detailed above), single-fiber tension (force/CSA) should reflect force producing capacity per unit myofilament.
Fig. 3.
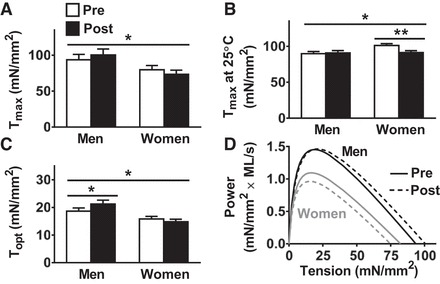
Training-induced adaptations in single MHC I fiber maximal Ca2+-activated tension (Tmax; A and B), tension at maximum power production (Topt, C), and power-tension relationships (D). All measurements were conducted at 15°C, except for Tmax at 25°C (B). Tension = force/CSA. Data represent means ± SE. *P ≤ 0.05, **P < 0.01 for training × sex effect (bar across all groups/time points) or post hoc comparison for training effect within each sex (bar across each sex separately).
Myosin-actin cross-bridge mechanics and kinetics [n = 188 (77 men, 111 women) pretraining fibers and n = 159 (55 men, 104 women) posttraining fibers] showed training × sex interaction effects (P < 0.05) for B and C (Fig. 4, C and E), parameters that reflect the number of strongly bound myosin-actin cross bridges and cross-bridge stiffness. The mechanism underlying training-induced differences in B and C in men and women may be explained, in part, by a trend toward a training × sex interaction effect for MHC I ton (P = 0.06, Fig. 4F), with this interaction effect being driven by an increase in ton in men (P < 0.05). This notion is reinforced by the strong relationship between training-induced changes in B and ton [r = 0.810, P < 0.001, Fig. 4G; note that a similar relationship was observed between MHC I C and ton (r = 0.802, P < 0.001)]. As the number of strongly bound cross bridges is equivalent to the total population of myosin heads multiplied by the fraction of time a cross bridge is formed (51), training-induced increases in B and C are due to an increased number of strongly bound cross bridges, secondary to an increased ton. Other cross-bridge mechanic (A-elastic/viscous) and kinetic variables (2πb) did not change with training and were similar in men and women (Fig. 4, A, B, and D, respectively).
Fig. 4.
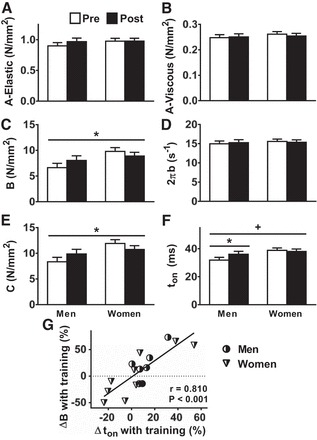
Training-induced adaptations in cross-bridge mechanics and kinetics in single MHC I fibers (A–F) and the relationship between training-induced changes in the number of strongly bound cross bridges (mechanics variable B in C) and ton (G). All measurements were conducted at 25°C. Details of the physiological interpretation of each parameter are provided in Molecular-Level Contractile Function Measurements. Data represent means ± SE. *P < 0.05 for training × sex effect (bar across all groups/time points) or post hoc comparison for training effect within each sex (bar across each sex separately); +P = 0.06 for training × sex effect.
MHC IIA.
Measurements of cellular contractility at 15°C (n = 33 pretraining fibers and n = 34 posttraining fibers) showed no training effects for Tmax (Pre 120.4 ± 7.5 vs. Post 122.5 ± 7.6 mN/mm2), Vmax (1.41 ± 0.10 vs. 1.49 ± 0.10 ML/s), Pmax (7.09 ± 0.56 vs. 7.00 ± 0.57 mN/mm2 × ML/s), Topt (25.1 ± 1.5 vs. 24.3 ± 1.5 mN/mm2), or Vopt (0.28 ± 0.01 vs. 0.29 ± 0.01 ML/s) and no training × sex interaction effects. Measurements of Tmax at 25°C [n = 62 (30 men, 32 women) pretraining fibers and n = 57 (34 men, 23 women) posttraining fibers], however, showed a trend (P = 0.07) toward a training × sex interaction effect, with values reduced in men and increased in women (Fig. 5A). At the molecular level [n = 62 (30 men, 32 women) pretraining fibers and n = 57 (34 men, 23 women) posttraining fibers], these reciprocal changes in MHC IIA Tmax were accompanied by a similar training × sex interaction effect for A-elastic (Fig. 5B) and changes in A-elastic were strongly correlated to changes in tension (r = 0.758; P < 0.01; Fig. 5C). As A-elastic can be affected by the number of attached cross bridges, we cannot discount that this relationship is influenced by changes in the number of attached cross bridges. Although not significant, there were minor changes in B (men: Pre = 24.7 ± 2.0 vs. Post = 21.9 ± 2.0; women: Pre = 25.4 ± 2.0 vs. Post = 25.0 ± 2.3 N/mm2) and C (men: Pre = 25.8 ± 2.1 vs. Post = 22.9 ± 2.1; women: Pre = 26.6 ± 2.1 vs. Post = 26.0 ± 2.3 N/mm2), and changes in both parameters were related to tension changes (B: r = 0.642, P < 0.05; C: r = 0.644, P < 0.05). To dissect the contribution of bound cross bridges and their stiffness from the relationship between changes in A-elastic and tension, we statistically controlled for changes in B or C and found that the relationship between changes in A-elastic and tension persisted (B: r = 0.611, P < 0.05; C: r = 0.609, P < 0.05). Conversely, statistical control for the effects of changes in A-elastic eliminated the relationship of changes in B or C to tension (B: r = 0.097, P = 0.78; C: r = 0.102, P = 0.77). These analyses show that the relationship between changes in A-elastic and tension is independent of the effect of alterations in the number of bound cross bridges or their stiffness (i.e., parameters B and C), indicating that the changes in A-elastic are likely due to altered non-cross-bridge myofilament stiffness. As for other cross-bridge mechanic/kinetic parameters, there was a trend (P = 0.07) toward a training effect for A-viscous (men: Pre = 0.26 ± 0.02 vs. Post = 0.24 ± 0.02; women: Pre = 0.29 ± 0.02 vs. Post = 0.26 ± 0.02 N/mm2), whereas B (men: Pre = 24.7 ± 2.0 vs. Post = 21.9 ± 2.0; women: Pre 25.4 ± 2.0 vs. Post = 25.0 ± 2.3 N/mm2), C (men: Pre = 25.8 ± 2.1 vs. Post = 22.9 ± 2.1; women: Pre = 26.6 ± 2.1 vs. Post = 26.0 ± 2.3 N/mm2), and 2πb (men: Pre = 49.5 ± 1.2 vs. Post = 51.6 ± 1.1; women: Pre = 48.3 ± 1.2 vs. Post = 48.4 ± 1.3 s−1) were not affected by training and did not show training × sex interactions.
Fig. 5.
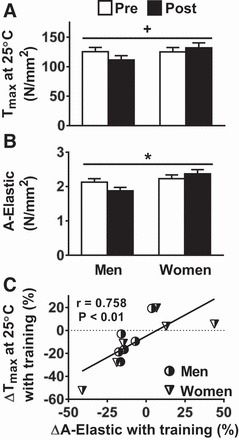
Training-induced adaptations in single-MHC IIA fiber Tmax at 25°C (A), A-elastic (B), and the relationship between changes in Tmax and A-elastic (C). Tension = force/CSA. Data represent means ± SE. *P ≤ 0.05 for training × sex effect; +P = 0.07 for training effect.
MHC IIAX.
Measurements of cellular contractile function at 15°C [n = 28 pretraining (10 men, 18 women) and n = 31 posttraining (15 men, 16 women) fibers] showed no training effects for Tmax (Pre 114.2 ± 7.7 vs. Post 111.1 ± 7.0 mN/mm2), Vmax (Pre 1.34 ± 0.12 vs. Post 1.31 ± 0.05 ML/s), Pmax (Pre 7.95 ± 0.94 vs. Post 8.77 ± 0.89 mN/mm2 × ML/s), Topt (26.6 ± 2.2 vs. 28.1 ± 2.0 mN/mm2), or Vopt (0.31 ± 0.02 vs. 0.31 ± 0.02 ML/s) and no training × sex interaction effects, with the exception of Vopt (P < 0.01; Fig. 6A). Changes in Vopt were due to an increase in men (P < 0.05) and a decrease in women (P < 0.05). A similar pattern of sex differences in the training response was apparent in Vmax (men: Pre = 1.27 ± 0.21, Post = 1.42 ± 0.06; women: Pre = 1.40 ± 0.11, Post = 1.20 ± 0.09 ML/s) but did not reach significance (P = 0.30). Power-velocity curves illustrate these changes in Vopt (Fig. 6B). Interestingly, there was a trend toward a training × sex interaction effect (P = 0.07) in the fractional expression of MHC IIX isoform in MHC IIAX fibers (men: Pre = 41.7 ± 5.3, Post = 48.9 ± 5.1%; women: Pre = 41.0 ± 3.7, Post = 40.8 ± 3.5% of MHC IIX), with an increase in men (P < 0.05) and no change in women. To further assess this interaction, we pooled MHC IIAX fibers from both cellular and molecular functional assessments [n = 55 (25 men, 30 women) pretraining fibers and n = 76 (41 men, 35 women) posttraining fibers] and found a training × sex interaction effect (P < 0.05), due to an increase in men (P < 0.05; Fig. 6C).
Fig. 6.
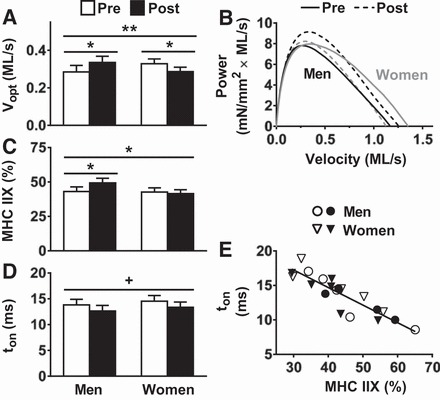
Training-induced adaptations in MHC IIAX fiber velocity at maximum power output (Vopt; A), power-velocity relationships (B), the fractional expression of MHC IIX in MHC IIAX fibers pooled from cellular and molecular functional assessments (C), myosin attachment time (ton; D), and the relationship between ton and the fractional expression of MHC IIX in single MHC IIAX fibers assessed for molecular function (E). Measurements were conducted at 15°C for A and B and at 25°C for D and E. Data represent means ± SE. *P ≤ 0.05 for training × sex effect (bar across all groups/time points) or training effect (across each sex separately); **P < 0.01 for training × sex effect; +P = 0.06 for training effect.
At the molecular level [n = 27 (15 men, 12 women) pretraining fibers and n = 45 (26 men, 19 women) posttraining fibers], there was a trend (P < 0.06) toward a training effect to reduce ton in MHC IIAX fibers (Fig. 6D). A shift toward a greater fractional expression of MHC IIX would be expected to reduce ton (44, 46), and we noted a relationship between these parameters (r = −0.888; P < 0.01; Fig. 6E). This relationship was diminished slightly when we examined training-induced changes in ton and MHC IIX fractional expression, albeit a trend was still observed (r = −0.705; P = 0.08). In addition to ton, there was a training effect (P = 0.05) to increase A-viscous (men: Pre = 0.27 ± 0.02 vs. Post = 0.33 ± 0.02; women: Pre = 0.30 ± 0.03, Post = 0.31 ± 0.02 N/mm2). There were no training or training × sex interaction effects for A-elastic (men: Pre = 1.88 ± 0.16, Post = 2.21 ± 0.15; women: Pre = 2.11 ± 0.17, Post = 2.17 ± 0.14 N/mm2), B (men: Pre = 19.6 ± 2.1 vs. 17.9 ± 2.0; women: Pre = 8.4 ± 2.2 vs. Post = 19.1 ± 1.9 N/mm2), C (20.3 ± 2.2, 18.6 ± 2.1; 19.3 ± 2.3, 19.8 ± 2.0 N/mm2) or 2πb (64.8 ± 5.8, 68.6 ± 5.8; 58.7 ± 5.8, 65.2 ± 5.4 s−1).
DISCUSSION
To our knowledge, this is the first study to probe the mechanisms underlying morphological and functional adaptations to moderate-intensity REX at the subcellular and molecular levels, a regimen that is attractive for older adults with functional limitations (59) such as knee OA. What stands out most from our data is the fact that cellular and molecular morphological and functional adaptations were absent or minimal. Together with knee extensor measures, our results suggest that much of the improvement in whole muscle performance with moderate-intensity REX may be due to factors extrinsic to muscle, such as changes in neural activation (13). Thus, while REX is recommended in knee OA patients (40) to correct muscle deficits, such as atrophy (10), these beneficial effects may not be forthcoming with moderate-intensity programs. Accordingly, further knowledge is needed regarding the dose-response relationships of the intensity and volume of REX in volunteers with knee OA (58), as well as other disabled older adult populations (59), to correct deficits in muscle cell size and function. This is not to suggest that moderate-intensity REX is without benefits, such as pain mitigation (39), but our data would suggest that its effects on intrinsic muscle properties within the time frame of most rehabilitation programs (e.g., 12 wk) are minimal.
Structural Adaptations to Moderate-Intensity Training
Moderate-intensity REX increased quadriceps muscle CSA (~7%) measured by CT, but these gains were not apparent from DEXA-derived measures of thigh fat-free tissue mass. Considering the commonly accepted hypertrophic effects of REX in older adults (15, 22) and the notion that CT can more precisely track such changes (18), one might conclude that DEXA was unable to detect muscle hypertrophy. However, analysis of single muscle fibers with two independent techniques found no change in average fiber CSA. This is not to suggest that moderate-intensity REX had no impact, as IHC showed a mild hypertrophy of MHC IIA fibers that was offset by decreased CSA in MHC I fibers, but the net effect was no change in muscle fiber size (Fig. 1, C and E). Shifts in hydration status with training can impact noninvasive, imaging-based measures of muscle tissue CSA (5, 17, 55). Thus training-induced shifts in fluid volume may increase CT-derived quadriceps CSA (17), but DEXA may not detect these changes (18) and these shifts would not be evident in single-fiber measures. We should acknowledge that the smaller number of fibers measured from muscle biopsies may fail to manifest changes at the whole muscle level (37). In particular, because of reciprocal changes in MHC I and IIA CSA (Fig. 1), bias toward MHC I fibers (37) might limit the extent of hypertrophy given their larger CSA, albeit this bias is likely minor (6). On balance, overall muscle fiber hypertrophy was modest or absent in this cohort in response to moderate-intensity REX.
This absence of hypertrophy is not unprecedented. Despite the widely held notion that REX leads to fiber hypertrophy (15, 22), REX-induced changes in single-fiber CSA in older adults are variable. Recent work has shown wide variation in single-muscle fiber CSA changes in older adults with moderate- to high-intensity REX (60–75% 1 RM) (67), including fiber atrophy in a substantial proportion of individuals (~25%), similar to MHC I fibers in the present study. While we noted hypertrophy in MHC IIA fibers (+8.5%), it was modest compared with high-intensity regimens (15, 22, 75, 76). A simple explanation for these findings is the moderate intensity of the training program (60% 1 RM) and the lower training volume (2 sets/exercise), although increasing training volume is difficult in knee OA because of joint pain and greater aggregate training volume with longer interventions does not yield hypertrophy in older women (52). Moreover, prior studies from our laboratory have similarly shown no change in fiber CSA in response to high-intensity REX in sedentary healthy control volunteers and volunteers with heart failure (3 sets of 80% 1 RM) (73). One commonality across cohorts studied in our laboratory is their muscle disuse. In fact, direct measures of weight-bearing activity from accelerometry showed similar values among volunteers with knee OA and heart failure and healthy older adults recruited to have activity levels that match heart failure patients (9, 47), raising the intriguing hypothesis that muscle disuse may render muscle resistant to the hypertrophic effects of REX of any intensity within the confines of standard-length rehabilitation programs (~12 wk).
The most prominent structural alteration with moderate-intensity REX was the increase in IMF mitochondrial content, which was driven by increased mitochondrial size in men. Although REX is not typically thought to alter mitochondrial content/function (30), a growing body of evidence supports an effect on mitochondria in healthy and diseased older adults (3, 32, 41, 53). In fact, the long-held notion that REX does not impact mitochondria is likely incorrect, as mitochondrial biogenesis occurs with REX-induced hypertrophy in younger individuals to maintain the proportional content of mitochondria (81), an adaptation that is likely necessary to maintain energy supply as the contractile machinery expands. Notably, this proportional increase in mitochondrial content in younger adults (81) was due to increased mitochondrial size, similar to adaptations in men in the present study. In this context, increased IMF mitochondria content may accommodate greater ATP demands with training in older men via mass action (32), which is in contrast with the upregulation of ATP production per unit mitochondria in young men with REX training (56). The reasons for sex differences in older men and women in our study are not readily apparent, but our results are not unique, as studies in younger adults have shown a greater mitochondrial biogenic response to interval training in men vs. women (66). One potential explanation is that older adult men have greater fractional ATP production from SS mitochondria (43). Thus sex-specific adaptations in IMF mitochondria may reflect the need to increase ATP production in the IMF compartment in men. Moreover, men had a trend (P = 0.10) toward lower mitochondrial size compared with women at baseline, and the increase in IMF mitochondrial size may serve to remediate this deficit and increase IMF oxidative capacity.
Building on this point, increased IMF mitochondrial content contrasts with greater responsivity of SS mitochondria to aerobic exercise (AEX) regimens in older adults (42). Adaptations in SS mitochondria may accommodate AEX training-induced changes in gene transcription and/or increased nutrient delivery secondary to increased muscle blood flow, as SS mitochondria reside in close proximity to myonuclei and capillaries (26). In our study, there may be less demand for expansion of SS mitochondria to support transcription in the absence of hypertrophy and because short bouts of REX yield less vascular response. In contrast to SS mitochondria, those in the IMF are positioned in the I-band region (8) and provide ATP for myosin and sarcoplasmic reticulum Ca2+-ATPases. While studies have shown increased mitochondrial content in older adults with REX training, SS and IMF compartments were not delineated (32). Thus our data show a novel compartment-specific mitochondrial response and, in light of recent studies highlighting functional specialization of SS and IMF subfractions in generating and utilizing the proton motive force, respectively (25), suggest that REX may differ from AEX in its effects on mitochondrial compartments, with implications for training-specific energy provision within the muscle cell.
Functional Adaptations to Moderate-Intensity Training
Moderate-intensity REX provoked substantial improvements in 1 RM strength in both men and women (Table 2), but these gains were less apparent when assessed by isometric/kinetic dynamometry (e.g., isotonic leg extension 1 RM vs. isometric/isokinetic knee extensor strength). This variation in task-specific performance suggests that pronounced functional gains represented by 1 RM assessments are likely related to adaptations extrinsic to muscle, that is, not related to changes in muscle size/structure, excitation-contraction coupling, or myofilament function, with the most likely mediator being improved neural activation (14). Parsing out the relative importance of the regulators of contraction with tissue-level measurements is complicated by the number of factors, as well as potential limitations in their assessment, such as those detailed above for muscle size. Our assessments of single-fiber function isolate the effects of training on myofilaments, the end effectors of muscle contraction, and suggest fiber type- and sex-specific adaptations in cellular function and its molecular determinants.
In MHC I fibers, Tmax increased in men and decreased in women, with corresponding shifts in the power-tension curves, such that Topt occurred at a greater tension in men compared with women. These results in men agree with the effects of standard, high-intensity, progressive REX training in older adults to increase whole muscle force at peak power (65). However, this training × sex interaction varied based on the temperature (15°C vs. 25°C), with the effect being more apparent in men or women depending on temperature. At a fundamental level, fibers from the two sexes appear to vary with temperature independent of training, with Tmax being higher in women at greater temperatures while tension is relatively constant across temperatures in men. Although two different fiber populations were studied at these different temperatures on different experimental equipment, temperature-related differences in Tmax agree with data in women at baseline (9) and data from other laboratories from serial measurements in young men (7). The cause of the sex-specific temperature response is unclear, but one possibility is differential posttranslational modification of myofilament proteins, as the functional effects of some modifications are temperature dependent (27). We have previously observed sex-specific differences in posttranslational modifications in older adults, such as myosin light chain (MLC) phosphorylation (45), a modification with temperature-dependent effects (27). However, because of the directionality of its effect and group differences, MLC phosphorylation is an unlikely cause of the differences observed in our study (27). Nonetheless, it highlights the potential for posttranslational modifications to drive temperature-specific responses in men and women, and possibly differential functional responses to training observed in our study.
At the molecular level, training increased the number of strongly bound cross bridges in MHC I fibers in men, a primary determinant of single-fiber tension (44). The increased number of strongly bound cross bridges, in turn, was accompanied by a slowing of myosin-actin cross-bridge kinetics (i.e., increased ton). That increased ton contributed to the greater number of strongly attached cross bridges was supported by the robust correlation between changes in B and ton. Thus our results define a potential molecular mechanism underlying REX-induced adaptations in single-MHC I fiber tension in men. The absence of training-induced adaptations in strongly bound cross bridges and ton in women may relate to a ceiling effect, as ton was already increased in women at baseline (9), a finding that is in keeping with our results showing slowed cross-bridge kinetics in a separate cohort of healthy, moderately active, older adults (45).
Sex-specific training effects in velocity were also found for MHC IIAX hybrid fibers, with an increase in Vopt in men. These functional changes in men were accompanied by their greater relative expression of MHC IIX and a corresponding reduction in ton. Changes in these two variables were strongly correlated, implicating altered MHC IIX expression as a molecular mechanism explaining REX-induced velocity adaptations in men. Interestingly, changes in ton did not differ by sex. In fact, the training effect on ton was due to decreases in both sexes. In the absence of altered MHC IIA and IIX proportions in women, their reduction in ton may relate to alterations in the intrinsic function of IIA or IIX myosin or both, possibly secondary to posttranslational modifications, such as phosphorylation (45) or oxidation (9). A recent report showed that aging increases posttranslational modification of MHC IIX (38), but this report included only men. Regardless of the precise molecular explanation for sex-specific changes in cross-bridge kinetics in women or why they did not translate into changes in velocity, our results suggest that men produce a more powerful contractile phenotype in MHC IIAX fibers via greater MHC IIX expression with moderate-intensity REX.
In MHC IIA fibers, training × sex interactions were apparent for Tmax at 25°C, with a reduction in Tmax in men and an increase in women. At the molecular level, these changes in Tmax were accompanied by a similar pattern of changes in A-elastic, a parameter that represents the underlying stiffness of the myofilament lattice and attached myosin-actin cross bridges in series, with a strong correlation between training-induced changes in tension and A-elastic. Although there were minor nonsignificant changes in B and C, statistical control for these parameters did not diminish the relationship between changes in A-elastic and tension, leading us to interpret these results as evidence that sex-specific changes in MHC IIA tension relate to alterations in non-cross-bridge myofilament force transmissibility, not changes in cross-bridge number or stiffness (as reflected in B and C). Our results are similar to those of Ochala et al. (50), who found increased stiffness in fibers from older compared with younger men, but differ inasmuch as they did not observe relationships between the increased stiffness and contractile characteristics. That myofilament force transmission may alter single-fiber tension was suggested by our prior work, where we found that greater A-elastic with age in older women was accompanied by increased single-fiber tension in both MHC I and IIA fibers (45). Differences between our work (45) and that of Ochala et al. (50) may relate to the different approaches to measuring stiffness (step release vs. small-amplitude sinusoidal length perturbation) and the fact that our studies of age-related changes matched young and older individuals for physical activity level to better discern primary aging effects. Collectively, our results suggest changes in myofilament stiffness as a novel mechanism through which single-fiber Tmax can be modulated in response to age and/or REX training. Why this mechanism would be specific to women and MHC IIA fibers is unclear, but baseline MHC IIA function was impaired in women with knee OA (9), suggesting that adaptations in myofilament stiffness may remediate functional deficits in MHC IIA fibers in women.
Sex Differences
Sex-specific differences occurred across structural and functional variables, with men generally showing more beneficial adaptations, such as increased mitochondrial content and improved contractility. Our results agree with those of Trappe et al. (75, 76), who found more hypertrophy and greater functional improvements in healthy older men compared with women in response to high-intensity REX. Adaptations may be blunted in women, and longer durations of moderate-intensity training may be required to achieve adaptations in muscle fiber function (52). The mechanisms underlying sex-specific differences are not apparent but indicate that the response to REX of any intensity should be considered in each sex independently, as different conclusions could be drawn without including sex in the analytical model (e.g., Fig. 2). From a clinical perspective, diminished muscle morphological and functional responses in women with knee OA may partially explain their decreased whole muscle/body functional recovery with clinical rehabilitation (28) and their need to rely more on neural adaptations (54). More broadly, together with our findings in these knee OA volunteers at baseline (9, 10) and in other cohorts with aging (8, 45), these data suggest that unique cellular-, organellar-, and molecular-level muscle structural and functional adaptations in women to altered muscle use may explain their greater disposition toward disability (35, 49).
Study Limitations
Several limitations to our study deserve discussion. We did not include a nondiseased control group because of problems equating exercise intensity between groups. As maximal exercise in knee OA will be influenced by joint pain, intensities for REX based on 1 RM will underestimate actual physiological capacity of their muscles, whereas healthy elderly without signs or symptoms of knee OA would perform a 1 RM closer to their physiological maximum and, in turn, exercise at a higher fractional intensity of that maximum during training. Building on this discussion, we must acknowledge that some of the lack of response to REX in knee OA might relate to the reduced exercise intensity (60% 1 RM), as studies of high-intensity training in healthy elderly subjects have shown more robust improvements in muscle fiber size and contractility (12, 15, 16, 21, 76). However, our recent studies in similarly aged, sedentary healthy older adults and older adults with heart failure found no improvements in fiber CSA and modest improvements in function with high-intensity training (80% of 1 RM) (73), implying that factors other than exercise intensity may explain the diminished response to REX. One factor that is common among these training studies in our laboratory is muscle disuse. In fact, we specifically recruited volunteers with knee OA who were free of comorbidities that could confound the response to training, to further isolate the effects of disuse on the exercise response. While we acknowledge that the knee OA disease process could affect muscle, studies that suggest such effects [e.g., inflammation (36)] have failed to exclude comorbidities that could illicit these muscle phenotypes. Moreover, as the muscle disuse that accompanies knee OA and its comorbidities could provoke some of these adaptations [e.g., inflammation (31)], parsing out the influence of knee OA per se, vs. the muscle disuse itself or its comorbidities, is exceedingly difficult, as we have discussed previously (9). Finally, we cannot discount that the impaired response to moderate-intensity REX in knee OA reflects sarcopenia that is refractory to such interventions, as these volunteers have extensive muscle atrophy and functional impairment (9, 10).
In summary, our results show that moderate-intensity REX has minimal effects to improve intrinsic muscle atrophy and contractile abnormalities in older adult volunteers with advanced-stage knee OA at the cellular and molecular levels, particularly women. Considering that knee OA is the leading cause of disability in older adults, and other prevalent pathologies in older adults share the magnitude of muscle disuse found in knee OA (e.g., heart failure, as described above), our results have clear clinical relevance and reasonably wide applicability with respect to the possible effects of muscle disuse. Together with whole muscle functional data, our results suggest that the majority of functional benefits likely derive from adaptations extrinsic to muscle, such as task-specific neural adaptations. While these gains are not without benefit to the patient, their durability after cessation of training is unclear. Thus moderate-intensity REX may not be effective at remediating intrinsic skeletal muscle atrophy and contractile dysfunction in knee OA that lie at the root of functional disability.
GRANTS
This study was funded by a National Institutes of Health grant (AG-033547), an Institutional National Research Service Award (HL-007647), and a Mentored Research Scientist Development Award (AG-031303).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.S.M., D.M.C., T.W.T., J.R.S., A.K., B.R.F., P.D.S., and M.J.T. performed experiments; M.S.M., D.M.C., T.W.T., J.R.S., A.K., B.R.F., P.D.S., and M.J.T. analyzed data; M.S.M., D.M.C., T.W.T., J.R.S., A.K., B.R.F., P.D.S., and M.J.T. interpreted results of experiments; M.S.M., D.M.C., and M.J.T. prepared figures; M.S.M., D.M.C., and M.J.T. drafted manuscript; M.S.M., D.M.C., T.W.T., J.R.S., A.K., B.R.F., P.D.S., P.A.A., B.D.B., and M.J.T. edited and revised manuscript; M.S.M., D.M.C., T.W.T., J.R.S., A.K., B.R.F., P.D.S., P.A.A., B.D.B., and M.J.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all the volunteers who dedicated their valuable time to these studies.
REFERENCES
- 1.Ades PA, Savage PD, Brochu M, Tischler MD, Lee NM, Poehlman ET. Resistance training increases total daily energy expenditure in disabled older women with coronary heart disease. J Appl Physiol (1985) 98: 1280–1285, 2005. doi: 10.1152/japplphysiol.00360.2004. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous Prevalence and most common causes of disability among adults—United States, 2005. Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep 58: 421–426, 2009. [PubMed] [Google Scholar]
- 3.Balakrishnan VS, Rao M, Menon V, Gordon PL, Pilichowska M, Castaneda F, Castaneda-Sceppa C. Resistance training increases muscle mitochondrial biogenesis in patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 996–1002, 2010. doi: 10.2215/CJN.09141209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15: 1833–1840, 1988. [PubMed] [Google Scholar]
- 5.Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 148: 379–385, 1993. doi: 10.1111/j.1748-1716.1993.tb09573.x. [DOI] [PubMed] [Google Scholar]
- 6.Blomstrand E, Ekblom B. The needle biopsy technique for fibre type determination in human skeletal muscle—a methodological study. Acta Physiol Scand 116: 437–442, 1982. doi: 10.1111/j.1748-1716.1982.tb07163.x. [DOI] [PubMed] [Google Scholar]
- 7.Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495: 573–586, 1996. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan DM, Bedrin NG, Subramanian M, Berking J, Ades PA, Toth MJ, Miller MS. Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex specific: relationship to single-fiber function. J Appl Physiol (1985) 116: 1582–1592, 2014. doi: 10.1152/japplphysiol.01362.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callahan DM, Miller MS, Sweeny AP, Tourville TW, Slauterbeck JR, Savage PD, Maugan DW, Ades PA, Beynnon BD, Toth MJ. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol 592: 4555–4573, 2014. doi: 10.1113/jphysiol.2014.279034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callahan DM, Tourville TW, Miller MS, Hackett SB, Sharma H, Cruickshank NC, Slauterbeck JR, Savage PD, Ades PA, Maughan DW, Beynnon BD, Toth MJ. Chronic disuse and skeletal muscle structure in older adults: sex-specific differences and relationships to contractile function. Am J Physiol Cell Physiol 308: C932–C943, 2015. doi: 10.1152/ajpcell.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan DM, Tourville TW, Slauterbeck JR, Ades PA, Stevens-Lapsley J, Beynnon BD, Toth MJ. Reduced rate of knee extensor torque development in older adults with knee osteoarthritis is associated with intrinsic muscle contractile deficits. Exp Gerontol 72: 16–21, 2015. doi: 10.1016/j.exger.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canepari M, Rossi R, Pellegrino MA, Orrell RW, Cobbold M, Harridge S, Bottinelli R. Effects of resistance training on myosin function studied by the in vitro motility assay in young and older men. J Appl Physiol (1985) 98: 2390–2395, 2005. doi: 10.1152/japplphysiol.01103.2004. [DOI] [PubMed] [Google Scholar]
- 13.Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol (1985) 101: 1514–1522, 2006. doi: 10.1152/japplphysiol.00531.2006. [DOI] [PubMed] [Google Scholar]
- 14.Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol 544: 641–652, 2002. doi: 10.1113/jphysiol.2002.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charette SL, McEvoy L, Pyka G, Snow-Harter C, Guido D, Wiswell RA, Marcus R. Muscle hypertrophy response to resistance training in older women. J Appl Physiol (1985) 70: 1912–1916, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Claflin DR, Larkin LM, Cederna PS, Horowitz JF, Alexander NB, Cole NM, Galecki AT, Chen S, Nyquist LV, Carlson BM, Faulkner JA, Ashton-Miller JA. Effects of high- and low-velocity resistance training on the contractile properties of skeletal muscle fibers from young and older humans. J Appl Physiol (1985) 111: 1021–1030, 2011. doi: 10.1152/japplphysiol.01119.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damas F, Phillips SM, Lixandrão ME, Vechin FC, Libardi CA, Roschel H, Tricoli V, Ugrinowitsch C. Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur J Appl Physiol 116: 49–56, 2016. doi: 10.1007/s00421-015-3243-4. [DOI] [PubMed] [Google Scholar]
- 18.Delmonico MJ, Kostek MC, Johns J, Hurley BF, Conway JM. Can dual energy X-ray absorptiometry provide a valid assessment of changes in thigh muscle mass with strength training in older adults? Eur J Clin Nutr 62: 1372–1378, 2008. doi: 10.1038/sj.ejcn.1602880. [DOI] [PubMed] [Google Scholar]
- 19.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263: 3029–3034, 1990. doi: 10.1001/jama.1990.03440220053029. [DOI] [PubMed] [Google Scholar]
- 20.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256, 2011. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve 28: 601–608, 2003. doi: 10.1002/mus.10480. [DOI] [PubMed] [Google Scholar]
- 22.Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol (1985) 64: 1038–1044, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA, Peterson CA. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol 592: 2625–2635, 2014. doi: 10.1113/jphysiol.2014.271288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine . American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43: 1334–1359, 2011. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 25.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, Balaban RS. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523: 617–620, 2015. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glancy B, Hsu LY, Dao L, Bakalar M, French S, Chess DJ, Taylor JL, Picard M, Aponte A, Daniels MP, Esfahani S, Cushman S, Balaban RS. In vivo microscopy reveals extensive embedding of capillaries within the sarcolemma of skeletal muscle fibers. Microcirculation 21: 131–147, 2014. doi: 10.1111/micc.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg MJ, Mealy TR, Watt JD, Jones M, Szczesna-Cordary D, Moore JR. The molecular effects of skeletal muscle myosin regulatory light chain phosphorylation. Am J Physiol Regul Integr Comp Physiol 297: R265–R274, 2009. doi: 10.1152/ajpregu.00171.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustavson AM, Wolfe P, Falvey JR, Eckhoff DG, Toth MJ, Stevens-Lapsley JE. Men and women demonstrate differences in early functional recovery after total knee arthroplasty. Arch Phys Med Rehabil 97: 1154–1162, 2016. doi: 10.1016/j.apmr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hootman JM, Macera CA, Ham SA, Helmick CG, Sniezek JE. Physical activity levels among the general US adult population and in adults with and without arthritis. Arthritis Rheum 49: 129–135, 2003. doi: 10.1002/art.10911. [DOI] [PubMed] [Google Scholar]
- 30.Hoppeler H, Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med Sci Sports Exerc 35: 95–104, 2003. doi: 10.1097/00005768-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Jackman RW, Cornwell EW, Wu CL, Kandarian SC. Nuclear factor-κB signalling and transcriptional regulation in skeletal muscle atrophy. Exp Physiol 98: 19–24, 2013. doi: 10.1113/expphysiol.2011.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol (1985) 90: 1663–1670, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16: 494–502, 1957. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange AK, Vanwanseele B, Fiatarone Singh MA. Strength training for treatment of osteoarthritis of the knee: a systematic review. Arthritis Rheum 59: 1488–1494, 2008. doi: 10.1002/art.24118. [DOI] [PubMed] [Google Scholar]
- 35.Leveille SG, Penninx BW, Melzer D, Izmirlian G, Guralnik JM. Sex differences in the prevalence of mobility disability in old age: the dynamics of incidence, recovery, and mortality. J Gerontol B Psychol Sci Soc Sci 55: S41–S50, 2000. doi: 10.1093/geronb/55.1.S41. [DOI] [PubMed] [Google Scholar]
- 36.Levinger I, Levinger P, Trenerry MK, Feller JA, Bartlett JR, Bergman N, McKenna MJ, Cameron-Smith D. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis Rheum 63: 1343–1348, 2011. doi: 10.1002/art.30287. [DOI] [PubMed] [Google Scholar]
- 37.Lexell J, Taylor C, Sjöström M. Analysis of sampling errors in biopsy techniques using data from whole muscle cross sections. J Appl Physiol (1985) 59: 1228–1235, 1985. [DOI] [PubMed] [Google Scholar]
- 38.Li M, Ogilvie H, Ochala J, Artemenko K, Iwamoto H, Yagi N, Bergquist J, Larsson L. Aberrant post-translational modifications compromise human myosin motor function in old age. Aging Cell 14: 228–235, 2015. doi: 10.1111/acel.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Su Y, Chen S, Zhang Y, Zhang Z, Liu C, Lu M, Liu F, Li S, He Z, Wang Y, Sheng L, Wang W, Zhan Z, Wang X, Zheng N. The effects of resistance exercise in patients with knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil 30: 947–959, 2016. doi: 10.1177/0269215515610039. [DOI] [PubMed] [Google Scholar]
- 40.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Roos EM, Underwood M. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 22: 363–388, 2014. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Melov S, Tarnopolsky MA, Beckman K, Felkey K, Hubbard A. Resistance exercise reverses aging in human skeletal muscle. PLoS One 2: e465, 2007. doi: 10.1371/journal.pone.0000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61: 534–540, 2006. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab 288: E818–E825, 2005. doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- 44.Miller MS, Bedrin NG, Ades PA, Palmer BM, Toth MJ. Molecular determinants of force production in human skeletal muscle fibers: effects of myosin isoform expression and cross-sectional area. Am J Physiol Cell Physiol 308: C473–C484, 2015. doi: 10.1152/ajpcell.00158.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME 2nd, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol (1985) 115: 1004–1014, 2013. doi: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller MS, VanBuren P, LeWinter MM, Braddock JM, Ades PA, Maughan DW, Palmer BM, Toth MJ. Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans. J Physiol 588: 4039–4053, 2010. doi: 10.1113/jphysiol.2010.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller MS, Vanburen P, Lewinter MM, Lecker SH, Selby DE, Palmer BM, Maughan DW, Ades PA, Toth MJ. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ Heart Fail 2: 700–706, 2009. doi: 10.1161/CIRCHEARTFAILURE.109.876433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulieri LA, Barnes W, Leavitt BJ, Ittleman FP, LeWinter MM, Alpert NR, Maughan DW. Alterations of myocardial dynamic stiffness implicating abnormal crossbridge function in human mitral regurgitation heart failure. Circ Res 90: 66–72, 2002. doi: 10.1161/hh0102.103221. [DOI] [PubMed] [Google Scholar]
- 49.Newman AB, Brach JS. Gender gap in longevity and disability in older persons. Epidemiol Rev 23: 343–350, 2001. doi: 10.1093/oxfordjournals.epirev.a000810. [DOI] [PubMed] [Google Scholar]
- 50.Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci 62: 375–381, 2007. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- 51.Palmer BM, Suzuki T, Wang Y, Barnes WD, Miller MS, Maughan DW. Two-state model of acto-myosin attachment-detachment predicts C-process of sinusoidal analysis. Biophys J 93: 760–769, 2007. doi: 10.1529/biophysj.106.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parente V, D’Antona G, Adami R, Miotti D, Capodaglio P, De Vito G, Bottinelli R. Long-term resistance training improves force and unloaded shortening velocity of single muscle fibres of elderly women. Eur J Appl Physiol 104: 885–893, 2008. doi: 10.1007/s00421-008-0845-0. [DOI] [PubMed] [Google Scholar]
- 53.Parise G, Brose AN, Tarnopolsky MA. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp Gerontol 40: 173–180, 2005. doi: 10.1016/j.exger.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Petterson SC, Raisis L, Bodenstab A, Snyder-Mackler L. Disease-specific gender differences among total knee arthroplasty candidates. J Bone Joint Surg Am 89: 2327–2333, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Ploutz-Snyder LL, Convertino VA, Dudley GA. Resistance exercise-induced fluid shifts: change in active muscle size and plasma volume. Am J Physiol Regul Integr Comp Physiol 269: R536–R543, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 47: 1922–1931, 2015. doi: 10.1249/MSS.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ratamess N, Alvar B, Evetoch T, Housh T, Kibler W, Kraemer W. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41: 687–708, 2009. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 58.Regnaux JP, Lefevre-Colau MM, Trinquart L, Nguyen C, Boutron I, Brosseau L, Ravaud P. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev 10: CD010203, 2015. doi: 10.1002/14651858.CD010203.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rejeski WJ, Axtell R, Fielding R, Katula J, King AC, Manini TM, Marsh AP, Pahor M, Rego A, Tudor-Locke C, Newman M, Walkup MP, Miller ME, LIFE Study Investigator Group . Promoting physical activity for elders with compromised function: the lifestyle interventions and independence for elders (LIFE) study physical activity intervention. Clin Interv Aging 8: 1119–1131, 2013. doi: 10.2147/CIA.S49737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rejeski WJ, Brawley LR, Ettinger W, Morgan T, Thompson C. Compliance to exercise therapy in older participants with knee osteoarthritis: implications for treating disability. Med Sci Sports Exerc 29: 977–985, 1997. doi: 10.1097/00005768-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Rengo JL, Callahan DM, Savage PD, Ades PA, Toth MJ. Skeletal muscle ultrastructure and function in statin-tolerant individuals. Muscle Nerve 53: 242–251, 2016. doi: 10.1002/mus.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rengo JL, Savage PD, Toth MJ, Ades PA. Statin therapy does not attenuate exercise training response in cardiac rehabilitation. J Am Coll Cardiol 63: 2050–2051, 2014. doi: 10.1016/j.jacc.2014.02.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhea MR, Alvar BA, Burkett LN, Ball SD. A meta-analysis to determine the dose response for strength development. Med Sci Sports Exerc 35: 456–464, 2003. doi: 10.1249/01.MSS.0000053727.63505.D4. [DOI] [PubMed] [Google Scholar]
- 64.Roubenoff R, Walsmith J, Lundgren N, Snydman L, Dolnikowski GJ, Roberts S. Low physical activity reduces total energy expenditure in women with rheumatoid arthritis: implications for dietary intake recommendations. Am J Clin Nutr 76: 774–779, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Sayers SP, Gibson K. High-speed power training in older adults: a shift of the external resistance at which peak power is produced. J Strength Cond Res 28: 616–621, 2014. doi: 10.1519/JSC.0b013e3182a361b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Klochak AL, Lonac MC, Paris HL, Szallar SE, Wood LM, Peelor FF 3rd, Holmes WE, Hellerstein MK, Bell C, Hamilton KL, Miller BF. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28: 2705–2714, 2014. doi: 10.1096/fj.13-246595. [DOI] [PubMed] [Google Scholar]
- 67.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310: E652–E661, 2016. doi: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults: a meta-analysis. Med Sci Sports Exerc 42: 902–914, 2010. doi: 10.1249/MSS.0b013e3181c34465. [DOI] [PubMed] [Google Scholar]
- 69.Suetta C, Frandsen U, Jensen L, Jensen MM, Jespersen JG, Hvid LG, Bayer M, Petersson SJ, Schrøder HD, Andersen JL, Heinemeier KM, Aagaard P, Schjerling P, Kjaer M. Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS One 7: e51238, 2012. doi: 10.1371/journal.pone.0051238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor-Piliae RE, Norton LC, Haskell WL, Mahbouda MH, Fair JM, Iribarren C, Hlatky MA, Go AS, Fortmann SP. Validation of a new brief physical activity survey among men and women aged 60-69 years. Am J Epidemiol 164: 598–606, 2006. doi: 10.1093/aje/kwj248. [DOI] [PubMed] [Google Scholar]
- 71.Thomas SG, Pagura SM, Kennedy D. Physical activity and its relationship to physical performance in patients with end stage knee osteoarthritis. J Orthop Sports Phys Ther 33: 745–754, 2003. doi: 10.2519/jospt.2003.33.12.745. [DOI] [PubMed] [Google Scholar]
- 72.Toth MJ, Miller MS, Callahan DM, Sweeny AP, Nunez I, Grunberg SM, Der-Torossian H, Couch ME, Dittus K. Molecular mechanisms underlying skeletal muscle weakness in human cancer: reduced myosin-actin cross-bridge formation and kinetics. J Appl Physiol (1985) 114: 858–868, 2013. doi: 10.1152/japplphysiol.01474.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, Palmer BM. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol 590: 1243–1259, 2012. doi: 10.1113/jphysiol.2011.219659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toth MJ, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Maughan DW, Ades PA. Reduced knee extensor function in heart failure is not explained by inactivity. Int J Cardiol 143: 276–282, 2010. doi: 10.1016/j.ijcard.2009.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol 281: C398–C406, 2001. [DOI] [PubMed] [Google Scholar]
- 76.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol (1985) 89: 143–152, 2000. [DOI] [PubMed] [Google Scholar]
- 77.Trappe TA, Liu SZ. Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise. J Appl Physiol (1985) 115: 909–919, 2013. doi: 10.1152/japplphysiol.00061.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trappe TA, Ratchford SM, Brower BE, Liu SZ, Lavin KM, Carroll CC, Jemiolo B, Trappe SW. COX inhibitor influence on skeletal muscle fiber size and metabolic adaptations to resistance exercise in older adults. J Gerontol A Biol Sci Med Sci 71: 1289–1294, 2016. doi: 10.1093/gerona/glv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Gool CH, Penninx BW, Kempen GI, Rejeski WJ, Miller GD, van Eijk JT, Pahor M, Messier SP. Effects of exercise adherence on physical function among overweight older adults with knee osteoarthritis. Arthritis Rheum 53: 24–32, 2005. doi: 10.1002/art.20902. [DOI] [PubMed] [Google Scholar]
- 80.Walker DJ, Heslop PS, Chandler C, Pinder IM. Measured ambulation and self-reported health status following total joint replacement for the osteoarthritic knee. Rheumatology (Oxford) 41: 755–758, 2002. doi: 10.1093/rheumatology/41.7.755. [DOI] [PubMed] [Google Scholar]
- 81.Wang N, Hikida RS, Staron RS, Simoneau JA. Muscle fiber types of women after resistance training--quantitative ultrastructure and enzyme activity. Pflugers Arch 424: 494–502, 1993. doi: 10.1007/BF00374913. [DOI] [PubMed] [Google Scholar]


