Abstract
We measured human immunodeficiency virus (HIV) ribonucleic acid (RNA) in paired cerebrospinal fluid (CSF) and plasma samples in a prospective study of 91 HIV-infected, antiretroviral therapy-naive patients with cryptococcal meningitis. Cerebrospinal fluid HIV RNA was lower than in plasma (median 4.7 vs 5.2 log10 copies/mL, P < .0001) and positively correlated with plasma HIV RNA, peripheral CD4+ T-cell percentage, and CSF CXCL10. Plasma/CSF ratio of HIV RNA ranged widely from 0.2 to 265.5 with a median of 2.6. Cerebrospinal fluid quantitative cryptococcal culture positively correlated with CSF CCL2 and CCL3. CSF-plasma viral discordance was not associated with cryptococcal-associated immune reconstitution inflammatory syndrome.
Keywords: cerebrospinal fluid, cryptococcal meningitis, HIV
Infection of the central nervous system (CNS) occurs early in the course of human immunodeficiency virus (HIV)-1 infection [1, 2]. Presence of HIV-1 in the CNS has been shown to contribute to HIV-associated neurological disorders—adversely impacting on patient memory, daily activities, medication adherence, and quality of life [3]. The immune privileged status of the CNS, immune cell, and HIV target cell compartmentalization and reduced penetrance of antiretroviral therapy (ART) contribute to the phenomena of discordant cerebrospinal fluid (CSF)-plasma HIV viral load and ART resistance [4, 5]. Progressive neurological dysfunction has been reported in patients who are aviremic in plasma but demonstrate escape of HIV-1 from suppression by ART in the CSF [4, 6, 7].
Although plasma HIV-1 viral load is commonly measured as an indicator of clinical response in resource-rich settings, viral load measurements in the CSF are usually limited to patients presenting with neurological problems. There are limited data on CSF HIV-1 viral loads in HIV-infected patients from sub-Saharan Africa, where the high rates of CNS co-infection such as cryptococcal meningitis (CM), coupled with delayed clinical presentation, advanced immunosuppression, and limited access to ART, are likely to contribute to higher CNS HIV-1 burden. Meningeal infection may be associated with breaches in the blood-brain-barrier (BBB) and increased CNS inflammation, thereby leading to increased HIV-1 penetrance into the CNS. It remains unclear whether CNS HIV-1 viral load may predict, associate with, or exacerbate neurological deterioration (ND) after treatment initiation in co-infected individuals. A small study previously demonstrated that HIV-infected patients coinfected with CM have higher CSF viral loads compared with those monoinfected with HIV [8]. Another found no correlation between CSF cryptococcal antigen and CSF HIV viral load [9].
We measured HIV ribonucleic acid (RNA) in paired CSF and blood samples, as a nested substudy of a prospective study of cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS) previously described [10]. We hypothesized that increased CSF viral burden before ART initiation may contribute to ND post-ART commencement and that increased cellularity and proinflammatory cytokine patterns correlate with higher CNS HIV viral load. Our aims were to determine whether HIV RNA was disproportionately higher in CSF compared with blood and the frequency of post-ART CNS viral escape (where HIV RNA is suppressed in the plasma but not in the CSF) in the setting of HIV-CM coinfection. We sought to also explore immune markers associated with high CSF HIV and cryptococcal burden and to assess whether high CSF HIV RNA predicts for subsequent C-IRIS.
METHODS
One hundred and six HIV-infected, ART-naive patients experiencing their first CM episode were treated with antifungal agents and ART and observed for 24 weeks after ART, in Durban, South Africa, as previously described [10]. Neurological deterioration events post-ART commencement were classified as C-IRIS (probable or possible) or not-C-IRIS. Ethics approval was granted by the Biomedical Research and Ethics Committee of the University of KwaZulu-Natal (Reference number BF053/09). Peripheral blood CD4+ T-cell counts were recorded.
Cerebrospinal fluid was centrifuged at 750× for 10 minutes, and cell-free CSF and blood plasma were stored within 4 hours of collection in −80°C freezers. Paired CSF and plasma samples collected at ART initiation were available for 91 patients, at 38 post-ART ND events. Human immunodeficiency virus-1 RNA levels were measured using the COBAS TaqMan HIV-1 test (Hoffmann-La Roche, Basel, Switzerland), with a limit of detection of 34 copies/mL. Paired samples were run in the same polymerase chain reaction run.
We used the proposed CNS penetration effectiveness (CPE) scores, developed and revised by Letendre et al [3] in 2010, to approximate expected effectiveness of ART. We calculated plasma-to-CSF HIV RNA ratio and defined post-ART CSF viral escape using the following strict definition: CSF HIV-1 RNA >1 log10 copies/mL greater than plasma HIV-1 RNA [4] and CSF HIV-1 RNA >200 copies/mL in cases where plasma HIV-1 RNA was undetectable (<34 copies/mL) [6].
We also assessed the relationship of plasma and CSF HIV-1 RNA levels (copies/mL) with CSF concentrations of CXCL10 (important in T-cell recruitment), CCL2, and CCL3 (both involved in myeloid cell trafficking) determined by customized multiplex Bio-Plex Pro assays (Bio-Rad, Gladesville, Australia), CSF CD4+ and CD8+ T-cell percentages measured by flow cytometry, and CSF quantitative cryptococcal culture (QnCC) as previously reported [10, 11].
Human immunodeficiency virus-1 RNA concentrations were log-transformed. Continuous variables were assessed for skew and summarized using mean and standard error or median and interquartile range (IQR) as appropriate and analyzed using either a t test or a Wilcoxon rank-sum test. P values are 2-tailed, and P values <.05 were considered significant. Spearman non-parametric correlation was used to correlate between 10 measured parameters and after Bonferroni correction for multiple comparison (P values <.0011 were considered significant). All analyses were performed using GraphPad Prism, version 6.
RESULTS
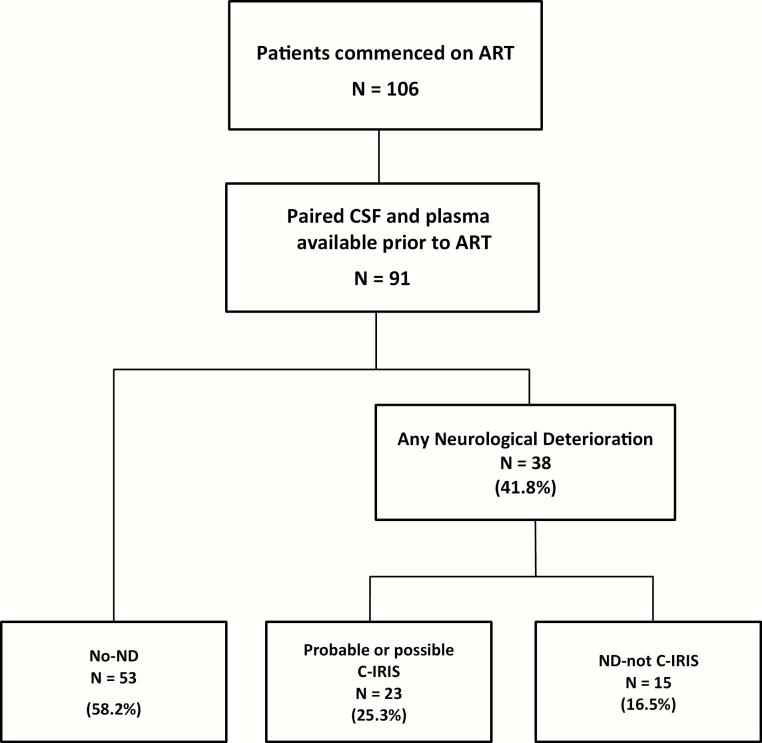
Cerebrospinal fluid-plasma paired samples collected at ART initiation were available from 91 patients with a median age of 34 years (IQR, 29–40), CD4+ T-cell count of 36 cells/μL (IQR, 16–78), and CD4+ T-cell percentage of 5.5% (IQR, 2.3–8.6), and 54 (59.3%) of whom were males. At ART initiation, median CSF protein was 0.92 g/L (IQR, 0.64–1.39), CSF neutrophils 4 cells/µL (IQR, 0–10), and CSF lymphocytes 24 cells/µL (IQR, 10–69). Twenty-three patients (25.3%) developed probable or possible C-IRIS, 15 patients (16.5%) experienced ND that was not due to C-IRIS, and 53 patients (58.2%) had no-ND in the first 24 weeks after ART initiation (Figure 1).
Figure 1.
Cohort flowchart. ART, antiretroviral therapy; C-IRIS, cryptococcosis-associated immune reconstitution inflammatory syndrome; CSF, cerebrospinal fluid; ND, neurological deterioration.
Plasma-to-Cerebrospinal Fluid Ratio of Human Immunodeficiency Virus RNA Varied Greatly at Antiretroviral Therapy Initiation
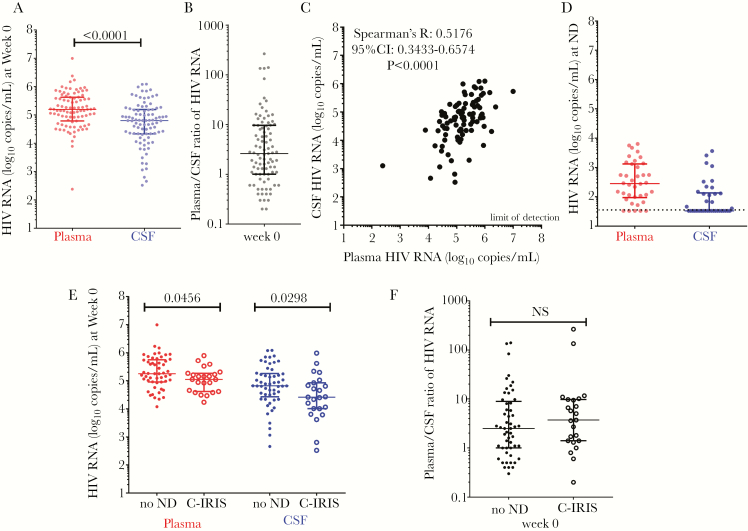
Overall, plasma HIV-1 RNA level was significantly higher than CSF HIV-1 RNA level ([median 5.2, IQR 4.8–5.6 vs median 4.7, IQR 4.3–5.2] log10 copies/mL, P < .0001) (Figure 2A). The median plasma-to-CSF log HIV-1 RNA ratio was 2.6 (IQR, 1–9.6) with a very large range of values from a low of 0.2 to a high of 265.5 (Figure 2B). Human immunodeficiency virus-1 RNA in CSF was positively correlated with that in plasma (Spearman’s R, 0.5176; 95% confidence interval, 0.3433–0.6574; P < .0001) (Figure 2C). Twenty-one patients (23.1%) had a higher HIV-1 RNA level in the CSF compared with plasma.
Figure 2.
Relationship of human immunodeficiency virus (HIV) ribonucleic acid (RNA) measured in blood and cerebrospinal fluid (CSF) in patients with HIV and cryptococcal meningitis coinfection. (A) Human immunodeficiency virus RNA measured in plasma (in red) and in CSF (in blue) before antiretroviral therapy (ART) commencement. (B) Plasma/CSF ratio of HIV RNA and (C) correlation of plasma to CSF HIV RNA before ART commencement. (D) Human immunodeficiency virus RNA measured in plasma (in red) and in CSF (in blue) at time of neurological deterioration post-ART commencement. (E) Human immunodeficiency virus RNA measured in plasma (in red) and in CSF (in blue), and (F) plasma/CSF HIV RNA ratio (in black), before ART-commencement in those with future cryptococcosis-associated immune reconstitution inflammatory syndrome ([C-IRIS] large circles) and those with no neurological deterioration (ND) (small circles) during the 24-week follow-up period post-ART initiation. CI, confidence interval.
Human Immunodeficiency Virus and Cryptococcal Infections Exert Differential Effects on Markers of Immune Dysfunction and/or Inflammation in the Cerebrospinal Fluid
Both HIV and cryptococcal infection of the CNS are associated with markers of immune dysfunction and/or inflammation in the CSF. To examine the relationship between these markers and either infection, we correlated these with CSF HIV-1 RNA levels and QnCC (Supplementary Table). The most notable finding was that CSF levels of HIV-1 RNA and CXCL10 correlated positively (R = 0.5616, P < .0001), whereas CSF QnCCs demonstrated a positive correlation with levels of CCL2 (R = 0.3718, P = .0005) and CCL3 (R = 0.3532, P = .0009). Cerebrospinal fluid HIV-1 RNA levels also positively correlated with blood CD4+ T-cell percentage (R = 0.5183, P < .0001) and CSF white cell count (R = 0.3683, P = .0003).
No Cerebrospinal Fluid Viral Escape Occurred at Neurological Deterioration Event
Of the 91 patients, 51 (56.0%) patients commenced on tenofovir-lamivudine-efavirenz (CPE = 6), 39 (42.9%) patients commenced on stavudine-lamivudine-efavirenz (CPE = 7), and 1 patient (1.1%) commenced on stavudine- lamivudine-nevirapine (CPE = 8) [3]. At 24-weeks post-ART commencement, 41 (45.1%) patients had undetectable plasma HV RNA of <34copies/mL (median < 34, IQR < 34–260), and the median CD4+ T-cell count and percentage were 138 cells/µL (IQR, 106–226) and 10.8% (IQR, 6.9–13.9), respectively.
Thirty-eight paired CSF and plasma samples were collected at ND event, which occurred at a median time of 7 weeks (IQR, 4–18) after commencing ART. At ND event, HIV RNA was undetectable in 5 (13.2%) plasma samples with a median of 2.45 log10 copies/mL; (IQR, 2.00–3.09) HIV RNA was undetectable in 21 (55.3%) CSF samples (Figure 2D) of which 12 patients on an ART regimen with a CPE score of 7, and 9 patients with a CPE score of 6. Thirty-three patients recorded higher HIV-1 RNA in plasma than in CSF, whereas 4 patients (10.5%) had no detectable HIV-1 RNA in either plasma or CSF. One patient had undetectable HIV-1 RNA in plasma and 46 copies/mL in CSF but did not make the definition of CSF viral escape.
Plasma-to-Cerebrospinal Fluid Human Immunodeficiency Virus-1 RNA Ratios at Antiretroviral Therapy Initiation Were Not Different in Patients Who Developed Probable or Possible Cryptococcosis-Associated Immune Reconstitution Inflammatory Syndrome Compared With Those With No Neurological Deterioration
The C-IRIS group compared with the no-ND group had lower HIV-1 RNA levels in plasma ([median 5.0, IQR 4.6–5.3 vs median 5.2, IQR 5.0–5.8] log10 copies/mL, P = .0456) and in the CSF ([median 4.4, IQR 4.0–4.9 vs median 4.8, IQR 4.4–5.3] log10 copies/mL, P = .0298) at ART initiation (Figure 2E). The plasma-to-CSF HIV RNA ratio was not different between groups ([median 3.7, IQR 1.4–9.7 vs median 2.5, IQR 1.0–8.9], P = .3667) (Figure 2F). The plasma-to-CSF HIV RNA ratio calculated at C-IRIS event and at ART initiation were not different (P = .8412) (data not shown).
DISCUSSION
As part of an investigation of the immunopathogenesis of C-IRIS in patients co-infected with CM, we have shown that plasma-to-CSF ratios of HIV-1 RNA at ART initiation or at ND were not associated with the occurrence of C-IRIS but that CSF HIV-1 RNA levels correlated with CSF CXCL10 levels, whereas CSF cryptococcal burden, measured by CSF quantitative culture counts, correlated weakly with CSF levels of CCL2 and CCL3. Together, these data suggest that in the context of CM co-infection, the amount of HIV-1 replication in the CNS relative to the plasma does not directly influence the development of C-IRIS and that production of pro-inflammatory chemokines is affected differently by HIV-1 and cryptococcosis. The association between CSF HIV-1 RNA and CSF CXCL10 has previously been reported [12]. Our finding that residual CNS cryptococcal burden is not associated with CXCL10 levels but rather with CSF CCL2 and CCL3 levels provides evidence that HIV-1 and cryptococcosis are driving different inflammatory mechanisms that might impact on the outcome of advanced HIV-CM co-infection. Advanced HIV infection leads not only to T-cell depletion but also a progressively dysfunctional innate system, which is a vital defense against fungal infection.
We have previously shown the importance of CSF cryptococcal sterility at ART initiation in reducing future ND events, C-IRIS, and cryptococcal relapse [10], and we suggested the importance of CD8+ T-cell and myeloid cell trafficking into the CNS in the development of C-IRIS [11, 13]. Our findings here reiterate the possibility of an imbalance in cellular trafficking into the CNS, which defaults towards non-CD4+ T-cell-mediated immune responses, including innate immune responses, as is increasingly recognized in C-IRIS [11, 13, 14].
Higher CNS fungal burden was not associated with CSF viral burden—this undermines the simplistic hypothesis of passive leakage of Cryptococcus spp and HIV through a breached BBB. However, HIV-induced immune activation may attract cell types important also in fungal clearance, thereby outcompeting one for the other, or, alternatively, different cell types and signaling pathways are involved with resisting HIV and cryptococcal infections.
Despite CM being a severe CNS opportunistic infection, we found that overall, HIV-1 RNA is not higher in CSF than in plasma at CM presentation and at time of ND, suggesting that there is no distinguishable CNS viral escape from immune response nor ART when measured by quantitative viral burden. Furthermore, higher CSF HIV-1 RNA levels were not associated with subsequent C-IRIS. This suggests that immune dysfunction and cryptococcal factors, rather than greater HIV replication, are more likely to contribute to the development of C-IRIS. Further work will need to address whether the anti-cryptococcal immune environment predisposes less favorably to HIV-1 replication. Although there is a wide range of plasma-to-CSF HIV-1 RNA ratios in our cohort, overall this is low (median 2.6)—consistent with previous findings in which HIV-infected patients with CNS co-infections and those with neurological symptoms recorded lower ratios compared with neurologically asymptomatic patients [8]. Central nervous system involvement in newly diagnosed HIV-infected individuals is underappreciated. It is worth noting that all of our patients have measurable HIV virus in the CSF, although it is perhaps surprising that despite their advanced meningeal infection, overall, their CSF viral load was still lower than their plasma viral load. Cerebrospinal fluid HIV-1 escape from the effects of ART is rare when measured longitudinally [15].
Limitations of our study include the lack of HIV-1- and CM-mono-infected individuals as control groups and the lack of subsequent time-defined matched samples to measure viral and QnCC kinetics. It is unfortunate that we were not able to perform an analysis at the time of CM diagnosis—antifungal agents may have had some variable impact on the chemokines and QnCC measured.
CONCLUSIONS
In summary, our study suggests that disproportionately high viral burden in the CSF compared with blood is not commonly seen in HIV-CM co-infection and does not predict for C-IRIS. Future studies are needed to better understand virus characteristics and the immune microenvironment such as mechanistic real-time viral killing assays, CSF microarray analyses for gene expression, and HIV viral genotypic and phenotypic characterization.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We acknowledge the patients and their families and staff at HIV Pathogenesis Programme and King Edward VIII Hospital.
Author contributions. C. C. C., R. K., and T. N. designed the experiments. C. C. C., R. K., K. S., and T. N. analyzed the data. C. C. C., M. A. F., and T. N. wrote the manuscript. R. K., S. O., and K. H. performed the virology assay. C. C. C. and B. G. conducted the clinical study. C. C. C., T. N., M. A. F., S. R. L., and M.-Y. S. M. were members of the protocol steering committee. All authors read and approved the final manuscript.
Financial support. This work was funded by the REACH Initiative ([Research and Education in HIV/AIDS for Resource Poor Countries] to M. A. F.) and Pfizer Neuroscience (Grant NS052.10; to C. C. C.). C. C. C. is supported by the Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship APP1092160. M. A. F. was supported by NHMRC Grant 510448. S. R. L. is an NHMRC practitioner fellow APP1042654. T. N. was supported in part by an International Early Career Scientist grant from the Howard Hughes Medical Institute and by the South African Department of Science and Technology/National Research Foundation Research Chairs Initiative. Open Access publication of this article has been made possible through support from the Victor Daitz Information Gateway, an initiative of the Victor Daitz Foundation and the University of KwaZulu-Natal.
Potential conflicts of interest. All authors: No reported conflicts.All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012; 206:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goudsmit J, de Wolf F, Paul DA, et al. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet 1986; 2:177–80. [DOI] [PubMed] [Google Scholar]
- 3. Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med 2010; 18:45–55. [PMC free article] [PubMed] [Google Scholar]
- 4. Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010; 50:773–8. [DOI] [PubMed] [Google Scholar]
- 5. Strain MC, Letendre S, Pillai SK, et al. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol 2005; 79:1772–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edén A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 2010; 202:1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012; 26:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christo PP, Greco DB, Aleixo AW, Livramento JA. Factors influencing cerebrospinal fluid and plasma HIV-1 RNA detection rate in patients with and without opportunistic neurological disease during the HAART era. BMC Infect Dis 2007; 7:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cecchini DM, Cañizal AM, Rojas H, et al. Variables that influence HIV-1 cerebrospinal fluid viral load in cryptococcal meningitis: a linear regression analysis. J Int AIDS Soc 2009; 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang CC, Dorasamy AA, Gosnell BI, et al. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS 2013; 27:2089–99. [DOI] [PubMed] [Google Scholar]
- 11. Chang CC, Omarjee S, Lim A, et al. Chemokine levels and chemokine receptor expression in the blood and the cerebrospinal fluid of HIV-infected patients with cryptococcal meningitis and cryptococcosis-associated immune reconstitution inflammatory syndrome. J Infect Dis 2013; 208:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cinque P, Bestetti A, Marenzi R, et al. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol 2005; 168:154–63. [DOI] [PubMed] [Google Scholar]
- 13. Naranbhai V, Chang CC, Durgiah R, et al. Compartmentalization of innate immune responses in the central nervous system during cryptococcal meningitis/HIV coinfection. AIDS 2014; 28:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meya DB, Okurut S, Zziwa G, et al. Cellular immune activation in cerebrospinal fluid from ugandans with cryptococcal meningitis and immune reconstitution inflammatory syndrome. J Infect Dis 2015; 211:1597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edén A, Nilsson S, Hagberg L, et al. Asymptomatic cerebrospinal fluid HIV-1 viral blips and viral escape during antiretroviral therapy: a longitudinal study. J Infect Dis 2016; 214:1822–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.