Abstract
Background
Nucleic acid amplification (NAA) testing for Mycobacterium tuberculosis (MTB) offers improved diagnostic accuracy, compared with smear microscopy, in differentiating MTB from other mycobacteria. We aimed to evaluate the reliability and projected impact of NAA testing in patients with acid-fast bacilli (AFB) smear-positive respiratory samples.
Methods
We identified a retrospective cohort of all patients with AFB smear-positive respiratory specimens at Henry Ford Hospital from January 1, 2001 through December 31, 2011. We examined the association between patients’ sociodemographic factors and clinical comorbidities with the likelihood of being diagnosed with MTB. We evaluated the projected change in duration of airborne isolation and unnecessary MTB treatment with introducing NAA testing into clinical decision making for AFB smear-positive patients.
Results
One hundred thirty patients had AFB smear-positive respiratory specimens, 80 of these patients had a positive NAA test result, and 82 patients grew MTB on culture. Nucleic acid amplification testing had a sensitivity and specificity of 97.6% and 100%, respectively. Integrating NAA testing into clinical decision making for patients with AFB-positive smears was associated with a significantly shorter time in airborne isolation (6.0 ± 7.6 vs 23.1 ± 38.0, P < .001) and 9.5 ± 11.32 fewer days of unnecessary MTB treatment in patients with negative NAA test.
Conclusions
Nucleic acid amplification testing provided a rapid and accurate test in the diagnosis of MTB while significantly reducing the duration of isolation and unnecessary medications in patients with negative NAA test.
Keywords: diagnostic testing, Mycobacterium tuberculosis, nucleic acid tests
The global disease burden caused by Mycobacterium tuberculosis (MTB) remains substantial with an estimated 10.4 million new cases and 1.4 million deaths in 2015 [1]. Early detection of MTB and prompt initiation of therapy is crucial for disease control. Historically, diagnosis of MTB relied on microscopic evaluation of the obtained sample, most commonly sputum, for the presence of acid-fast bacilli (AFB). This was then confirmed by isolating MTB in cultures. However, AFB smears have a low sensitivity and specificity [2] and do not distinguish MTB from nontuberculosis mycobacteria (NTM) [3]. The increasing incidence of NTM has further decreased the reliability of AFB smear as a rapid screening test for diagnosing MTB.
In 1995, nucleic acid amplification tests (NAATs) were initially approved to be performed on respiratory specimens from patients with smear-positive AFB and clinical features of tuberculosis (TB). Nucleic acid amplification test sensitivity and specificity in diagnosing MTB has been reported to be as high as 99% and 98%, respectively [4, 5], resulting in a higher MTB detection rate and shorter time to treatment initiation [6]. Nucleic acid amplification tests of respiratory specimens from patients with suspected pulmonary TB has been recommended by the US Centers for Disease Control and Prevention (CDC) [7]. The utility and cost effectiveness of NAAT for the rapid diagnosis of MTB have been evaluated in populations with high rates of human immunodeficiency virus (HIV) infection, in whom NTM is prevalent [8, 9]. However, the utility of NAAT has not been evaluated in other immunocompromised patient populations with high rates of NTM such as cancer patients and transplant recipients. We aimed to evaluate the reliability of NAAT, implemented in 2001 at our institution, among various hospitalized patient populations, and its expected impact on the duration of airborne isolation and unnecessary anti-MTB therapy.
METHODS
Henry Ford Hospital is a 900-bed teaching hospital in Detroit, Michigan, which serves a predominately urban population with a high prevalence of HIV infection and also serves as a tertiary care center for cancer therapy and transplantation. We used our institution’s medical and laboratory data repository to retrospectively identify the cohort of patients hospitalized from January 1, 2001 through December 31, 2011 with an AFB smear-positive respiratory specimen. The study was approved by the Henry Ford Health System Institutional Review Board, and the requirement for an informed consent was waived. All patient records and pertinent information were anonymized and deidentified before the analysis.
Isolation Policy
Our hospital’s Infection Control Isolation policy, based on the CDC recommendations, requires that all patients with suspected MTB, or patients in whom an AFB smear and culture is ordered, be placed in airborne isolation. Placement in airborne isolation is continued until 3 sputum or respiratory specimens are AFB smear negative or an alternate diagnosis of the pulmonary illness is confirmed. If the sputum is AFB smear positive, the patient is started on empiric anti-MTB therapy until the mycobacterial cultures results are available. The AFB smear-positive patient is removed from airborne isolation after receiving at least 2 weeks of anti-MTB therapy, shows improvement of symptoms, and has 3 negative AFB smears. In addition, all AFB smear-positive patients are reported to the local health department for contact investigation. Since 2001, AFB smear-positive respiratory specimens were further evaluated with direct NAAT. Our Infection Control Isolation policy was modified in 2001 to permit the removal of AFB smear-positive patients from airborne isolation if the NAAT was negative for MTB.
Microbiology Methodology
Respiratory samples were decontaminated and concentrated for acid-fast smear (auramine rhodamine) and culture using standard procedures [10]. Both solid and liquid culture media were performed on all samples. Isolates from positive cultures were sent to the local health department for drug sensitivity testing and deoxyribonucleic acid fingerprinting.
Between 2001 and 2007, NAAT was performed daily using an in-house Amplified Mycobacterium Tuberculosis Direct Test ([MTD] Gen-Probe, San Diego, CA) on AFB smear-positive specimens. After 2007, all AFB smear-positive sputum and bronchoalveolar lavage (BAL) samples were sent to the Michigan State Tuberculosis Laboratory, where samples were analyzed using the MTD Gen-Probe assay. The turnaround time (TAT) for both the in-house and the state NAAT testing was comparable (24–48 hours). The TAT for both NAAT and cultures was measured as the time from obtaining a positive AFB smear to the time for corresponding final test result.
Clinical Data
Demographic variables including date of birth, gender, race, employment status, and housing conditions, and social history including drug use were abstracted from the medical records and contingent on patient self-reporting and physician documentation. Clinical variables evaluated included presenting signs and symptoms along with clinical risk factors associated with pulmonary TB (history of HIV, cancer, or transplant and positive tuberculin skin testing with purified protein derivative [PPD]).
For the AFB smear-positive group, we examined the association between patients’ sociodemographic factors and clinical comorbidities with the likelihood of being diagnosed with MTB. The actual duration of airborne isolation and days of unnecessary MTB treatment (for AFB smear-positive patients with NTM) based on NAAT was compared with the expected duration of these endpoints had the culture results been used for removal from airborne isolation and clinical decision making as in the pre-NAAT period before 2001.
Statistical Analysis
Numeric study variables were summarized using means and standard deviations, whereas categorical study variables were summarized using frequency counts and percentages. The study groups were compared using 2-sample t tests for normally distributed numeric data, Wilcoxon rank-sum tests for nonnormally distributed numeric data, χ2 tests for nonsparse categorical data, and Fisher exact tests for sparse categorical data. Categorical data sparsity was defined as the presence of expected cell counts less than 5. Statistical significance was defined as P < .05 for all comparison test results.
RESULTS
Patient Characteristics
Patient demographics and clinical characteristics are shown in Table 1. Over a 10-year period, 130 patients with positive AFB smear of sputum or respiratory specimens including BAL samples had NAAT done and were included in our analysis. Only the index admission during which an AFB smear was positive and NAAT was performed was included in the analysis. Patients had a mean age of 55 ± 18.2 years. The majority were male (59%) and African American (69%). Of the 88 patients in whom HIV status was available, 22 (25%) were HIV seropositive. Fifteen patients (11.6%) had a history of malignancy and/or had undergone transplantation.
Table 1.
Patient Demographics and Clinical Characteristics
| Variable | Overall N (%) |
|
|---|---|---|
| Age in years (mean ± SD) | 55.0 ± 18.2 | |
| Gender | Female | 53 of 130 (40.8%) |
| Male | 77 of 130 (59.2%) | |
| Race | White | 26 of 130 (20.0%) |
| African American | 90 of 130 (69.2%) | |
| Other | 14 of 130 (10.8%) | |
| PPD test positive | 29 of 57 (50.9%) | |
| HIV seropositive | 22 of 88 (25.0%) | |
| Cancer or transplant patient | 15 of 130 (11.5%) | |
| Drug abuse | 32 of 130 (24.6%) | |
| Homeless | 9 of 130 (6.9%) | |
| Healthcare worker | 2 of 130 (1.5%) | |
Abbreviations: HIV, human immunodeficiency virus; PPD, purified protein derivative; SD, standard deviation.
Characteristics of Acid-Fast Bacilli Testing
The positive predictive values (PPVs) of AFB smears in diagnosing MTB across different clinical settings are shown in Table 2. Overall, the PPV of AFB smear to detect MTB was 63.1% because MTB was isolated from 82 of the 130 specimens. The PPV of an AFB smear was significantly lower for HIV-seropositive patients (45.5%) and patients with history of malignancy or transplantation (26.7%). In contrast, the PPV of an AFB smear was significantly higher for patients with positive PPD test (86.2%) and patients with a prior history of TB (62.5%).
Table 2.
Positive Predictive Value of AFB Across Different Clinical Characteristics
| Clinical Characteristics | Total AFB Smear Specimens | Positive Culture for TB | Negative Culture for TB | AFB PPV |
|---|---|---|---|---|
| Total | 130 | 82 | 48 | 63.1% |
| Immunocompromised | 37 | 14 | 23 | 37.8% |
| HIV seropositive | 22 | 10 | 12 | 45.5% |
| Cancer and/or transplant patient | 15 | 4 | 11 | 26.7% |
| Previous history of TB | 24 | 15 | 9 | 62.5% |
| Non-immunocompromised | 102 | 71 | 31 | 69.6% |
| PPD positive | 29 | 25 | 4 | 86.2% |
| Drug abuse | 32 | 24 | 8 | 51.8% |
Abbreviations: AFB, acid-fast bacilli; HIV, human immunodeficiency virus; PPD, purified protein derivative; PPV, positive predictive value; TB, tuberculosis.
Characteristics of Nucleic Acid Amplification Tests
Among the 130 patients with positive AFB smear, 80 patients had a positive NAAT (61.5%) and the remainder were negative. All patients with positive NAAT and 2 of the 50 patients with negative NAAT had MTB isolated on culture. In the remaining 48 patients, the cultures grew Mycobacterium avium complex (60%), Mycobacterium kansasii (19 %), Mycobacterium chelonae (6%), and Mycobacterium xenopi (14%). Based on the culture results, NAAT had a sensitivity and specificity of 97.6% and 100%, respectively, for the detection of MTB. The NAAT had a negative predictive value of 96.0% and PPV of 100%. Mean TAT for the NAAT was 1.43 ± 0.90 days, compared with 12.7 ± 7.6 days for the mycobacterial culture (Table 3).
Table 3.
Difference in Turnaround Time for NAAT and Mycobacterial Culture, Duration of Isolation, and Length of Hospitalization
| Variable | Overall (N = 130) | NAAT Negative (N = 50) |
NAAT Positive (N = 80) |
P Value |
|---|---|---|---|---|
| Turnaround time for NAAT in days, mean ± SD (median) |
1.43 ± 0.90 (1.00) | 1.38 ± 1.00 (1.00) | 1.46 ± 0.83 (1.00) | .312 |
| Turnaround time for mycobacterial culture, mean ± SD (median) |
12.7 ± 7.6 (11.0) |
12.1 ± 9.8 (8.0) |
13.0 ± 6.0 (11.5) |
.017 |
| Airborne infection isolation days, mean ± SD (median) |
16.8 ± 31.5 (7.0) |
6.0 ± 7.6 (3.0) |
23.1 ± 38.0 (10.0) | <.001 |
| Days in hospital, mean ± SD (median) |
20.5 ± 31.4 (11.0) | 12.0 ± 8.5 (9.0) |
25.4 ± 38.2 (13.0) | .044 |
Abbreviations: NAAT, nucleic acid amplification tests; SD, standard deviation.
Clinical Impact of Nucleic Acid Amplification Tests
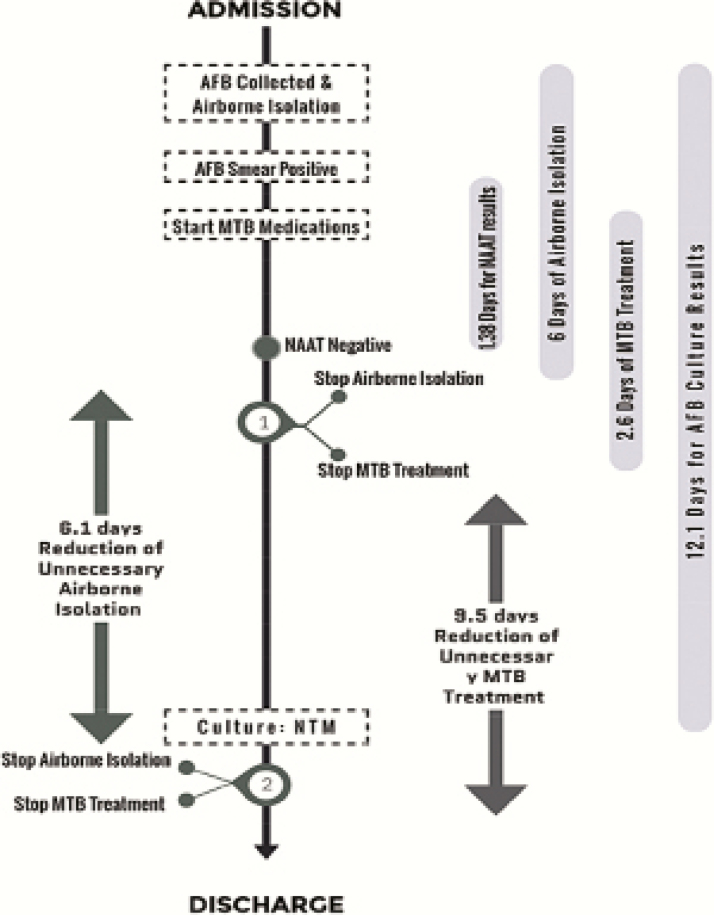
Patients with positive AFB smear but negative NAAT spent a significantly shorter time in airborne isolation (6.0 ± 7.6 days vs 23.1 ± 38.0 days, P < .001). The estimated mean duration in airborne isolation was reduced by 6.1 days (difference between actual duration based on NAAT and expected duration based on culture results) (Figure 1). Similarly, patients with negative NAAT had a significantly shorter hospital stay (12.0 ± 8.5 days vs 25.4 ± 38.2 days, P = .044) (Table 3). For the 48 patients with negative NAAT results, 20 were started on MTB therapy for an average duration of 2.6 ± 5.4 days. The mean duration of unnecessary anti-TB therapy was reduced by 9.5 ± 11.32 days (difference between actual duration of unnecessary anti-MTB therapy received and expected duration based on culture results) (Figure 1).
Figure 1.
Projected impact of nucleic acid amplification test (NAAT) on reducing duration of airborne isolation and duration of unnecessary Mycobacterium tuberculosis (MTB) treatment for patients with nontuberculous mycobacteria (NTM). 1 denotes that actual time of discontinuing airborne isolation and stopping MTB treatment. 2 denotes the expected time discontinuing airborne isolation and stopping MTB treatment, if NAAT was not available. AFB, acid-fast bacilli.
DISCUSSION
The implementation of NAAT of AFB smear-positive respiratory specimens at our institution had a significant positive impact on the clinical management of patients with suspected pulmonary TB. In our 10-year retrospective cohort, NAAT has proved to be a reliable test, especially in the immunocompromised patient population, in differentiating MTB from NTM with a sensitivity and specificity of 97.6% and 100%, respectively. In addition, NAAT allowed for rapid identification of patients with NTM, thereby resulting in significantly shorter duration of airborne isolation and decreased unnecessary exposure to anti-MTB therapy.
Our results highlight the accuracy of NAAT in the diagnosis of MTB in patients with AFB smear-positive specimens. The sensitivity and specificity of the test, when applied in our health system, were comparable to those reported in the literature [5, 8, 9, 11, 12]. Among patients with smear-positive respiratory specimens, AFB smears had an overall PPV of 63.1% compared with a PPV of 100% for NAAT. It is notable that the PPV of a positive AFB smear was much lower in our immunocompromised HIV-infected, cancer, and transplant subpopulations who have a high likelihood of NTM infection. Nucleic acid amplification tests will be most useful and have a greater impact in clinical and infection control decision making in these populations.
To prevent transmission of MTB in healthcare facilities, patients with suspected pulmonary TB are placed in negative-pressure airborne isolation rooms [13]. In areas with low TB prevalence, isolating all patients with pulmonary symptoms and chest radiograph suspicious for TB leads to overuse of airborne isolation rooms [14, 15]. Similarly, solely using an AFB smear-positive result to determine the need for airborne isolation may be unreliable given the increasing incidence of NTM [16]. Nucleic acid amplification tests resulted in a mean reduction of 6.1 days of airborne isolation among patients who had a positive AFB smear but negative NAAT.
Our study highlights that patients with positive AFB smears but negative NAAT receive unnecessary anti-MTB therapy for 2.6 days. This is significantly lower treatment than what would be expected if clinicians were to rely solely on culture results (12.1 days). Accordingly, NAA testing provides both cost savings and decreases unnecessary exposure and potential adverse effects related to TB therapy.
There are multiple other benefits from performing NAAT in smear-positive patients. Several reports have evaluated the overall cost effectiveness of NAAT [9, 17] , with a recent study reporting a cost reduction of up to $2000 per NAAT performed [9]. The cost reduction in smear-positive patients was mostly related to shorter hospitalizations, shorter length of isolation time, patients taking fewer MTB medications, and fewer contact and exposure investigations [9].
Our study has several limitations. We estimated the impact of NAAT in reducing length of airborne isolation and duration of unnecessary MTB therapy based on a hypothetical algorithm that reflected the clinical management before the implementation of NAAT in 2001. Because our study was a retrospective chart review, the variables studied, particularly those related to social history and drug use, were dependent on patient reporting and proper documentation. In addition, we did not have information regarding the HIV status for all of the subjects. Because our institution does not perform NAAT on smear-negative samples, we were unable to assess the clinical impact of NAAT under these conditions.
CONCLUSIONS
Through this 10-year longitudinal study, we highlight multiple advantages NAAT has introduced in the routine management of AFB smear-positive patients, especially in the immunocompromised patient populations. The availability of the rapid and accurate NAATs resulted in a shorter period of airborne isolation and reduced duration of unnecessary anti-MTB medications. Recent advances in molecular testing for MTB include Expert MTB/Rif assay, which allows for both MTB detection and rifampin resistance testing. Although patients with negative Expert MTB/Rif assay results can avoid prolonged airborne isolation and MTB treatment, those with positive assay can be started on effective treatment much sooner than waiting for culture-based drug susceptibility results [18].
Acknowledgments
We thank Drs. Pablo Buitron de la Vega, Anuradha Sreenivasan, Sagar Patel, and John Robert Koethe for assistance with research and comments that greatly improved the manuscript.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Global Tuberculosis Report 2016. Available at: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1 Accessed 14 Febuary 2017. [Google Scholar]
- 2. Davis JL, Cattamanchi A, Cuevas LE, et al. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catanzaro A, Perry S, Clarridge JE, et al. The role of clinical suspicion in evaluating a new diagnostic test for active tuberculosis: results of a multicenter prospective trial. JAMA 2000; 283:639–45. [DOI] [PubMed] [Google Scholar]
- 4. Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007; 11:1–196. [DOI] [PubMed] [Google Scholar]
- 5. Guerra RL, Hooper NM, Baker JF, et al. Use of the amplified Mycobacterium tuberculosis direct test in a public health laboratory: test performance and impact on clinical care. Chest 2007; 132:946–51. [DOI] [PubMed] [Google Scholar]
- 6. Peralta G, Barry P, Pascopella L. Use of nucleic acid amplification tests in tuberculosis patients in California, 2010–2013. Open Forum Infect Dis 2016; 3:ofw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention (CDC). Update: nucleic acid amplification tests for tuberculosis. MMWR Morb Mortal Wkly Rep 2000; 49:593–4. [PubMed] [Google Scholar]
- 8. Bergmann JS, Yuoh G, Fish G, Woods GL. Clinical evaluation of the enhanced gen-probe amplified Mycobacterium tuberculosis direct test for rapid diagnosis of tuberculosis in prison inmates. J Clin Microbiol 1999; 37:1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adelman MW, Kurbatova E, Wang YF, et al. Cost analysis of a nucleic acid amplification test in the diagnosis of pulmonary tuberculosis at an urban hospital with a high prevalence of TB/HIV. PLoS One 2014; 9:e100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leber AL. Clinical Microbiology Procedures Handbook. 4th ed Washington, DC: American Society of Microbiology; 2016. [Google Scholar]
- 11. Coll P, Garrigó M, Moreno C, Martí N. Routine use of gen-probe amplified Mycobacterium tuberculosis direct (MTD) test for detection of Mycobacterium tuberculosis with smear-positive and smear-negative specimens. Int J Tuberc Lung Dis 2003; 7:886–91. [PubMed] [Google Scholar]
- 12. Gamboa F, Fernandez G, Padilla E, et al. Comparative evaluation of initial and new versions of the Gen-Probe amplified Mycobacterium tuberculosis direct test for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol 1998; 36:684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141. [PubMed] [Google Scholar]
- 14. Leonard MK, Egan KB, Kourbatova E, et al. Increased efficiency in evaluating patients with suspected tuberculosis by use of a dedicated airborne infection isolation unit. Am J Infect Control 2006; 34:69–72. [DOI] [PubMed] [Google Scholar]
- 15. Chitnis AS, Davis JL, Schecter GF, et al. Review of nucleic acid amplification tests and clinical prediction rules for diagnosis of tuberculosis in acute care facilities. Infect Control Hosp Epidemiol 2015; 36:1215–25. [DOI] [PubMed] [Google Scholar]
- 16. Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 2015; 36:13–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dowdy DW, Maters A, Parrish N, et al. Cost-effectiveness analysis of the gen-probe amplified Mycobacterium tuberculosis direct test as used routinely on smear-positive respiratory specimens. J Clin Microbiol 2003; 41:948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]