Abstract
Background
Cutaneous melanoma (CM) incidence rates continue to increase, and the reasons are unknown. Previously, we reported a unique age-specific gender difference in melanoma that suggested additional causes other than solar UV radiation.
Objective
This study attempted to understand whether and how UV radiation differentially impacts the CM incidence in men and women.
Methods
CM data and daily UV index (UVI) from 31 cancer registries were collected for association analysis. A second dataset from 42 states of the U.S.A. was used for validation.
Results
There was no association between log-transformed female CM rates and levels of UVI but there was a significant association between male rates and UVI and a significant association between overall rates and UVI. The 5 year age-specific rate-UVI association levels (represented by Pearson’s coefficient ρ) increased with age in men, but age-specific ρ levels remained low and unchanged in women. The significant rate-UVI association in men and non-association in women was validated in the US white population.
Limitations
Confounders including temperature and latitude are difficult to separate from UVI Conclusions: Ambient UVI appears to be associated with melanoma incidence in males but not in females.
INTRODUCTION
Incidence rates of cutaneous melanoma (CM) have been increasing in the past few decades in US and European countries [1]. The causation of melanomagenesis remains under debate [2, 3], especially the role of ultraviolet radiation (UVR), which is the major known environmental risk factor for CM and non-melanoma skin cancer (NMSC). CMs frequently occur on the trunk where UVR does not usually reach, while NMSCs are mostly found on sun-exposed body sites such as the head, neck and limbs [4]. Unlike NMSCs, CMs are not associated with cumulative UV exposure [5]. Furthermore, there are less UV-signature mutations in CMs than in NMSCs [6]. Hence, in contrast to NMSC, the involvement and effect of UVR in CM is much more complex [7]. It is now generally accepted that CM is associated with intermittent UV exposure [5]. Based on this concept, the primary melanoma preventive measure is the application of sunscreen. Though the use of sunscreen began as early as the 1930s and has boomed since the 1950s, the incidence of CM has continued to increase during this time period [8].
Our previous publication indicated that women from the US and the Nordic countries had higher CM incidence rates than men up until age 45 with a peak difference at age 20–24 years [9]. There was no evidence of such a pattern for NMSC [9]. Basically, men and women were at equal risk of developing NMSC at a young age, although elderly men were at a higher risk, as was true for melanoma. Based on this comparison, we speculated that the etiology of melanoma, as for NMSC [9], in older age groups was largely attributable to cumulative UV exposure, but causative factors in younger females required further investigation.
The purpose of our current investigation is to understand the heterogeneous etiological factors that may contribute to gender and age differences in CM. In this study, we collected cancer registry data for melanoma and computed daily average UV index (UVI) for that registry area. The association between UVI and gender- and age-specific rates was analyzed.
Methods
Data collection, inclusion and exclusion criteria
Melanoma tumor classification was based on the standard of the International Classification of Diseases for Oncology, ICD-O-3, with code C43. Cancer registries were selected primarily based on availability of data and majority of Caucasian populations, which include selected European countries, United States, Australia and New Zealand. The data from Northern Territory in Australia (which contains considerable population of indigenous Australians) were extracted to contain only non-indigenous population. The European registry selection is mainly based on light eye color which was reported before [10]. Countries with more than 50% of population with light eye color were selected, hence France, Italy and most of southern European countries are excluded.
For primary analysis US data were retrieved from the SEER18 database using 2013 database (including data from 1973 to 2011), with all cutaneous melanomas (site group: 7.1 Melanoma; ICDO-3 behavior recode: 3; primary site C000-C809, histology type 8720–8723, 8726, 8728, 8730, 8740–8746, 8761, 8770–8774, 8780). Only white race (Race=1) data were included for analysis. Registries 27, 37 and 47 (Atlanta Metropolitan, Rural Georgia and Georgia excluding Atlanta/Rural Georgia) were pooled as “Georgia”, and Registries 1 and 31 (San Francisco-Oakland and San Jose-Monterey) were pooled as “SFSJ” area as the UVI is the same for these areas. Therefore, US SEER generated a total of 13 areas. The age-standardized incidence rates are calculated according to the world standardized population for 2000–2025 (NCI SEER website).
For validation data set, information was extracted from International Agency for Research of Cancer (IARC) CI5 volume X which contains data for 2003 to 2007 only. To ensure homogeneity of the data, only US data of white population was used. This US dataset contained some overlapping period and regions from the SEER data entries; but even within the same SEER region, the data collected in this set was limited to 2003–2007 which was different from the SEER dataset where data was collected since the establishment of the registry. The source of data is listed in Supplemental Table S1.
UVI calculation and estimation
Daily average UVI was calculated based on records from July 1, 2002 to June 30, 2014 from satellite database: http://www.temis.nl/uvradiation/SCIA/stations_uv.html. For country UVI estimation, normally data obtained from the station in the center of the country will be used; or average data from stations on the borders of the country is calculated and used. For US and Australian registries, data from a satellite station within the registry area was used for that registry, or a location with similar latitude was used if no station was found within the registry region. For example, UVI for Louisiana (29°N to 33°N) was estimated to be 8.9, which was extrapolated from the monitoring data from a station in the Everglades National Park (25°N) in Florida. Denmark did not have a monitoring station; therefore, data from Manchester, UK was used because it has the closest latitude to Denmark. The average daily UV indices for selected countries are listed in the Supplemental Table S2.
Statistical Analysis
All data were processed by SAS 9.3 if not specified. The age-standardized rates are calculated according to the world-standardized population for 2000–2025 (source: NCI SEER website). The association between UVI and melanoma rates or the risk ratio was analyzed by a simple linear regression model and Pearson product-moment correlation method, as well as Spearman’s correlation analysis. The normality test was carried out by all three defaulted test methods in SAS 9.3 (Kolmogorov-Smirnov, Cramer-von Mises, Anderson-Darling), the results are shown in Supplemental Table S3. All p values were obtained for two-sided tests, with a significance level at 0.05.
RESULTS
The association of gender-specific melanoma incidence rates with UVI
To investigate the UVR impact on genders, we calculated age-standardized rates (ASR) of melanoma in men and women from 31 cancer registries in the United States, European and Australia continents (Table 1, Supplemental Table S1). Daily average UVI was calculated using data collected by the GOME-2 satellite stations (Table 1 and Supplemental Table S2). Ambient UVI was modeled with data collected at noon each day with consideration for the local cloud conditions and was well correlated with ground erythemal UV dose, without distinction of UVA or UVB [11]. The UVI ranged from 1.8 (Finland) to 12.0 (Australia, Northern Territory) and roughly demonstrated a normal distribution (Supplemental Table S3).
Table 1.
Crude and Age-Adjusted Melanoma Incidence Rates and Rate Ratios
| Crude Rate | ASR | |||||||
|---|---|---|---|---|---|---|---|---|
| Registry/Country | UVI | Male | Female | All | Male | Female | All | RR |
| Australia Capital Territory | 7.2 | 44.2 | 35.9 | 39.8 | 39.8 | 29.8 | 34.2 | 0.75 |
| Australia New South Wales | 7.2 | 61.1 | 41.9 | 51.4 | 46.0 | 31.3 | 37.9 | 0.68 |
| Australia NT non-indigenous | 12.0 | 35.8 | 27.9 | 32.1 | 31.7 | 25.3 | 28.9 | 0.80 |
| Australia Queensland | 9.4 | 74.2 | 53.2 | 63.7 | 59.2 | 42.5 | 50.3 | 0.72 |
| Australia Tasmania | 5.6 | 54.5 | 46.5 | 50.4 | 40.5 | 34.5 | 37.0 | 0.85 |
| Australia Victoria | 6.3 | 45.4 | 36.3 | 40.8 | 34.8 | 27.1 | 30.6 | 0.78 |
| Western Australia | 7.7 | 60.0 | 41.6 | 50.8 | 48.7 | 32.8 | 40.4 | 0.67 |
| South Australia | 7.0 | 48.5 | 37.0 | 42.6 | 35.0 | 26.7 | 30.4 | 0.76 |
| Austria | 4.1 | 19.1 | 18.8 | 19.0 | 10.2 | 9.2 | 9.7 | 0.90 |
| Belgium | 3.4 | 25.5 | 35.3 | 30.5 | 18.7 | 26.6 | 22.7 | 1.43 |
| Connecticut-US | 4.9 | 22.2 | 17.3 | 19.7 | 17.6 | 13.3 | 15.4 | 0.75 |
| Denmark | 3.0 | 21.0 | 25.1 | 23.0 | 15.5 | 19.1 | 17.3 | 1.23 |
| Detroit-US | 4.9 | 16.8 | 13.1 | 14.9 | 14.4 | 10.8 | 12.6 | 0.75 |
| Finland | 1.8 | 15.3 | 14.1 | 14.7 | 11.3 | 9.5 | 10.4 | 0.84 |
| Georgia-US | 6.7 | 29.0 | 21.7 | 25.3 | 25.6 | 17.9 | 21.7 | 0.7 |
| Germany | 3.8 | 18.1 | 18.8 | 18.5 | 12.0 | 12.9 | 12.5 | 1.08 |
| Hawaii-US | 10.5 | 52.5 | 35.0 | 44.4 | 45.9 | 28.5 | 37.2 | 0.62 |
| Iowa-US | 4.9 | 15.5 | 13.4 | 14.4 | 12.7 | 11.0 | 11.8 | 0.86 |
| Kentucky-US | 5.7 | 28.6 | 21.0 | 24.7 | 22.0 | 16.3 | 19.1 | 0.74 |
| Los Angeles-US | 6.9 | 18.1 | 12.5 | 15.3 | 17.5 | 10.7 | 14.1 | 0.61 |
| Louisiana-US | 8.9 | 22.7 | 14.6 | 18.6 | 16.9 | 11.1 | 14.0 | 0.66 |
| Netherlands | 3.2 | 28.7 | 38.4 | 33.5 | 20.6 | 29.9 | 25.2 | 1.46 |
| New Jersey-US | 5.6 | 31.9 | 23.3 | 27.5 | 22.9 | 16.5 | 19.7 | 0.72 |
| New Mexico-US | 6.7 | 17.7 | 13.1 | 15.3 | 15.6 | 11.2 | 13.4 | 0.72 |
| Norway | 1.9 | 22.7 | 25.3 | 24.0 | 17.3 | 18.7 | 18.0 | 1.08 |
| New Zealand non-Maori | 5.4 | 64.7 | 56.4 | 60.5 | 46.5 | 40.2 | 42.9 | 0.87 |
| Seattle-US | 4.3 | 23.2 | 20.3 | 21.8 | 20.0 | 16.9 | 18.5 | 0.85 |
| SFSJ-US | 5.7 | 23.7 | 18.1 | 20.9 | 20.0 | 14.3 | 17.1 | 0.72 |
| Sweden | 2.2 | 21.4 | 21.5 | 21.5 | 14.6 | 15.0 | 14.8 | 1.03 |
| United Kingdom | 3.3 | 40.9 | 43.2 | 42.0 | 27.2 | 30.1 | 28.7 | 1.11 |
| Utah-US | 6.1 | 18.8 | 14.5 | 16.7 | 20.9 | 15.0 | 17.9 | 0.72 |
Histograms ASRs from the 31 registries showed that these rates did not follow normal distributions (data not shown). Log transformation was then carried out for ASRs for men, women and both; and none of the log-transformed rates was significant different from a normal distribution (Supplemental Table S3), which enabled us to fit data into a linear regression model. Log-transformed rates were then used for regression analysis against UVI. Both Pearson’s and Spearman’s linear regression models were used to analyze the association of ASRs from each area and local UVIs. Pearson’s method is based on the actual number and Spearman’s method is based on the rank of the data. As shown in Table 2, male rates (log-transformed, same for all the following rates) showed moderate (Pearson’s ρ= 0.61) but significant (p=0.0003) association with UVI, but females rates showed non-significant low level of association (ρ= 0.31 and p=0.09). When both genders were considered, the association was moderate but significant (ρ= 0.49 and p=0.005). The results from Spearman’s association analysis were similar (Table 2).
Table 2.
Association between log-transformed rates with UVI
| Male | Female | All | |
|---|---|---|---|
| Pearson’s ρ | 0.61 | 0.31 | 0.49 |
| p | 0.0003 | 0.09 | 0.005 |
| R2 | 0.37 | 0.09 | 0.24 |
| Spearman’s ρ | 0.63 | 0.28 | 0.48 |
| p | 0.0002 | 0.13 | 0.006 |
| R2 | 0.39 | 0.076 | 0.23 |
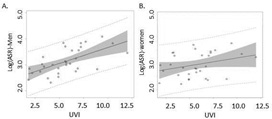
As shown in Figure 1, the log-transformed rates in men showed a moderate regression line with UVI (Fig 1A) but the regression line in log-transformed female rates was much flatter, and the distribution was more scattered (Fig 1B); indicating non-association between the two variables. In addition, coefficients of determination R2 calculated from Pearson’s regression model for men, women and both sexes were 0.37, 0.09 and 0.24 (Table 2), suggesting that the UVI only explained 9% of the melanoma incidence rates in women, but 37% of the incidence rates in men.
Figure 1. Melanoma incidence rates: Linear regression of log-transformed age-standardized melanoma incidence rates from 31 registries with average local daily UVI.
A, males; B, females. Solid lines represent the fitted line; shaded areas represent 95% confidence interval.
Levels of ASR-UVI association increase with age in men
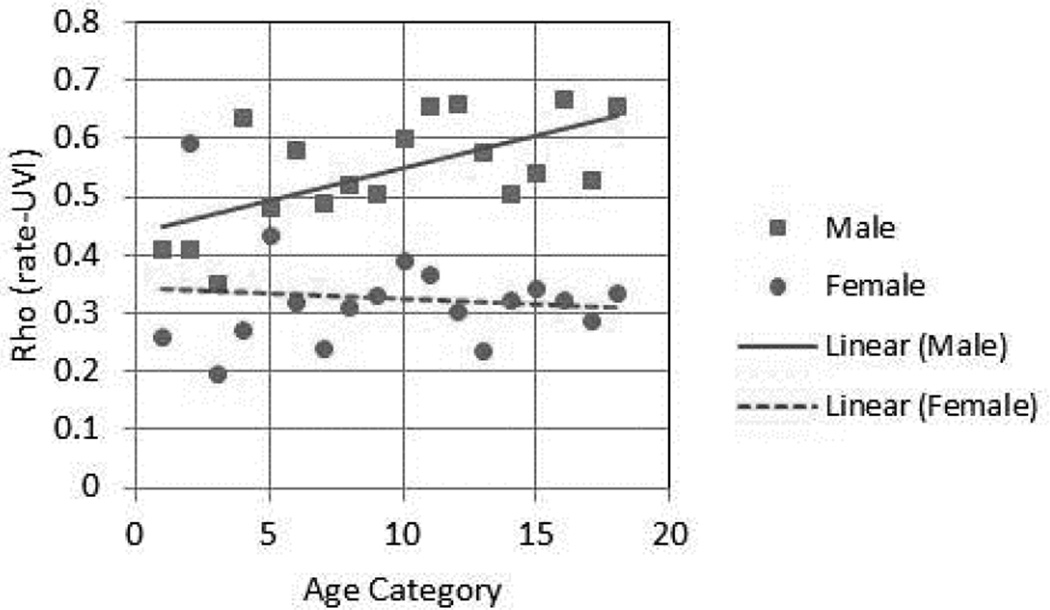
Since melanoma incidence rates increase with age in both genders, we hypothesized that perhaps the CM rates in older females would show a better association with UVI. To test this hypothesis, the 5-year age specific rates in each registry were calculated. Age category (Agecat) 1 represents age 0–4, Agecat 2 represents age 5 to 9 and so on. The rates in each agecat were log-transformed and then fitted into Pearson’s regression model with UVI as dependable variables, and the association levels (ρ and corresponding p values) for each agecat were computed and listed in Table 3. As shown in Figure 2, there was a significant linear increase of ρ (association levels) with age in men, e.g., the rate-UVI association became stronger in older age groups (ρm1=0.62, p=0.006). Again, no association of ρ with age was observed in women (Fig 2B) (ρf1 = −0.11, p = 0.67).
Table 3.
Association between age-specific rates and UVI based on 31 registries
| Male | Female | ||||
|---|---|---|---|---|---|
| agecat | age | rho | p | rho | p |
| 1 | 0–4 | 0.41 | 0.071 | 0.26 | 0.286 |
| 2 | 5–9 | 0.41 | 0.053 | 0.59 | 0.004 |
| 3 | 10–14 | 0.35 | 0.074 | 0.20 | 0.332 |
| 4 | 15–19 | 0.64 | <.0001 | 0.27 | 0.141 |
| 5 | 20–24 | 0.48 | 0.0051 | 0.43 | 0.013 |
| 6 | 25–29 | 0.58 | 0.0005 | 0.32 | 0.075 |
| 7 | 30–34 | 0.49 | 0.0045 | 0.24 | 0.184 |
| 8 | 35–39 | 0.52 | 0.0023 | 0.31 | 0.081 |
| 9 | 40–44 | 0.51 | 0.0031 | 0.33 | 0.064 |
| 10 | 45–49 | 0.60 | 0.0003 | 0.39 | 0.027 |
| 11 | 50–54 | 0.66 | <.0001 | 0.37 | 0.039 |
| 12 | 55–59 | 0.66 | <.0001 | 0.31 | 0.089 |
| 13 | 60–64 | 0.58 | 0.0006 | 0.24 | 0.190 |
| 14 | 65–69 | 0.51 | 0.0031 | 0.32 | 0.072 |
| 15 | 70–74 | 0.54 | 0.0013 | 0.34 | 0.055 |
| 16 | 75–79 | 0.67 | <.0001 | 0.32 | 0.072 |
| 17 | 80–84 | 0.53 | 0.0018 | 0.29 | 0.110 |
| 18 | 85+ | 0.66 | <.0001 | 0.34 | 0.064 |
Figure 2. Melanoma rates and age: Levels of rate-UVI association increase with age in men but not in women.
Five-year age-specific rates were calculated for each registry for each gender, association of these rates (log-transformed) with UVI was analyzed to obtain Pearson’s ρ for each age category. These ρ values were plotted against age categories. Squares and solid line: ρ for males in each age categories and the regression line. Filled circles and dotted line: ρ for females in each age categories and the regression line.
Validation of the observation with a second dataset
in order to validate our observation of the different association of CM rates with UVI, a separate dataset was obtained from IARC CI5 Volume X, where 42 states from US provided cancer data from 2003 to 2007 with race information (California counted for 2 registries therefore a total of 43 registries) (Table 4). Only white race data was used for analysis, hence this dataset contained more homogeneous data on race (white race) and data collection period (2003–2007). For this dataset 12 registries (28%) overlapped with the primary dataset used in the main analysis, but the data collection period was shorter as compared to the one used in the main analysis. Pearson’s regression analysis indicated that in this new dataset the log-transformed rates in men again showed a moderate but significant association with UVI (ρ = 0.34 and p = 0.02) while that in women did not (ρ = 0.08 and p = 0.68) (Table 4). When both genders were combined, the rates-UVI association was no longer significant (Table 4).
Table 4.
Melanoma age-adjusted incidence rates in US white population from 2003–2007 and their association with UVI
| USWhite2003_2007 | Male | Female | All | UVI |
|---|---|---|---|---|
| Alabama | 18.9 | 13.4 | 16.2 | 6.7 |
| Arizona | 13.9 | 9.0 | 11.5 | 6.7 |
| Arkansas | 13.0 | 9.0 | 11.0 | 6.7 |
| Los Angeles | 15.9 | 9.8 | 12.9 | 5.7 |
| San Francisco | 20.3 | 14.4 | 17.4 | 5.6 |
| Colorado | 17.7 | 14.5 | 16.1 | 5.9 |
| Connecticut | 20.6 | 16.1 | 18.4 | 4.9 |
| Delaware | 22.8 | 16.2 | 19.5 | 5.6 |
| Florida | 16.5 | 11.6 | 14.1 | 8.7 |
| Georgia | 23.3 | 17.1 | 20.2 | 6.7 |
| Hawaii | 55.3 | 36.9 | 46.1 | 10.5 |
| Idaho | 20.1 | 14.8 | 17.5 | 4.7 |
| Illinois | 13.9 | 10.8 | 12.4 | 4.9 |
| Indiana | 13.9 | 11.1 | 12.5 | 4.9 |
| Iowa | 15.0 | 12.8 | 13.9 | 4.9 |
| Kentucky | 17.8 | 14.2 | 16.0 | 5.7 |
| Louisiana | 14.9 | 9.7 | 12.3 | 8.9 |
| Maine | 18.2 | 14.7 | 16.5 | 4.2 |
| Massachusetts | 18.5 | 14.3 | 16.4 | 4.9 |
| Michigan Detroit | 16.8 | 14.2 | 15.5 | 4.9 |
| Mississippi | 12.3 | 8.6 | 10.5 | 6.7 |
| Missouri | 15.3 | 11.1 | 13.2 | 5.7 |
| Montana | 13.5 | 12.2 | 12.9 | 4.3 |
| Nebraska | 13.0 | 10.0 | 11.5 | 4.9 |
| New Jersey | 19.5 | 15.1 | 17.3 | 5.0 |
| New Mexico | 15.4 | 11.0 | 13.2 | 6.7 |
| New York State | 14.9 | 10.7 | 12.8 | 4.9 |
| North Carolina | 19.3 | 15.0 | 17.2 | 6.0 |
| North Dakota | 12.0 | 12.7 | 12.4 | 4.3 |
| Ohio | 15.4 | 12.9 | 14.2 | 5.6 |
| Oklahoma | 17.4 | 13.0 | 15.2 | 5.7 |
| Oregon | 19.9 | 18.1 | 19.0 | 4.7 |
| Pennsylvania | 15.2 | 12.4 | 13.8 | 5.0 |
| Rhode Island | 18.7 | 14.1 | 16.4 | 4.9 |
| South Carolina | 23.4 | 18.1 | 20.8 | 6.8 |
| Tennessee | 17.9 | 12.9 | 15.4 | 6.3 |
| Texas | 13.2 | 8.4 | 10.8 | 6.8 |
| Utah | 22.4 | 16.2 | 19.3 | 6.1 |
| Vermont | 23.3 | 21.3 | 22.3 | 4.2 |
| Virginia | 20.2 | 14.6 | 17.4 | 5.5 |
| Washington Seattle | 20.7 | 18.3 | 19.5 | 4.3 |
| West Virginia | 15.1 | 11.5 | 13.3 | 5.3 |
| Wisconsin | 14.0 | 11.6 | 12.8 | 4.9 |
| log(rates) vs UVI ρ(p) | 0.34 (0.02) | 0.075 (0.63) | 0.23 (0.14) | - |
DISCUSSION
This ecological study provides evidence that 1) there is a significant difference in the association of geographical UVI with male and female CM incidence rates. CM rates in men are associated with UVI but not that in women; and 2) the levels of association between male rates and UVI increase with age. It was previously reported that the mean daily UVR accounted for 82% of BCC and 85% of SCC incidence rates [12]. In our study, UVI only accounted for approximately 1–2% of melanoma rates in women, approximately 1/3 in men, and approximately 13–15% for the whole population (Table 2). While it was known that UV radiation played a very complex role in melanoma etiology, our observation on gender difference is quite interesting.
Potential major confounders include temperature and latitude. Higher UVIs are usually associated with higher temperatures and lower latitudes, both of which may be associated with different sun behaviors, e.g., how much individuals wear and how long they stay outdoors [13]. Limited information is available on how external ambient temperatures impact CM incidence [14], and further investigations are needed to address this question [3]. Estimated solar UV radiation (as well as latitude) was shown to be strongly associated with NMSC, but data from 10 US Metropolitan populations from 1977–1978 showed very limited association with melanoma rates [15]. Armstrong and Kricker concluded in 2001 that “inconsistencies have been observed in these patterns (association of incidence rates with geographical UV) depending on the populations studied, particularly for melanoma” [15]. Furthermore, not much analysis was based on separated genders and age categories. Our analysis included more registries with relatively homogenous population backgrounds, and used more updated incidence rates.
Nevertheless a limitation of this study is still the heterogeneous population from different countries. One would argue that because non-Caucasians usually are a minority population and exhibit much lower rates of melanoma, the overall error may not affect our conclusion. Validation from US white population from the 2003 to 2007 dataset thus provides important support to our conclusion.
The weakness of this study, due to its ecological nature, is that the conclusion is not applicable to individuals, e.g., an individual’s risk for CM may not have such a linear correlation with individual UV exposure because we cannot assume all people are exposed to the same level of UVR in the same area. Also, intermittent sun exposure which is a known risk factor for melanoma [16], cannot be assessed from UVI and therefore not a variable in this study.
The underlying mechanisms as to why women did not show an association with UV index is likely complex, reflecting either difference in sun behavior or physiological difference, or both. One immediate argument is that use of cosmetics and/or sunscreen in females provides protection against UV radiation, therefore cosmetics use masks the UV effect. A recent survey showed that compared to men, women were more likely to use sunscreen and hence were protected [17]. However, our previous study showed that younger women exhibited significantly higher (not lower) CM rates than younger men [9]. Therefore, using more cosmetics or sunscreen by women does not completely explain the non-association between UVI and rates, at least for younger age.
Several lines of evidence suggest that physiological difference in genders may play crucial roles in melanoma development. First, male and female skins differ substantially in their structure and biology [18]. Male skins are thicker, richer in collagen and elastin, secrete more sebum, have less sub-cutaneous fat, more hair and different hair patterns due to androgen stimulation and estrogen suppression [18, 19]. These differences lead to differences in skin responses to environmental stress including UVR [19]. Potentially, female skins may naturally be more protected from UVR or may be equally prone to UV-induced damage but more capable of repair. Evidence from the literature supports both hypotheses [20, 21]. A Netherlands study revealed that male subjects had a lower minimal erythema dose than female volunteers [20] suggesting that male skin was more sensitive to UVR. A similar study using simulated solar UVR revealed that the doses required to elicit a similar immunosuppression response in men were three times lower than those in women [21]. Additionally, studies in animal models have suggested that hormones play a role in UV-induced skin responses. The female hormone 17-β-estradiol inhibits UVR-induced immunosuppression in female mice [22], and the male hormone androgen impedes acute skin wound healing in male rats [23]. Lastly, melanomas have been shown to clinically respond to treatment with tamoxifen, follicle-stimulating, melatonin, and nerve growth factor hormones [24, 25]. The outcome of melanoma treatment is different in men and women [26], which also implies a role for hormones in response to treatment.
In summary, we found no association between UVI and female CM incidence rates. In contrast, there was a significant association between male CM incidence rates and UVI, and this association showed a moderate increase with age. Our results reveal a previously under-appreciated area of gender-specific causative factors in melanoma development, which may potentially lead to novel gender-specific research on etiology and possibly preventive strategies in the future.
Supplementary Material
Capsule Summary.
The influence of gender on the association of melanoma incidence with UV index (UVI) is uncertain.
Our study shows that melanoma rates are associated with UVI in males but not in females.
The differential effect on UVI on melanoma incidence suggests the possibility of gender-specific prevention strategies.
Acknowledgments
This study was supported by NCI K07 award (CA160756) to FLS, the Waltmar Foundation (to FLM and FLS) and the Alan Hubbell Education Grant (CSUF and UCI-CFCCC Partnership for Cancer Health Disparities Research P20 CA174188 to Hubbell and subgrant to FLS and AJM).
Abbreviation and acronym
- UV
ultra-violet
- NMSC
non-melanoma skin cancer
- UVI
ultra-violet index
- RR
relative risk
- CI
confidence interval
- ASR
age-standardized rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests: There is no conflict of interest for authors.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015 doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Reichrath J, Reichrath S. Sunlight, vitamin D and malignant melanoma: an update. Adv Exp Med Biol. 2014;810:390–405. doi: 10.1007/978-1-4939-0437-2_22. [DOI] [PubMed] [Google Scholar]
- 3.Christophers AJ. Melanoma is not caused by sunlight. Mutat Res. 1998;422(1):113–117. doi: 10.1016/s0027-5107(98)00182-1. [DOI] [PubMed] [Google Scholar]
- 4.Kricker A, Armstrong BK, English DR. Sun exposure and non-melanocytic skin cancer. Cancer Causes Control. 1994;5(4):367–392. doi: 10.1007/BF01804988. [DOI] [PubMed] [Google Scholar]
- 5.Gandini S, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41(1):45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Maddodi N, Setaluri V. Role of UV in cutaneous melanoma. Photochem Photobiol. 2008;84(2):528–536. doi: 10.1111/j.1751-1097.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 7.Elwood JM, Hislop TG. Solar radiation in the etiology of cutaneous malignant melanoma in Caucasians. Natl Cancer Inst Monogr. 1982;62:167–171. [PubMed] [Google Scholar]
- 8.Urbach F. The historical aspects of sunscreens. J Photochem Photobiol B. 2001;64(2–3):99–104. doi: 10.1016/s1011-1344(01)00202-0. [DOI] [PubMed] [Google Scholar]
- 9.Liu F, et al. A unique gender difference in early onset melanoma implies that in addition to ultraviolet light exposure other causative factors are important. Pigment Cell Melanoma Res. 2013;26(1):128–135. doi: 10.1111/pcmr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frost P. European hair and eye color - A case of frequency-dependent sexual selection? Evolution and Human Behavior. 2006;27(2):85–103. [Google Scholar]
- 11.Jegou F, et al. Validity of satellite measurements used for the monitoring of UV radiation risk on health. Atmospheric Chemistry and Physics. 2011;11(24):13377–13394. [Google Scholar]
- 12.Xiang F, et al. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012: empirical relationships. JAMA Dermatol. 2014;150(10):1063–1071. doi: 10.1001/jamadermatol.2014.762. [DOI] [PubMed] [Google Scholar]
- 13.Fabbrocini G, et al. Epidemiology of skin cancer: role of some environmental factors. Cancers (Basel) 2010;2(4):1980–1989. doi: 10.3390/cancers2041980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharath AK, Turner RJ. Impact of climate change on skin cancer. J R Soc Med. 2009;102(6):215–218. doi: 10.1258/jrsm.2009.080261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. Journal of Photochemistry and Photobiology B-Biology. 2001;63(1–3):8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 16.Elwood JM, et al. Cutaneous melanoma in relation to intermittent and constant sun exposure--the Western Canada Melanoma Study. Int J Cancer. 1985;35(4):427–433. doi: 10.1002/ijc.2910350403. [DOI] [PubMed] [Google Scholar]
- 17.Holman DM, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73(1):83 e1–92 e1. doi: 10.1016/j.jaad.2015.02.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. J Dermatol Sci. 2009;55(3):144–149. doi: 10.1016/j.jdermsci.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Oblong JE. Comparison of the impact of environmental stress on male and female skin. Br J Dermatol. 2012;166(Suppl 2):41–44. doi: 10.1111/j.1365-2133.2012.10928.x. [DOI] [PubMed] [Google Scholar]
- 20.Broekmans WM, et al. Determinants of skin sensitivity to solar irradiation. Eur J Clin Nutr. 2003;57(10):1222–1229. doi: 10.1038/sj.ejcn.1601672. [DOI] [PubMed] [Google Scholar]
- 21.Damian DL, et al. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol. 2008;128(2):447–454. doi: 10.1038/sj.jid.5701058. [DOI] [PubMed] [Google Scholar]
- 22.Hiramoto K, et al. Effect of 17beta-estradiol on immunosuppression induced by ultraviolet B irradiation. Arch Dermatol Res. 2004;295(8–9):307–311. doi: 10.1007/s00403-003-0437-0. [DOI] [PubMed] [Google Scholar]
- 23.Gilliver SC, et al. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119(Pt 4):722–732. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- 24.Meyskens FL, Jr, Voakes JB. Tamoxifen in metastatic malignant melanoma. Cancer Treat Rep. 1980;64(1):171–173. [PubMed] [Google Scholar]
- 25.Meyskens FL, Jr, Salmon SE. Modulation of clonogenic human melanoma cells by follicle-stimulating hormone, melatonin, and nerve growth factor. Br J Cancer. 1981;43(1):111–115. doi: 10.1038/bjc.1981.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nosrati A, Wei ML. Sex disparities in melanoma outcomes: the role of biology. Arch Biochem Biophys. 2014;563:42–50. doi: 10.1016/j.abb.2014.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.