Abstract
Background
Persistent immune activation and inflammation in virologically suppressed HIV infection are linked to excess cardiovascular risk.
Objective
To evaluate atorvastatin as a strategy to reduce cardiovascular risk.
Methods
A5275 was a multicenter, prospective, randomized, double-blind, placebo-controlled, cross-over pilot study of atorvastatin (10mg/day for 4 weeks then 20mg/day for 16 weeks) with a planned enrollment of 97 HIV-infected participants ≥18 years old, receiving boosted protease inhibitor-based ART for ≥6 months, with plasma HIV-1 RNAs below limits of quantification ≥180 days, and fasting LDL-C ≥70 mg/dL and <130 mg/dL. Primary endpoints were differences of changes ([week 44 – week 24] - [week 20 - baseline]) in CD4+ and CD8+ T-lymphocyte activation (% CD38+/DR+) and plasma levels of IL-6 and D-dimer. Arms were compared using Wilcoxon rank sum tests and also summarized changes pre-to-post atorvastatin treatment. Analyses were as-treated.
Results
Ninety-eight participants were enrolled at 31 U.S. sites and 73 completed study treatment. Atorvastatin treatment did not decrease T-lymphocyte or monocyte activation, circulating biomarker levels (interleukin-6, D-dimer, soluble CD14, soluble CD163, monocyte chemoattractant protein-1, interferon-γ-induced protein-10, high sensitivity C-reactive protein, CD40L, P-selectin) or white blood cell Krüppel-like Factor 2/4 mRNA levels. Pre-to-post atorvastatin reductions in calculated LDL (−38%), oxidized-LDL (−33%), and lipoprotein-associated phospholipase A2 (−31%) were significant (p<0.01).
Conclusion
In virologically suppressed individuals with HIV infection, atorvastatin did not significantly decrease levels of soluble or cellular biomarkers of immune activation and inflammation, but resulted in robust reductions in LDL-C, oxLDL, and LpPLA2, biomarkers associated with cardiovascular risk.
Keywords: HIV, Statin, Immune Activation, Inflammation, Oxidized LDL, Cardiovascular
INTRODUCTION
In persons with HIV infection, cardiovascular disease (CVD) has become a leading cause of morbidity and mortality, and excess CVD risk has been identified after controlling for traditional risk factors [1,2]. CT angiography demonstrates increased non-calcified plaque in coronary arteries and PET-CT shows a similar process in the aortas of HIV-infected individuals compared with -uninfected controls [3,4]. At the core of the atherosclerotic lesion are activated T lymphocytes and monocyte/macrophages, chronically stimulated by microbial translocation, HIV and other viral elements, and lipids including oxidized low-density lipoprotein (oxLDL) [4,5,6,7,8,9,10,11,12,13, 14]. Chronic immune cell activation is also at the heart of a broader systemic inflammatory response, manifested by several immune activation biomarkers (including Interleukin-6 and D-dimer) that are positively correlated with morbidity and mortality [15, 16, 17, 18]. CVD risk and inflammatory biomarkers may be partially reduced by antiretroviral therapy (ART), but to further attenuate this risk other interventions are needed [19,20,21].
Statins reduce CVD risk by decreasing LDL cholesterol (LDL-C) levels and possibly via lipid-independent, pleotropic anti-inflammatory effects – though the later remains somewhat controversial [22]. In the JUPITER trial, which randomized 17,802 healthy subjects with high-sensitivity C-reactive protein (hs-CRP) >2 mg/dL and LDL-C <130mg/L to rosuvastatin or placebo, rosuvastatin administration resulted in a 44% reduction in vascular events, greater than expected based on LDL-C lowering alone [23]. Here, we examined atorvastatin's effect on immune activation, inflammatory biomarkers and lipoproteins in HIV-infected individuals with suppressed HIV-1 RNA and LDL-C <130 mg/dL.
MATERIALS and METHODS
Study design and population
A5275 was a multicenter, prospective, randomized, double-blind, placebo-controlled, cross-over pilot study of atorvastatin among HIV-infected participants ≥18 years old, receiving boosted protease inhibitor (PI)-based ART for ≥6 months, who had plasma HIV-1 RNA below limits of quantification ≥180 days and fasting LDL-C ≥70 mg/dL and <130 mg/dL. The target sample size was 97. An initial eligibility requirement of plasma D-dimer >0.34 μg/mL (QUEST Diagnostics) was included to enrich for subjects with increased inflammation/coagulopathy; this limit was lowered then removed due to a prohibitive number of screening failures. Overall, 52% of enrolled participants had a screening plasma D-dimer >0.34 μg/mL. Exclusion criteria included: pregnancy, malignancy, known CVD, diabetes mellitus, or calculated 10-year CVD risk >20% (National Cholesterol Education Programs Adult treatment Panel Guidelines III), known cirrhosis or chronic active viral hepatitis; other known inflammatory or infectious conditions; serious illness within 4 weeks; and anticoagulation, chemotherapy, lipid-lowering, or immunosuppressant therapy within 45 days of study entry. All participants provided written informed consent. The study was reviewed by the institutional review boards of each participating site and was registered on clinicaltrials.gov (NCT01351025).
Arm A (atorvastatin/placebo) initiated atorvastatin for 20 weeks followed by a 4 week washout, then placebo for 20 weeks and another washout. Arm B (placebo/atorvastatin) started placebo first, followed by washout, atorvastatin, then washout. Atorvastatin/placebo was started at 10mg/day and increased at week 4 to 20mg/day if no symptoms or laboratory findings suggestive of atorvastatin toxicity were found.
Laboratory assessments
For all visits, fasting plasma and serum were stored at −80°C, then thawed once for batched analysis. Peripheral blood mononuclear cells (PBMCs) were cryopreserved for long-term storage at −130°C. Plasma interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), interferon-γ-induced protein-10 (IP-10), soluble CD40 ligand (sCD40L), soluble CD14 (sCD14), P-selectin, lipoprotein-associated phospholipase A2 (Lp-PLA2), hs-CRP, soluble CD163 (sCD163; all R&D Systems, Minneapolis, MN), oxLDL (Mercodia, Uppsala, Sweden), and D-dimer (Diagnostica Stago, Asnières sur Seine, France) were measured by ELISA. Intra-assay CV for replicates of these assays was typically <5-7%; inter-assay CV was <10-15%. LD-C measurements were performed in real time at each site's clinical laboratory.
Krüppel-like Factor 2 (KLF2) and 4 (KLF4) were measured in RNA extracted from PBMCs using the RNeasy® RNA Mini Kit (Qiagen, Hilden, Germany). Complementary DNA (cDNA) was transcribed from total RNA using the High CapacityRNA to cDNA Kit (Invitrogen Life Technologies, Carlsbad, CA). Transcript levels of KL2 and KLF4 were quantified by Taqman Gene expression based real-time PCR (Invitrogen Life Technologies Carlsbad, CA). CD4+ and CD8+ T-lymphocyte activation was measured on thawed PBMCs (dead cells excluded) using a BD Fortessa flow cytometer. Lymphocytes were identified by forward and side light scatter, and by staining with anti-CD3 (Pacific Blue, BioLegend) and anti-CD4 (PE-Cy7) or anti-CD8 (APC-H7, BD Biosciences). Anti-HLA-DR PE (BD Biosciences) and anti-CD38 APC (BD Biosciences) were used to monitor T-lymphocyte activation. Gates were determined by staining with isotype controls (BD Biosciences) [24]. Monocyte activation was measured on thawed PBMCs using a BD LSRII flow cytometer. Monocytes were selected based on scatter characteristics and their subsets were enumerated by reactivity with antibodies (anti-CD14, Pacific Blue, BD Biosciences; anti-CD16 PE, BD Biosciences) using gating strategies, as reported previously [9]. Cells expressing CD3, CD19, and CD56 (BD Biosciences) were excluded from the analysis using PE-Cy7 conjugated antibodies (BD Biosciences). Anti-CCR2 PerCP-Cy5.5 (BioLegend), anti-CD40 AF700 (BD Biosciences), and anti-CX3CR1 APC (BioLegend) were used to further characterize monocyte subsets, and gates were established using isotype controls.
Statistical analysis
Soluble biomarkers were log10 transformed and presented as relative (percentage) changes. The primary endpoints are differences of changes ([week 44 – week 24] - [week 20 - baseline]) in: CD4+ and CD8+ T-lymphocyte activation (% CD38+/DR+) and plasma levels of IL-6, and D-dimer, which were compared between arms using Wilcoxon rank sum tests [25]. Analyses also summarized changes pre-to-post atorvastatin treatment. Analyses were as-treated, limited to participants with data at baseline, weeks 20, 24, and 44 who remained on study treatment through week 44 (allowing treatment interruption <4 weeks) and who did not use prohibited medications or have virologic failure. Safety analyses evaluated all participants who initiated study treatment. Spearman correlations evaluated associations between baseline biomarkers. We conducted several post hoc exploratory analyses using the median value of selected baseline biomarkers to stratify. Statistical tests were two-sided (α=0.05), without adjustment for multiple testing.
RESULTS
Study Enrollment, Baseline Characteristics, and Follow-up
Five hundred thirty-two participants screened; most screen-failures (66%) were due to D-dimer results below the threshold prior to removal of this exclusion criterion. Between April 14, 2011 and May 31, 2013, 98 participants (49 in each arm) were enrolled at 31 U.S. sites. Four participants did not start study treatment (1 enrollment error, 3 due to non-compliance). Of the 94 participants who started study treatment, 73 (78%) completed study treatment, 37 in arm A and 36 in arm B. There were 21 participants (22%) who discontinued study treatment prematurely, 9 from arm A and 12 from arm B. Of these, 6 had confirmed virologic failure, 5 took disallowed medications, 3 were lost to follow-up, and 7 failed to complete study treatment. Table 1 summarizes baseline demographic and biologic characteristics of the 94 eligible participants who initiated study treatment. Table 2 provides a summary (median, Q1-Q3) of the baseline biomarker data for the as-treated study population and comparable values for HIV-negative populations. The as-treated analysis for the soluble biomarkers includes 34 subjects in arm A and 36 in arm B. For the cellular activation markers, 65 participants had analyzable data, reflecting an insufficiency of viable cells for 5 participants. All participants received the 20mg atorvastatin dose, which is the maximum recommended dose when given with a boosted-PI.
Table 1.
Baseline characteristics of the study participants by study group
| Treatment arm | ||||
|---|---|---|---|---|
| Characteristic | Total (N=94) | A (Atorvastatin / Placebo) (N=46) | B (Placebo / Atorvastatin) (N=48) | |
| Age (yrs) | Median (Q1, Q3) | 48 (41, 55) | 47 (39, 55) | 50 (42, 55) |
| Race/Ethnicity | White Non-Hispanic | 23 (24%) | 10 (22%) | 13 (27%) |
| Black Non-Hispanic | 43 (46%) | 21 (46%) | 22 (46%) | |
| Hispanic (Regardless of Race) | 27 (29%) | 14 (30%) | 13 (27%) | |
| Asian, Pacific Islander | 1 (1%) | 1 (2%) | 0 (0%) | |
| Sex | Male | 64 (68%) | 29 (63%) | 35 (73%) |
| Entry HIV-1 RNA | < 40 copies/mL | 93 (99%) | 46 (100%) | 47 (98%) |
| ≥ 40 copies/mL | 1 (1%) | 0 (0%) | 1 (2%) | |
| CD4+ T lymphocyte (cells/mm3) | Median (Q1, Q3) | 552 (412, 714) | 587 (373, 732) | 545 (424, 689) |
| ≤ 350 | 20 (21 %) | 11 (24%) | 9 (19%) | |
| > 350 | 74 (79%) | 35 (76%) | 39 (81 %) | |
| Years since first HIV-1 RNA below assay limit prior to study entry | Median (Q1, Q3) | 6 (4, 12) | 6 (4, 11) | 5 (3, 12) |
| Fasting total cholesterol [mg/dL] | Median (Q1, Q3) | 181 (159, 200) | 180 (160, 197) | 181 (157, 203) |
| Fasting triglycerides [mg/dL] | Median (Q1, Q3) | 108 (88, 150) | 112 (92, 150) | 106 (87, 149) |
| Fasting HDL [mg/dL] | Median (Q1, Q3) | 46 (38, 61) | 46 (39, 60) | 47 (35, 62) |
| Fasting (calculated) LDL [mg/dL] | Median (Q1, Q3) | 107 (91, 117) | 107 (94, 117) | 106 (86, 117) |
| Oxidized LDL [U/L] | Median (Q1, Q3) | 45 (37, 51) | 43 (37, 48) | 46 (41, 51) |
| Smoking (cigars or cigarettes) | Yes | 32 (34%) | 17 (37%) | 15 (31%) |
| No | 62 (66%) | 29 (63%) | 33 (69%) | |
| Body Mass Index [kg/m2] | Underweight: <18.5 | 3 (3%) | 2 (4%) | 1 (2%) |
| Normal : 18.5 - <25 | 32 (34%) | 12 (26%) | 20 (43%) | |
| Overweight : 25 - <30 | 34 (37%) | 20 (43%) | 14 (30%) | |
| Obese : ≥30 | 24 (26%) | 12 (26%) | 12 (26%) | |
Table 2.
Baseline markers summary and comparable values for HIV-negative population
| Marker | Baseline Median (Q1, Q3) | Non-HIV Median (Q1, Q3) |
|---|---|---|
| (CD4+) CD38+/DR+ [%] | 9.5 ( 7.6, 13.0) | 1.8 (1.5, 2.4)a; 2.0 (2.0, 3.0)b |
| (CD8+) CD38+/DR+ [%] | 14.9 ( 10.7, 19.5) | 4.1 (2.7, 6.0)a; 7.0 (5.0, 10.0)b |
| IL-6 [pg/mL] | 1.6 ( 1.1, 2.8) | 0.9 (0.6, 1.4)a; 1.0 (0.6, 1.2)b |
| D-dimer [ng/mL] | 178 ( 121, 272) | 192 (157, 240)a; 112 (83, 145)b |
| MCP-1 [pg/mL] | 229 ( 167, 276) | |
| IP-10 [pg/mL] | 146 ( 122, 210) | |
| CD40L [pg/mL] | 6450 ( 4402, 8912) | |
| sCD14 [ng/mL] | 1701 ( 1459, 2113) | 1271 (1100, 1464)a; 1326 (1186, 1599)b |
| P-Selectin [ng/mL] | 82 ( 55, 104) | |
| sCD163 [ng/mL] | 571 ( 462, 796) | 658 (610, 772)a |
| (CD14+/CD16−) CCR2+ [%] | 100 ( 99, 100) | |
| (CD14+/CD16−) CD40+ [%] | 32 ( 25, 42) | |
| (CD14+/CD16−) CX3CR1+ [%] | 4 ( 2, 7) | |
| (CD14+/CD16+) CCR2+ [%] | 94 ( 84, 97) | |
| (CD14+/CD16+) CD40+ [%] | 55 ( 39, 69) | |
| (CD14+/CD16+) CX3CR1+ [%] | 13 ( 8, 19) | |
| (CD14dim/CD16+) CCR2+ [%] | 9 ( 6, 13) | |
| (CD14dim/CD16+) CD40+ [%] | 76 ( 61, 84) | |
| (CD14dim/CD16+) CX3CR1+ [%] | 69 ( 51, 81) | |
| Fasting total cholesterol [mg/dL] | 182 ( 160, 202) | |
| Fasting triglycerides [mg/dL] | 114 ( 91, 155) | |
| Fasting HDL [mg/dL] | 48 ( 38, 63) | |
| Fasting LDL [mg/dL] | 104 ( 91, 117) | |
| Oxidized LDL [U/L] | 45 ( 38, 50) | |
| Lp-PLA2[ng/mL] | 146 ( 93, 179) | |
| CRP[ng/mL] | 2862 ( 1376, 9928) | 1127 (3467, 3176)a |
Atorvastatin's Effects on Biomarkers and Krüppel-like factor (KLF) mRNA expression
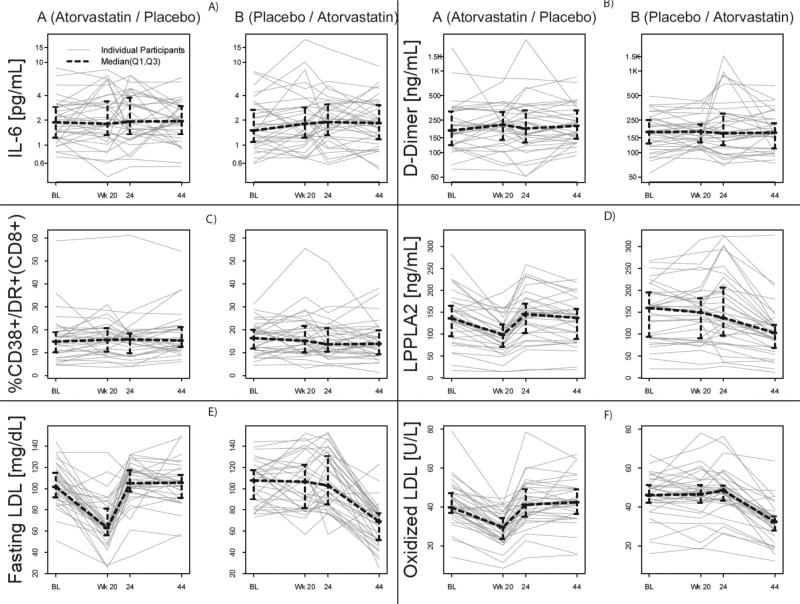
There were no significant atorvastatin treatment effects in any of the primary endpoints; CD4+ and CD8+ T-lymphocyte activation, IL-6, and D-dimer (all p>0.05) (Figure 1A-C). Secondary endpoints included plasma levels of MCP-1, IP-10, hs-CRP, CD40L, P-selectin, sCD14, and sCD163. Exploratory analyses examined atorvastatin's effects on KLF-2 and KLF-4 mRNA expression, proportional representation of monocyte subsets: CD14+CD16−, CD14+CD16+ and CD14dimCD16+, and expression of CCR2, CD40, or CX3CR1 among these cells. There were no significant treatment effects on any of these secondary or exploratory measurements (all p>0.05, data not shown), except a modest treatment effect on sCD163, with median difference of changes −9% for arm A and +8% for arm B (p=0.05), indicating a marginal increase during atorvastatin treatment. Further analyses assessed the pre-to-post atorvastatin change, defined as the combined change from baseline to week 20 for arm A and the change from week 24 to week 44 for arm B, and found non-significant changes for all primary and secondary outcome biomarkers except for sCD14 (median decrease −2%, 95% CI (−6%, −0.2%).
Figure 1. Atorvastatin treatment reduces plasma levels of LDL, oxidized LDL, and Lp-PLA2.
LPPLA2 = lipoprotein-associated phospholipase A2, IL-6 = Interleukin-6. Plasma samples from study participants were thawed and levels of A) IL-6, B) D-dimer, D) LpPLA2, E) fasting LDL cholesterol, and F) fasting oxidized LDL cholesterol were measured. IL-6 and D-dimer plots are log-spaced with y-axis labels showing the original scale of the data to better show any treatment effect. C) CD8+ T-lymphocyte activation (% CD38+/DR+ CD8+) was measured on thawed PBMCs using flow cytometery.
When stratified by either higher or lower screening D-dimer (≤/> 0.34 μg/mL), pre-entry IL-6 (≤/> 1.50pg/mL), or baseline CD8+ T lymphocyte activation (≤/> 19%), no significant effect was observed for any of the pre-to-post atorvastatin (log10 change) biomarkers, except the modest increase in sCD163 noted above was seen only with D-dimer >0.34 μg/mL, and a modest increase in CD4+ T lymphocyte activation in the high IL-6 group (data not shown). Baseline IL-6 levels correlated with D-dimer (r=0.41, p<0.001), hs-CRP (r=0.41, p<0.001), and weakly with MCP-1 (r=0.27, p=0.013); hs-CRP correlated with D-dimer (r=0.53, p<0.001); IP-10 correlated with CD8+ T lymphocyte activation (r=0.42, p<0.001) and weakly with MCP-1 (r=0.21, p=0.048) and D-dimer (r=0.23, p=0.03).
Atorvastatin Effects on Lipoproteins and Related Proteins
Atorvastatin treatment resulted in significant declines in fasting total cholesterol, calculated LDL-C, oxLDL, and Lp-LPA2 (all p<0.01, Figure 1D-F). The median changes (from baseline to week 20) in fasting total cholesterol in the atorvastatin vs. the placebo group were −35.5 vs. −2.5 mg/dL, LDL-C −36.0 vs. −1.0 mg/dL, and oxLDL −13.6 vs. −1.4 U/L. The median pre-to-post atorvastatin (95% CI) changes for LDL-C when treatment periods were combined were −39 (−46, −31) mg/dL, and for oxLDL were −14 (−16, −12) U/L, representing 38% and 33% reductions, respectively. There were no significant changes in triglycerides or calculated high density lipoprotein cholesterol (HDL-C). For Lp-PLA2, the median pre-to-post atorvastatin change was −35.8 ng/mL (95% CI: −68.6, −15.6), a 31% reduction. Baseline Lp-PLA2 correlated with oxLDL (r=0.32, p=0.003), but not with LDL, with %CCR2+ and %CX3CR1+(CD14dim/CD16+) monocytes (r=0.30, p=0.006 and r=0.22, p=0.041, respectively), and weakly and inversely with D-dimer (r=−0.21, p=0.05) Pre-to-post atorvastatin changes in Lp-PLA2 correlated strongly with changes in LDL (r=0.56, p<0.001) and oxLDL (r=0.49, p<0.001).
Safety
The majority of toxicities (43% and 46% in the atorvastatin and placebo arms before crossover) were grade 2. None was attributed to atorvastatin treatment. No deaths were reported during the study.
DISCUSSION
In this trial, HIV RNA-suppressed individuals on a boosted-PI containing regimen randomized to the maximum recommended dose of atorvastatin for 20 weeks did not demonstrate significantly decreased levels of soluble or cellular biomarkers of immune activation and inflammation, but demonstrated robust reductions in LDL-C, oxLDL, and LpPLA2. OxLDL activates the “inflammasome” within foamy macrophages in the vascular intima, which is central to atherosclerosis pathophysiology and linked to vascular events in general and HIV-infected populations [13,14,26,27,28,29,30]. Plasma oxLDL levels are increased significantly with HIV infection and have correlated with markers of monocyte activation, including sCD14 and tissue factor expression [14]. Lp-PLA2 is a pro-inflammatory enzyme produced by monocytes that binds to LDL cholesterol particles, hydrolyzing oxidized phospholipids into free fatty acids and lysophosphatidylcholine metabolites within the vascular intima, contributing to focal vascular intimal inflammation [31]. In the general population, Lp-PLA2 levels are predictive of CVD [32]. Lp-PLA2 levels in HIV-infected individuals are higher than in HIV-uninfected, and, in a study of 341 HIV-infected patients, Lp-PLA2 levels were associated with abnormal carotid intima medial thickness and elevated coronary artery calcium scores [33].
In individuals with untreated HIV-1 viremia or suboptimal T-lymphocyte reconstitution on ART, atorvastatin 80mg/day has been associated with reduced CD4+ and CD8+ T-lymphocyte activation [34,35]. This was not observed in our study that excluded viremic participants, but we did find potent effects on oxLDL and Lp-PLA2 levels (−31%). In the SATURN-HIV study, rosuvastatin 10mg/day decreased Lp-PLA2 (−12%), sCD14, and the proportions of activated T lymphocytes and tissue factor-positive monocytes at 48 weeks [36]. Of note, the SATURN-HIV trial enrolled some participants with active Hepatitis B or C, HIV-1 viremia (22%), and had a greater percentage of cigarette/cigar-smoking participants (66% vs 34%). These factors along with higher baseline sCD14 levels (performed in the same lab), and longer study duration may have accounted for the greater observed reductions in sCD14. In another randomized, placebo-controlled study in 40 HIV-infected participants (85% virologically suppressed) atorvastatin 40mg/day was associated with a reduction in non-calcified coronary plaque progression that correlated with reductions in oxLDL and Lp-PLA2 (−18%) but not sCD14 or sCD163, which, as in the present study, were unaffected [37]. These and other recent studies [21,38] suggests that biomarkers that more closely reflect events within the vascular intima such as oxLDL and Lp-PLA2 may prove more useful in predicting CVD clinical outcomes with statin therapy and related strategies than more systemic biomarkers of immune activation and inflammation.
Strengths of this pilot study include examining a wide array of the cellular, soluble, and lipoprotein biomarkers and the use of a homogeneous participant population without confounding sources of inflammation such as active hepatitis or other inflammatory conditions, all with fasting LDL-C <130 mg/dL and fully suppressed HIV-1 RNA using a boosted-PI – for much more uniform statin pharmacodynamics than mixed ART regimens. The effects of statin drugs may differ in individuals with HIV viremia, active hepatitis, or other inflammatory conditions but current treatment guidelines call for addressing these issues as soon as feasible. Limitations include a predetermined inability to generalized these results to hyperlipidemic or viemic individuals, a lack of any clinical or surrogate CVD markers, and a relatively high dropout rate (driven by relatively stringent restrictions on viremia and disallowed medications) which may have reduced power, though atorvastatin effects on inflammatory biomarkers were quite flat. Though ART reduces many biomarkers of inflammation, we attempted to enrich the study population for post ART residual inflammation. We chose to focus on elevated D-dimer as a screen for inflammation and cardiovascular risk because it was available as a well validated, commercial clinical lab assay with quick turn around, and because it strongly and consistently correlates with cardiovascular disease and mortality in the setting of HIV infection [15,16,17,18]. We were unable to recruit sufficient participants at a higher screening D-dimer value, so we may not have enriched for post ART inflammation. Indeed, the baseline D-dimer range for our study participants did not appear greatly different than other studies have observed in non HIV-infected individuals (see Table 2). Even when we stratified participants for higher baseline levels of D-dimer, IL-6, or CD8+ T lymphocyte activation, we did not observe significant differences in any of the pre-to-post atorvastatin biomarker measurements.
Conclusions
In HIV RNA-suppressed individuals on a boosted-PI containing regimen with LDL <130mg/dL, atorvastatin for 20 weeks did not significantly decrease levels of soluble or cellular biomarkers of immune activation and inflammation, but resulted in robust reductions in LDL-C, oxLDL, and LpPLA2. Statin mediated reductions in these lipoprotein related biomarkers suggest possible reductions in CVD risk, a strategy that is currently being tested in REPRIEVE (NCT02344290), a large multi-center clinical outcome study using pitavastatin in HIV-infected individuals at low risk for CVD by traditional risk factor assessment.
Highlights.
-
1)
Atorvastatin for 20 weeks was studied in 98 individuals with suppressed HIV infection
-
2)
Atorvastatin did not decrease biomarkers of immune activation and inflammation
-
3)
Atorvastatin treatment resulted in robust reductions LDL-C, oxLDL, and LpPLA2
-
4)
Atorvastatin was well tolerated with no significant toxicity over 20 weeks
ACKNOWLEDGEMENTS
The AIDS Clinical Trials Group Study A5275 Team, additional members: Katherine Bergstrom M.S., Linda Boone M.S., Laurie Myers M.S., Debra Meres Pharm.D., Katherine Shin Pharm.D., Francesca Aweeka Pharm.D. Karen Cavanagh R.N., Kathleen A. Medvik B.S., M.T. (A.S.C.P.), Laura Hovind M.S., Karl Shaw, Amanda Zadzilla B.S. We also thank Zhaohui Su for input on the study design.
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636 and UM1 AI106701. The ACTG Specialty Laboratory was supported under award numbers AI 069501, AI 036219, and AI 068636. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Study drug and matching placebo was generously provided by Pfizer Pharmaceuticals. The study team is grateful to the study participants and to individuals at the following participating clinical trial sites:
Eric Helgeson RN and Christine Jonsson - University of Washington ACTU (Site 1401) Grant UM AI69481
Baljinder Singh and Christie Lyn Costanza - Rutgers New Jersey Medical School Clinical Research Center (Site 31786) Grant U01 AI069503-01
Dan Lee MD and Leticia Muttera PharmD - UCSD Antiviral Research Center (Site 701) Grant AI069432
Ruben Lopez and Eric S Daar - Harbor-UCLA (Site 603) Grant AI 069424, CTSI UL1TR000124
Kristen Allen BSN RN CCRP and Jane Baum BSN RN CCRP - Case CRS (Site 2501) Grant AI69501
Michelle Cespedes MD and Karen Cavanagh RN - New York University/Bellevue ACTU (Site 401) Grant UM1 AI069532
Jordan Lake MD and Vanessa Cajahuaringa - UCLA Care Center CRS (Site 601) Grant AI069424
Margarita Aguilar and Baiba Berzins - Northwestern University CRS (Site 2701) Grant AI 069471
Christine Griesmer and Graham Ray - University of Colorado Hospital CRS (Site 6101) Grant 2UM1AI069432, 2UM1AI069432
Pablo Tebas MD and Carol Digiorgio – Penn Therapeutics CRS (Site 6201) ACTG Grant: UM1-AI069534-09, CFAR Grant: UM1-AI069534-09
The Wayne State University ACTG Clinical Research Site
Mary Albrecht MD and Andrea Kershaw NP - Beth Israel Deaconess (Partners/Harvard) CRS (Site 103) Grant UM1 AI069472-08
Princy N Kumar MD and Joseph G Timpone Jr MD - Georgetown University CRS (Site 1008) Grant 5U01 AI069494
Roberto C Arduino MD and Martine M Diez - Houston AIDS Research Team CRS (Site 31473) Grant 2 UM1 AI069503, 5 UM1 AI068636
Carol Clark RN and Vicky Watson RN - Virginia Commonwealth University CRS (Site 31475) Grant UM-A!069503
Deborah McMahon MD and Lisa Klevens RN BSN - University of Pittsburgh CRS (Site 1001) Grant UM1AI069494
Susanna Naggie MD MHS and Heather Adams RN BSN - Duke University CRS (Site 1601) Grant 5U01HL12333
Jenifer Baer RN BSN and Carl J Fichtenbaum MD - University of Cincinnati CRS (Site 2401) Grant UM1AI068636
John Baxter MD and Yolanda Smith BA - Cooper University Hospital CRS (Site 31476) Grant AI069503-01
Alicarmen Alvarez RN and Tamara James Data Manager - Alabama CRS (Site 31788) Grant UM1AI069452
The Massachusetts General Hospital ACTG Clinical Research Site
Paul Sax MD and Cheryl Keenan RN BC - Brigham and Women's Hospital (Site 107) Grant UM1AI069412
Lisa Kessels BSN RN BS and Teresa Spitz BSN RN - Washington University CRS (Site 2101) Grant AI69439
Dr Michael Para and Heather Harber RN - Ohio State University (Site 2301) Grant UM1AI069494
Kim Whitely and Julie Ziegler – MetroHealth CRS (Site 2503) Grant AI 69501
Susan Blevins RN MS ANP-C and Catherine Kronk BA - Chapel Hill CRS (Site 3201) Grant UM1 AI069423, CTSA: 1UL1TR001111, CFAR: P30 AI50410
Jorge L Santana Bagur MD FIDSA and Ileana Boneta RN BSN – Puerto Rico AIDS CTU (Site 5401) Grant 5UM1AI069415
Valery Hughes NP and Todd Stroberg RN - Weill Cornell-Chelsea CRS (Site 7804) Grant UM1AI069419, UL1TR000457 (CTSC)
Linda Makohon RN BSN and Leslie Faber RN BSN - Henry Ford Hospital CRS (Site 31472) Grant B40465
Mary Adams RN and Christine Hurley RN - University of Rochester CRS (Site 31787) Grant UM1 AI069511, UL1 TR000042
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
All authors played a role in editing the manuscript and approving the text as submitted
DN, SH, and JA were responsible for study conceptualization.
DN, SH, JA and RB designed the study.
ML and NF performed the laboratory assays
RB and EC performed the primary statistical analysis.
DN wrote the manuscript.
DN, JA, SH, RB, EC, ML, NF, JL, and KK, revised the manuscript critically for important intellectual content, and approved the final version.
DN, JA, RB, and EC take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest:
NF has served as a paid consultant for Gilead Science Inc. Other authors report no conflict of interest
REFERENCES
- 1.Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013 Apr 22;173(8):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010 Jan 16;24(2):243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012 Jul 25;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 6.Marchetti G, Bellistrì GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008 Oct 1;22(15):2035–8. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009 Apr 15;199(8):1177–85. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115(2):161–7. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012 Nov 29;120(23):4599–608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merlini E, Bai F, Bellistri GM, et al. Evidence for polymicrobic flora translocating in peripheral blood of HIV-infected patients with poor immune response to antiretroviral therapy. PLoS One. 2011 Apr 11;6(4) doi: 10.1371/journal.pone.0018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longenecker CT, 1, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014 Apr 24;28(7):969–77. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tincati C, Biasin M, Bandera A, et al. Early initiation of highly active antiretroviral therapy fails to reverse immunovirological abnormalities in gut-associated lymphoid tissue induced by acute HIV infection. Antivir Ther. 2009;14(3):321–30. [PubMed] [Google Scholar]
- 13.Zanni MV, Schouten J, Grinspoon SK, et al. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014 Dec;11(12):728–41. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 14.Zidar DA, Juchnowski S, Ferrari BJ, et al. Oxidized LDL Levels Are Increased in HIV Infection and May Drive Monocyte Activation. Acquir Immune Defic Syndr. 2015 Jun 1;69(2):154–60. doi: 10.1097/QAI.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grund B, Baker JV, Deeks SG, et al. Relevance of Interleukin-6 and D-Dimer for Serious Non-AIDS Morbidity and Death among HIV-Positive Adults on Suppressive Antiretroviral Therapy. PLoS One. 2016 May 12;11(5) doi: 10.1371/journal.pone.0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordell AD, McKenna M, Borges ÁH, et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014 May 28;3(3):e000844. doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014 Oct 15;210(8):1248–59. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson TA, Maki KC, Orringer CE, et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part. 2. J Clin Lipidol. 2015 Nov-Dec;9(6 Suppl):S1–S122. doi: 10.1016/j.jacl.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Zanni MV, Toribio M, Robbins GK. Effects of Antiretroviral Therapy on Immune Function and Arterial Inflammation in Treatment-Naive Patients With Human Immunodeficiency Virus Infection. JAMA Cardiol. 2016;1(4):474–480. doi: 10.1001/jamacardio.2016.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson JG, Smith B, Maheshwari, et al. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 2005 Nov 15;46(10):1855–62. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 24.Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011 Oct 15;204(8):1217–26. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch GG. The use of non-parametric methods in the statistical analysis of the two-period change-over design. Biometrics. 1972 Jun;28(2):577–84. [PubMed] [Google Scholar]
- 26.Itabe H, Ueda M. Measurement of plasma oxidized low-density lipoprotein and its clinical implications. J Atheroscler Thromb. 2007;14:1–11. doi: 10.5551/jat.14.1. [DOI] [PubMed] [Google Scholar]
- 27.Nishi K, Itabe H, Uno M, et al. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol. 2002;22:1649–1654. doi: 10.1161/01.atv.0000033829.14012.18. [DOI] [PubMed] [Google Scholar]
- 28.Bamberg F, Truong QA, Koenig W, et al. Differential associations between blood biomarkers of inflammation, oxidation, and lipid metabolism with varying forms of coronary atherosclerotic plaque as quantified by coronary CT angiography. Int J Cardiovasc Imaging. 2012;28:183–192. doi: 10.1007/s10554-010-9773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 30.Parra S, Coll B, Aragones G, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–231. doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 31.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25:923–31. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 32.White HD, Simes J, Stewart RA, et al. Changes in lipoprotein-Associated phospholipase A2 activity predict coronary events and partly account for the treatment effect of pravastatin: results from the Long-Term Intervention with Pravastatin in Ischemic Disease study. J Am Heart Assoc. 2013 2013 Oct 23;2(5) doi: 10.1161/JAHA.113.000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangili A, Ahmad R, Wolfert RL, et al. Lipoprotein-associated phospholipase A2, a novel cardiovascular inflammatory marker, in HIV-infected patients. Clin Infect Dis. 2014 Mar;58(6):893–900. doi: 10.1093/cid/cit815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganesan A, Crum-Cianflone N, Higgins J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011 Mar 15;203(6):756–64. doi: 10.1093/infdis/jiq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanjako D, Ssinabulya I, Nabatanzi R, et al. Atorvastatin reduces T-cell activation and exhaustion among HIV-infected cART-treated suboptimal immune responders in Uganda: a randomized crossover placebo-controlled trial. Trop Med Int Health. 2015 Mar;20(3):380–90. doi: 10.1111/tmi.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015 Apr 1;68(4):396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nou E, Lu MT, Looby SE, et al. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relatesindependently to reductions in cor onary plaque in patients with HIV. AIDS. 2016 Feb 20;30(4):583–90. doi: 10.1097/QAD.0000000000000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hileman CO, Turner R, Funderburg NT. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS. 2016;30:65–73. doi: 10.1097/QAD.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenorio AR, Chan ES, Bosch RJ, et al. Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy - ACTG A5286. J Infect Dis. 2015 Mar 1;211(5):780–90. doi: 10.1093/infdis/jiu515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funderburg NT, Stubblefield Park SR, Sung HC, et al. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology. 2013 Sep;140(1):87–97. doi: 10.1111/imm.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]