Abstract
Discussions about disclosing individual genetic research results include calls to consider participants’ preferences. In this study, parents of Boston Children’s Hospital patients set preferences for disclosure based on disease preventability and severity, and could exclude mental health, developmental, childhood degenerative, and adult-onset disorders. Participants reviewed hypothetical reports and reset preferences, if desired. Among 661 participants who initially wanted all results (64%), 1% reset preferences. Among 336 participants who initially excluded at least one category (36%), 38% reset preferences. Participants who reset preferences added 0.9 categories, on average; and their mean satisfaction on 0–10 scales increased from 4.7 to 7.2 (p<0.001). Only 2% reduced the number of categories they wanted disclosed. Findings demonstrate the benefits of providing examples of preference options and the tendency of participants to want results disclosed. Findings also suggest that preference-setting models that do not provide specific examples of results could underestimate participants’ desires for information.
Keywords: Return of research results, preferences, biobanking, pediatrics, ethics
Introduction
The controversy surrounding the return of individual research results includes questions about whether and how to incorporate participant preferences. Many commentators argue that researchers should respond to preferences for individual result return out of principles of respect (Kohane et al., 2007; Ravitsky & Wilfond, 2006; Wolf et al., 2015), and on various surveys, the overwhelming majority of respondents report a desire to have some choice or control over which results are returned (Breitkopf et al., 2015; E. D. Harris et al., 2012; Murphy Bollinger, Bridges, Mohamed, & Kaufman, 2014; Ziniel et al., 2014). Others have reservations about whether patients can realistically be expected to define and articulate the kinds of results they would want disclosed and withheld, and whether such preferences would be stable over time (Parker, 2012). Research evaluating the ability of research participants to set preferences for the return of individual results and how they might respond to information disclosed is limited.
Biobanking of pediatric samples provides a critical context for collecting this data. The inclusion of children in genetics research can facilitate prospective studies of genetic, epigenetic, and gene-environment contributors to disease (Hens et al., 2013). Health-relevant variants may be identified in pediatric biobank samples more often than other contexts, given how genetics frequently plays a stronger role in early-onset disorders (Colclough, Saint-Martin, Timsit, Ellard, & Bellanne-Chantelot, 2014; Lynch & de la Chapelle, 2003; Stiller, 2004). Moreover, at least one pediatric biobank has proposed an open-ended genetic research repository that incorporates patient and parental preferences into return-of-results decision making (Holm et al., 2014). Developing effective strategies for returning individual results from pediatric biobank research will be especially important as the pace of genetic discovery accelerates.
To better understand parents’ abilities to set preferences for the return of pediatric biobank results, we conducted a web-based survey. We first present descriptive data about preference-setting patterns, and how often participants reset those preferences after viewing examples of potential results. We then examine anticipated satisfaction with the return of results, hypothesizing that providing examples of potential results and an opportunity to reset preferences would enhance satisfaction. Finally, we describe why participants would be satisfied with disclosed and omitted results.
Method
Overview
Data were collected through a web-based survey, approved by the Boston Children’s Hospital (BCH) Institutional Review Board (IRB-P00006896), designed to compare preference-setting models for individual results return. Participants were randomized to one of four study arms presenting hypothetical biobanks, including one where participants used a step-wise process to designate granular preferences about the types of conditions for which they would want research results returned. Primary findings showed that participants favored a biobank that permitted granular preferences over a biobank that simply provided an “all or none” option for disclosure or biobanks that disclosed everything or nothing without considering parent preferences. The analyses that follow are restricted to participants who were randomized to the “granular arm.”
Before starting the survey, participants watched a 5-minute educational video about the role of genetics in health (included in Supplementary Materials). After reporting demographic information and attitudes about genetic research and the return of results, participants completed the preference-setting module. First, participants indicated if they would want individual results about their children returned for conditions that were preventable, non-preventable, both, or neither. Participants who wanted at least some results returned then indicated if they would want results about conditions classified as severe, non-severe, or both (participants who chose to receive neither preventable nor non-preventable results were presented no additional options). Finally, participants reported if they would want any of four specific categories of results excluded from disclosure: mental illness and psychological conditions; developmental disorders and learning disabilities; childhood-onset degenerative conditions; or adult-onset conditions lacking prevention options during childhood. The structure of the preference-setting model was modeled from a popular framework for prioritizing the return of whole genome sequencing results (Berg, Khoury, & Evans, 2011), although the specific disease characteristics we used were different and informed by a literature review and semi-structured interviews with parents of BCH patients (Bacon et al., 2015). The preference-setting model that was presented to participants is included in the Supplementary Materials.
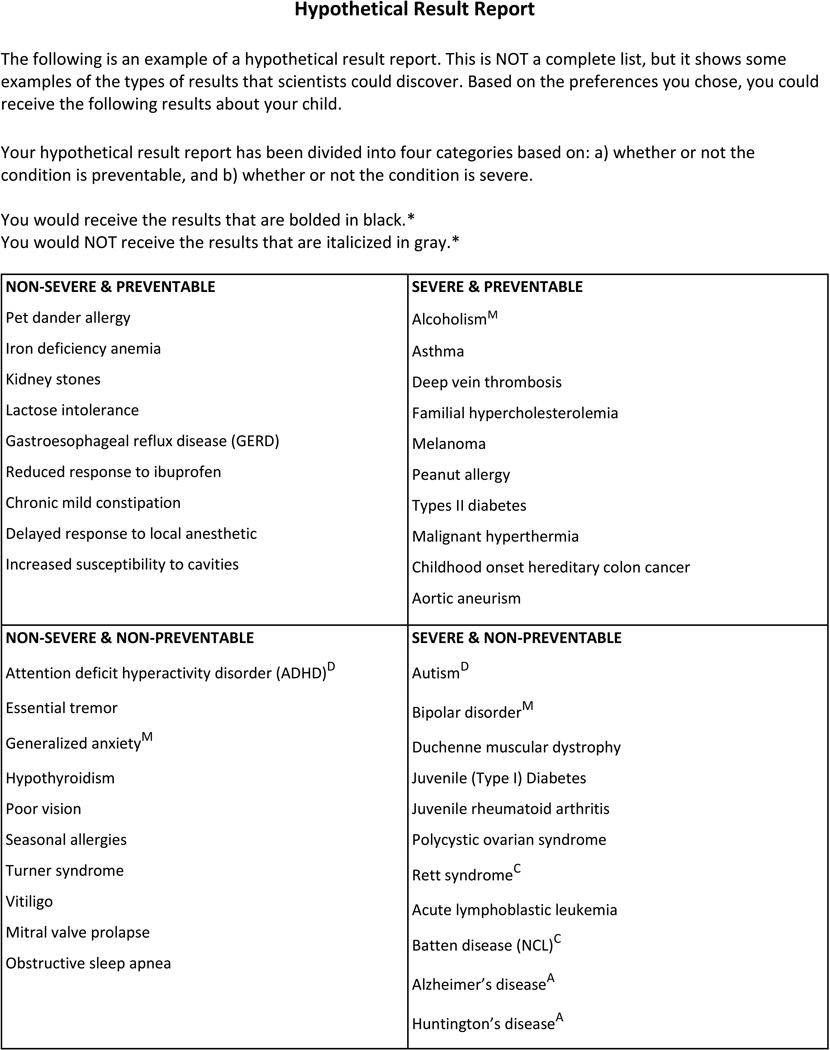
Participants then viewed a hypothetical results report listing 41 conditions, organized into four cells based on preventability and severity, that specified which individual results would be disclosed or withheld based on stated preferences. See Figure 1. Respondents were given the option to view a glossary of genetic conditions which provided brief descriptions of each example condition. Afterwards, participants responded to a series of survey items asking about satisfaction with the hypothetical results and the preference-setting process overall. Additional questions assessed rationales for feeling satisfied and dissatisfied about results. Participants were then given the option to change their preferences. Those who wanted to change their preferences were presented with the same preference-setting module as before and presented with an updated hypothetical results report. Finally, they again reported their satisfaction with the updated reports and process.
Figure 1.
Hypothetical Results Report template. Sentences with asterisks are omitted if applicable. Superscripts are not shown, but indicate conditions classified as mental illness (‘M’), developmental disorders (‘D’), childhood onset degenerative conditions (‘C’), and adult-onset conditions (‘A’). Disclosed conditions are bolded, while omitted conditions are shaded and italicized.
Development, Testing, and Dissemination of Survey
Survey questions were based on a literature review and refined by a multidisciplinary team of genomics researchers, health psychologists, genetic counselors, survey methodologists, and individuals knowledgeable in medical genetics. Existing guidelines and recommendations for preference setting and incidental findings disclosure typically focus on the preventability and severity of the conditions in questions (Fabsitz et al., 2010; Holm & Taylor, 2012; Kollek & Petersen, 2011; Wolf, 2013). Additional factors that matter to patients or are of concern to ethicists include the disease domain (e.g., developmental disorders) (Wade et al., 2012) and age of onset (American Academy of Pediatrics Committee on Bioethics & Committee on Genetics, 2013; Clayton et al., 2014). We choose and assigned conditions as either preventable or non-preventable and either severe or non-severe based on input from 20 genetic counselors and geneticists at BCH and other Boston area hospitals (including Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center) (Bacon et al., 2015). Early versions of the survey were edited based on feedback from cognitive interviews conducted with parents of inpatients at BCH to test the preferences-setting strategy and this survey for comprehension and ease of administration. The survey was programmed into REDCap (P. A. Harris et al., 2009) for web administration, and revised after pilot-testing on 500 participants selected randomly from the same sampling frame used in the main study.
Study Population
Participants were parents of BCH patients. Eligible individuals were parents or guardians of children who had received care at BCH within the 2 years preceding the study and who had an e-mail address listed in their medical record. Inclusion criteria included living in the United States, English-speaking, age 18 years or older, and having at least one child under 18 years of age at the time of participation. Parents were excluded if either the parent or their child was currently or previously enrolled in the Gene Partnership, a BCH pediatric biobank that offers the return of research results.
Potential participants were mailed a pre-notification letter that explained the background and process of the study and included the email address we planned to use for recruitment. The letter also indicated that participants who completed the survey would be entered into a raffle for one $100 Visa gift card for every 100 completed surveys. Nine days after the pre-notification letter was mailed, the survey invitation email was sent to parents. During the three months that the survey was open, participants were sent a maximum of three reminder emails.
Measures
Demographic Characteristics
Participants self-reported demographic information including age, race, ethnicity, gender, education, family history of disease, and experiences with healthcare, research, and genetic testing.
Preferences
Eight categories of disease were classified as either disclosed or omitted from reports depending on the preferences participants set: (1) Preventable conditions, (2) non-preventable conditions, (3) severe conditions, (4) non-severe conditions, (5) mental illness and psychological conditions, (6) developmental disorders and learning disabilities, (7) childhood-onset degenerative conditions, and (8) adult-onset conditions without prevention options during childhood. Number of categories was calculated as the total number of categories selected for disclosure. For the purpose of calculating the number of categories selected, participants who indicated that they wanted no results while setting preventability preferences were classified as declining all eight categories.
Satisfaction
Participants reported their overall process satisfaction (“How satisfied or dissatisfied would you be with this biobank's process for determining which results would or would not be returned to you?”) and overall results satisfaction (“How satisfied or dissatisfied would you be with the information that would or would not be returned to you in your hypothetical result report?”) on 0 to 10 scales, with 0 indicating “very dissatisfied” and 10 indicating “very satisfied.” Overall satisfaction measures were collected immediately after participants viewed the initial and updated hypothetical results reports.
Rationales
Following the first set of satisfaction items, participants responded to statements explaining why they would be satisfied or dissatisfied with their results. Participants rated their agreement with up to 20 statements about disclosed and omitted information, using 5-point scales ranging from strongly disagree to strongly agree. Those participants who set preferences to receive no results of any kind were not queried about disclosed results. Likewise, participants who set preferences to receive all results were not queried about omitted results. Responses to individual rationale items were dichotomized into agreed (strongly agree and agree) versus did not agree (strongly disagree, disagree, and neither agree nor disagree) to simplify the presentation of data.
Data Analysis
We used t-tests, chi-squared tests, Fisher’s exact test, and Wilcoxon Rank Sum tests to analyze drop out in the study before the option to reset preferences. We used paired t-tests to compare the number of categories selected before and after the option to reset preferences, and McNemar tests to compare preferences for specific categories of results before and after the opportunity to reset them. Paired t-tests compared overall satisfaction of process and information. Fisher’s exact test compared the association between rationales and preference changes, organized into four groups: (1) revised preferences to receive fewer categories of results, (2) did not change their preferences, (3) revised preferences, but added as many categories of results as they removed (balanced), and (4) revised preference to receive more categories of results. P values for Fisher’s exact tests were computed using Monte Carlo simulation on 2,000 replicates. We considered associations between preference changes and rationales significant only if comparisons were observed in statistical analyses that both included and omitted “neither agree nor disagree” responses, given that such responses often reflect confusion rather than ambivalence (Sturgis, Roberts, & Smith, 2014). All analyses excluded participants who dropped out of the study before the option to reset preferences. Given the exploratory nature and large number of analyses, statistical significance was set at p=0.001.
Results
Profile of respondents
Of 11,394 invited parents, 2,718 (23.9%) participated in the survey, including 1,285 randomized into the granular arm. Respondents who were under age 18 or did not provide age data were excluded from analyses (n=100). Final analyses omitted an additional 158 respondents who dropped out of the survey before the option to reset preferences. Characteristics of the analytic set are summarized in Table 1. Participants who dropped out of the study before the opportunity to reset preferences tended to want fewer categories of results. They were less likely to have set preferences to receive results about non-preventable conditions (53.5% vs 79.7%, p<0.001), mental health conditions (66.2% vs 87.9%, p<0.001), developmental delays (75.0% vs 90.8%, p<0.001), degenerative conditions (76.1% vs 89.4%, p<0.001) and adult-onset conditions (59.7% vs 76.1%, p<0.001).
Table 1.
Participant characteristics
| Retained in Final Analysis (n=1,027) |
Dropped before Option to Reset (n=158) |
||||
|---|---|---|---|---|---|
| n | % | n | % | p | |
| Age | |||||
| 30 or less | 38 | 3.7 | 8 | 5.1 | 0.688 |
| 31–40 | 347 | 33.8 | 50 | 31.6 | |
| 41–50 | 490 | 47.7 | 76 | 48.1 | |
| 51–60 | 139 | 13.5 | 24 | 15.2 | |
| 61+ | 13 | 1.3 | 0 | 0.0 | |
| Gender | 0.018 | ||||
| Female | 953 | 92.8 | 138 | 87.3 | |
| Male | 74 | 7.2 | 20 | 12.7 | |
| Ethnicity | 0.964 | ||||
| White non-Hispanic | 885 | 86.2 | 130 | 82.3 | |
| Hispanic | 27 | 2.6 | 4 | 2.5 | |
| Black | 14 | 1.4 | 3 | 1.9 | |
| Asian | 32 | 3.1 | 4 | 2.5 | |
| Other/Unspecified | 51 | 5.0 | 13 | 8.2 | |
| Multiple | 18 | 1.8 | 4 | 2.5 | |
| Education | 0.789 | ||||
| Less than college degree | 160 | 15.9 | 23 | 15.5 | |
| Undergraduate degree | 384 | 38.1 | 60 | 40.5 | |
| More than college degree | 464 | 46.0 | 65 | 43.9 | |
| Work in healthcare | 226 | 22.0 | 39 | 24.7 | 0.452 |
| Prior participation in research | 372 | 36.2 | 41 | 26.3 | 0.015 |
| Prior genetic testing experience | 415 | 40.4 | 52 | 32.9 | 0.073 |
| Child or self has genetic disorder | 241 | 23.5 | 33 | 20.9 | 0.474 |
Preferences Before and After the Option to Reset
Table 2 summarizes participants’ preferences before and after the option to reset them. The strongest preference, expressed by 64% of participants before the option to reset preferences and 69% of participants afterwards, was to receive all types of results. In contrast, 2% wanted no results both before and after the option to reset preferences. Age of disease onset and disease preventability were important criteria for many participants: 12% participants before and after the option to reset wanted all results except adult-onset conditions and/or non-preventable conditions. All other preference combinations represented 1% or less of the study population each, both before and after the option to reset preferences.
Table 2.
Initial and final preferences for the return of results about participants’ children. Only selections that represented over 1% of the study population after the option to reset preferences are shown (n=1,027).
| Prior to Option to Reset |
Final Selection |
|||
|---|---|---|---|---|
| n | % | n | % | |
| All conditions | 661 | 64.4 | 704 | 68.5 |
| All but adult-onset conditions | 51 | 5.0 | 82 | 8.0 |
| All but non-preventable and adult-onset conditions | 33 | 3.2 | 22 | 2.1 |
| No conditions | 18 | 1.8 | 19 | 1.9 |
| All but non-preventable | 40 | 4.0 | 18 | 1.8 |
| All but mental illnesses | 13 | 1.3 | 13 | 1.3 |
Prior to the option to reset preferences, participants on average elected to receive 7.1 of 8 categories of results. After being given an opportunity to change preferences, 148 participants (14.4%) did so. Twenty two participants (2.1%) ultimately elected fewer categories of results, 116 (11.3%) elected more categories, and 10 (1.0%) added as many categories for disclosure as they removed. Among the 661 participants who initially set preferences to receive all results, only 9 (1.4%) reset their preferences after viewing the hypothetic report. In contrast, among the 366 participants who set preferences to omit at least one category of results, 139 (38.0%) reset their preferences, including 3 of 18 (16.7%) of those who originally set preferences to receive no results. Those who reset their preferences elected to receive on average 6.5 categories of conditions after the reset versus 5.6 before the option (p<0.001), with changes most frequently made to add severe conditions and non-preventable (see Table 3). In fact, 46.7% of individuals who initially omitted severe conditions and 38.9% of individuals who initially omitted non-preventable conditions reset their preferences to receive them.
Table 3.
Preferences to receive specific types of individual research results.
| Prior to Option to Reset |
Final Selection |
|||||
|---|---|---|---|---|---|---|
| n | % | n | % | %Δ | p | |
| Preventable conditions | 1,009 | 98.2 | 1,005 | 97.9 | −0.4 | 0.343 |
| Severe conditions | 952 | 92.7 | 977 | 95.1 | +2.4 | <0.001* |
| Non-severe conditions | 946 | 92.1 | 957 | 93.2 | +1.1 | 0.072 |
| Developmental disorders | 933 | 90.8 | 937 | 91.1 | +0.3 | 0.571 |
| Childhood degenerative conditions | 918 | 89.4 | 91.0 | 91.0 | +1.7 | 0.007 |
| Mental illness | 903 | 87.9 | 88.8 | 88.8 | +0.9 | 0.188 |
| Non-preventable conditions | 819 | 79.7 | 86.6 | 86.6 | +6.8 | <0.001* |
| Adult-onset conditions | 782 | 76.1 | 76.2 | 76.2 | +0.1 | 1.000 |
Denotes significance at α=0.001
Satisfaction with Potential Results
Overall satisfaction prior to being given the option to reset preferences was moderately high for both the process of setting preferences (mean 7.1 on 0–10 scale) and for potential results received (mean: 6.7 on 0–10 scale). Among those who reset their preferences, mean satisfaction scores increased for both the overall process of setting preferences (from 5.1 to 7.3, p<0.001) and potential results received (from 4.7 to 7.2, p<0.001).
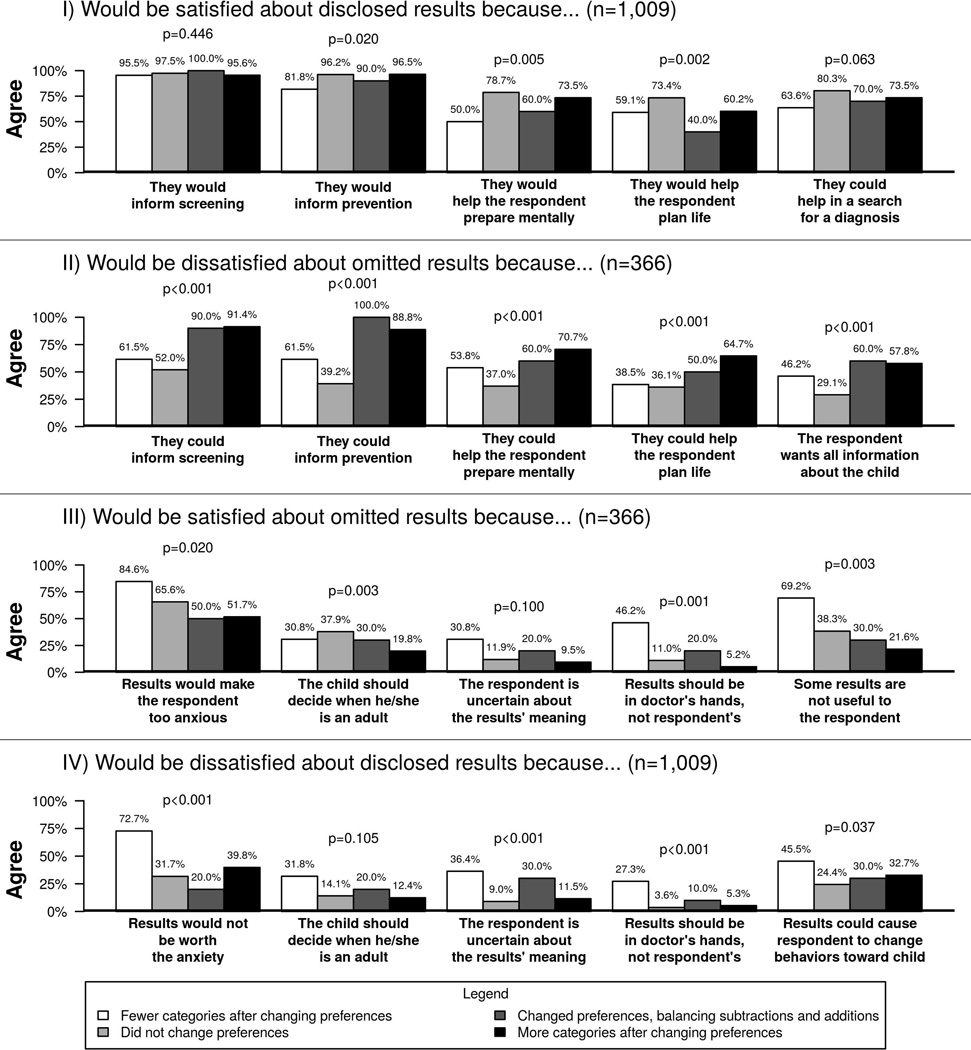
Rationales for satisfaction and dissatisfaction with results are summarized in Figure 2. Upon reviewing the initial hypothetical results report, participants were overall most likely to report satisfaction that disclosed results could inform screening and prevention strategies (endorsed by 97.2% and 95.8% of respondents, respectively) and most likely to report dissatisfaction that disclosed results were not worth the potential anxiety (33.4% of) or could change their behavior towards their children (25.9% of respondents). The most popular reason for satisfaction with omitted results was that the omitted results would not be worth the anxiety (61.5% of respondents), while over half of participants reported that they would be dissatisfied because the information could have informed screening and prevention practices (65.8% and 57.4% of respondents, respectively). Participants who revised their preferences to have more categories of results disclosed were more likely to endorse rationales for dissatisfaction with omitted results than participants who did not change their preferences or revised them to have fewer categories of results disclosed. In contrast, participants who revised their preferences to have fewer categories of results disclosed were more likely to report satisfaction about omitted results and dissatisfaction with disclosed results because they believed results should be in their doctors’ hands rather than their own. They were also more likely to report satisfaction about omitted results because they perceived some results as not useful, and were more likely to report dissatisfaction about disclosed results because they were uncertain about the results’ meaning and because some results would not be worth the anxiety.
Figure 2.
Rationales for satisfaction and dissatisfaction about the initial hypothetical results report. P-values represent analyses about whether rationales differed among participants who changed preferences to receive fewer categories of results, participants who did not change preferences, participants who changed preferences but balanced subtractions and additions, and participants who changed preferences to receive more categories of results.
 Fewer categories after changing preferences
Fewer categories after changing preferences
 Did not change preferences
Did not change preferences
 Changed preferences, balancing subtractions and additions
Changed preferences, balancing subtractions and additions
 More categories after changing preferences
More categories after changing preferences
Discussion
This study provides much-needed insight into how parents might set preferences for the return of individual research results from a pediatric biobank. We found that about two thirds of participants wanted all categories of results about their child, including non-preventable conditions. Moreover, very few of those who wanted all results reset preferences after viewing hypothetical results. Almost 40% of participants who initially chose to omit at least one category of results, in contrast, reset preferences after viewing the hypothetical results report, tending to want more categories of conditions disclosed. Revisions to add categories of results appeared to be motivated by dissatisfaction about omitted results, whereas revisions to remove categories of results appeared to be motivated by concerns about the limited utility and uncertainty of disclosed results, and beliefs that physicians were better qualified than the respondents to have the information. Findings suggest the kinds of preferences that parents will set for the return of results about their children will depend on the way the preference-setting model is presented. Specifically, most parents are likely to want to be informed about their child’s risk for different types of disease when they see examples of specific conditions.
It is also notable that more than one third of respondents initially, and 30% after the option to reset, established more nuanced preferences than receiving all or no results. In a small study of healthy adults receiving whole genomic sequencing, 94% of participants wanted all types of results disclosed (Sanderson et al., 2016). Fewer participants in our study wanted all results, suggesting that parents of potential research participants are likely to have more nuanced preferences about genetic information disclosure than patients who agree to undergo clinical sequencing personally. In contrast, only 30% of participants in a national survey about preferences for the return of clinical test results indicated that they would want results from all of 18 different hypothetical testing scenarios (Graves et al., 2015), suggesting that our clinic-based participants had more favorable attitudes towards the return of results than the general population.
Our study also highlights the challenges of using granular preferences to determine results disclosure. Not only was the percentage of participants who reset their preferences after viewing potential results high, but those preference revisions were also associated with large increases (over 2 points on 10-point scales) in satisfaction. Discordance between scientists’ and research participants’ classifications of conditions may explain some changes, as participants may not have anticipated how a particular condition would be categorized by researchers. However, we observed across-the-board trends for more information; and revisions to add severe conditions was not balanced by subtraction of non-severe conditions.
More likely, seeing specific examples of conditions that would be omitted may have prompted participants to think more about how results might be useful. Participants who revised preferences to receive more categories of results were much more likely to endorse rationales for dissatisfaction about omitted results than participants who retained their preferences or revised them to receive fewer categories of results. Given prior research showing great variability in the way patients classify scenarios as actionable (Graves et al., 2015), it is also likely that some participants in our study conceptualized categories such as “preventable,” and “severe” more broadly than our clinicians. The examples provided in the hypothetical results report may have been more informative to participants about how conditions would be classified than the abstract definitions provided in the preference-setting module. Alternatively, specific conditions presented on sample results may have corresponded to participants’ personal or family histories of disease, motivating them to change their minds about receiving certain categories of disease.
Our results also showed a minority of participants who wanting fewer categories of results disclosed after viewing potential results. These participants tended to believe that results should be in the doctor’s hands rather than their own, and also tended to feel uncertain about the meaning of results and that results would not be worth the anxiety. Many of the conditions we presented to exemplify preference categories were rare conditions for which the respondent may have been unfamiliar. When research participants feel they are unlikely to understand the value of the information they may receive, they appear to be more willing to defer to experts such as doctors about whether or not the results should be in their own hands. Whatever the explanation, prior qualitative work has shown that research participants’ preferences for the return of results can change when participants consider a wider range of factors, such as the certainty of results (Bollinger, Scott, Dvoskin, & Kaufman, 2012). Our study builds on those findings by demonstrating the impact of providing or withholding examples of conditions in the return of results model.
Of additional note, over ¾ of participants in our study wanted findings about adult-onset conditions disclosed. Current clinical policies discourage testing children for genetic predispositions towards adult-onset conditions unless interventions in childhood exist to reduce morbidity or mortality (American Academy of Pediatrics Committee on Bioethics & Committee on Genetics, 2013), but arguments in favor of identifying predispositions in children for adult-onset conditions are becoming more common (Mand, Gillam, Delatycki, & Duncan, 2012). These arguments are stronger for testing that can provide unexpected results, given that individuals may be unlikely to pursue targeted testing in the absence of clinical or family history indications (Clayton et al., 2014). The ACMG, for instance, recommended that laboratories routinely screen for variants in 56 genes and disclose secondary findings whenever clinical genome or exome sequencing is performed, regardless of patients’ ages (Green et al., 2013). Although our participants in our study were least likely to set preferences to receive results about adult-onset conditions, our data still show that a large majority of parents would want such information disclosed.
A number of limitations merit mention. BCH does not systematically collect demographic information about the parents of patients, so we cannot ascertain the representativeness of respondents relative to the full set of patients’ parents. Participants had sought care for their children, tended to be well-educated, non-Hispanic white women, and were parents of patients from a single health system. Given differential dropout among those who wanted fewer results, this analysis likely overstates the amount of information parents would want to receive. The return-of-results model did not make participants weigh many of the ethical and practical considerations that might affect their decisions and often discourage individuals from pursuing genetic testing altogether (Clayton et al., 2014; Robinson et al., 2016). Such considerations include the potential for results to affect access to life and disability insurance; the rights of children to make their own decisions about receiving results; obligations to share findings with relatives who may also have inherited risks; the potential need to confirm results through CLIA-approved laboratories, and more (Murphy Bollinger et al., 2014; Thorogood et al., 2014; Wolf, 2013). The number of conditions presented on hypothetical reports may have inflated participants’ expectations about the likelihood they would receive results, and stated preferences may not reflect participants’ actual preferences. In addition, many preference categories overlapped conceptually. Participants could have chosen to receive non-preventable conditions knowing they could omit adult-onset conditions lacking prevention options in childhood.
Of particular note, our preference setting model did not address how to incorporate the preferences of the children or how participants’ preferences change over time (Yu, Jamal, Tabor, & Bamshad, 2013). Regulations generally require child assent in addition to parental consent for research participation unless potential direct benefits exist only in the research context (Office of the Secretary, 1979). Assuming similar arguments apply to preference setting, researchers and families will need to decide how best to involve children in the process. Analyses of assent of minors provides some guidance, and suggests that the degree to which children can be expected to participate in preference setting decisions may be a function of their ability to understand the context and potential outcomes (e.g., potential harms, rights to revise preferences), their skills in decision making (e.g., weighing alternatives and considering new evidence), and the degree to which they exercise autonomy (Miller, Drotar, & Kodish, 2004). As a minimum consideration, commentators generally agree that capacity is better assessed through informal assessments of maturity than using age cutoffs (Hens, Cassiman, Nys, & Dierickx, 2011).
There is also consensus that minors should be offered the opportunity to re-consent to biobank research once they are capable of providing consent, understanding that investigators’ abilities to recontact them years after biobank samples were provided may be problematic. Similar arguments apply to preference setting. “Dynamic consent” models may be one avenue by which researchers enhance the ability of biobank participants to modify preferences over time. Already, investigators and institutions are testing platforms that provide research participants with the ability to change what kinds of research may be conducted on their biobank samples (Kaye et al., 2015; Thiel et al., 2015). These platforms can likely be modified to improve the ability of children and adults alike to also revise preferences about the return of individual research results.
As the pace of genetic discovery accelerates it becomes increasingly important to develop effective strategies for returning individual results. Our work demonstrates how providing concrete examples of conditions that would be disclosed and withheld under specific preference selections can help ensure that parents receive all the information they want about their children.
Best Practices
While many commentators and policymakers have called for the consideration of participant preferences when deciding about the disclosure of individual research results, few research studies have examined how to do so. Our previously-published results suggested that allowing research participants to set preferences about the kinds of conditions they would want disclosed would lead to greater satisfaction than providing no options for disclosure or providing simple yes-or-no options about whether disclosure should occur at all (Holm et al., 2015). The findings we report here expand from those findings by suggesting that participants may classify disease characteristics such as actionability and severity differently than researchers and healthcare providers. Researchers can improve the accuracy of participants’ expectations about disclosure by providing examples of conditions that match specific preference-setting options.
Our findings also suggest that research participants are more concerned about important health-relevant results being withheld from them than the potential harms of receiving unexpected, unsolicited information. Satisfaction is only one of many benefits and harms that need to be considered when deliberating about various disclosure approaches (Wolf et al., 2012), and ethical arguments for and against the use of participant preferences may carry as much influence as utilitarian ones (Kollek & Petersen, 2011). In addition, research participants are only one of many stakeholder groups impacted by the way disclosure protocols are implemented, and must be balanced against the needs of researcher, healthcare providers, and research and clinical institutions. Nevertheless, our findings suggests that patients may appreciate if research program err or the side of inclusion rather than exclusion when making decisions about disclosing research results of potential importance.
Research Agenda
This research study examined responses to a hypothetical preference-setting and disclosure protocol. While small studies have examined how research participants respond to disclosure of actual individual results from genomic research (Christensen et al., 2011), large, collaborative efforts such as those of the Electronic Medical Records and Genomics (Kullo et al., 2014) promise to provide data about how the impact of disclosing a variety of research-derived genomic findings on individuals, families, physicians and healthcare systems. In addition, these and other efforts will provide “real world” findings about how actual clinical research participants set preferences for the return of results (Brothers et al., 2016), how preferences change over longer periods of time (Simon, Shinkunas, Brandt, & Williams, 2012), and how disclosure of personal results differ from the disclosure of results about offspring.
Educational Implications
Findings from this study are of greatest relevance to genomic research programs that are establishing protocols for the disclosure of individual results. Individual researchers who solicit preferences for disclosure will need to be aware of the ways that participants’ perceptions about disease might differ from their own, and will need to develop educational strategies to help align expectations about disclosure. Oversight boards will also need to be aware how participants may benefit from provision of specific examples of diseases that fall into specific preference categories, and encourage researchers to implement such approaches into educational protocols for their participants.
Supplementary Material
Acknowledgments
Sources of Support
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (NIH) grants F32HG006993 (Christensen [PI]), R01HG006615 (Holm [PI]), U01HG006500 (Green [PI]), and U19HD077671 (Green [mPI], Holm, Christensen).
Footnotes
Disclaimers
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Academy of Pediatrics Committee on Bioethics, & Committee on Genetics, Committee on Genetics, the American College of Medical Genetics and Genomics Social, Ethical, and Legal Issues Committe. Ethical and policy issues in genetic testing and screening of children. Pediatrics. 2013;131(3):620–622. doi: 10.1542/peds.2012-3680. [DOI] [PubMed] [Google Scholar]
- Bacon PL, Harris ED, Ziniel SI, Savage SK, Weitzman ER, Green RC, Holm IA. The development of a preference-setting model for the return of individual genomic research results. Journal of Empirical Research on Human Research Ethics. 2015;10(2):107–120. doi: 10.1177/1556264615572092. [DOI] [PubMed] [Google Scholar]
- Berg JS, Khoury MJ, Evans JP. Deploying whole genome sequencing in clinical practice and public health: meeting the challenge one bin at a time. Genetics in Medicine. 2011;13(6):499–504. doi: 10.1097/GIM.0b013e318220aaba. [DOI] [PubMed] [Google Scholar]
- Bollinger JM, Scott J, Dvoskin R, Kaufman D. Public preferences regarding the return of individual genetic research results: findings from a qualitative focus group study. Genetics in Medicine. 2012;14(4):451–457. doi: 10.1038/gim.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkopf CR, Petersen GM, Wolf SM, Chaffee KG, Robinson ME, Gordon DR, Koenig BA. Preferences regarding return of genomic results to relatives of research participants, including after participant death: empirical results from a cancer biobank. The Journal of Law, Medicine & Ethics. 2015;43(3):464–475. doi: 10.1111/jlme.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers K, Clayton E, Cooper G, East K, Amaral M, Bowling K, Westbrook M. Eliciting preferences on secondary findings: the preferences instrument for genomic secondary results (PIGSR) Tampa, FL: Paper presented at the 2016 ACMG Annual Clinical Genetics Meeting; 2016. [Google Scholar]
- Christensen KD, Roberts JS, Shalowitz DI, Everett JN, Kim SYH, Raskin L, Gruber SB. Disclosing individual CDKN2A research results to melanoma survivors: interest, impact, and demands on researchers. Cancer Epidemiology, Biomarkers & Prevention. 2011;20(3):522–529. doi: 10.1158/1055-9965.EPI-10-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EW, McCullough LB, Biesecker LG, Joffe S, Ross LF, Wolf SM For the Clinical Sequencing Exploratory Research Consortium Pediatrics Working G. Addressing the ethical challenges in genetic testing and sequencing of children. The American Journal of Bioethics. 2014;14(3):3–9. doi: 10.1080/15265161.2013.879945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colclough K, Saint-Martin C, Timsit J, Ellard S, Bellanne-Chantelot C. Clinical utility gene card for: maturity-onset diabetes of the young. European Journal of Human Genetics. 2014;22(9) doi: 10.1038/ejhg.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabsitz RR, McGuire A, Sharp RR, Puggal M, Beskow LM, Biesecker LG, Burke GL. Ethical and practical guidelines for reporting genetic research results to study participants. Circulation: Cardiovascular Genetics. 2010;3(6):574–580. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves KD, Sinicrope PS, McCormick JB, Zhou Y, Vadaparampil ST, Lindor NM. Public perceptions of disease severity but not actionability correlate with interest in receiving genomic results: nonalignment with current trends in practice. Public Health Genomics. 2015;18(3):173–183. doi: 10.1159/000375479. [DOI] [PubMed] [Google Scholar]
- Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, Biesecker LG. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in Medicine. 2013;15(7):565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ED, Ziniel SI, Amatruda JG, Clinton CM, Savage SK, Taylor PL, Holm IA. The beliefs, motivations, and expectations of parents who have enrolled their children in a genetic biorepository. Genetics in Medicine. 2012;14(3):330–337. doi: 10.1038/gim.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens K, Cassiman J-J, Nys H, Dierickx K. Children, biobanks and the scope of parental consent. European Journal of Human Genetics. 2011;19(7):735–739. doi: 10.1038/ejhg.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens K, Van El CE, Borry P, Cambon-Thomsen A, Cornel MC, Forzano F, Dierickx K. Developing a policy for paediatric biobanks: principles for good practice. European Journal of Human Genetics. 2013;21(1):2–7. doi: 10.1038/ejhg.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm IA, Iles BR, Ziniel SI, Bacon PL, Savage SK, Christensen KD, Huntington NL. Participant satisfaction with a preference-setting tool for the return of individual research results in pediatric genomic research. Journal of Empirical Research on Human Research Ethics. 2015;10(4):414–426. doi: 10.1177/1556264615599620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm IA, Savage SK, Green RC, Juengst E, McGuire A, Kornetsky S, Taylor P. Guidelines for return of research results from pediatric genomic studies: deliberations of the Boston Children's Hospital Gene Partnership Informed Cohort Oversight Board. Genetics in Medicine. 2014;16(7):547–552. doi: 10.1038/gim.2013.190. [DOI] [PubMed] [Google Scholar]
- Holm IA, Taylor PL. The Informed Cohort Oversight Board: from values to architecture. Minnesota Journal of Law, Science & Technology. 2012;13(2):669–690. [PMC free article] [PubMed] [Google Scholar]
- Kaye J, Whitley EA, Lund D, Morrison M, Teare H, Melham K. Dynamic consent: a patient interface for twenty-first century research networks. Eur J Hum Genet. 2015;23(2):141–146. doi: 10.1038/ejhg.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane IS, Mandl KD, Taylor PL, Holm IA, Nigrin DJ, Kunkel LM. Reestablishing the researcher-patient compact. Science. 2007;316(5826):836–837. doi: 10.1126/science.1135489. [DOI] [PubMed] [Google Scholar]
- Kollek R, Petersen I. Disclosure of individual research results in clinico-genomic trials: challenges, classification and criteria for decision-making. Journal of Medical Ethics. 2011;37(5):271–275. doi: 10.1136/jme.2009.034041. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Haddad Ra, Prows CA, Holm I, Sanderson SC, Garrison NA, Jarvik GP. Return of genomic results in the genomic medicine projects of the eMERGE Network. Frontiers in Genetics. 2014;5 doi: 10.3389/fgene.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. New England Journal of Medicine. 2003;348(10):919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- Mand C, Gillam L, Delatycki MB, Duncan RE. Predictive genetic testing in minors for late-onset conditions: a chronological and analytical review of the ethical arguments. Journal of Medical Ethics. 2012;38(9):519–524. doi: 10.1136/medethics-2011-100055. [DOI] [PubMed] [Google Scholar]
- Miller VA, Drotar D, Kodish E. Children's competence for assent and consent: a review of empirical findings. Ethics & Behavior. 2004;14(3):255–295. doi: 10.1207/s15327019eb1403_3. [DOI] [PubMed] [Google Scholar]
- Murphy Bollinger J, Bridges JFP, Mohamed A, Kaufman D. Public preferences for the return of research results in genetic research: a conjoint analysis. Genetics in Medicine. 2014;16(12):932–939. doi: 10.1038/gim.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Secretary, Department of Health, Education and Welfare. The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. Washington, DC: U.S. Government Printing Office; 1979. [PubMed] [Google Scholar]
- Parker LS. Returning individual research results: what role should people’s preferences play? Minnesota Journal of Law, Science & Technology. 2012;13(2):449–484. [Google Scholar]
- Ravitsky V, Wilfond BS. Disclosing individual genetic results to research participants. American Journal of Bioethics. 2006;6(6):8–17. doi: 10.1080/15265160600934772. [DOI] [PubMed] [Google Scholar]
- Robinson JO, Carroll TM, Feuerman LZ, Perry DL, Hoffman-Andrews L, Walsh RC, McGuire AL. Participants and study decliners' perspectives about the risks of participating in a clinical trial of whole genome sequencing. Journal of Empirical Research on Human Research Ethics. 2016 doi: 10.1177/1556264615624078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson SC, Linderman MD, Suckiel SA, Diaz GA, Zinberg RE, Ferryman K, Schadt EE. Motivations, concerns and preferences of personal genome sequencing research participants: baseline findings from the HealthSeq project. European Journal of Human Genetics. 2016;24(1):14–20. doi: 10.1038/ejhg.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Shinkunas LA, Brandt D, Williams JK. Individual genetic and genomic research results and the tradition of informed consent: exploring US review board guidance. J Med Ethics. 2012;38(7):417–422. doi: 10.1136/medethics-2011-100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller CA. Epidemiology and genetics of childhood cancer. Oncogene. 2004;23(38):6429–6444. doi: 10.1038/sj.onc.1207717. [DOI] [PubMed] [Google Scholar]
- Sturgis P, Roberts C, Smith P. Middle alternatives revisited: how the neither/nor response acts as a way of saying "I don’t know". Sociological Methods & Research. 2014;43(1):15–38. [Google Scholar]
- Thiel DB, Platt J, Platt T, King SB, Fisher N, Shelton R, Kardia SLR. Testing an online, dynamic consent portal for large population biobank research. Public Health Genomics. 2015;18(1):26–39. doi: 10.1159/000366128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorogood A, Joly Y, Knoppers B, Nilsson T, Metrakos P, Lazaris A, Salman A. An implementation framework for the feedback of individual research results and incidental findings in research. BMC Medical Ethics. 2014;15(1):88. doi: 10.1186/1472-6939-15-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CH, Shiloh S, Woolford SW, Roberts JS, Alford SH, Marteau TM, Biesecker BB. Modelling decisions to undergo genetic testing for susceptibility to common health conditions: an ancillary study of the Multiplex Initiative. Psychology & Health. 2012;27(4):430–444. doi: 10.1080/08870446.2011.586699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM. Return of individual research results and incidental findings: facing the challenges of translational science. Annual Review of Genomics and Human Genetics. 2013;14(1):557–577. doi: 10.1146/annurev-genom-091212-153506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Branum R, Koenig BA, Petersen GM, Berry SA, Beskow LM, Wilfond BS. Returning a research participant's genomic results to relatives: analysis and recommendations. The Journal of Law, Medicine & Ethics. 2015;43(3):440–463. doi: 10.1111/jlme.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, Wolf WA. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genetics in Medicine. 2012;14(4):361–384. doi: 10.1038/gim.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J-H, Jamal SM, Tabor HK, Bamshad MJ. Self-guided management of exome and whole-genome sequencing results: changing the results return model. Genet Med. 2013;15(9):684–690. doi: 10.1038/gim.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziniel SI, Savage SK, Huntington N, Amatruda J, Green RC, Weitzman ER, Holm IA. Parents' preferences for return of results in pediatric genomic research. Public Health Genomics. 2014;17(2):105–114. doi: 10.1159/000358539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.