Abstract
Background
Brain estrogen receptor-α (ERα) is essential for estrogenic regulation of energy homeostasis and reproduction. We previously showed that ERα expressed by pro-opiomelanocortin (POMC) neurons mediates estrogen’s effects on food intake, body weight, negative regulation of hypothalamic–pituitary–gonadal axis (HPG axis) and fertility.
Results and conclusions
We report here that global deletion of a key downstream receptor for POMC peptide, the melanocortin 4 receptor (MC4R), did not affect normal negative feedback regulation of estrogen on the HPG axis, estrous cyclicity and female fertility. Furthermore, loss of the MC4R did not influence estrogenic regulation on food intake and body weight. These results indicate that the MC4R is not required for estrogen’s effects on metabolic and reproductive functions.
Key terms: Estrogen, MC4R, Energy homeostasis, Reproduction
Introduction
The ovarian hormone, estrogen, produces important anti-obesity benefits through regulating food intake, physical activity, energy expenditure and fat distribution [1–4]. Estrogenic regulation on energy homeostasis is primarily mediated by estrogen receptor-α (ERα) in both sexes [4], while other ERs including ERβ and GPR30 have been shown to regulate female body weight when fed on high fat diet (HFD) [5–7]. Accumulating evidence indicates that multiple ERα neural populations in the brain are required to maintain normal body weight [8–11]. We previously demonstrated that two populations of ERα-expressing neurons in the hypothalamus are essential for different aspects of estrogenic regulation of energy homeostasis in females. ERα expressed by pro-opiomelanocortin (POMC) neurons in the arcuate nucleus (ARC) regulates food intake and glucose homeostasis, while ERα in steroidogenic factor 1 (SF1) neurons in the ventral medial hypothalamus (VMH) is responsible for energy expenditure [8]. Later Correa et al. demonstrated that a subset of ventral lateral VMH (VMHvl) neurons marked by ERα, NK2 homeobox transcription factor 1 (NKX2-1), and neuropeptide-encoding gene tachykinin 1 (Tac1), regulate estrogen-dependent fluctuations in physical activity in females [10]. Recently, we identified the first ERα site in male brain that mediates the anti-obesity effects of this “female sex” hormone. We found that ERα in an extra-hypothalamic brain region called medial amygdala (MeA) mediates estrogenic regulation of physical activity in both female and male mice [9]. These findings significantly enhanced our understanding on the mechanism by which estrogen/ERα regulate body weight.
While the metabolic effects of different ERα neural populations have been investigated, the downstream neural signaling underlying the regulatory effects of ERα neurons on energy balance are unknown. Previously we demonstrated that loss of ERα specifically in POMC neurons (ERα-POMC-KO) leads to chronic hyperphagia and body weight gain [8] and that the anorexigenic effects of estrogen is attenuated in ERα-POMC-KO females [12]. These results indicate that estrogenic regulation on food intake is at least partially mediated by ERα in POMC neurons. It has been shown that melanocortin 4 receptor (MC4R)-expressing neurons in the paraventricular hypothalamus (PVN) are downstream of hypothalamic POMC neurons and mediate food intake inhibition by binding with α-melanocyte stimulating hormone (α-MSH), a peptide processed from the POMC precursor [13]. These observations led to the prediction that the MC4R is required to mediate estrogen-induced anorexia. Additionally, we also observed ERα-POMC-KO females showed inhibited negative feedback regulation of estrogens and impaired fertility [8]. Since MC4R is also involved in modulating reproductive function [14–16], here we tested if the MC4R is required for estrogenic regulations on energy homeostasis and reproduction by using MC4R null mice in which MC4R expression is globally disrupted.
Material and methods
Mouse
All animal cares and procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Mice were housed in a temperature controlled (22–24°C) room on a 12-hour light, 12-hour dark cycle (lights on at 06:00am). The mice were maintained ad libitum on standard mouse chow with minimal phytoestrogens (6.5% fat, no. 2920; Harlan-Teklad, Madison, WI) and tap water. MC4R null mice were previously generated by inserting the loxTB in the MC4R gene to disrupt MC4R expression globally [17], and have been backcrossed onto C57BL/6 background for more than 12 generations. Heterozygous MC4R null mice were bred to each other in order to generate study mice: female homozygous MC4R null mice and their wild type (WT) female littermates.
Vaginal opening and estrus cycle assessment
Female mice were weaned at 3–4 weeks of age. Body weight was monitored twice a week after weaning. Vaginal opening was checked every day after weaning according to published protocol [18] and the first day of vaginal opening was recorded. After vaginal opening, vaginal smears were gently performed at 1 pm every day to determine the estrous state based on microscopic cytology. At least 3 complete estrous cycles were observed from each mouse.
Fertility assessment
Sexually naïve female WT and MC4R null mice (9 week of age, chow-fed) were bred with proven male breeders until obvious pregnancy was observed or for at least 2 months. Breeding success rate was determined as the percentage of female mice producing one or more pups. The latency of birth was determined as the gap between the mating date and the date of birth of first litter. Litter sizes was determined as the number of pups in each litter.
Effects of subcutaneous 17β-estradiol in ovariectomized mice
The effects of subcutaneous 17β-estradiol treatment was evaluated in both WT and MC4R null mice. Female mice (at the age of 12 weeks) were anesthetized with inhaled isoflurane, and received bilateral ovariectomy (OVX). These mice were then randomly divided into two groups per mouse line to receive subcutaneous implantations of pellets containing 17β-estradiol (0.5 µg/d for 90 days, OVX+E; Innovative Research of America, Sarasota, FL) or vehicle pellets (OVX+V), as previously described [8, 19]. Body composition (fat mass and lean mass) was measured by quantitative magnetic resonance (QMR) two day prior to the surgery. On the next day, mice were shortly fasted for 2 hours (from 8am to 10am) and fed glucose levels were measured in tail blood using a One-Touch glucometer. Body length was measured during anesthesia. Body weight and food intake were monitored every day after the surgery. Feed efficiency was calculated as the ratio between the changes in body weight and cumulative food intake.
Four weeks after surgery, body composition and fed glucose were measured again as described above. Then mice were deeply anesthetized and sacrificed. Gonadal WAT (gWAT) and uterus were isolated and weighed. Blood was collected and processed to measure serum insulin using the mouse insulin ELISA kit (#90080, Crystal Chem). Pituitary tissues were isolated and quickly stored at −80°C. Expression of LHβ and FSHβ in pituitary was measured using the quantitative real-time PCR as described before [8]. Briefly, total mRNA from pituitary was extracted using the RNeasy Mini Kit (#74104, Qiagen, Valencia, CA) according to the instructions provided by the manufacturer. The total mRNA was reverse-transcribed to cDNA using the SuperScript III First-Strand Synthesis System (#18080-051, Invitrogen, Carlsbad, CA) according the instructions provided by the manufacturer. SYBR Green Real-Time PCR was performed according to published protocols [20]. Results were normalized by the expression of house-keeping gene Cyclophilin. The primer sequences are as follow. Cyclophilin (Cyclo): F-TGGAGAGCACCAAGACAGACA and R-TGCCGGAGTCGACAATGAT; follicle-stimulating hormone (FSHβ): F-TTCTGGTGCTGGAGAGCA and R-GCCGAGCTGGGTCCTTAT; luteinizing hormone (LHβ): F-CTGAGCCCAAGTGTGGTGTG, R-GACCATGCTAGGACAGTAGCC.
Statistics
Statistical analyses were performed using GraphPad Prism. Data were compared by non-paired student’s t test or one-way ANOVA, followed by post hoc Bonferroni tests. The data were presented as mean±SEM. P≤0.05 was considered to be statistically significant.
Results
MC4R null mice developed early onset obesity and hyperglycemia
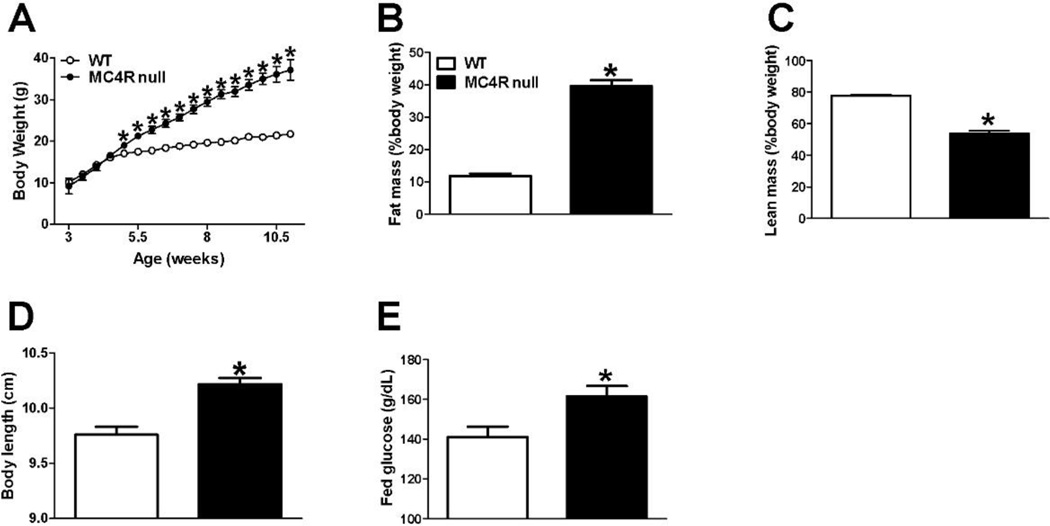
The body weights started to diverge at 5 weeks of age between female WT and MC4R null mice and the difference reached to more than 15g after 11 weeks of normal chow diet feeding (Fig. 1A). Consistently with previous report [17], at 12 weeks of age, female MC4R null showed significantly higher fat mass percentage and lower lean mass percentage (Fig. 1B–1C), indicating an early onset obesity phenotype. Additionally, the body length and fed glucose were significantly higher in MC4R null females than in WT females (Fig. 1D–1E).
Figure 1.
MC4R null mice developed early on-site obesity and impaired fed glucose. (A) Bi-weekly body weight in female mice weaned on regular chow. N=15/genotype. Results are presented as mean ± SEM. *, P<0.05 in two way ANOVA analyses followed by post hoc Bonferroni tests. (B–E) Fat mass percentage (B), lean mass percentage (C), body length (D) and fed glucose (E) in 12-week-old female mice fed with regular chow. N=12/genotype. Results are presented as mean ± SEM. *, P<0.05 in non-paired student’s t test.
MC4R null females have normal estrous cyclicity and fertility
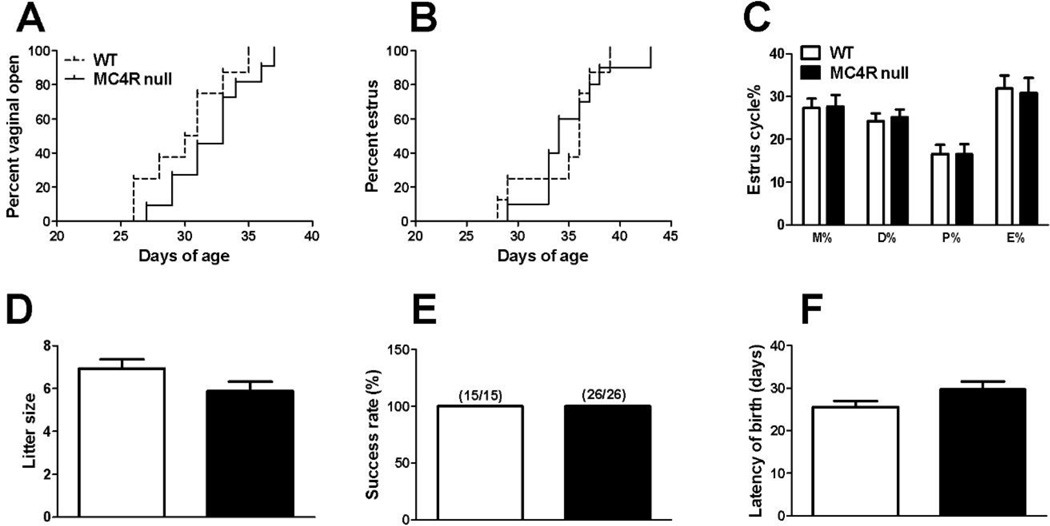
We found that the gonad-intact MC4R null females had normal vaginal opening and first estrous time (Fig. 2A–2B). No differences were observed in the length of the each estrous phase (Fig. 2C). Furthermore, female MC4R null mice (before 12 weeks of age) had normal reproductive capacity as indicated by comparable litter size, success rate to deliver first litter and latency of birth (Fig. 2D–2F). Collectively, these findings indicate that the MC4R is not required to maintain normal estrous cyclicity and female fertility at least during the early adulthood.
Figure 2.
MC4R null females have normal estrous cyclicity and fertility. (A) Vaginal open rate in female mice. N=8 or 11/genotype. Data were compared by Log-rank and Wilcoxon test. (B) Estrus rate in female mice. N=8 or 10/genotype. Data were compared by Log-rank and Wilcoxon test. (C) Length of metoestrus, diestrus, proestrus and estrus relative to the entire etrus cycles. n = 10 or 13/genotype. (D) Averaged litter size. N=15 or 26/genotype. (E) Percentage of mice that successfully delivered pups. N = 15 or 26/genotype. (F) Averaged time period between mating day and birth day of pups. N = 15 or 26/genotype. Results are presented as mean ± SEM. Data were compared by non-paired student’s t test.
Effects of estrogen replacement in MC4R global null female mice
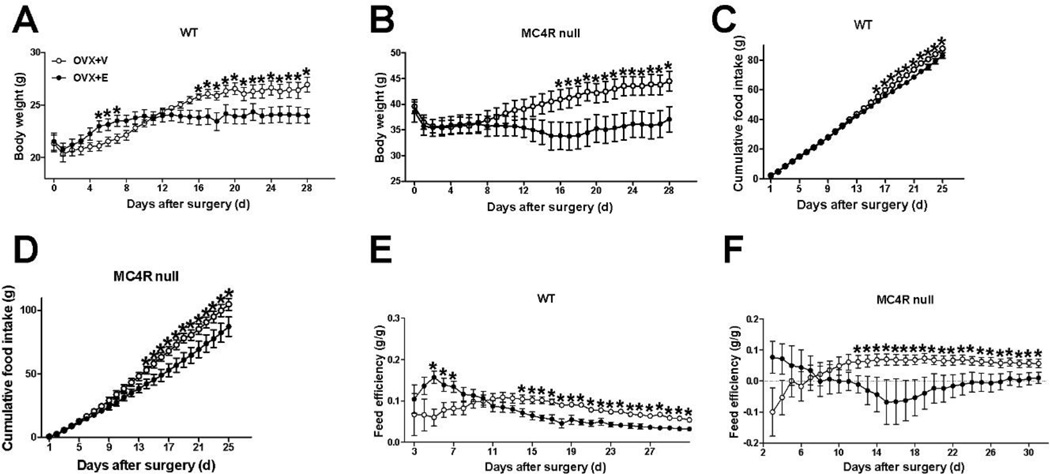
Here we examined the effects of chronic estrogen replacement on food intake and body weight in WT and MC4R null female mice. As expected, OVX+V WT female mice consumed significantly more food and quickly gained body weight after estrogen depletion, and estrogen replacement effectively prevented the increases of both food intake and body weight (Fig. 3A and 3C). To our surprise, food intake and body weight of OVX+E MC4R null female were also significantly reduced compared to OVX+V MC4R null mice (Fig. 3B and 3D), to a similar degree as seen in OVX+E WT female mice (Fig. 3A and 3C). Energy expenditure was also indirectly evaluated as feed efficiency. Consistently with previous reports on stimulatory effects of estrogen on energy expenditure [11, 12], we found that OVX+E treatment significantly increased energy expenditure as indicated by decreased of feed efficiency in WT female mice (Fig. 3E). Similar responses in energy expenditure was also observed in OVX+V and OVX+E MC4R null female mice (Fig. 3F).
Figure 3.
MC4R null female mice have normal estrogenic responses on food intake and body weight. (A–B) Daily body weight of WT (A) and MC4R null (B) female mice after receiving ovariectomy plus estradiol-17β replacement (0.5 µg/day/mouse, OVX+E) or plus vehicle (OVX+V). N = 6/group. (C–D) Cumulative food intake of WT (C) and MC4R null (D) female mice after receiving OVX+E or OVX+V. N = 6/group. (E–F) Feed efficiency of WT (E) and MC4R null (F) female mice after receiving OVX+E or plus OVX+V. N = 6/group. Results are presented as mean ± SEM. *, P<0.05 in two way ANOVA analyses followed by post hoc Bonferroni tests.
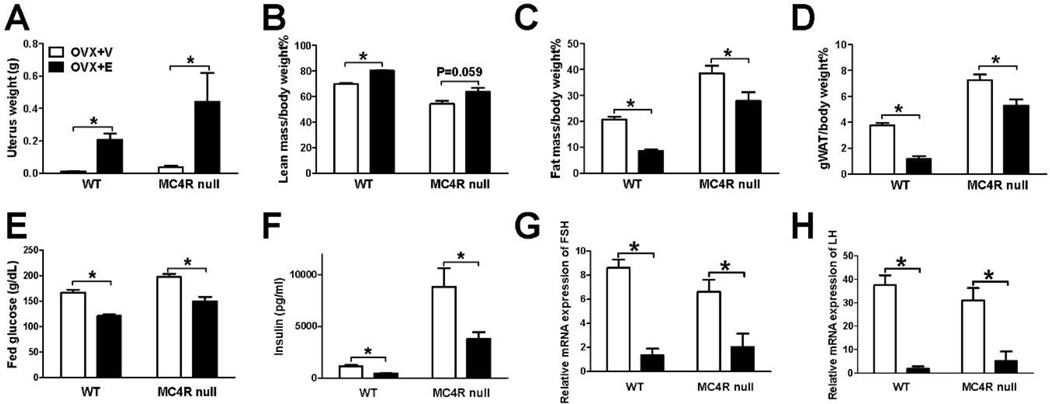
Terminal analyses demonstrated that in both WT and MC4R null mice, OVX+V group showed expected uterine atrophy suggesting successful estrogen depletion (Fig. 4A). On the other hand, OVX+E group had significantly heavier uterus weight compared to OVX+V group, indicating sufficient levels of estrogen replacement (Fig. 4A). Consistent with changes in food intake and body weight, OVX+E treatment significantly increased lean mass and reduced fat mass and gWAT weight in both WT and MC4R mice (Fig. 4B–4D). Glucose homeostasis was further characterized by measuring fed glucose and insulin levels. Consistent with early reports [12, 21], OVX+E significantly decreased fed glucose and insulin levels compared to the levels in OVX+V-treated WT female mice (Fig. 4E–4F). The similar improvement was also observed in OVX+E-treated MC4R null females (Fig. 4E–4F). Together, these results indicate that female mice lacking the MC4R has normal metabolic responses to estrogen replacement treatment.
Figure 4.
MC4R null female mice have normal estrogenic regulation on body fat, blood glucose and HPG axis. (A) Uterus weight measured 30 days after receiving OVX+E or OVX+V. N = 6/group. (B–D) Lean mass percentage (B), fat mass percentage (C) and gWAT weight (D) measured 30 days after receiving OVX+E or OVX+V. N = 6/group. (E–F) Fed glucose (E) and blood insulin (F) measured 30 days after receiving OVX+E or OVX+V. N = 6/group. (G–H) Relative mRNA levels of FSHβ (G) or LHβ (H) in the pituitary measured 30 days after receiving OVX+E or OVX+V. N = 6/group. Results are presented as mean ± SEM. *, P<0.05 in non-paired student’s t test.
Additionally, subcutaneous implantation of 17β-estradiol significantly suppressed the mRNA expression of FSHβ and LHβ subunits in the pituitary of both WT and MC4R null females (Fig. 4G–4H), suggesting that the MC4R is not required for the negative feedback regulation of estrogen on LH and FSH synthesis.
Discussion
Using a MC4R null mouse model that globally disrupts MC4R expression, we demonstrated that loss of the MC4R induced a severe early onset obesity and hyperglycemia in female mice, which is consistent with previous reports [17]. We further provided evidence that MC4R null mice have normal negative feedback regulation of estrogen on LH and FSH synthesis, estrous cyclicity and female fertility. Surprisingly, we also found that loss of the MC4R did not influence estrogenic regulation on food intake, energy expenditure and body weight. These findings implicate that the MC4R is not required to mediate estrogenic regulations on negative feedback, fertility and energy homeostasis.
We previously observed that deletion of ERα from POMC neurons leads to chronic hyperphagia and body weight gain [8], suggesting POMC neurons play an important role in mediating estrogenic inhibition on food intake. Given that anorexigenic effects of POMC neurons are primarily mediated by the MC4R [17], the MC4R is predicted to mediate the estrogenic effects on food intake. However, we showed that the reductions in both body weight and food intake following estradiol supplementation were similar in WT and MC4R null female mice. Thus, our findings support an alternative model in which POMC neurons mediate estrogen-induced anorexia through MC4R-independent mechanisms.
Estrogen has been showed to primarily act through estrogen receptors (ER) in both central and peripheral system to decrease body weight by inhibiting food intake and stimulating energy expenditure [8, 22–24]. The anorexigenic effects of estradiol are primarily mediated by central, but not peripheral estrogen receptor [25]. The ERα expressed by POMC neurons in ARC regions has been shown to mediate estrogenic inhibition on food intake [8]. These results provide a useful context to consider our key observation. Because estrogenic regulation on food intake is primarily through central mechanism, estrogenic inhibition of food intake in MC4R null mice cannot be explained by override of central estrogen signals by peripheral effects of estradiol, but nevertheless involves activation of other essential ER sites in the brain. On the other hand, the stimulatory effects of estrogen on energy expenditure include both central and peripheral mechanism. Central estrogen activates ERα expressed in VMH [8, 11] or MeA [9] to increase BAT thermogenesis or spontaneous physical activity to increase energy expenditure, while peripheral estrogen directly activate ERs in fat to regulate distribution pattern of adipose tissue [26] and increase BAT thermogenesis [27]. Based on these observations, we speculate that both central and peripheral estrogen actions were involved in the regulatory effects of estradiol on feed efficiency and body weight, which are independent from MC4R.
Although the MC4R plays an essential role in mediating anorexigenic effects of POMC neurons, other studies have also suggested a role for MC3R in the regulation of food intake and body weight [28–30]. For example, peripherally administration of a specific MC3R agonist, γ-MSH, stimulates feeding in freely moving animals, presumably through an autoinhibitory effect on POMC neurons [29]. Such observations suggest that not only the MC4R but also the MC3R play a role in food intake regulation. This mechanism of appetite regulation is further supported by observations that anorexigenic responses of a mixed MC3/4R agonist, MTII, were attenuated in both MC3R null and MC4R null mice [31]. Further, it has been reported that leptin-induced anorexia is blocked in MC3R null mice, but remains intact in MC4R null mice [30], which indicates that the anorexigenic effects of leptin require the MC3R, but not the MC4R. Additionally, a recent study demonstrated that the MC3R within the ventral tegmental area regulates the incentive motivation for food, which is an essential component of feeding behavior [32]. Combined the negative observation in MC4R null mice in our study and previous reports on anorexigenic effects of the MC3R, the MC3R is likely to contribute to estrogenic regulation of food intake in female mice.
Besides α-MSH, POMC neurons also give rise to other neural peptides, including β-endorphin, which was previously showed to be orexigenic. For example, intracerebroventricular injection of β-endorphin increases food intake in rats [33] and antagonizes the effects of α-MSH on food intake and body weight [34]. Recent studies also showed that cannabinoids increase food intake by stimulating POMC neurons to release β-endorphin [35]. In addition to neural peptide products, POMC neurons also express and release both glutamate and GABA neural transmitters to acutely stimulate or inhibit downstream neurons [36, 37], which are thought to contribute to the regulation of food intake. These findings raise the possibility that estrogen acts through POMC neurons to regulate release of β-endorphin, glutamate and/or GABA, which in turn regulates food intake and body weight. These possibilities warrant further investigations.
It has been increasingly evident that POMC neurons are a critical neural node where metabolic signals interplay with the reproduction system [38–40]. We previously demonstrated that the estrogen-induced suppressions of FSH and LH synthesis were blunted in ERα-POMC-KO females [8]. This result indicated that besides metabolic functions, ERα in POMC neurons also mediates the regulatory effects of estrogen on the hypothalamic-pituitary-gonadal (HPG) axis. MC4R neurons has been identified as candidate mediators of POMC regulation on reproduction, in part because they lie downstream of hypothalamic POMC neurons and project to the medial preoptic area (MPOA) containing gonadotropin-releasing hormone (GnRH) [41]. Consistent with this view, several studies provided additional evidences for a significant participation of MC4R in HPG axis. For example, in vitro cell culture showed that hypothalamic GT1-1 cells express a functional MC4R receptor that couples to GnRH release [42], while in vivo rodent studies showed that MC4R mediates the stimulatory effects of leptin on LH and PRL secretion [15] and the preovulatory surge in PRL [16]. Genetic mouse models further demonstrated global deletion of MC4R leads to an increase cystic follicles, which results from an inadequate gonadotrophic stimulation [14]. These observations support a model that MC4R increases normal estrogen production by activating HPG axis and is likely to mediate estrogenic regulation on HPG axis. However, our finding that young MC4R null mice have normal estrogenic HPG negative feedback, estrous cyclicity and female fertility forces a reconsideration of this perception.
It is well known that MC4R null mouse line has breeding difficulty [14, 43]. We postulated that the normal fertility is observed in MC4R null mice because we used young female mice below 12 weeks of age. This is further supported by the previous report that reduced female fertility is observed in MC4R null mice older than 3 months and a reduction in ovulation rate is observed in aged females [14]. Another possibility for breeding difficulty is primarily due to male infertility. Consistent with this view, erectile dysfunction is observed in male MC4R null mice [43] and can be rescued by voluntary exercise [44].
Another POMC gene product, β-endorphin may contribute to the negative feedback of estrogen on the HPG axis. For example, in vitro electrophysiological recording has shown that estrogens increase the secretion of β-endorphin [45], which inhibits GnRH neurons [46]. Such observations suggest a model that estrogens act on ERα expressed by POMC neurons to increase secretion of β-endorphin, which in return act through GnRH neurons to regulation HPG axis. This model is further supported by evidence that central infusion of β-endorphin strongly suppress hypothalamic GnRH gene expression [47] and secretion [48]. Of note, electron microscopic evidence also showed that β-endorphin-immunoreactive terminals directly synapse on the GnRH neurons soma in MPOA region [49]. Therefore, it is likely that β-endorphin mediates the regulatory effects of ERα expressed by POMC neurons on HPG negative feedback.
In conclusion, by using loss-of-function model, we provided strong genetic evidence that the MC4R is not required for estrogenic regulations on food intake, body weight and HPG negative feedback. Our findings suggest alternative mechanisms by which estrogen/POMC regulate energy homeostasis and reproduction. These results enhanced our understanding of estrogen/POMC regulatory pathway and warrant further studies on other alternative mechanisms.
Acknowledgments
The authors wish to thank Xiaorui Zhang for invaluable help in mouse colony maintenance.
Funding information: This work was supported by grants from the NIH (K99DK107008 to PX; R01DK093587 and R01DK101379 to YX; R01DK092605 to QT), American Diabetes Association (#1-15-BS-184 to QT), American Heart Association postdoctoral fellowship (PX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No potential conflicts of interest relevant to this article were reported.
Contributions of authors: Pingwen Xu is the main contributor in the conduct of the study, data collection and analysis, data interpretation and manuscript writing; Liangru Zhu, Kenji Saito, Yongjie Yang, Chunmei Wang, Yanlin He, Xiaofeng Yan and Ilirjana Hyseni contributed to the conduct of the study; Qingchun Tong contributed to the manuscript writing and data interpretation; Yong Xu contributed to the study design, data interpretation and manuscript writing.
Reference
- 1.Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2194–R2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 3.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang A, Luo J, Moore W, Alkhalidy H, Wu L, Zhang J, et al. GPR30 regulates diet-induced adiposity in female mice and adipogenesis in vitro. Sci Rep. 2016;6:34302. doi: 10.1038/srep34302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, Veverka KA, et al. Estrogen receptor-{beta}-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem. 2010;285:31292–31303. doi: 10.1074/jbc.M110.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu P, Cao X, He Y, Zhu L, Yang Y, Saito K, et al. Estrogen receptor-alpha in medial amygdala neurons regulates body weight. J Clin Invest. 2015;125:2861–2876. doi: 10.1172/JCI80941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, et al. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L, Xu P, Cao X, Yang Y, Hinton AO, Jr, Xia Y, et al. The ERalpha-PI3K Cascade in Pro-opiomelanocortin Progenitor Neurons Regulates Feeding and Glucose Balance in Female Mice. Endocrinology. 2015 doi: 10.1210/en.2015-1660. en20151660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millington GW. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab (Lond) 2007;4:18. doi: 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandrock M, Schulz A, Merkwitz C, Schoneberg T, Spanel-Borowski K, Ricken A. Reduction in corpora lutea number in obese melanocortin-4-receptor-deficient mice. Reprod Biol Endocrinol. 2009;7:24. doi: 10.1186/1477-7827-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanobe H, Schioth HB, Wikberg JE, Suda T. The melanocortin 4 receptor mediates leptin stimulation of luteinizing hormone and prolactin surges in steroid-primed ovariectomized rats. Biochem Biophys Res Commun. 1999;257:860–864. doi: 10.1006/bbrc.1999.0547. [DOI] [PubMed] [Google Scholar]
- 16.Watanobe H, Yoneda M, Kakizaki Y, Kohsaka A, Suda T, Schioth HB. Further evidence for a significant participation of the melanocortin 4 receptor in the preovulatory prolactin surge in the rat. Brain Res Bull. 2001;54:521–525. doi: 10.1016/s0361-9230(01)00442-7. [DOI] [PubMed] [Google Scholar]
- 17.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L, Zou F, Yang Y, Xu P, Saito K, Othrell Hinton A, Jr, et al. Estrogens prevent metabolic dysfunctions induced by circadian disruptions in female mice. Endocrinology. 2015;156:2114–2123. doi: 10.1210/en.2014-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 22.Saito K, He Y, Yang Y, Zhu L, Wang C, Xu P, et al. PI3K in the ventromedial hypothalamic nucleus mediates estrogenic actions on energy expenditure in female mice. Sci Rep. 2016;6:23459. doi: 10.1038/srep23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 24.Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 25.Rivera HM, Eckel LA. Activation of central, but not peripheral, estrogen receptors is necessary for estradiol's anorexigenic effect in ovariectomized rats. Endocrinology. 2010;151:5680–5688. doi: 10.1210/en.2010-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 27.Monjo M, Rodriguez AM, Palou A, Roca P. Direct effects of testosterone, 17 beta-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology. 2003;144:4923–4930. doi: 10.1210/en.2003-0537. [DOI] [PubMed] [Google Scholar]
- 28.Shaw AM, Irani BG, Moore MC, Haskell-Luevano C, Millard WJ. Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice. Peptides. 2005;26:1720–1727. doi: 10.1016/j.peptides.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–264. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Kilroy GE, Henagan TM, Prpic-Uhing V, Richards WG, Bannon AW, et al. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J. 2005;19:1482–1491. doi: 10.1096/fj.05-3851com. [DOI] [PubMed] [Google Scholar]
- 31.Rowland NE, Schaub JW, Robertson KL, Andreasen A, Haskell-Luevano C. Effect of MTII on food intake and brain c-Fos in melanocortin-3, melanocortin-4, and double MC3 and MC4 receptor knockout mice. Peptides. 2010;31:2314–2317. doi: 10.1016/j.peptides.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandit R, Omrani A, Luijendijk MC, de Vrind VA, Van Rozen AJ, Ophuis RJ, et al. Melanocortin 3 Receptor Signaling in Midbrain Dopamine Neurons Increases the Motivation for Food Reward. Neuropsychopharmacology. 2016;41:2241–2251. doi: 10.1038/npp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKay LD, Kenney NJ, Edens NK, Williams RH, Woods SC. Intracerebroventricular beta-endorphin increases food intake of rats. Life Sci. 1981;29:1429–1434. doi: 10.1016/0024-3205(81)90006-0. [DOI] [PubMed] [Google Scholar]
- 34.Dutia R, Meece K, Dighe S, Kim AJ, Wardlaw SL. beta-Endorphin antagonizes the effects of alpha-MSH on food intake and body weight. Endocrinology. 2012;153:4246–4255. doi: 10.1210/en.2012-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch M, Varela L, Kim JG, Kim JD, Hernandez-Nuno F, Simonds SE, et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci. 2012;32:4042–4048. doi: 10.1523/JNEUROSCI.6032-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wittmann G, Hrabovszky E, Lechan RM. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013;521:3287–3302. doi: 10.1002/cne.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiesner JB, Koenig JI, Krulich L, Moss RL. Possible delta receptor mediation of the effect of beta-endorphin on luteinizing hormone (LH) release, but not on prolactin (PRL) release, in the ovariectomized rat. Endocrinology. 1985;116:475–477. doi: 10.1210/endo-116-1-475. [DOI] [PubMed] [Google Scholar]
- 39.Backholer K, Smith J, Clarke IJ. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology. 2009;150:5488–5497. doi: 10.1210/en.2009-0604. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Faulkner LD, Hill JW. Cross-Talk between Metabolism and Reproduction: The Role of POMC and SF1 Neurons. Front Endocrinol (Lausanne) 2011;2:98. doi: 10.3389/fendo.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward DR, Dear FM, Ward IA, Anderson SI, Spergel DJ, Smith PA, et al. Innervation of gonadotropin-releasing hormone neurons by peptidergic neurons conveying circadian or energy balance information in the mouse. PLoS One. 2009;4:e5322. doi: 10.1371/journal.pone.0005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khong K, Kurtz SE, Sykes RL, Cone RD. Expression of functional melanocortin-4 receptor in the hypothalamic GT1-1 cell line. Neuroendocrinology. 2001;74:193–201. doi: 10.1159/000054686. [DOI] [PubMed] [Google Scholar]
- 43.Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, et al. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci U S A. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irani BG, Xiang Z, Moore MC, Mandel RJ, Haskell-Luevano C. Voluntary exercise delays monogenetic obesity and overcomes reproductive dysfunction of the melanocortin-4 receptor knockout mouse. Biochem Biophys Res Commun. 2005;326:638–644. doi: 10.1016/j.bbrc.2004.11.084. [DOI] [PubMed] [Google Scholar]
- 45.Lagrange AH, Ronnekleiv OK, Kelly MJ. The potency of mu-opioid hyperpolarization of hypothalamic arcuate neurons is rapidly attenuated by 17 beta-estradiol. J Neurosci. 1994;14:6196–6204. doi: 10.1523/JNEUROSCI.14-10-06196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lagrange AH, Ronnekleiv OK, Kelly MJ. Estradiol-17 beta and mu-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- 47.Ciechanowska MO, Lapot M, Malewski T, Mateusiak K, Misztal T, Przekop F. The central effect of beta-endorphin and naloxone on the expression of GnRH Gene and GnRH receptor (GnRH-R) gene in the hypothalamus, and on GnRH-R gene in the anterior pituitary gland in follicular phase ewes. Exp Clin Endocrinol Diabetes. 2008;116:40–46. doi: 10.1055/s-2007-990299. [DOI] [PubMed] [Google Scholar]
- 48.Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology. 1995;136:2412–2420. doi: 10.1210/endo.136.6.7750462. [DOI] [PubMed] [Google Scholar]
- 49.Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Immunohistochemical evidence for synaptic connections between pro-opiomelanocortin-immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Res. 1988;449:167–176. doi: 10.1016/0006-8993(88)91035-9. [DOI] [PubMed] [Google Scholar]