Abstract
Introduction
Use of a ureteral access sheath (UAS) within flexible ureteroscopy (fURS) for the management of kidney and ureteral stones has shown improvements in its effectiveness, but it is also associated with increased risk of ureteral injury. Use of ureteral stent (US) after fURS is recommended by some authors, because of its role in reducing postoperative pain and preventing complications. Our objective is to determine if postoperative stenting is necessary in pre-stented patients that underwent fURS using UAS.
Material and methods
A retrospective history review of patients who underwent fURS using UAS at our hospital between July 1st 2013 and May 31st 2016 was performed. Only pre-stented patients were included. All procedures were performed using the same UAS (Boston Navigator TM., 11–13 Fr.). Patients were separated according to the use or not of postoperative US. The same US (26 cm 6 Fr percuflex, Boston Scienfic) was used for all stented patients. Clinical parameters, stone demographics, operative time and postoperative events were analyzed.
Results
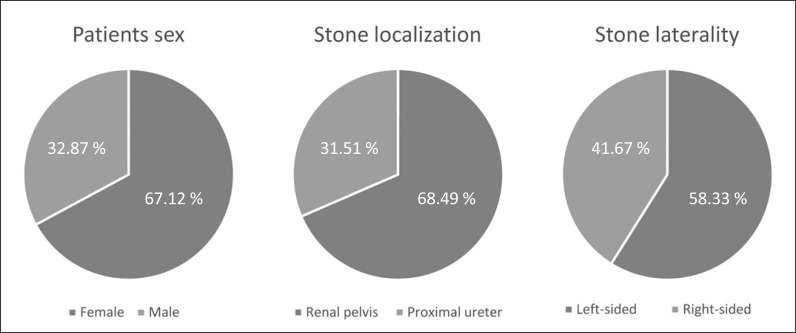
Seventy patients met the inclusion criteria. Mean stone size was 8.5 mm (SD 7.06), 68.49% were located in the renal pelvis and 31.51% were in the proximal ureter. Reasons of preoperative stenting were: 14 (19.18%) ureteral stricture, 17 (23.29%) urosepsis, 29 (39.73%) residual stones after a first intervention (stage procedure) and 13 (17.8%) unsuccessful extracorporeal shockwave lithotripsy. Mean operative time was 88 minutes (SD 37.20); 32 patients (45.71%) were stented and 38 (54.28%) were not. There were no significant differences in operative time (p = 0.85) or postoperative outcomes (p = 1).
Conclusions
A postoperative ureteral stent is not necessary after fURS using UAS in pre-stented patients.
Keywords: ureteroscopy, ureteral stones, ureteral access sheath, ureteral stent
INTRODUCTION
Flexible ureteroscopy (fURS) is nowadays a common intervention for the management of kidney and ureteral stones. It is associated with lower morbidity, shorter hospital stay and higher stone-free rates (SFRs) in comparison to traditional procedures [1, 2]. The use of a ureteral access sheath (UAS) during the procedure is becoming a very popular practice among urologists. Studies have shown improvements in the effectiveness of surgery, mainly due to a reduction in operative time and overall costs, as it enables repeated passage of the ureteroscope, while minimizing damage to inner structures and to the ureteroscope itself [3]. It also decreases intrarenal pressure, leading to an optimization of the flow of irrigation fluid and less damage to the renal pelvis, even using a small diameter sheath [4]. Nevertheless, the use of UAS is not exempt of complications and has been associated with increased risk of ureteral injury, such as abrasion of the urothelium to ischaemia or even avulsion [5].
Use of a ureteral stent [US] after fURS is known to reduce postoperative pain and to prevent complications such as hydronephrosis, ureteral obstructions and strictures [3]. However, the criteria for postoperative stenting are not well defined for all clinical scenarios and most guidelines do not contain recommendations [6]. Although some authors recommend it, in order to prevent potential obstructions associated with postoperative ureteral edema or residual stones [7], the presence of US is not totally free of complications and it is associated with higher costs due to a second necessary procedure for its removal.
The presence of a US prior to the procedure is involved in passive dilation of the ureter and is associated with a decrease in ureteral injuries [5]. Some have suggested that pre-stented patients might have better outcomes, in terms of pain and complication rates [8].
Our objective is to determine if postoperative stenting is necessary in pre-stented patients that underwent fURS using UAS.
MATERIAL AND METHODS
Patient demographics
Once approved by the local ethics committee, we retrospectively reviewed clinical records from all patients who had undergone fURS with use of UAS at our hospital between July 1st 2013 and May 31st 2016. Our inclusion criteria were: presented patients who underwent uncomplicated fURS with use of UAS, thus patients who suffered intraoperative complications were excluded from the study. Ureteral injury and fornix rupture, demonstrated in retrograde ureteropyelography at the end of the procedure, were considered as intraoperative complications. Clinical parameters such as gender, age and comorbidities were retrieved. All the presented patients were selected and separated into two main groups, according to the use or not of postoperative US
Procedure information
All procedures were performed using the same UAS (Boston Navigator TM., 11–13 Fr.). The decision to stent or not to stent the patient was made by discretion of the urologist. Stone characteristics (diameter, localization), preoperative stenting information, operative time, post-operative events (urinary tract infections (UTIs), renal colic), emergency room (ER) visits and need of hospital readmission were recorded. Postoperative outcomes and complications were compared in both groups.
Statistical analysis
Statistical analysis was performed using Stata 12.0v. After descriptive statistics for the variables referred, categorical variables were compared with exact Fisher test. T-test was used to analyze continuous variables. P-value significance was set at <0.05.
RESULTS
From 115 patients who underwent fURS, we selected 73 patients who had a US at the moment of surgery as the main study group. Mean age of the patients was 51 years old (SD 12.8). Mean stone size was 8.5 mm (SD 7.06) and the stones were classified according to their localization and laterality (Figure 1). The reasons of preoperative stenting were: 14 (19.18%) because of ureteral stricture, 17 (23.29%) because of urosepsis, 29 (39.73%) because of residual stones after a first intervention and 13 (17.8%) because of unsuccessful extracorporeal shockwave lithotripsy (SWL).
Figure 1.
Patients and stones demographics.
From the seventy-three patients, only two suffered intraoperative complications and were not included in the data analysis. One of them suffered a ureteral injury during the procedure, classified as grade 3 in the ureteral wall injury scale, and the other patient presented a fornix rupture. A third patient suffered from acute urine retention and was also excluded, because of his need of being catheterized, which would modify further postoperative outcomes. At the end of the surgery, out of 70 patients, 32 (45.71%) were stented and 38 (54.28%) were not. The same US (26 cm 6 Fr percuflex, Boston Scienfic) was used for all stented patients. Mean operative time for the stented group was 88 minutes (SD 6.13) and 87 minutes for the not stented group (SD 6.27), with no significant differences between both groups (p = 0.85).
Regarding postoperative outcomes we compared both groups (stented vs. not stented) based on different parameters: postoperative events (UTIs and renal colic), visits to the ER and need for hospital readmission. There were 4 patients per group with postoperative events (p = 1). Four stented patients visited the ER and 2 were readmitted, while 3 non-stented patients visited the ER and 1 was readmitted (Table 1).
Table 1.
Postoperative outcomes: comparison between two groups of patients, with and without postoperative ureteral stent
| Stented patients (n = 32) | Non-stented patients (n = 38) | P-value | |
|---|---|---|---|
| ER visits | 4 | 3 | 0.695 |
| UTIs | 0 | 3 | 0.245 |
| Renal colic | 4 | 1 | 0.171 |
| Hospital readmission | 2 | 1 | 0.589 |
ER – emergency room, UTI – urinary tract infection
DISCUSSION
Flexible ureteroscopy is widely known as an effective treatment for proximal ureteral or renal stones. Utilization of UAS during the procedure has been associated with some benefits, such as a decrease in intrarenal pressure, allowance of repeated passage of the ureteroscope, reduction of ureteral damage, improvement of visibility and SFR [3, 9, 10, 11]. Miernik et al. have suggested using UAS in all patients undergoing fURS because of their superior clinical results: high stone clearance and low complication rate [12]. The UAS used in our study is the smallest that is capable of allowing the insertion of all flexible ureteroscopes [13].
The use of a US after performing a semi-rigid ureteroscopy to manage medial or distal ureteral stones is not recommended [9, 14]. However, it is still controversial after fURS. Traxer et al. have investigated the incidence of complications and possible ureteral damage after the use of a UAS and postoperative US. They found that US would have a positive role in avoiding initial ureteral edema and directly minimizing pain secondary to residual stone fragments and blood clots [5]. On the same subject, Rapoport et al. have published that patients in whom UAS was used and US was placed were less likely to return to the ER [15]. Kawahara et al. recommend catheterization after fURS with UAS in uncomplicated cases, in which early catheter removal is also suggested and demonstrated to be safe, except in patients with potential risk for the development of ureteral strictures, for instance those with presence of impacted stones, pre-operative ureteral stricture, intra-operative ureteral injuries and longer operation time [9]. Although postoperative stenting is linked to the reduction in the pressure of the collecting system and in minimizing ureteral mucosa damage after instrumentation, it is not free of risks. Potential risks include prolonged hydronephrosis, higher pain scores, stent migration, incrustation and discomfort [16, 17]. Studies have shown that these complications are related to bladder and lower urinary tract symptoms and can cause decreased quality of life [18, 19]. In addition, a second necessary procedure for its removal increases the costs and morbidity for the patient. These are some of the reasons why some authors have argued that postoperative US is not always necessary. However, this is not a completely clear matter, as they have failed to demonstrate exactly in which particular situations it should be avoided [20].
Hollenbeck et al. recommend not using US after fURS, independent of the presence or absence of preoperative stents, which are known to passively dilate the ureter and an important factor to consider, in as much as the absence of postoperative US does not augment complications [21]. These findings are described in fURS without use of UAS. With a preoperative stent, the ureter is wider during the surgery, allowing for a faster and easier procedure. This could potentially decrease the ischaemia associated with UAS, causing fewer complications after its use [5, 22]. In this context, we suggest not using postoperative US, which is supported by our results.
Advantages of stentless URS are decreasing urinary symptoms and no need for additional procedures [16]. The need of a complementary procedure to remove the stent, suggests that proper criteria for postoperative stenting need to be defined. In this context, according to our findings, we compared postoperative outcomes between both groups of patients, with and without postoperative US, after undergoing fURS with UAS. There were no statistical differences between stented and not stented patients in those terms (p = 1). Our findings are similar to the conclusion of the study performed by Torricelli et al., which concluded that it is not necessary to re-stent presented patients after surgery, given that they are a safer group to perform fURS on, independently of the use of UAS [8, 23]. This would lower overall costs and morbidity, as it would not be necessary for the patient to undergo a second procedure for stent removal, which also is not exempt of risks.
The limitations of this article need to be discussed. The number of patients included is not very high, given that the study was developed in one single hospital. As this is a retrospective study, registration bias could be present. Selection bias could also be present as the urologists were the ones to decide whether to stent or not, without guidelines or previous agreement. Only one kind of US was used. Patients who suffered intraoperative complications were excluded because of their need for postoperative US. Costs of a second procedure to remove postoperative stents were not included in the analysis. A clinical randomized study is necessary to confirm our findings.
CONCLUSIONS
Postoperative ureteral stenting is not always necessary after using UAS during flexible ureteroscopy in a pre-stented patient. There were no significant differences in postoperative events, ER visits or need of hospital readmission. However, a prospective randomized clinical trial would be necessary to support our findings with a higher level of evidence. We consider that this is a subject worth further investigation because of its impact on costs and morbidity.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Hyams ES, Monga M, Pearle MS, et al. A prospective, multi-institutional study of flexible ureteroscopy for proximal ureteral stones smaller than 2 cm. J Urol. 2015;193:165–169. doi: 10.1016/j.juro.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geraghty RM, Aboumarzouk OM, Rai B, Somani BK. Evidence for ureterorenoscopy and laser fragmentation (URSL) for large renal stones in the modern era. Curr Urol Rep. 2015;16:54. doi: 10.1007/s11934-015-0529-3. 1-6. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan AG, Lipkin ME, Scales CD, Jr, Preminger GM. Use of ureteral access sheaths in ureteroscopy. Nat Rev Urol. 2016;13:135–140. doi: 10.1038/nrurol.2015.271. [DOI] [PubMed] [Google Scholar]
- 4.Monga M, Bodie J, Ercole B. Is there a role for small-diameter ureteral access sheaths? Impact on irrigant flow and intrapelvic pressures. Urology. 2004;64:439–441. doi: 10.1016/j.urology.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 5.Traxer O, Thomas A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarrenal surgery. J Urol. 2013;189:580–584. doi: 10.1016/j.juro.2012.08.197. [DOI] [PubMed] [Google Scholar]
- 6.Chen YT, Chen J, Wong WY, Yang SS, Hsieh CH, Wang CC. Is ureteral stenting necessary after uncomplicated ureteroscopic lithotripsy? A prospective, randomized controlled trial. J Urol. 2002;167:1977–1980. [PubMed] [Google Scholar]
- 7.Ozyuvali E, Resorlu B, Oguz U, et al. Is routine ureteral stenting really necessary after retrograde intrarenal surgery? Arch Ital Urol Androl. 2015;87:72–75. doi: 10.4081/aiua.2015.1.72. [DOI] [PubMed] [Google Scholar]
- 8.Torricelli F, De S, Hinck S, Noble M, et al. Flexible ureteroscopy with a ureteral access sheath: when to stent. Urology. 2014;83:278–281. doi: 10.1016/j.urology.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Geavlete P, Georgescu D, Nita G. Complications of 2735 retrograde semirrigid ureteroscopy procedures: a single center experience. J Endourol. 2006;20:179–185. doi: 10.1089/end.2006.20.179. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara T, Ito H, Terao H. Ureteral stent encrustation, incrustation and coloring: morbidity related to indwelling times. J Endourol. 2012;26:309–313. doi: 10.1089/end.2011.0385. [DOI] [PubMed] [Google Scholar]
- 11.L’esperance JO, et al. Effect of ureteral access sheath on stone-free rates in patients undergoing ureteroscopic management of renal calculi. Urology. 2005;66:252–255. doi: 10.1016/j.urology.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Miernik A, Wilhelm K, Ardelt PU, et al. Standardized flexible ureteroscopic technique to improve stone free rates. Urology. 2012;80:1198–1202. doi: 10.1016/j.urology.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Al-Qahtani S, Letendre J, Thomas A, et al. Which ureteral access sheath is compatible with your flexible ureteroscope? J Endourol. 2014;28:286–290. doi: 10.1089/end.2013.0375. [DOI] [PubMed] [Google Scholar]
- 14.Geraghty Robert M, Hiro Ishii, Somani Bhaskar K. Outcomes of flexible ureteroscopy and laser fragmentation for treatment of large renal stones with and without the use of ureteral access sheaths: Results from a university hospital with a review of literature. Scand J Urol. 2016;50:216–219. doi: 10.3109/21681805.2015.1121407. [DOI] [PubMed] [Google Scholar]
- 15.Rapoport D, Perks AE, Teichman JM. Ureteral access sheath use and stenting in ureteroscopy: effect on unplanned emergency room visits and cost. J Endourol. 2007;21:993–998. doi: 10.1089/end.2006.0236. [DOI] [PubMed] [Google Scholar]
- 16.Kawahara T, Ito H, Terao H, et al. Early ureteral catheter removal after ureteroscopic lithotripsy using ureteral access sheath. Urolithiasis. 2013;41:31–35. doi: 10.1007/s00240-012-0518-7. [DOI] [PubMed] [Google Scholar]
- 17.Ahallal Y, Khallouk A, El Fassi MJ, Farih MH. Risk Factor Analysis and Management of Ureteral Double-J Stent Complications. Rev Urol. 2010;12:e147–151. [PMC free article] [PubMed] [Google Scholar]
- 18.Haleblian G, Kijvikai K, de la Rosette J, et al. Ureteral stenting and urinary stone management: a systematic review. J Urol. 2008;179:424–430. doi: 10.1016/j.juro.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Leibovici D, Cooper A, Lindner A, et al. Ureteral Stents: Morbidity and Impact on Quality of Life. IMAJ. 2005;7:491–494. [PubMed] [Google Scholar]
- 20.Nabi G, Cook J, N'Dow J, McClinton S. Outcomes of Stenting after Uncomplicated Ureteroscopy: Systematic Review and Meta-Analysis. BMJ. 2007;334:572–575. doi: 10.1136/bmj.39119.595081.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollenbeck BK, Schuster TG, Faerber GJ, et al. Routine placement of ureteral stents is unnecessary after ureteroscopy for urinary calculi. Urology. 2001;57:639–643. doi: 10.1016/s0090-4295(01)00917-7. [DOI] [PubMed] [Google Scholar]
- 22.Assimos D, Crisci A, Culkin D, et al. Preoperative JJ stent placement in ureteric and renal stone treatment: results from the Clinical Research Office of Endourological Society (CROES) ureteroscopy (URS) Global Study. BJU Int. 2016;117:648–654. doi: 10.1111/bju.13250. [DOI] [PubMed] [Google Scholar]
- 23.Lei Chu, Sternberg Kevan M, Averch Timothy D. Preoperative Stenting Decreases Operative Time and Reoperative Rates of Ureteroscopy. J Endourol. 2011;25:751–754. doi: 10.1089/end.2010.0400. [DOI] [PubMed] [Google Scholar]