Abstract
Introduction
Fatigue and depression are commonly attributed to malignant and chronic benign diseases. However, these phenomena have been little investigated to date in prostatic diseases. Our aim was to compare fatigue and depression in prostate cancer patients treated with Androgen Deprivation Therapy (ADT) and in patients with Lower Urinary Tract Symptoms (LUTS) / Benign Prostatic Syndrome.
Material and methods
100 patients each with PCa (prostate cancer) and BPS (Benign Prostatic Syndrome) were surveyed using the Brief Fatigue Inventory (BFI), EORTC-QLQ C30 [1], and Beck Depression Inventory (BDI). EORTC-QLQ-C30 was analyzed by the Mann-Whitney-U-Test. Results were analyzed using the MWUT, CST and ST.
Results
No differences were found between both groups in terms of fatigue (BFI). The prostate cancer group showed a significantly higher impairment in the EORTC-QLQ-C30 role function and fatigue score. We found differences on the BDI in regards to self-criticism with higher mean scores for LUTS patients, whereas loss of energy and loss of sexual interest were more relevant in prostate cancer patients. However, the overall mean score of both groups showed no difference.
Conclusions
This study compared fatigue, depression, and the quality of life in prostate cancer patients treated with ADT and patients with BPS/LUTS. The two groups do not differ in fatigue and depression levels.
Keywords: prostate cancer, BPS, fatigue, depression
INTRODUCTION
Prostate cancer is the most common malignant tumor in men. Nine percent of patients with prostate cancer have metastases at the time of diagnosis, and up to 54% of the curatively treated patients develop a relapse [2, 11]. ADT BPS is the first choice treatment for patients with metastases or relapse after a presumed cure.
Many patients suffering from prostate cancer and treated with ADT report physical and mental exhaustion. These problems can restrict their quality of life. Often it is difficult to distinguish this exhaustion or fatigue from clinical depression.
Surprisingly, no studies have been conducted to scrutinize the concept of fatigue in these types of patients. Fatigue seems to be taken as self-evident; no one questions what it really is or the source of it. This study surveyed the commonly accepted clinical phenomena of fatigue.
The aim of this study was to evaluate fatigue in patients with prostate cancer treated with ADT in comparison to men with benign prostate syndrome and without ADT treatment. It was our hypothesis that there is no difference between these two groups of patients concerning the existence of fatigue.
The basis for this presumption was the daily observation in our office, that patients with BPS had a reduced quality of life and suffered from fatigue as well. Moreover, we hypothesized that the fatigue would be correlated with depression.
MATERIAL AND METHODS
The study was approved by the ethical review committee (Landesärztekammer Hessen FF 104/2012). We included 100 consecutive male patients in each group. These patients suffered from prostate cancer (Group 1) and from BPS, respectively (Group 2). We collected demographic data such as education level completed, age, and the family status of the patients.
We used three validated questionnaires: The Brief Fatigue Inventory (BFI), the Quality of life Questionnaire EORTC QLQ-C30 (Version 3.0), and the Beck Depression Inventory II (BDI-II). The BFI was validated in German in 2003 [3]. It is a unidimensional questionnaire with ten items, which measures the severity of fatigue and impairment. The EORTC QLQ-C30 is a questionnaire with 30 items, which measures the quality of life of patients with a tumor during and after treatment [4, 12]. The 30 items can be related to one of the scales: the global health status/QoL scale, the functional scale, or the symptom scale. The BDI-II measures the severity of depression. The BDI-II is a patient-reported measure [5].
The statistical analysis was performed with SPSS version 20.0 (IBM; Armonk, NY, USA). The data of the EORTC-QLQ-C30 questionnaire was analyzed using the Mann-Whitney-U-Test. Categorical data was evaluated with a chi-square test. Correlation coefficients (r) were calculated with the Spearman-test. Statistical significance was set at p ≤0.05 for all analyses.
RESULTS
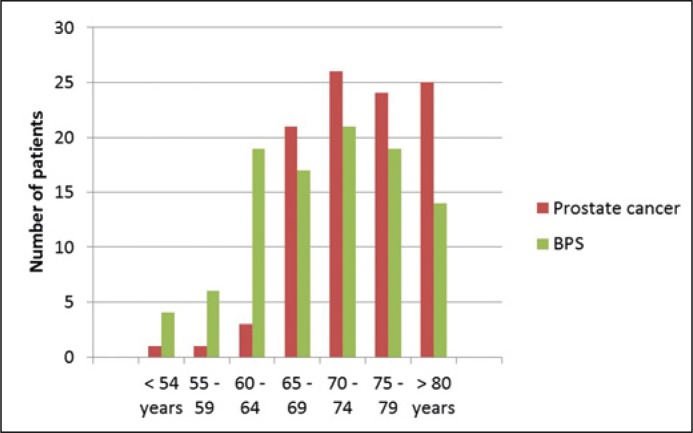
Analyzable data was available for 102 patients with prostate cancer and 100 patients with BPS. The differences in graduation and family status were not significant whereas there was a significant difference in the age of the subjects. The age distribution of all included patients ranged from 48 years to 90 years. The mean age was 74.2 years in the prostate cancer group and 70.1 years in the BPS group (Figure 1).
Figure 1.
Age distribution of all included patients.
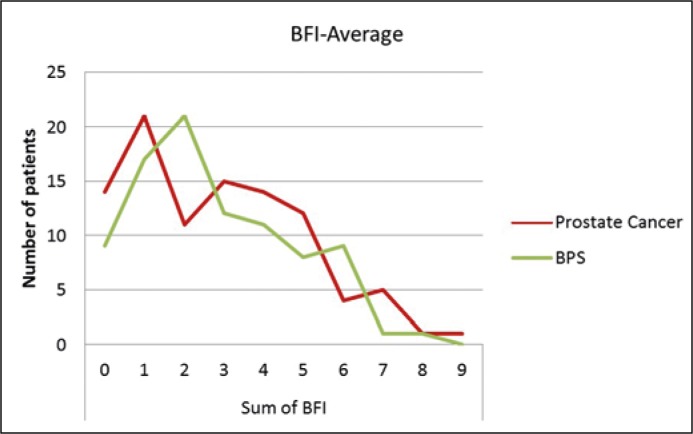
The average of BFI score was 2.86 in the prostate cancer group and 2.79 in the BPS group. There was no significant difference between the groups concerning the BFI-score (p = 0.255) (Figure 2). Clinically relevant fatigue requiring treatment is above 3 points on the BFI [6]. The rate of clinically relevant fatigue in the prostate cancer group (36.7%) and the BPS group (33.7%) was not statistically significant (p = 0.3).
Figure 2.
Distribution of BFI-Average.
The prostate cancer group showed a significantly higher impairment in the EORTC-QLQ-C30 role function (70.1 vs. 77.5 points) (p = 0.038), physical function (71.0 vs. 78.4 points (p = 0.020), and fatigue score (38.3 vs. 31.4 points) (p = 0.047).
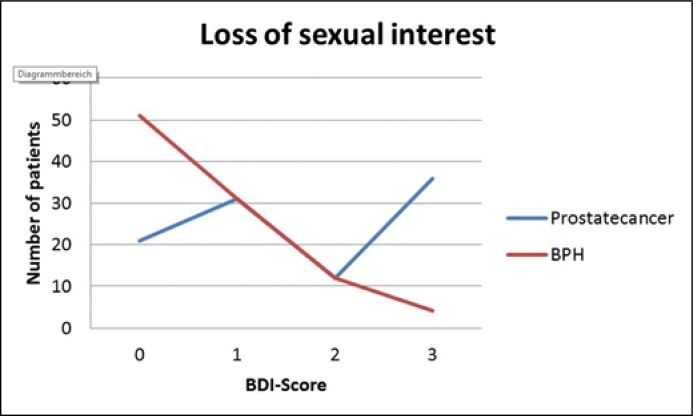
A BDI-II score over 14 is defined as clinically relevant depression. A moderate/severe depression was found in 10.1% of the prostate cancer patients versus 6.3% of the BPS patients. On the BDI there were statistically significant differences for self-criticism with higher mean scores for BPS Patients (0.44 vs. 0.23 points, p = 0.015), loss of energy with higher mean scores for prostate cancer patients (1.01 vs. 0.71 points, p = 0.020), and loss of sexual interest with higher mean scores for prostate cancer patients (1.65 vs. 0.69 points, p <0.001) (Figure 3). However, the mean total BPI-II score of the prostate cancer patients (10.3) and the BPS patients (8.6) was not statistically significantly different (p = 0.6).
Figure 3.
Loss of sexual interest in BDI.
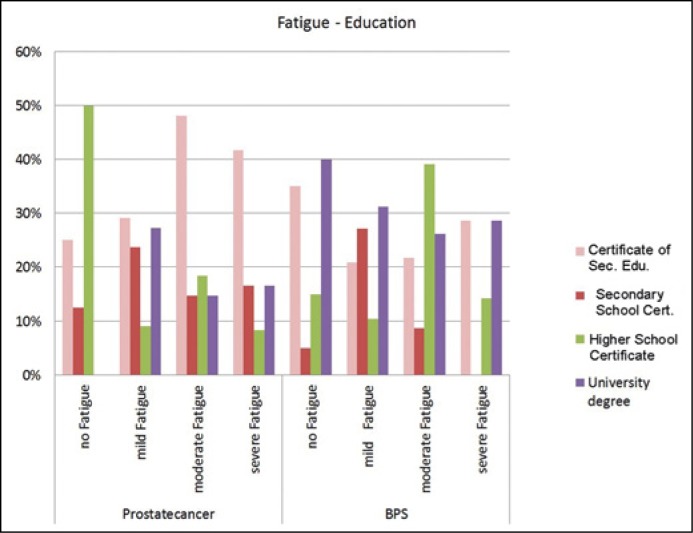
There was no difference between the age or marital status between either of the groups. A significant difference was observed in the education level in prostate cancer patients and fatigue measured with the FA-score whereas lower education was correlated with higher FA-score levels (p = 0.029, Figure 4). A correlation between depression and fatigue could not be calculated due to the insufficient sample size. In summary, there was no statistically significant difference between the two groups regarding fatigue by the BFI or regarding depression as measured by the BDI-II.
Figure 4.
Fatigue FA Score – education level.
DISCUSSION
Fatigue is by far the most common symptom affecting people with cancer. It is a subjective feeling of tiredness that can be alleviated by periods of rest. Fatigue can have physical or mental causes. Physical causes can be serious diseases such as advanced tumor disease. Fatigue at this level impacts the emotional and psychological well-being too.
In summary, in our study there was no statistically significant difference between the two groups regarding fatigue as measured by the BFI or by depression as measured by the BDI-II.
It is arguable, whether a questionnaire with only a few questions measures the degree of fatigue more imprecisely than a more comprehensive questionnaire. Since the BFI-questionnaire includes the most questions, one can suppose that the BFI is the most comprehensive questionnaire The ASCPRO (Assessing Symptoms of Cancer Using Patient-Reported Outcomes study group does not give a recommendation concerning this question [7]. A single-item questionnaire has a better rate of return and is therefore more suitable for longitudinal studies with surveys at multiple time points. There exists a high correlation concerning the degree of fatigue between single-item and multiple-item measures of fatigue. It is recommended to use both single-item and multiple-item questionnaires, especially in longitudinal studies [7].
A problem is still the conceptual inaccuracy of the term fatigue and the consequential difficult classification of the particular questionnaire items and the clinical relevant differentiation of the degree of particular fatigue. The study group of Knobel et al. examined the question of whether the FA-score out of the EORTC-QLQ-C30 questionnaire can measure the multidimensional construct of fatigue at all [8]. Higher fatigue scores in EORTC-QLQ-C30 are associated with higher impairment due to fatigue. It was shown that the FA-score has the limitation of measuring only physical symptoms of fatigue. A floor/ceiling effect resulted, which means that comparatively more patients with a tumor scored higher, whereas a survivor scored a lower value when compared to the fatigue questionnaire. It seems preferable to interpret the FA-score as only representing physical fatigue. This point is correspondent to our observation: it might be the case that the FA-score was significantly different because the prostate cancer patients in the present study had a significantly worse physical function when compared to BPS patients (71.0 vs. 78.4, p = 0.020). This would explain why the BF-score, which measures more dimensions of fatigue besides physical did not show any difference between the two groups.
Clinically relevant fatigue independent of age was reported by the research group of Storey et al. [9]. Their investigation also used the BFI and was consistent with our results. Thus it can be ruled out that age differences between our two study groups were the cause of differences in fatigue scores.
Regarding the floor/ceiling effect of the EORTC-QLQ-C30 FA-Score which was described by Knobel et al., our study shows a comparable distribution. The grouping due to the degree of fatigue of the FA Score in prostate cancer patients compared to the BFI score shows obvious discrepancies. The concordance in lower scores (no fatigue on both measuring methods) was 85.7%; by contrast, the concordance in severe fatigue was only 41.7%. This circumstance can be explained by the ceiling effect, described by Knobel et al. In contrast, healthy BPS-patients show a floor effect comparable to this study: only 47.4% of all BPS patients had a consistent result in the class ‘no fatigue’. However 42.1% of all patients who had ‘no fatigue’ according to the FA-score were classified in the category ‘mild fatigue’ according to the BFI-score. The reason for this is that the FA-score only measures physical symptoms. Physical symptoms are normally more distinct in tumor patients than in healthy persons, as such was confirmed in our study.
Conclusions from our data may be limited, as this was a retrospective study consisting of heterogeneous patient groups. Important clinical data are missing in order to appropriately characterize the patients of both groups. In the prostate cancer group for instance, we have only incomplete data with respect to tumor stage, administration of chemotherapy, use of analgesics such as morphine, and the presence of bone metastases.
Similarly, in the BPS group, clinical data regarding the severity of symptoms, previous prostate surgery and medication such as finasteride are missing. Importantly, further details such as the comorbidities in both patient groups would undoubtedly strengthen the results of the study.
We assume that there are selection and reporting biases in the patients who responded to the questionnaires; this may have skewed the results to depict a population with less severe symptoms of fatigue and depression. Moreover, we have little information on and about the reasons regarding the patients who did not participate. We do not know if patients with more advanced disease did not participate in the study at a higher rate.
CONCLUSIONS
Our study compared fatigue and depression in prostate cancer patients treated with ADT and patients with BPS-associated LUTS. Prostate cancer patients have levels of fatigue and depression comparable to patients with non-malignant conditions. Concerning fatigue and its pathophysiology, there is no agreed upon scientific explanation why fatigue occurs in the surprisingly high levels in LUTS patients as observed in our study. One explanation could be the interruption of sleep at night due to nocturia, which then leads to day-time fatigue.
Patients with fatigue due to tumor disease are remarkably compromised by these symptoms which cause limitations in daily routine and social interaction [10].
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Knobel H, Loge JH, Brenne E, Fayers P, Hjermstad MJ, Kaasa S. The validity of EORTC QLQ-C30 fatigue scale in advanced cancer patients and cancer survivors. Pallia Med. 2003;17:664–672. doi: 10.1191/0269216303pm841oa. [DOI] [PubMed] [Google Scholar]
- 2.Thalgott M, Gschwend JE, Retz M. Hormonablative Therapie des Prostatakarzinoms. UroNews. 2011;3:42–45. [Google Scholar]
- 3.Radbruch L, Sabatowski R, Elsner F, Everts J, Mendoza T, Cleeland C. Validation of the German Version of the Brief Fatigue Inventory. J Pain Symptom Manage. 2003;25:449–458. doi: 10.1016/s0885-3924(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 4.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Nat Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 5.Hautzinger M, Bailer M, Worall H, Keller F. Beck-Depression-Inventar (BDI manual). Bern: Hans Huber; 1996. [Google Scholar]
- 6.Storey DJ, McLaren DB, Atkinson MA, et al. Clinically relevant fatigue in men with hormon-sensitive prostate cancer on long-term androgen deprivation therapy. Ann Oncol. 2012;23:1542–1549. doi: 10.1093/annonc/mdr447. [DOI] [PubMed] [Google Scholar]
- 7.Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO Recommendations for the Assessment of Fatigue as an Outcome in Clinical Trials. J Pain Symptom Manage. 2010;39:1086–1099. doi: 10.1016/j.jpainsymman.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knobel H, Loge JH, Brenne E, Fayers P, Hjermstad MJ, Kaasa S. The validity of EORTC QLQ-C30 fatigue scale in advanced cancer patients and cancer survivors. Palliat Med. 2003;17:664–672. doi: 10.1191/0269216303pm841oa. [DOI] [PubMed] [Google Scholar]
- 9.Storey DJ, McLaren DB, Atkinson MA, et al. Clinically relevant fatigue in men with hormon-sensitive prostate cancer on long-term androgen deprivation therapy. Ann Oncol. 2012;23:1542–1549. doi: 10.1093/annonc/mdr447. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in On-cology. Cancer-Related Fatigue. 2015. s.l.: nccn.org, Version 2.
- 11.Golabek T, Belsey J, Drewa T, et al. Evidence-based recommendations on androgen deprivation therapy for localized and advanced prostate cancer. Cent European J Urol. 2016;69:131–138. doi: 10.5173/ceju.2016.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutwin P, Konecki T, Jabłonowski Z, Wolski Z, Sosnowski M. Quality of life in a population of Polish patients with prostate cancer. Cent European J Urol. 2016;69:53–56. doi: 10.5173/ceju.2016.633. [DOI] [PMC free article] [PubMed] [Google Scholar]