Abstract
Objective
Thiol isomerases facilitate protein folding in the endoplasmic reticulum, and several of these enzymes, including PDI and ERp57, are mobilized to the surface of activated platelets, where they influence platelet aggregation, blood coagulation and thrombus formation.
In this study we examined for the first time the synthesis and trafficking of thiol isomerases in megakaryocytes, determined their subcellular localization in platelets and identified the cellular events responsible for their movement to the platelet surface upon activation.
Approach and Results
Immunofluorescence microscopy (IFM) imaging was used to localize PDI and ERp57 in murine and human megakaryocytes at various developmental stages. IFM and subcellular fractionation analysis were used to localize these proteins in platelets to a compartment distinct from known secretory vesicles that overlaps with an inner cell surface membrane region defined by the endoplasmic/sarcoplasmic reticulum proteins calnexin and SERCA3. IFM and flow cytometry were used to monitor thiol isomerase mobilization in activated platelets in the presence and absence of actin polymerization (inhibited by latrunculin), and in the presence or absence of membrane fusion mediated by Munc 13-4 (absent in platelets from Unc13dJinx mice).
Conclusions
Platelet-borne thiol isomerases are trafficked independently of secretory granule contents in megakaryocytes, and become concentrated in a subcellular compartment near the inner surface of the platelet outer membrane corresponding to the sarco/endoplasmic reticulum of these cells. Thiol isomerases are mobilized to the surface of activated platelets via a process that requires actin polymerization but not SNARE/Munc 13-4-dependent vesicular-plasma membrane fusion.
Keywords: platelets, megakaryocytes, thiol isomerases, trafficking, granules
Introduction
Thiol isomerases facilitate the reduction, oxidation and rearrangement of disulfide bonds essential for correct folding of secreted proteins in the endoplasmic reticulum (ER).1, 2 Some cells also have thiol isomerases in granular structures3 or at the cell surface,4, 5 and in platelets protein disulfide isomerase (PDI), ERp57 and other thiol isomerases are mobilized to the cell surface with procoagulant activation. Inhibition of platelet-borne thiol isomerases reduces aggregation, granule secretion, receptor activation and thrombus formation in vitro and in vivo.3, 4, 6-11 As with platelets, release of thiol isomerases by vascular endothelial cells has been linked to de-encryption of tissue factor, fibrin formation10, 12, 13 and neutrophil recruitment to the vessel wall during inflammation.8, 14
Elucidating the role of thiol isomerases in platelet function, blood coagulation and thrombus formation requires understanding how they are packaged and mobilized.6, 8, 10, 15-19 In this study we tracked PDI and ERp57 through development of precursor megakaryocytes (MK) and platelets, where we observed that these enzymes are concentrated in a subcellular compartment distinct from known secretory vesicles that overlaps with the dense tubular system (DTS) derived from MK smooth ER, and with the inner surface of the platelet outer membrane where the sarco/endoplasmic reticulum calcium ATPase SERCA3 is known to be localized.20 During platelet activation, actin polymerization facilitates the movement of secretory vesicles to the cell plasma membrane surface,21 where the interaction of soluble N-ethylmaleimide-sensitive fusion protein attachment receptors (SNAREs) facilitates membrane fusion mediated by Munc 13-4.22-26 We observed that surface mobilization of thiol isomerases within activated platelets also requires actin polymerization, but proceeds in the absence of Munc13-4-mediated membrane fusion.
Material and Methods
Materials and methods are available in the online-only Data Supplement
Results
Expression and distribution of PDI and ERp57 during MK development
Unlike other mammalian cells, platelets are not derived from individual precursors via cell fission. Their development begins in the bone marrow with MK progenitors that undergo endomitosis to produce large cells (>20 μM diameter) with multi-lobed nuclei. Maturing MKs synthesize large amounts of proteins and develop elaborate intracellular membrane systems. The synthesized secretory cargo (e.g. Von Willebrand Factor, VWF) moves from the Golgi to multivesicular bodies (MVBs), where it is joined by endocytosed proteins (e.g. fibrinogen) prior to packaging in secretory granules. The platelet DTS is derived from MK smooth ER, while cell surface and open canalicular system (OCS) membranes originate in the MK demarcation membrane system (DMS), composed of flattened cisternae and tubules that develop by processes that involve dynamin 2-dependent endocytosis,27 surface membrane invagination and PACSIN2-mediated membrane tubulation.28 MK development culminates in the production of proplatelet extensions that protrude into the vasculature and release hundreds or thousands of platelets from each cell into blood circulation.29
We examined the intracellular distribution of PDI and ERp57 in developing cultured mouse MKs via high resolution immunofluorescence microscopy (IFM; Figure 1). MKs were identified by positive staining for CD41/integrin αIIb30 and staged by size, shape and nuclear morphology. In early (<10 μM diameter) and mitotic cells (Fig. 1A; Video 1) both thiol isomerases were extensively distributed through the cell, a pattern that persisted in later cells (i.e. synthetic cells; Fig.1B; Video 2), where ERp57 was sometimes concentrated in perinuclear ribbon-like structures (Fig.1B inset detail). Late-stage cultured MKs typically flatten out prior to producing proplatelet extensions, and in these extending cells (Fig.1C; Video 3) both PDI and ERp57 were observed throughout the extranuclear region and in proplatelet extensions (Fig.1D; Video 4) including the terminal buds, where PDI appeared to be concentrated near the outer membrane. While throughout MK development colocalization of PDI and ERp57 was observed (white in merged images), substantial proportions of these proteins were not in the same locations. Similar subcellular distributions of these thiol isomerases were observed in cultured human MKs, where proplatelet development is not as readily observed (data not shown).
Figure 1. Intracellular distribution of PDI and ERp57 during MK development.
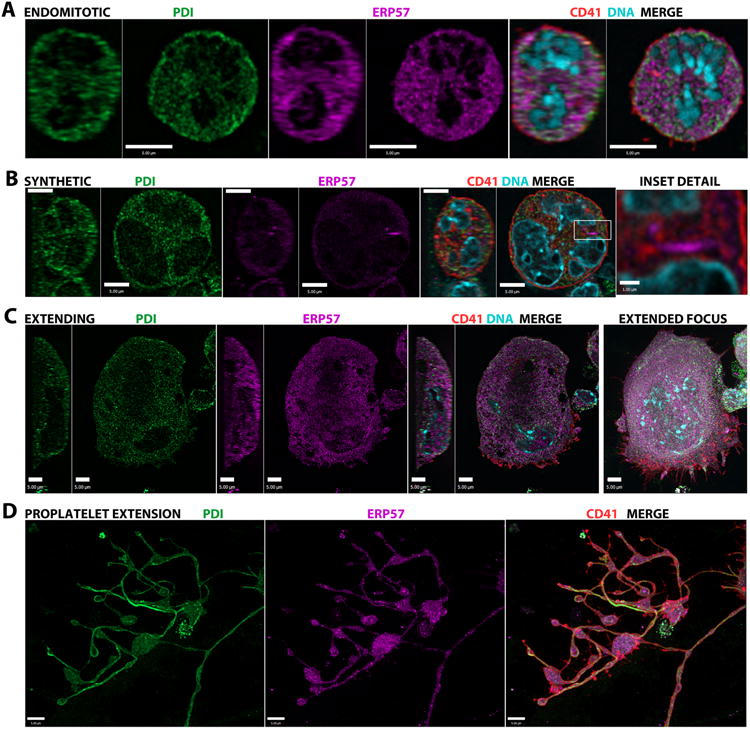
IFM images (mid-cell Z-sections with YZ and XY profiles or extended focus renders) are shown for representative cultured mouse MK stained for ERp57 (magenta), PDI (green), CD41 (red) and DNA (blue; bars = 5μm except where noted). A) In early endomitotic cells ERp57 and PDI show a similar extranuclear distribution (see Video 1). B) In larger cells undergoing maximal protein synthesis PDI remains widely distributed, and in some cells ERp57 is highly concentrated in ribbon-like structures that may represent ER (inset detail bar = 1μm; see Video 2). C) As late-stage MKs flatten out, both thiol isomerases are present throughout the extranuclear region (see Video 3) and D) in proplatelet extensions (main cell body at right out of field), where they are present throughout the shafts and in terminal buds (see Video 4).
MK trafficking of thiol isomerases is distinct from secretory granules and cargo
Platelet secretory granule protein cargo is synthesized in the MK ER and transported via vesicles derived from the trans-Golgi network31 to early MVBs, which according to current models also receive cargo in endocytic vesicles as they mature and give rise to platelet lysosomes and α and dense32 granules.31, 33, 34 Transferrin receptor (TFR; CD71) undergoes clathrin-mediated endocytosis and is frequently used as marker of the MK recycling endocytic compartment,35 while TGN46 is a marker of the trans-Golgi network. IFM examination of thiol isomerase expression in human MKs in serum-free culture revealed little overlap between ERp57, TFR and TGN46 (Figure 2 A; Video 5) or between PDI and the α-granule cargo proteins VWF (Figure S1A; Video 6) and thrombospondin-1 (TSP-1; not shown). At no point did we observe thiol isomerases distributed in granular puncta (e.g. VWF in Figure S1A) or structures resembling MVBs, rather their distribution pattern was consistent with association with intracellular membranes. Thus our observations indicate that throughout MK and proplatelet development thiol isomerases are localized in a cellular compartment that is distinct from secretory granules and their cargo, and that these enzymes are likely associated with future platelet cell surface and/or non-granular membranes.
Figure 2. Thiol isomerases are not associated with MK trans-Golgi network or recycling endosomal compartments, or with platelet α-granules.
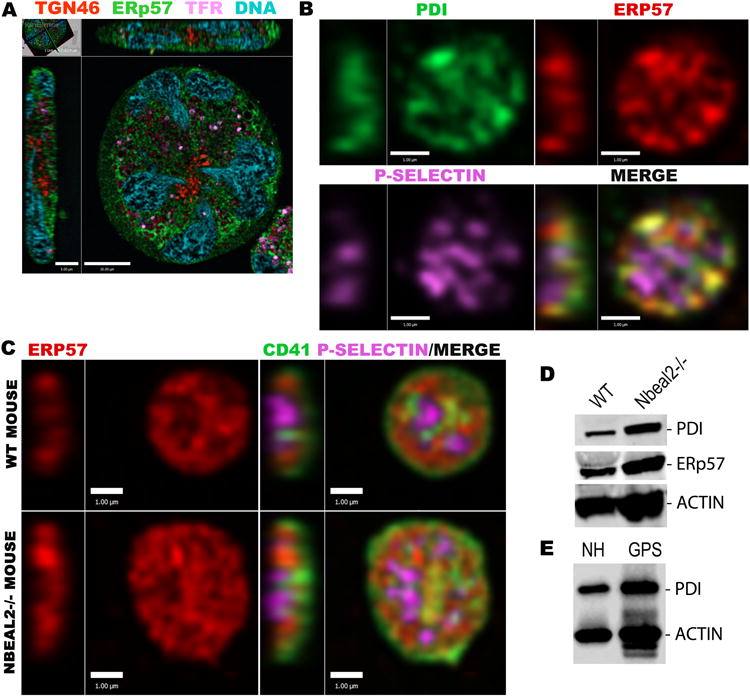
A) IFM imaging of a representative mature human MK shows ERp57 (green) distributed throughout the cell having little overlap with trans-Golgi defined by TGN46 (red) or recycling endosomes containing TFR (magenta; mid-cell Z-section, bar = 10 μm). B) In a human platelet, PDI (green) and ERp57 (red) overlap with each other but not with P-selectin (magenta; mid-cell Z-sections, bars = 1μm). C) Platelets stained for CD41/integrin αIIb (green), ERp57 (red) and P-selectin (magenta) from WT and Nbeal2-/- mice (the latter lacking α-granule cargo) show similar intracellular distributions of thiol isomerase (mid-cell Z-sections, bars = 1 μM). D) Immunoblot analysis shows similar levels of PDI and ERp57 in WT and Nbeal2-/- mouse platelet lysates, and E) similar expression of PDI in lysates from normal human (NH) and Gray platelet syndrome (GPS) patient platelets (actin used as loading control).
The trafficking of thiol isomerases within MKs should determine their distribution within platelets. IFM observations showed consistent association of the α-granule membrane protein P-selectin with cargo proteins (e.g. VWF, Figure S1B) but not with PDI or ERp57 (Figure 2B), which, as observed in MKs (Figure 1), partially overlap with each other (mean Pearson's correlation coefficient = 0.6; sd = 0.08, n=12 cells). Similar relationships for PDI, ERp57 and P-selectin were observed in platelets from Nbeal2-/-mice lacking α-granules (Figure 2C), and thiol isomerase levels were similar in mouse and human platelets in the presence or absence of α-granules30, 36 (Figure 2D,E), further indicating their production and trafficking is independent of granules and their cargo.
Platelet thiol isomerases are localized to a non-granular compartment near the cell surface membrane defined by overlap with SERCA3
The association of thiol isomerases with platelet subcellular structures was investigated by fractionating cell lysates via sucrose gradient ultracentrifugation, and then probing fractions via immunoblotting for PDI, ERp57 and cell surface and interior membranes (β3 integrin), proteins associated with cytosol (Rab GDP Dissociation Inhibitor; RabGDI), α – granule cargo (TSP-1) and the DTS (calreticulin) (Figure 3A). As reported by Jonnalagadda et al37 β3 integrin was present in the lighter fractions (2-3), but also abundant in the more dense fractions (8-10); corresponding to the plasma membrane and to the membrane of granules and endosomal compartments, respectively. The soluble cytosolic marker RabGDI was mainly concentrated at the top of the gradient as were the fractions containing calreticulin, a marker for the DTS previously used to detect PDI in this compartment in platelets.19 TSP-1 was abundantly expressed in the heaviest fractions where α –granules are fractionated. Consistent with our imaging and previous results,38 PDI and ERp57 were concentrated in the lightest fractions, indicating association with cell-surface and/or near-surface membrane structures such as the DTS. While fractionation might suggest the presence of thiol isomerases in the platelet cytosol, this is less likely because these proteins are expected to be targeted to the secretory system of cells by virtue of their ER retention motifs (PDI: KDEL, ERp57: QEDL).
Figure 3. Platelet thiol isomerases associate with cell-surface membrane structures and overlap with SERCA3 on the inner surface of the cell outer membrane.
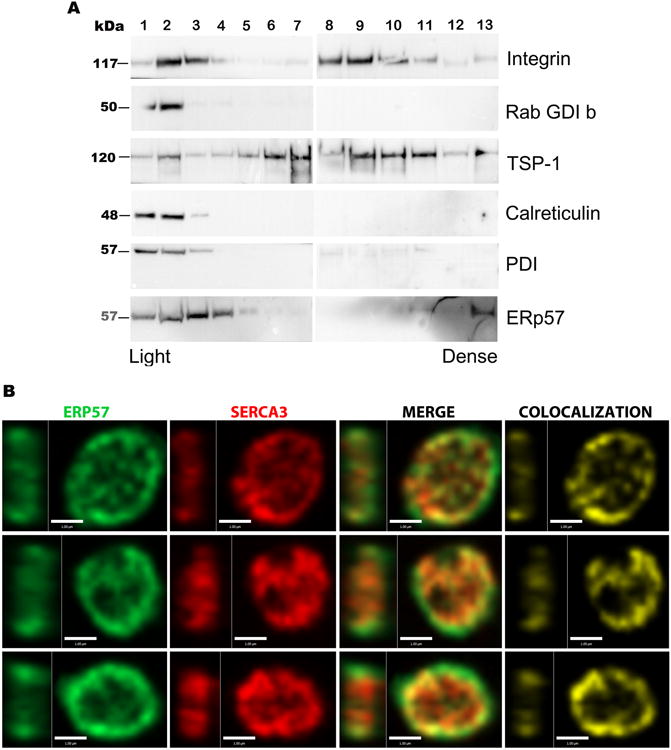
A) Human platelet lysates were fractionated by sucrose gradient and fractions (1-13) analyzed by immunoblotting for PDI, ERp57, β3 integrin, RabGDI b, TSP-1 and calreticulin (marker sizes in kilodaltons at left). Peak concentrations of PDI and ERp57 overlapped with β3 integrin corresponding to the cell surface membrane fraction, with cytosolic RabGDI b, and the DTS marker calreticulin and showed little overlap with α-granule borne TSP-1 (Figure representative of 3 experiments). B) IFM imaging of fixed resting human platelets stained for ERp57 (green) and SERCA3 (red) shows overlap in a compartment defined by the latter on the inner surface of the cell outer membrane, delineated by the colocalization channel (yellow; mid-cell Z-sections, bars = 1 μM).
We examined the potential interaction of ERp57 with the DTS in human MK co-stained for calnexin, which like thiol isomerases promotes protein folding,39, 40 but observed little apparent association (Figure S2A; Video 7). In platelets we observed that calnexin is associated with well-defined regions distinct from α-granules (Figure S2B) that likely represent the DTS, which showed partial overlap with ERp57 (Figure S2C), with most of the thiol isomerase detected outside the overlapping regions. Similar results were obtained (not shown) in colocalization analyses with other markers of the DTS (e.g. calreticulin), Golgi (e.g. TGN46) and endosomal compartments (e.g. EEA1), all of which were confined to intracellular locations distinct from those of thiol isomerases.
The sarcoplasmic/endoplasmic reticulum calcium ATPase SERCA3 is a well-studied regulator of platelet calcium loading that has been localized to the inner surface of the platelet outer membrane by cell fractionation, immunoprecipitation and IEM.20, 41 The main antibody employed in these studies was a mouse monoclonal (PL/IM430) raised against human platelet membranes that detects all 3 SERCA3 isoforms present in human platelets.41 Using the same antibody we examined the relationship of ERp57 with SERCA3 via IFM in human platelets, and the results (Figure 3B) show extensive overlap (mean Pearson's correlation coefficient = 0.794; sd = 0.067, n=11 cells) in a compartment near the inner surface of the cell outer membrane delineated by the colocalization of these proteins (Figure 3, colocalization channel). Thus platelet thiol isomerase appears to be concentrated in a compartment that may overlap with the DTS and OCS, but is largely located near the inner cell surface.
Platelet-borne PDI has been reported to associate with a T-granule system defined by toll-like receptor 9 (TLR9).18 We were unable to replicate this observation by IFM with the same anti-TLR9 antibody used in the original description of the T-granules (data not shown). Consistent with proteomics studies reporting low levels of TLR9 in platelets42, 43, we could not detect TLR9 in platelets by immunoblotting, and consequently, we did not further investigate the presence of thiol isomerases in putative T-granules.
Mobilization of thiol isomerases to the platelet surface requires actin polymerization but not Munc 13-4-mediated membrane fusion
The SERCA3 interaction partner STIM1 mobilizes to the surface of activated platelets via a process that is actin-dependent and associated with cytoskeletal and microdomain rearrangements, but is not thought to involve the fusion of vesicular and cell membranes.44 Like other platelet-borne thiol isomerases, PDI and ERp57 move to the surface upon activation,6, 17 as can be observed by IFM comparing their intracellular distribution with that of P-selectin (Figure S3A). We examined the role of actin polymerization in this process using latrunculin A, which prevents actin polymerization.45 We determined that 200 μM latrunculin A inhibits P-selectin mobilization in activated platelets by >50% (Figure S3B), and compared by IFM the intracellular/extracellular distributions of P-selectin and the α-granule borne protein VWF (Figure 4A) or of P-selectin and thiol isomerases (Figure 4B), in platelets activated in the presence or absence of this concentration of latrunculin A. The results show that, in the absence of actin polymerization, platelets underwent little or no mobilization of PDI and ERp57 or of P-selectin and VWF, and retained a resting morphology indicated by flat shape and intact tubulin cytoskeletal ring. Using flow cytometry, we observed an increased surface expression of thiol isomerases, as reported previously 10, 17. Blocking actin polymerization with latrunculin A significantly inhibited the movement to the surface of both PDI and ERp57 (Figure 4C).
Figure 4. Mobilization of thiol isomerases in activated platelets requires actin polymerization.
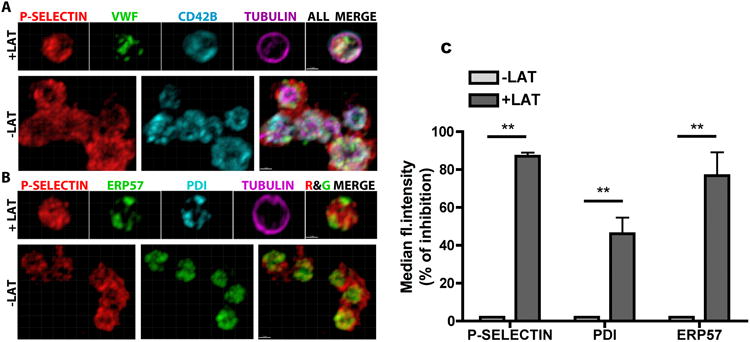
IFM imaging of human platelets activated with 4 μM U46619 in the presence (+LAT) or absence (-LAT) of latrunculin A, fixed and stained for tubulin (magenta), P-selectin (red) and either: A) CD42B/GP1b (light blue) and VWF (green), or B) PDI (light blue) and ERp57 (green). Reorganization of cytoskeletal tubulin and surface mobilization of P-selectin, VWF and ERp57 is evident in platelets not treated with latrunculin, while treated cells show intracellular protein distribution patterns similar to unactivated platelets (Fig.2B). Maximum intensity renders, bars = 1 μM. C) The effect of latrunculin treatment on the surface mobilization of thiol isomerases, as compared to that of P-selectin, was quantified by flow cytometry. Platelets were activated with 1U/ml of thrombin, the binding of the primary antibody for thiol isomerases was detected using a FITC-conjugated species-specific secondary antibody, the PE/Cy5 anti-human CD62P was used to detect P-selectin. The percentage of inhibition of the Median Fluorescence Intensity was calculated (**P<0.01, one-way ANOVA, followed by Dunnett's test, n=3).
When platelets are stimulated by agonists such as PAR4-activating peptide (PAR4-AP), Munc13-4 facilitates the pairing of SNAREs25, 26, 46 involved with the fusion of secretory vesicles with the cell surface membrane. ADP has also been observed to trigger Munc13-4-independent granule secretion.46 We investigated the role of Munc13-4-dependent membrane fusion in platelet thiol isomerase mobilization using Unc13dJinx mice, which lack Munc13-4 activity but show equivalent expression to wild type (WT) of platelet thiol isomerases (Figure 5A). Flow cytometry assessment of surface expression of P-selectin and thiol isomerases in resting and agonist-stimulated platelets showed that cells from Unc13dJinx mice had significantly less P-selectin surface expression than WT (Figure 5B) when stimulated with PAR4-AP, and there was partial rescue of response with ADP. When stimulated with PAR4-AP, Unc13dJinx platelets showed greater mobilization of PDI relative to WT (Figure 5B), and along with WT cells showed increased PDI mobilization in the presence of ADP. These results indicate that Munc13-4-mediated membrane fusion events are not required for the mobilization/release of platelet thiol isomerases during activation, which may involve a mechanism similar to that proposed for STIM1.44
Figure 5. Surface mobilization of platelet thiol isomerase is independent of Munc13-4.
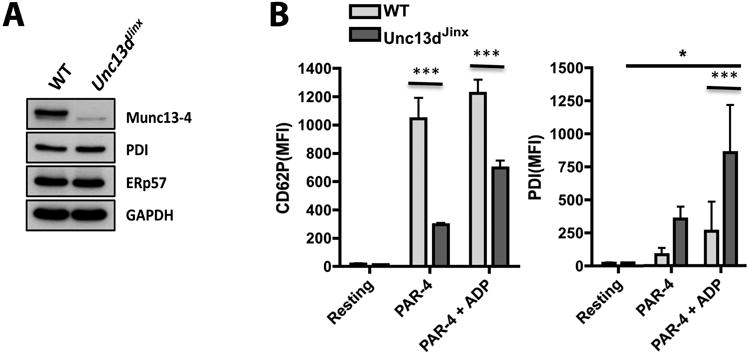
A) Immunoblot analysis of platelet lysates from wild type (WT) and Unc13dJinx mice shows lack of Munc13-4 in the latter and similar levels of PDI and ERp57 (GAPDH loading control). B) Flow cytometry measurement of surface P-selectin (CD62P) and PDI in platelets under resting conditions or after stimulation with PAR4-AP in the presence/absence of ADP shows that P-selectin mobilization is significantly impaired in Unc13dJinx platelets but PDI mobilization is greater than WT. ADP significantly increased PDI mobilization as compared to WT or resting Unc13dJinx platelets (*P<0.05, *** P<0.001, one-way ANOVA, followed by Dunnett's test or two-way ANOVA, followed by Bonferroni's test, n=4).
Discussion
In this study we began by tracing the synthesis, trafficking and packaging of platelet-borne thiol isomerases in cultured primary human and murine MKs. The use of murine MKs allowed us to follow platelet development to the proplatelet extension stage, where nascent platelets are loaded with secretory granules and other organelles.47 This approach has proven effective in elucidating the origin of other platelet proteins such as glycosyltransferases.48 While human MKs derived from peripherally-mobilized CD34+ cells cultured under serum-free conditions do not develop proplatelets as readily as mouse cells, they have also proven useful in studies of the biogenesis of platelet granules.49 Their use was important to confirm that, as was expected, thiol isomerases destined for platelets are synthesized by MKs, since they are present in cells grown under serum-free conditions and show little evident association with endocytic pathways (Figure 2A).
Our observations indicate that the bulk of MK ERp57 (and most likely PDI) synthesized by MKs is destined to become platelet cargo (Figure S2A), although we did observe an apparent transient strong association of ERp57 with the ER in mouse MKs undergoing peak protein synthesis (Figure 1B). Our observations also consistently indicate that the trafficking of thiol isomerases is distinct from α-granule cargo proteins at all stages of MK development, in terms of cellular localization (Figure 1 A-D, Videos 1-4), distribution pattern (Figure S1A, Video 5) and interaction with the endocytic and trans-Golgi compartments (Figure 2A). This indicates that thiol isomerase destined for platelets is not trafficked through the MVBs that process granule-bound cargo. Rather their intracellular distribution indicates association with another of the many MK membrane systems, as has been proposed for platelet Golgi-derived glycosyltransferases.48 The membrane system associated with thiol isomerases appears to elaborate with proplatelet extensions (Figure 1D).
In platelets, we observed that consistent with our MK observations PDI and ERp57 are localized in a subcellular compartment distinct from the α-granule matrix delineated by P-selectin (Figure 2B). This is consistent with the reported lack of overlap between PDI and lysosomes50 or hyaluronidase-2 in human platelets, where the latter enzyme has been localized to α-granules via IFM and subcellular fractionation.51 The thiol isomerase compartment appears to surround the P-selectin matrix (Figure 2B) and to be contained by the outer cell membrane (Figure 2C), a pattern that holds in GPS human and mouse Nbeal2-/- platelets where α-granule cargo is absent (Figure 2C). The observation that α-granule cargo-deficient platelets have normal levels of thiol isomerases (Figure 2D,E) further supports the independence of their trafficking from granule cargo.
Our observations are consistent with reports of thiol isomerase located near the platelet surface6, 8, 10, 15-17 and DTS.19 We saw little association of ERp57 with smooth ER defined by calnexin in mature human MKs (Figure S2A), although we did observe localized overlap between this marker of the platelet DTS and ERp57 (Figure S2C). According to the most recent proteomics data ERp57 is approximately twice as abundant as calnexin in platelets (1736 vs. 890 ppm42), consistent with our observation that the thiol isomerase is more widely distributed in these cells (Figure S2C). Subcellular fractionation data indicate that in addition to the DTS, ERp57 and PDI are also likely associated with the cell surface membrane (Figure 3A). This expectation was confirmed by the observation of extensive overlap between ERp57 and SERCA3, long known to be located on the inner surface of the human platelet outer membrane (Figure 3B)20. In addition, ERp57 has been reported to interact with STIM152, a transmembrane ER protein that upon calcium depletion translocates to subplasmalemmal punctae where it interacts with the calcium channel Orai 1 located in the plasma membrane and allows calcium influx into the cell53. Given that as PDI and ERp57, SERCA 3 is an ER-derived protein20, 41, we cannot exclude the possibility that thiol isomerases co-distribute with SERCA 3 in a non-calnexin subpopulation of DTS (or possibly ER remnant) that is localized at the inner surface of the platelet membrane.
The mobilization of thiol isomerases to the surface of activated platelets facilitates their involvement in clot formation,8-10 and studies have shown that PDI and ERp5 move to the platelet surface with similar kinetics to α-granule proteins.7 We have extended these observations by demonstrating that as with P-selectin and α-granule cargo,21 the mobilization of platelet thiol isomerases requires actin polymerization (Figure 4). Secretion by platelet granules also involves interactions between v-SNAREs present on their surface with t-SNAREs on the plasma membrane,54, 55 where membrane fusion is primed by Munc13-4, a limiting factor in platelet granule secretion.22-24, 26 We observed that compared to WT mice, platelet thiol isomerase mobilization is stronger in Unc13dJinx mice, where platelets lack Munc13-4 activity and have absent or severely impaired P-selectin mobilization (Figure 5B) and secretion from lysosomes and dense and α-granules, although they do show activation-associated changes in morphology.25 We also observed that thiol isomerase mobilization is enhanced in both mutant and wild type mouse platelets by ADP, which has been shown to stimulate Munc13-4-independent secretion of α-granule contents.46 Thus our observations indicate that while platelet thiol isomerase mobilization/secretion requires actin polymerization, it does not involve Munc13-4 mediated membrane fusion. It may be that the cellular rearrangements associated with platelet activation are sufficient to move thiol isomerases to the surface and facilitate their release, as has been proposed for STIM1.56 Increased surface exposure of PDI in the absence of Munc 13-4 could also indicate that this protein serves as a brake on the mobilization of platelet thiol isomerases, pointing to a novel aspect of regulation of platelet function.
The results presented here can be combined with current knowledge to produce a model of platelet thiol isomerase trafficking, packaging and mobilization in activated cells. In this scheme (Figure 6; for the sake of simplicity only MK-synthesized proteins are considered) protein destined for platelet α-granules (e.g. VWF, TSP-1) moves from the MK ER to the trans-Golgi network for sorting into MVBs that give rise to mature secretory vesicles. Following a different route, thiol isomerases destined for platelets move from the ER to the developing intracellular membranes. When MK extend proplatelets, secretory cargo is packaged into granules while thiol isomerases associate with membranes that give rise to the platelet surface and possibly the outer DTS and OCS. In platelets that later undergo activation, thiol isomerases are mobilized to the outer cell surface via a mechanism driven by actin polymerization and cytoskeletal/membrane rearrangements.
Figure 6. Model comparing synthesis, trafficking, packaging and mobilization of platelet thiol isomerases and α-granule cargo.
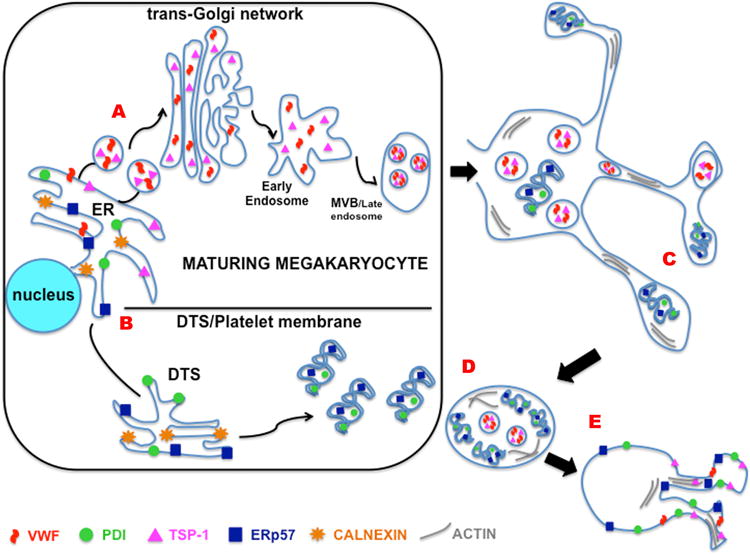
A) In the maturing MK, proteins destined for α-granules (e.g. VWF, TSP-1) are trafficked from the ER via a pathway that leads through the trans-Golgi network to multivesicular bodies. B) Thiol isomerases associate with membranes destined for the platelet DTS and cell surface. C) As proplatelet extensions form secretory granule cargo is packaged, while thiol isomerases move with membranes into forming platelets. D) In mature platelets, thiol isomerases are concentrated near the surface membrane and in the near-surface DTS. E) In activated platelets actin polymerization and membrane fusion facilitates the surface mobilization and secretion of granule cargo, while thiol isomerases mobilized without the need for membrane fusion.
Elucidation of the expression and function of platelet thiol isomerases is important because these enzymes have been shown to be key modulators of thrombus formation via their activity on the surface of platelets and vascular endothelial cells. The influence of PDI, ERp57 and ERp5 on platelet function has been primarily attributed to their ability to bind the platelet cell surface receptor integrin αIIbβ3 and thus favor its activation,6-10 and it is also likely that this receptor assumes a high-affinity conformation in activated platelets that facilitates surface binding of mobilized PDI and ERp57.57 A search for other substrates of thiol isomerases is ongoing, as are efforts to develop methods for the selective modulation of thiol isomerases as a therapeutic strategy against thrombotic disease.58
Supplementary Material
Highlights.
Platelet-borne thiol isomerases play important roles in hemostasis and in pathological thrombosis.
We examined for the first time the synthesis of thiol isomerases during megakaryocyte development showing that they are trafficked independently of granule cargo.
Within platelets thiol isomerases localize near the inner surface of the plasma membrane and partially overlap with the DTS
Thiol isomerases are released on the surface of activated platelets in an actin-dependent process that does not require Munc13-4 mediated membrane fusion.
Defining the synthesis, trafficking, location and mechanism of mobilization/secretion of platelet thiol isomerases may aid the development of new antithrombotic strategies.
Acknowledgments
We thank Dr Sidney W. Whiteheart for kindly providing us the cell-disruption apparatus and Mr Khaled Sahli for helping preparing the manuscript.
Sources of Funding: This work was supported by grants from the Medical Research Council (J002666/1) and British Heart Foundation (PG/11/125/29320) to J.M.G. and from the Canadian Institutes of Health Research to W.H.A.K. (MOP-259952).
Nonstandard Abbreviations and Acronyms
- PDI
protein disulfide isomerase
- ER
endoplasmic reticulum
- MK
megakaryocyte
- DTS
dense tubular system
- SNAREs
soluble N-ethylmaleimide-sensitive fusion protein attachment receptors
- MVBs
multivesicular bodies
- TFR
Transferrin receptor
- VWF
Von Willebrand factor
- TSP-1
thrombospondin-1
- PF4
Platelet Factor 4
- RabGDI
Rab GDP Dissociation Inhibitor
- SERCA3
sarco/endoplasmic reticulum calcium ATPase 3
- TLR9
toll-like receptor 9
- WT
wild type
Footnotes
Authorship: Contribution: M.C. and F.G.P. designed the research, performed the experiments, and wrote the manuscript; S.L., M.S.A., S.V., M.P.S, L.M.H., I.M.J. provided intellectual contribution, antibodies and contributed to writing the manuscript; L.L., R.W.L. and T.G.W designed and performed some experiments; H.F. performed some experiments and contributed to writing the manuscript; A.W.P., W.H.A.K. and J.M.G designed the research and contributed to writing the manuscript.
Disclosures: The authors declare no competing financial interests
References
- 1.Ferrari DM, Soling HD. The protein disulphide-isomerase family: Unravelling a string of folds. Biochem J. 1999;339(Pt 1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan PA, Gibbins JM. Extracellular disulfide exchange and the regulation of cellular function. Antioxid Redox Signal. 2006;8:312–324. doi: 10.1089/ars.2006.8.312. [DOI] [PubMed] [Google Scholar]
- 3.Jasuja R, Furie B, Furie BC. Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood. 2010;116:4665–4674. doi: 10.1182/blood-2010-04-278184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akagi S, Yamamoto A, Yoshimori T, Masaki R, Ogawa R, Tashiro Y. Distribution of protein disulfide isomerase in rat hepatocytes. J Histochem Cytochem. 1988;36:1533–1542. doi: 10.1177/36.12.3192937. [DOI] [PubMed] [Google Scholar]
- 5.Kroning H, Kahne T, Ittenson A, Franke A, Ansorge S. Thiol-proteindisulfide-oxidoreductase (proteindisulfide isomerase): A new plasma membrane constituent of mature human b lymphocytes. Scand J Immunol. 1994;39:346–350. doi: 10.1111/j.1365-3083.1994.tb03384.x. [DOI] [PubMed] [Google Scholar]
- 6.Holbrook LM, Sasikumar P, Stanley RG, Simmonds AD, Bicknell AB, Gibbins JM. The platelet-surface thiol isomerase enzyme erp57 modulates platelet function. J Thromb Haemost. 2012;10:278–288. doi: 10.1111/j.1538-7836.2011.04593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan PA, Stevens JM, Hubbard GP, Barrett NE, Sage T, Authi KS, Gibbins JM. A role for the thiol isomerase protein erp5 in platelet function. Blood. 2005;105:1500–1507. doi: 10.1182/blood-2004-02-0608. [DOI] [PubMed] [Google Scholar]
- 8.Kim K, Hahm E, Li J, Holbrook LM, Sasikumar P, Stanley RG, Ushio-Fukai M, Gibbins JM, Cho J. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122:1052–1061. doi: 10.1182/blood-2013-03-492504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passam FH, Lin L, Gopal S, Stopa J, Bellido-Martin L, Huang M, Furie BC, Furie B. Both platelet- and endothelial cell-derived erp5 supports thrombus formation in a laser-induced mouse model of thrombosis. Blood. 2015 doi: 10.1182/blood-2013-12-547208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Wu Y, Zhou J, Ahmad SS, Mutus B, Garbi N, Hammerling G, Liu J, Essex DW. Platelet-derived erp57 mediates platelet incorporation into a growing thrombus by regulation of the alphaiibbeta3 integrin. Blood. 2013;122:3642–3650. doi: 10.1182/blood-2013-06-506691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Ahmad SS, Zhou J, Wang L, Cully MP, Essex DW. The disulfide isomerase erp57 mediates platelet aggregation, hemostasis, and thrombosis. Blood. 2012;119:1737–1746. doi: 10.1182/blood-2011-06-360685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahm E, Li J, Kim K, Huh S, Rogelj S, Cho J. Extracellular protein disulfide isomerase regulates ligand-binding activity of alphambeta2 integrin and neutrophil recruitment during vascular inflammation. Blood. 2013;121:3789–3800. doi: 10.1182/blood-2012-11-467985. , s3781-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essex DW, Chen K, Swiatkowska M. Localization of protein disulfide isomerase to the external surface of the platelet plasma membrane. Blood. 1995;86:2168–2173. [PubMed] [Google Scholar]
- 16.Furie B, Flaumenhaft R. Thiol isomerases in thrombus formation. Circ Res. 2014;114:1162–1173. doi: 10.1161/CIRCRESAHA.114.301808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holbrook LM, Watkins NA, Simmonds AD, Jones CI, Ouwehand WH, Gibbins JM. Platelets release novel thiol isomerase enzymes which are recruited to the cell surface following activation. Br J Haematol. 2010;148:627–637. doi: 10.1111/j.1365-2141.2009.07994.x. [DOI] [PubMed] [Google Scholar]
- 18.Thon JN, Peters CG, Machlus KR, Aslam R, Rowley J, Macleod H, Devine MT, Fuchs TA, Weyrich AS, Semple JW, Flaumenhaft R, Italiano JE., Jr T granules in human platelets function in tlr9 organization and signaling. J Cell Biol. 2012;198:561–574. doi: 10.1083/jcb.201111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Nispen Tot Pannerden HE, van Dijk SM, Du V, Heijnen HF. Platelet protein disulfide isomerase is localized in the dense tubular system and does not become surface expressed after activation. Blood. 2009;114:4738–4740. doi: 10.1182/blood-2009-03-210450. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs T, Berger G, Corvazier E, Paszty K, Brown A, Bobe R, Papp B, Wuytack F, Cramer EM, Enouf J. Immunolocalization of the multi-sarco/endoplasmic reticulum ca2+ atpase system in human platelets. Br J Haematol. 1997;97:192–203. doi: 10.1046/j.1365-2141.1997.9982639.x. [DOI] [PubMed] [Google Scholar]
- 21.Flaumenhaft R, Dilks JR, Rozenvayn N, Monahan-Earley RA, Feng D, Dvorak AM. The actin cytoskeleton differentially regulates platelet alpha-granule and dense-granule secretion. Blood. 2005;105:3879–3887. doi: 10.1182/blood-2004-04-1392. [DOI] [PubMed] [Google Scholar]
- 22.Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue munc13-1 with the n terminus of syntaxin. The Journal of biological chemistry. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- 23.Feldmann J, Callebaut I, Raposo G, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (fhl3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 24.Madison JM, Nurrish S, Kaplan JM. Unc-13 interaction with syntaxin is required for synaptic transmission. Curr Biol. 2005;15:2236–2242. doi: 10.1016/j.cub.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 25.Ren Q, Wimmer C, Chicka MC, Ye S, Ren Y, Hughson FM, Whiteheart SW. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116:869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savage JS, Williams CM, Konopatskaya O, Hers I, Harper MT, Poole AW. Munc13-4 is critical for thrombosis through regulating release of adp from platelets. J Thromb Haemost. 2013;11:771–775. doi: 10.1111/jth.12138. [DOI] [PubMed] [Google Scholar]
- 27.Bender M, Giannini S, Grozovsky R, Jonsson T, Christensen H, Pluthero FG, Ko A, Mullally A, Kahr WH, Hoffmeister KM, Falet H. Dynamin 2-dependent endocytosis is required for normal megakaryocyte development in mice. Blood. 2015;125:1014–1024. doi: 10.1182/blood-2014-07-587857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begonja AJ, Pluthero FG, Suphamungmee W, Giannini S, Christensen H, Leung R, Lo RW, Nakamura F, Lehman W, Plomann M, Hoffmeister KM, Kahr WH, Hartwig JH, Falet H. Flna binding to pacsin2 f-bar domain regulates membrane tubulation in megakaryocytes and platelets. Blood. 2015;126:80–88. doi: 10.1182/blood-2014-07-587600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE, Jr, Shivdasani RA, von Andrian UH. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 30.Kahr WH, Lo RW, Li L, Pluthero FG, Christensen H, Ni R, Vaezzadeh N, Hawkins CE, Weyrich AS, Di Paola J, Landolt-Marticorena C, Gross PL. Abnormal megakaryocyte development and platelet function in nbeal2(-/-) mice. Blood. 2013;122:3349–3358. doi: 10.1182/blood-2013-04-499491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Larouziere V, Brouland JP, Souni F, Drouet L, Cramer E. Inverse immunostaining pattern for synthesized versus endocytosed alpha-granule proteins in human bone marrow megakaryocytes. Br J Haematol. 1998;101:618–625. doi: 10.1046/j.1365-2141.1998.00755.x. [DOI] [PubMed] [Google Scholar]
- 32.Ambrosio AL, Boyle JA, Di Pietro SM. Mechanism of platelet dense granule biogenesis: Study of cargo transport and function of rab32 and rab38 in a model system. Blood. 2012;120:4072–4081. doi: 10.1182/blood-2012-04-420745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blair P, Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91:2313–2325. [PubMed] [Google Scholar]
- 35.Beardmore J, Howell KE, Miller K, Hopkins CR. Isolation of an endocytic compartment from a431 cells using a density modification procedure employing a receptor-specific monoclonal antibody complexed with colloidal gold. J Cell Sci. 1987;87(Pt 4):495–506. doi: 10.1242/jcs.87.4.495. [DOI] [PubMed] [Google Scholar]
- 36.Kahr WH, Hinckley J, Li L, et al. Mutations in nbeal2, encoding a beach protein, cause gray platelet syndrome. Nat Genet. 2011;43:738–740. doi: 10.1038/ng.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonnalagadda D, Sunkara M, Morris AJ, Whiteheart SW. Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochim Biophys Acta. 2014;1841:1581–1589. doi: 10.1016/j.bbalip.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niessen J, Jedlitschky G, Grube M, Bien S, Strobel U, Ritter CA, Greinacher A, Kroemer HK. Subfractionation and purification of intracellular granule-structures of human platelets: An improved method based on magnetic sorting. J Immunol Methods. 2007;328:89–96. doi: 10.1016/j.jim.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Benham AM. Protein secretion and the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2012;4:a012872. doi: 10.1101/cshperspect.a012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caramelo JJ, Parodi AJ. Getting in and out from calnexin/calreticulin cycles. The Journal of biological chemistry. 2008;283:10221–10225. doi: 10.1074/jbc.R700048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovacs T, Felfoldi F, Papp B, Paszty K, Bredoux R, Enyedi A, Enouf J. All three splice variants of the human sarco/endoplasmic reticulum ca2+-atpase 3 gene are translated to proteins: A study of their co-expression in platelets and lymphoid cells. The Biochemical journal. 2001;358:559–568. doi: 10.1042/0264-6021:3580559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MS, Pinto SM, Getnet D, et al. A draft map of the human proteome. Nature. 2014;509:575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martens L, Van Damme P, Van Damme J, Staes A, Timmerman E, Ghesquiere B, Thomas GR, Vandekerckhove J, Gevaert K. The human platelet proteome mapped by peptide-centric proteomics: A functional protein profile. Proteomics. 2005;5:3193–3204. doi: 10.1002/pmic.200401142. [DOI] [PubMed] [Google Scholar]
- 44.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of stim1 with endogenously expressed human canonical trp1 upon depletion of intracellular ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 45.Spector I, Braet F, Shochet NR, Bubb MR. New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microsc Res Tech. 1999;47:18–37. doi: 10.1002/(SICI)1097-0029(19991001)47:1<18::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 46.Harper MT, van den Bosch MT, Hers I, Poole AW. Platelet dense granule secretion defects may obscure alpha-granule secretion mechanisms: Evidence from munc13-4-deficient platelets. Blood. 2015;125:3034–3036. doi: 10.1182/blood-2014-12-618439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wandall HH, Rumjantseva V, Sorensen AL, Patel-Hett S, Josefsson EC, Bennett EP, Italiano JE, Jr, Clausen H, Hartwig JH, Hoffmeister KM. The origin and function of platelet glycosyltransferases. Blood. 2012;120:626–635. doi: 10.1182/blood-2012-02-409235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo B, Li L, Gissen P, Christensen H, McKiernan PJ, Ye C, Abdelhaleem M, Hayes JA, Williams MD, Chitayat D, Kahr WH. Requirement of vps33b, a member of the sec1/munc18 protein family, in megakaryocyte and platelet alpha-granule biogenesis. Blood. 2005;106:4159–4166. doi: 10.1182/blood-2005-04-1356. [DOI] [PubMed] [Google Scholar]
- 50.Zbidi H, Jardin I, Woodard GE, Lopez JJ, Berna-Erro A, Salido GM, Rosado JA. Stim1 and stim2 are located in the acidic ca2+ stores and associates with orai1 upon depletion of the acidic stores in human platelets. J Biol Chem. 2011;286:12257–12270. doi: 10.1074/jbc.M110.190694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albeiroti S, Ayasoufi K, Hill DR, Shen B, de la Motte CA. Platelet hyaluronidase-2: An enzyme that translocates to the surface upon activation to function in extracellular matrix degradation. Blood. 2015;125:1460–1469. doi: 10.1182/blood-2014-07-590513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prins D, Groenendyk J, Touret N, Michalak M. Modulation of stim1 and capacitative ca2+ entry by the endoplasmic reticulum luminal oxidoreductase erp57. EMBO reports. 2011;12:1182–1188. doi: 10.1038/embor.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of orai1 with stim1 mediates orai1 channel activation. The Journal of biological chemistry. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 54.Rothman JE, Warren G. Implications of the snare hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 55.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. Snarepins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 56.Lopez JJ, Jardin I, Bobe R, Pariente JA, Enouf J, Salido GM, Rosado JA. Stim1 regulates acidic ca2+ store refilling by interaction with serca3 in human platelets. Biochem Pharmacol. 2008;75:2157–2164. doi: 10.1016/j.bcp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Bennett JS, Zigmond S, Vilaire G, Cunningham ME, Bednar B. The platelet cytoskeleton regulates the affinity of the integrin alpha(iib)beta(3) for fibrinogen. The Journal of biological chemistry. 1999;274:25301–25307. doi: 10.1074/jbc.274.36.25301. [DOI] [PubMed] [Google Scholar]
- 58.Flaumenhaft R, Furie B, Zwicker JI. Therapeutic implications of protein disulfide isomerase inhibition in thrombotic disease. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:16–23. doi: 10.1161/ATVBAHA.114.303410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


