Abstract
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in patients with chronic kidney disease (CKD) and end stage renal disease (ESRD). The rate of death in incident dialysis patients remains high. This has led to interest in the study of the evolution of CVD during the critical transition period from CKD to ESRD. Understanding the natural history and risk factors of clinical and subclinical CVD during this transition may help guide the timing of appropriate CVD therapies to improve outcomes in patients with kidney disease. This review will provide an overview of the epidemiology of subclinical and clinical CVD during the transition from CKD to ESRD and discuss clinical trials of CVD therapies to mitigate risk of CVD in CKD and ESRD patients.
Keywords: Cardiovascular disease, CKD, ESRD, transition
Introduction
In the United States, chronic kidney disease (CKD) affects 14% of the population, including approximately 500,000 patients with end-stage renal disease (ESRD), who receive dialysis or a kidney transplant. There have been many advancements in the care of kidney disease patients: there is greater recognition of CKD and incidence rates of treated ESRD are decreasing. However, the rate of death among incident dialysis patients remains unacceptably high. This has led to greater interest in the study of the critical transition period from CKD to ESRD. More specifically, understanding the evolution of cardiovascular disease (CVD), which remains the leading cause of morbidity and mortality in patients with kidney disease, during the transition from CKD to ESRD may lead to improved outcomes in this population.
Epidemiology of cardiovascular disease at advanced stages of kidney disease
CVD remains the leading cause of morbidity and mortality among patients with CKD. Studies have suggested that the risk of death from CVD exceeds the risk of progression to ESRD among CKD patients. Keith et al. analyzed outcomes of 27,998 patients with CKD and showed that the 5-year mortality rates for CKD stages 2, 3, and 4 were 19.5%, 24.3%, and 45.7%, respectively; while the percentages of patients with these stages who progressed to ESRD were much lower at 1.1%, 1.3%, and 19.9%.1 Among patients with CKD, lower estimated glomerular filtration rate (eGFR) (or more advanced CKD) is associated with even greater risk of CVD compared to mild or moderate stages of CKD. In a study of over 1.1 million adults, Go et al reported a step-wise graded increase in age-standardized rates of CVD events (which included hospitalizations for CHD, HF, ischemic stroke and PAD) across declining categories of eGFR.2 Age-standardized rates of CVD events were 21.80 (per 100 person-years) among patients with stage 4 CKD (eGFR 15–29 ml/min/1.73 m2) and 36.60 (per 100 person-years) for stage 5 CKD (<15, not on dialysis) compared with 11.29 (per 100 person-years) for stage 3b CKD. Similarly, in over 11,000 participants in the Atherosclerosis Risk in Communities (ARIC) Study, lower eGFR was associated with greater risk of CVD across all categories of age, race and gender.3 In a pooled analysis of 21 studies of over 1.2 million study participants, investigators reported a linear association between lower eGFR and risk of CVD mortality. Those with stage 5 CKD (eGFR 15–29 ml/min/1.73 m2) had up to 5 to 13 fold greater risk of CVD mortality compared to those with eGFR 90–104 ml/min/1.73 m2, which varied based on the level of albuminuria.4 In the same study population, the addition of eGFR and urine ACR significantly improved discrimination of heart failure, coronary disease and stroke risk, beyond traditional CVD risk factors.5
Coronary disease and heart failure (HF) are the leading types of CVD among patients with advanced CKD. Data from USRDS reports that among CKD patients who die, 91% have a diagnosis of CVD overall; and 57% have a diagnosis of coronary CVD and 63% have a diagnosis of HF.6
Epidemiology of cardiovascular disease at end-stage renal disease
CVD affects greater than 50% of patients receiving dialysis. In the Wave 2 Dialysis Morbidity and Mortality Study (DMMS), a prospective study of 4024 patients initiating dialysis therapy in the United States from 1996 and 1997, a history of HF was present in 35% of patients, coronary disease in 32%, peripheral vascular disease in 17%, and cerebrovascular disease in 10%– so that, overall, 52% had preexisting overt cardiovascular disease.7 In patients without prevalent CVD, rates of incident CVD were high—10.2% developed incident coronary disease, 13.6% incident HF, 2.2% incident stroke and 14% incident peripheral vascular disease over a mean of 2.2 years.7 Studies have also reported that CVD contributes significantly to morbidity among ESRD patients, leading to frequent hospitalizations and re-hospitalizations.8–10 Presence of CVD also adversely affects long-term survival in ESRD patients. CVD contributes to more than half of all deaths in patients with ESRD (Figure 1). Of the subtypes of CVD, arrhythmias and cardiac arrest account for the greatest proportion of deaths in patients with ESRD.11–13
Figure 1.
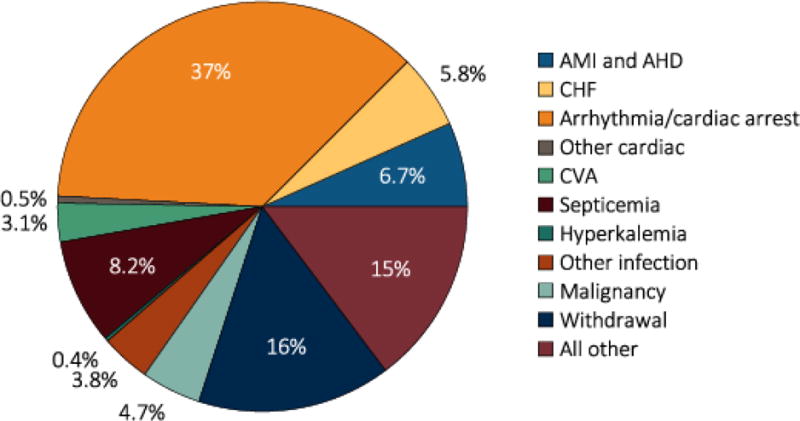
Causes of death in ESRD patients (source: USRDS)
Cardiovascular events and death during first 90 days of dialysis initiation
The period of incident dialysis is a physiologically complicated time with heightened risk for CVD and mortality. As patients initiate dialysis, there are significant changes in blood pressure, volume status and circulating solutes, all of which may contribute to increased risk of CVD. In a study of over 300,000 incident dialysis patients, the risk of death during the first two weeks after starting dialysis was 2.72-fold higher and the risk of hospitalization was 1.95-fold higher compared to the risk of death and hospitalization after the first year….14 The risk of mortality and hospitalization remained elevated through the initial 90 day period and decreased substantially between days 91 and 365.14 This study did not report rates of CVD related deaths or hospitalizations unfortunately. Data from the Dialysis Outcomes and Practice Patterns Study (DOPPS, 1996 to 2004) reported that the risk of death was elevated during the first 120 days after initiation of dialysis compared with 121 to 365 days (27.5 vs. 21.9 deaths per 100 person-years, p=0.002).15 The most common cause of death in the first 120 days was CVD and the greatest difference in rates of early deaths vs. late deaths was for CVD-related deaths versus other causes (Figure 2). Prevalent HF was one of the strongest predictors of early death after initiation of dialysis.15 Given the high rates of adverse events in the first few months after dialysis initiation, understanding the evolution of CVD during the transition from CKD to ESRD may ultimately improve outcomes among incident dialysis patients.
Figure 2.
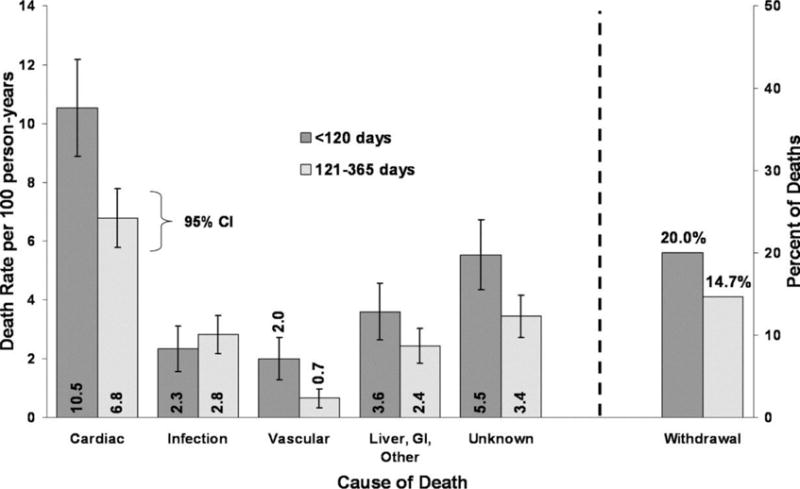
(Left) Cause-specific mortality rates and 95% confidence intervals for the <120-and 121- to 365-d periods. (Right) Percentage of all deaths during the <120 and 121- to 365-d periods that occur subsequent to withdrawal. (Bradbury et al. CJASN 2007;2:89–99)
Clinical cardiovascular disease during the transition from CKD to ESRD
While there have been several studies examining prevalence and incidence of CVD during CKD as well as at ESRD, there is limited data on the evolution of clinical CVD during the transition from CKD to ESRD. USRDS reports that at 18.5 months prior to ESRD, 50% of Medicare patients have evidence of CVD. The cumulative probability of CVD increases in the months prior to ESRD (Figure 3). By the time of ESRD, nearly 90% of patients have CVD. According to this data, rates of atherosclerotic CVD and HF are comparable. In a longitudinal analysis of a subset of participants in the Chronic Renal Insufficiency Cohort Study, 190 participants were followed from advanced CKD (mean eGFR 17 ml/min/173 m2) through initiation of dialysis. During the transition from CKD to ESRD, overall prevalence of CVD increased (from 51% to 64%). Specifically, prevalence of HF increased from 15% to 26%, stroke from 14% to 18%, myocardial infarction from 25% to 38% and peripheral vascular disease from 9% to 11%.16 These studies provide evidence that there is a cumulative increase in CVD during the transition from CKD to ESRD. Further studies are needed to characterize the risk factors, particularly those that are potentially modifiable, during this critical phase of disease.
Figure 3.

Cumulative probability of cardiovascular comorbidity during the transition to ESRD (data from USRDS)
Subclinical cardiovascular disease during the transition from CKD to ESRD
As a precursor to clinical CVD, investigation into the evolution of subclinical CVD from CKD to ESRD provides important insight on the natural history and risk factors that may contribute to the heightened risk of CVD during this transition period, which may ultimately guide the timing of interventions in patients with CKD. CVD imaging tools have been utilized in clinical studies to evaluate subclinical CVD during this transition.
Left ventricular structure and function
In patients with CKD and ESRD, abnormalities in left ventricular structure and function are common and are associated with adverse clinical outcomes.2,14,17 Approximately three-quarters of incident dialysis patients are reported to have left ventricular hypertrophy,18,19 which is an independent predictor of cardiovascular events and death after onset of ESRD.20,21 Similarly, low left ventricular ejection fraction (LVEF), even in the absence of clinical HF, has also been shown to be a risk factor for CVD and all-cause mortality among patients with CKD and ESRD.22,23
With declining eGFR and progression to ESRD, it has been hypothesized that there is progression of subclinical measures of CVD as measured by echocardiogram. We studied 190 participants from the multi-center observational study, the Chronic Renal Insufficiency Cohort (CRIC) study, who progressed to ESRD and initiated dialysis. We compared echocardiograms performed at incident dialysis to echocardiograms performed at stage 4 or 5 CKD (eGFR<20 ml/min1/73 m2). Overall, during the transition from CKD to ESRD over a mean of 2.0 years, the proportion of patients with LVH did not significantly change (85% at advanced CKD vs. 79% at ESRD, p=0.1). Mean LVMI did not change either (62 g/m2.7 at advanced CKD vs. 60 at ESRD, p=0.1). However, there was a modest, but statistically significant decline in LVEF from advanced CKD to ESRD (from 53% to 50%, p=0.002); and the proportion of patients with reduced LVEF (defined as LVEF<50%) increased from 29% to 48% from advanced CKD to ESRD (p<0.001).16 This study suggested that LVM is abnormally elevated but relatively “fixed” by advanced stages of CKD. In contrast, there is a deterioration of LVEF during the transition from CKD to ESRD. Physiologically, this is plausible as pathologic processes such as hypertension are initiated early in CKD, likely leading to LVH, an adaptive response that initially normalizes wall stress and maintains a normal LVEF. Over time, LVH may cause subendocardial ischemia and fibrosis, which can lead to systolic dysfunction.
The CASCADE study examined echocardiograms one-year apart in 278 patients with stages 3–5 CKD (of which 140 had stage 4–5 CKD).24 Among patients with stage 4 and 5 CKD at baseline, mean eGFR decreased from 18 to 15 ml/min/1.73 m2 (it is unknown whether any of these participants started dialysis) over one year, LVMI increased (from 115 to 123 g/m2), and left atrial volume increased (from 33 ml/m2 to 39 ml/m2). However the change in LVEF was not statistically significant. These results differ from the previous study done among CRIC participants. These findings also differ from a study of 41 heart failure patients, where LVMI decreased after initiation of HD.25 There are several possible explanations for the differing findings among these studies. There were key differences in the study populations as well as the timing of the echocardiograms. In the CRIC study, there was a greater difference in time between echocardiograms which may have allowed more time for significant detectable changes.
In contrast to the observational studies discussed above, the IDEAL trial randomized stage 5 CKD patients to early vs. late start dialysis. As part of the trial, serial echocardiograms were performed 12 months apart in a subset of 182 (out of 646 total) participants.26 After 12 months, 55.5% started HD, 41.2% started PD and 3.2% did not start dialysis. Primary outcomes for the echocardiogram substudy included change in clinically important echocardiogram measures. At baseline, echocardiogram measures were abnormal in both groups with no significant differences between the late vs. early start groups. The study found that there was no change in LVMI, LA diameter, diastolic dysfunction or LVEF over 12 months (Figure 4).26 These findings suggest that earlier initiation of dialysis does not result in differences in cardiac structure and function. This is interesting, particularly given the differences in findings compared with the previous observational studies. It should be noted that in the IDEAL study, over 40% initiated PD (vs. HD), which was not the case for the observational studies. Differences in dialysis modality may be one possible explanation for the differing results of the IDEAL trial versus the observational studies.
Figure 4.
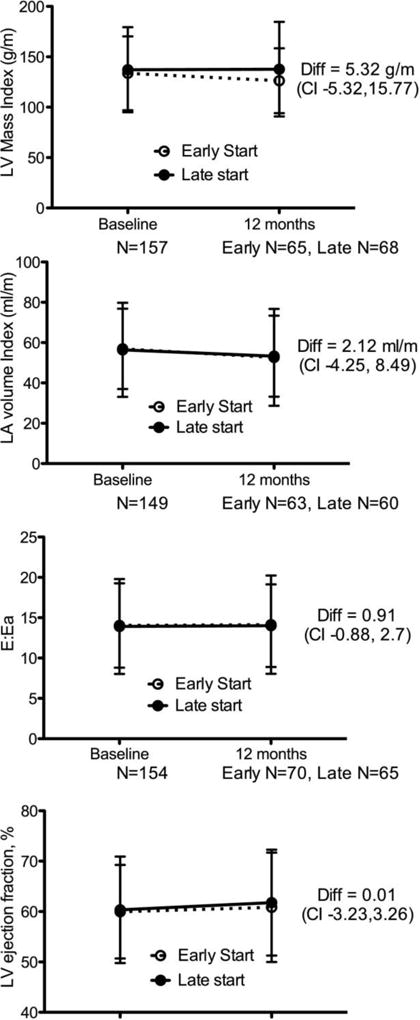
Primary echocardiography end points by randomly assigned group at baseline and 12 months in the IDEAL trial. Abbreviations: CI, confidence interval; diff, difference; LA, left atrium; LV, left ventricle. (Whalley et al, AJKD 2013)
Vascular calcification
Vascular calcification is strongly linked with coronary atherosclerosis and cardiac valvular disease. Vascular calcification is more common in patients with CKD and ESRD compared to the general population. An autopsy study of 56 patients on dialysis and 18 patients with CKD reported that calcification was present in 79% of dialysis patients and 44% of the CKD patients.27
Measurement of coronary artery calcium (CAC) by coronary tomography (CT) is one measure of vascular calcification. Several studies have demonstrated a graded relationship between lower estimated glomerular filtration rate (eGFR) and higher CAC.28–31 Among participants with CKD in the CRIC study, smoking, hypertension, diabetes, and serum phosphorus were associated with greater odds of CAC while there was an inverse association between eGFR and odds of CAC.32 Studies have reported that higher CAC predicts CVD events and mortality among patients with CKD.33
Among patients receiving dialysis, there appears to be progression of CAC over time. Among 213 dialysis patients, approximately half had CAC Agatston scores>400, placing them at high risk for CVD events.34 Over one year, CAC Agatston scores increased by 25% (mean Agatston score increased by 87).34 Progression of CAC did not vary by anti-hypertensive or statin use. In another study of 99 participants initiating dialysis, 37% had progression of CAC over 1 year.35
Non-coronary vascular calcification also progresses during the transition from CKD to ESRD. In a study of 134 patients, 60 on HD, 28 on PD and 46 with stage 4 CKD, patients were evaluated for progression of femoral vascular calcification by multi-slice spiral CT performed at baseline, 12 months and 24 months.36 Over the 24 months, approximately half of the stage 4 CKD patients initiated dialysis. Median calcium score increased over 24 months across all three groups of patients, with the largest increase among HD patients. Age and male gender were associated with greater progression of vascular calcification while higher serum albumin, use of lipid lowering agents and b-blockers were associated with less progression of vascular calcification. In survival analyses, change in calcium score was significantly associated with higher mortality.36 Although this study was small, the conclusions were interesting and relevant to CVD management during the transition from CKD to ESRD.
Arterial stiffness
Arterial stiffness is measured by pulse wave velocity (PWV). Abnormally high PWV is highly prevalent among patients with CKD and ESRD37 and there is a step-wise increase in PWV with progressive stages of CKD.38,39 In a study of over 2,500 CKD participants from the CRIC study, age, blood glucose concentrations, race, waist circumference, blood pressure and diabetes were positively associated with higher PWV while there was a negative association between PWV and level of kidney function.40 Higher PWV is associated with greater risk of CVD and death among patients with CKD and ESRD.41–45 In a study of 241 HD patients, higher PWV was associated with greater than 5-times odds for all-cause mortality and nearly 6-fold greater odds for CVD mortality.41 Among 2600 patients with CKD, those with the highest PWV had greater than 3-fold greater risk of incident HF. This association remained statistically significant even with adjustment for brachial blood pressure.44 Longitudinal studies have suggested that arterial stiffness worsens with progression of CKD. One study found that over 24 months, PWV increased among stage 4 CKD patients (of which approximately half initiated dialysis).36
Risk factors and therapies for cardiovascular disease during the transition from CKD to ESRD
Cardiovascular medication use
CVD medications are a mainstay of primary and secondary CVD prevention, however they are often underutilized in patients with CKD and ESRD. There are a few studies that have specifically examined use of these medications during advanced stages of CKD and through the transition to ESRD. Our previous study noted of almost 1800 participants with stage 4 and 5 CKD not on dialysis reported that 70% were using ACE/ARBs, 52% were taking calcium channel blockers, 59% B-blockers, 12% were on coronary vasodilators, 70% were on diuretics, 30% were on alpha-blockers and 64% were on statin therapy.46
One USRDS study examined use of anti-hypertensive medications during the transition from CKD to ESRD among patients >67 years.47 This study found that the number of anti-hypertensive medications increased as patients neared ESRD (peaking at 3.4 in the quarter preceding dialysis initiation), however declined to an average of 2.2 anti-hypertensive medications 2 years later. ACE and ARB use did not significantly change during the transition from CKD to ESRD, even among patients with coronary disease and HF.47
A recent study evaluated pre-dialysis CVD medication adherence (which included all blood pressure medications, statins, and aspirin) and mortality after transition to ESRD among over 32,000 U.S. Veterans.48 The authors reported that 76% of patients had over 80% adherence to CVD medications prior to initiating dialysis. Patients with high CVD medication adherence were older, more likely to be white and married, and were more likely to initiate dialysis with an arteriovenous fistula. In multivariable models, lower adherence to CVD medications in the year prior to dialysis initiation was associated with greater risk of all-cause and CVD mortality after dialysis initiation.48
Another group investigated patterns of medication use after initiation of dialysis. Among 12,000 HD patients, 59% were prescribed a beta-blocker, 45% a RAAS inhibitor, 49% a CCB and 28% a diuretic at 6 months post dialysis initiation.49 Among the 900 PD patients in the study, 55% were taking a beta-blocker, 52% a RAAS inhibitor, 50% a CCB and 41% a diuretic at 6 months post dialysis-initiation.49 From months 1 to 6, prescription of anti-hypertensive medications increased in HD patients, with the exception of diuretics, which declined. BP medication prescription was higher among patients with a pre-existing CVD or diabetes. RAAS inhibitor prescription was greater in patients with diabetes but did not differ by prevalent CVD status.49 In examining beta-blocker use in long-term survivors, of all patients prescribed a beta-blocker at 6 months, 89% and 76% of those who survived at 12 and 24 months, respectively, continued beta-blocker prescriptions.
Overall, the current studies suggest that there are opportunities to improve CVD medication prescription and adherence for the primary and secondary prevention of CVD during the transition from CKD to ESRD, which may improve outcomes, particularly in patients with prevalent CVD.
Hypertension
Prior observational studies have consistently noted a U-shaped association between level of systolic blood pressure (SBP) measured in the dialysis unit and risk of all-cause mortality among HD patients.46,50–60 HD patients with pre-dialysis systolic blood pressure less than 140 mmHg experience higher risk of mortality than those with SBP above 140 mmHg.46,52,53,61,62 Moreover, patients with pre-dialysis systolic blood pressure of 150–179 mmHg appear to be at similar, if not lower, adjusted risk for death compared to those with pre-dialysis SBP of 140–149 mmHg, even accounting for case-mix.52,63 We aimed to understand the association of blood pressure and risk of all-cause mortality among patients with stage 4 and 5 CKD (not on dialysis) and these same cohort of participants as they transitioned to ESRD and initiated dialysis in the CRIC study.62 We first examined the association of systolic blood pressure measured when the participants’ eGFR<30 ml/min/1.73 m2 and risk of all-cause mortality. We found that in multivariable models, the association between SBP and mortality was not statistically significant. In these same participants who survived to ESRD and initiated HD, we confirmed a U-shaped associated between level of systolic blood pressure and risk of all-cause mortality (with a nadir of 140–160 mmHg) as seen by other studies.62 Interestingly, in these same participants, when blood pressure was measured at a CRIC study visit (rather than in the dialysis-unit), we noted a strong, linear association between higher systolic blood pressure and greater risk of mortality.62
There are a limited number of clinical trials to guide blood pressure treatment among patients with CKD and ESRD. A recent landmark clinical trial, the SPRINT trial, demonstrated that targeting a systolic blood pressure less than 120 mm Hg resulted in lower rates of cardiovascular disease (CVD) and death in the general population and in the subgroup with mild to moderate CKD (which comprised 20% of trial population).64 However, few SPRINT participants had advanced CKD (e.g. stage 4 or 5, not requiring dialysis).65 In a meta-analysis of 8 such trials of HD patients, lowering of dialysis-unit BP was associated with 29% lower risk of CVD events and 29% lower risk of CVD mortality.66 Some trials have focused on specific agents of BP medications67–70 or specific groups of HD patients (e.g. those with HF).71,72 For example, one study randomized 366 HD patients to ARB versus no ARB. The trial found that ARB use was associated with nearly 50% reduction in CVD events.67 In another study of 251 hypertensive HD patients, investigators randomized participants to either amlodipine versus placebo. Participants randomized to amlodipine achieved lower blood pressure and had a 47% reduction in risk of all-cause mortality or CVD.69 Furthermore, the ongoing Blood pressure in Dialysis (BID) trial is testing pre-dialysis blood pressure targets in the HD population and will hopefully provide important information.73
One group of investigators evaluated whether blood pressure during CKD is associated with outcomes after progression to ESRD. The MDRD trial was a randomized controlled trial of the effect of strict blood pressure control and dietary protein restriction on the progression of CKD among CKD patients with GFR 13–55 ml/min/1.73 m2. In a post-hoc analysis of the MDRD trial, Ku et al examined risk of post-ESRD mortality in patients randomized to strict blood pressure control (MAP<92 mmHg, which corresponds to 125/75 mmHg) vs. usual blood pressure. Of the 840 enrollees, 627 developed ESRD over a median follow-up time of 19.3 years from randomization. Post ESRD, median follow-up time was 10.0 years. Participants randomized to strict blood pressure control during CKD had a lower risk of death after ESRD (adjusted HR 0.74, 95% CI: 0.59, 0.92).74
Collectively, the observational and clinical trial data suggest that lower blood pressure targets may reduce risk of CVD in patients with CKD and ESRD. Although further studies are needed to test these hypotheses in patients with advanced CKD and determine which blood pressures (pre-dialysis, home dialysis, etc) should be the target of therapy in HD patients and what those blood pressure targets should be.
Lipid lowering
Lipid lowering medications have been proven to reduce risk of CVD in the general population. The data on lipids and lipid lowering medications in patients with CKD and ESRD are less certain. A few trials have tested the efficacy of lipid lowering medications to improve outcomes in patients on dialysis. The 4D trial randomized over 1200 HD patients with diabetes mellitus to atorvastatin vs. placebo. The study found that atorvastatin had no statistically significant effect on the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and stroke in patients with diabetes receiving HD.75 In the AURORA trial of 2776 HD patients, those randomized to rosuvastatin (versus placebo) had lower LDL cholesterol levels however there was no significant effect on the composite primary end point of CVD death, non-fatal MI or non-fatal stroke.76 Subsequently, the Study of Heart and Renal Protection (SHARP) trial randomized over 9,000 patients with CKD and ESRD with no history of MI or coronary revascularization. Patients were randomly assigned to simvastatin plus ezetimibe vs. matching placebo. The primary outcome was first major atherosclerotic event. Overall, there was 17% risk reduction (95% CI: 6%, 26%) in major atherosclerotic events in patients randomized to simvastatin plus ezetimibe.77 Among the subgroup of patients with CKD not receiving dialysis, the findings were similar to the overall trial results. However, among patients receiving dialysis, there was no benefit of lipid lowering therapy.77 While no studies have been specifically conducted to evaluate the effects of lipid lowering medications during the transition from CKD to ESRD, the current body of literature suggests that lipid lowering medications may be appropriate in CKD, with less clear benefits in patients receiving dialysis.
Implantable cardioverter defibrillators
Patients with kidney disease are at high risk for sudden cardiac death.12,78 Rates of SCD rise with declining eGFR79 and the risk of SCD is particularly high during the first three months after dialysis initiation.6 Trials have demonstrated that implantable cardioverter defibrillators (ICDs) as a primary prevention strategy for SCD can reduce the risk of death in adults with HF and reduced left ventricular ejection fraction (LVEF) when compared against optimal medical therapy alone.80–82 Nearly all of the existing clinical trials have excluded patients with advanced CKD or ESRD.80–86 Existing post-hoc analyses of clinical trials have suggested no survival benefit with ICD implantation in patients with mild to moderate CKD.87,88 In an observational study of 108 patients with eGFR<30 ml/min/1.73 m2 who received a primary prevention ICD matched to patients without ICD, prophylactic ICD implantation did not confer a survival advantage.89 Previous observational studies of dialysis patients have suggested that ESRD patients may be “too sick” to benefit from primary prevention ICD placement due to high competing risks of death90,91 and post-ICD complications.90,92,93 While these observational data have extended our knowledge, residual confounding remains an issue with the interpretation of these data. Further clinical trials are needed to test whether ICD implantation improves outcomes in patients with advanced CKD and incident ESRD.
Timing of dialysis initiation
Given the high burden of CVD at the time of dialysis initiation, it is plausible that earlier dialysis initiation, which may lead to better volume control, blood pressure and solute clearance, may reduce the risk of CVD. The IDEAL trial tested this hypothesis. The IDEAL trial randomized 828 patients based on eGFR to start dialysis therapy early (eGFR 10–14 ml/min/1.73 m2) vs. late (eGFR 5–7 ml/min/1.73 m2). The primary outcome was all-cause mortality. Secondary outcomes included CVD events (CVD death, nonfatal MI, nonfatal stroke, transient ischemic attach or new-onset angina). The median time from randomization to initiation of dialysis was 1.8 months (95% CI: 1.60, 2.23) in the early start group compared with 7.40 (95% CI: 6.23, 8.27) in the late start group. There was no difference in survival between the early and late start groups (HR for death in the early start group 1.04, 95% CI: 0.83, 1.30, p=0.75). There was also no difference in CVD events between the early and late start groups (HR 1.23, 95% CI: 0.97, 1.56), p=0.09).94
Dialysis modality
A key decision during the transition from CKD to ESRD is choice of dialysis modality. Clearly, numerous considerations go into making this important decision. Studies have examined whether dialysis modality is associated with CVD after dialysis initiation. In one observational study of over 3000 incident HD and PD patients, there was no difference in risk of incident CVD between patients who initiated PD vs. HD (RR 1.06, 95% CI: 0.79, 1.43).95 Other studies have reported differing findings. In a retrospective study of 2,000 patients in the USRDS DMMS Wave 2 study, HD (vs. PD) was associated with greater rates of both de novo and recurrent HF.96 Similar results were reported in an analysis of 24,587 incident dialysis patients in Australia and New Zealand.97 Overall 21% died from CVD causes. The incidence rates of CVD events were higher in PD vs. HD patients (9.99 and 7.96 per 100 patient years, respectively). After 1 year of treatment, PD was significantly associated with increased rates of CVD death (IRR 1.25, 95% CI: 1.12, 1.32).97 In a study of over 6516 propensity score matched incident HD and PD patients without preexisting CVD in Taiwan, no difference was observed in the overall risk of de novo coronary disease between HD and PD groups (HD vs. PD, adjusted HR 1.03, 95% CI: 0.86, 1.22).98 However HD was associated with higher risk of de novo HF (adjusted HR 1.29, 95% CI: 1.13, 1.47), particularly in the first year of dialysis.98
Baseline presence of CVD may also affect the association of dialysis modality with survival. A study of 107,922 incident dialysis patients in USRDS from 1995–1997 examined the association of PD vs. HD on 2-year survival among patients with and without prevalent coronary disease. In patients with and without diabetes, patients with coronary disease treated with PD had poorer survival compared with HD. Among diabetics, patients with coronary disease treated with PD had 23% higher relative risk of mortality (95% CI: 1.12, 1.34) compared with HD patients. Among non-diabetics, patients with coronary disease treated with PD had 20% higher relative risk of mortality (95% CI: 1.10, 1.32) compared with those on HD. Patients without CAD had similar survival on HD or PD.99
Collectively the current body of observational data suggests that PD may be associated with better CVD outcomes compared with HD, except in certain subgroups, such as those with pre-existing CVD. However further clinical trials would be helpful to confirm these findings given the likely residual confounding in the selection of PD vs. HD patients.
Mineral metabolism
Disorders of mineral metabolism, which include calcium, phosphorus, vitamin D, parathyroid hormone (PTH) and fibroblast growth factor (FGF)-23,100–102 are common in CKD and ESRD, are linked with CVD and are potentially modifiable.103–108 Mineral metabolism abnormalities accelerate with progression of CKD. Few studies have focused mineral metabolism and associations with CVD in patients with late CKD or incident CKD. The HOST study was a multi-center, prospective RCT examining the effects of folate, vitamin B6 and vitamin B12 replacement on all-cause mortality and atherosclerotic CVD events in patients with advanced CKD (not on dialysis) with elevated plasma total homocysteine concentrations.109 Among these participants with advanced CKD (mean eGFR 18 ml/min/1.73 m2), over a median follow-up of 2.9 years, 41% of participants died from any cause, 20% had an atherosclerotic CVD event and 56% initiated dialysis. The top quartiles of FGF23 were significantly associated with greater risk of all-cause mortality and atherosclerotic CVD events.109 Similarly in the same study population, lower 1, 25-dihydroxyvitamin D was associated with greater risk of all-cause mortality, while there was not association of 25-hydroxyvitamin D with outcomes.110
Several trials have tested therapies to treat mineral metabolism disorders to improve outcomes in patients with CKD and ESRD.
In a meta-analysis of 11 randomized trials of calcium-based versus calcium-free phosphate binders in patients with CKD found that patients randomized to calcium-free phosphate binders had 22% reduction in all-cause mortality compared to those assigned to calcium-based binders.111 In a similar meta-analysis of 14 trials in dialysis patients, patients assigned to calcium-free binders (sevelamer) had significantly higher phosphate levels but lower serum calcium levels compared to patients assigned to calcium-based binders. In the 5 trials that examined all-cause mortality as an outcome, there was no difference among patients assigned to either calcium-based or calcium-free binders.112
A recent trial of HD patients randomized patients to 6 months of ergocalciferol treatment. After 6 months of therapy, there was no change in serum calcium, phosphorus, intact parathyroid hormone, or C-reactive protein levels, cinacalcet use, or phosphate binder or calcitriol dose in either study arm. Rates of all-cause, cardiovascular, and infection-related hospitalizations did not differ by study arm, although statistical power was limited for these outcomes.113 The Paricalcitol Capsule Benefits in Renal Failure–Induced Cardiac Morbidity (PRIMO) trial randomized 227 patients with CKD and LVH to receive oral paricalcitol vs. placebo. After 48 weeks, change in LVMI did not differ between the treatment groups. Doppler measures of diastolic function including peak early diastolic lateral mitral annular tissue velocity also did not differ.114
The Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial tested the hypothesis that treatment with cinacalcet would reduce the risks of death and nonfatal cardiovascular events among 3883 patients with secondary hyperparathyroidism who were undergoing dialysis.115 The trial found that cinacalcet did not reduce the risk of death or major CVD events.115
Therapies to target FGF-23 are currently under development116,117 and will hopefully lead to clinical trials in humans in the future.
Overall, the recent trials of mineral metabolism therapies have been largely negative in patients with CKD and ESRD. No trials have been conducted to study use of these therapies during the transition from CKD to ESRD.
Evolution of cardiovascular disease from CKD through kidney transplantation
Although dialysis remains the most common form of renal replacement therapy, the number of patients with kidney transplants continues to grow. CVD remains the leading cause of death among kidney transplant recipients. The 5- year survival of adult kidney transplant recipients (KTR) is 70%, with death from cardiovascular causes accounting for 30% of deaths, infectious causes accounting for 21% and malignancies for 9%.78 Risk factors for CVD among kidney transplant recipients are similar to those with the CKD population with the added effects of immunosuppressive agents, which are also linked to CVD risk factors such as hypertension and lipid abnormalities.
Few studies have examined the evolution of CVD from CKD through kidney transplant and have yielded differing results.118–123 In a study of 50 patients with CKD and ESRD, those who underwent kidney transplant did not have regression of LVMI as measured by cardiac MRI compared to those who remained on the waiting list.124 In a study of 232 patients, post-transplant echocardiograms showed improvement in ejection fraction, diastolic dysfunction and LVMI compared with pre-transplant echocardiograms.125 A study of 433 patients found that left ventricular contractility normalized, left ventricular hypertrophy regressed and left ventricular dilation improved after kidney transplant.119 Another study also showed improvement in left ventricular structure and function after kidney transplant and reported that improved ambulatory systolic blood pressure was one of the strongest predictors for this improvement.118
In addition to echocardiogram parameters, some studies have evaluated the evolution of other subclinical CVD measures from CKD to transplant. In a study of patients from Korea with ESRD, measures of arterial stiffness by PWV were compared pre and post kidney transplant. Investigators found that mean PWV decreased/improved after kidney transplantation (1418 vs. 1517 cm/s, p<0.05).126 In a study of 31 patients who underwent kidney transplantation, mean CAC Agatston scores significantly decreased from pre-transplantation to 6 months post transplant and correlated with a decrease in PTH and Ca × P product.127
Overall, the current body of literature suggests a possible improvement in subclinical CVD measures after kidney transplant. Further studies are needed to elucidate mechanisms and therapies to reduce risk of post-transplant CVD events and death.
Conclusions
The transition from CKD through ESRD is a physiologically complicated, important yet understudied period associated with overall progression and heightened risk of subclinical and clinical CVD. Much of the data on CVD risk is extrapolated from cross-sectional studies conducted in CKD and ESRD populations, study designs that are susceptible to survivor bias, rather than longitudinal studies of patients with CKD who progress to ESRD. Unfortunately, most of the clinical trials to mitigate CVD risk have been largely negative in patients with kidney disease. Thus, future directions should include further studies to more comprehensively characterize the evolution and risk factors of CVD as well as test therapies to improve CVD risk specifically during the transition from CKD to ESRD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Hui X, Matsushita K, Sang Y, Ballew SH, Fulop T, Coresh J. CKD and cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study: interactions with age, sex, and race. Am J Kidney Dis. 2013;62(4):691–702. doi: 10.1053/j.ajkd.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. The lancet. Diabetes & endocrinology. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.http://www.usrds.org
- 7.Foley RN, Herzog CA, Collins AJ. Smoking and cardiovascular outcomes in dialysis patients: the United States Renal Data System Wave 2 study. Kidney Int. 2003;63(4):1462–1467. doi: 10.1046/j.1523-1755.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- 8.Harel Z, Wald R, McArthur E, et al. Rehospitalizations and Emergency Department Visits after Hospital Discharge in Patients Receiving Maintenance Hemodialysis. J Am Soc Nephrol. 2015;26(12):3141–3150. doi: 10.1681/ASN.2014060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson KF, Winkelmayer WC, Chertow GM, Bhattacharya J. Physician visits and 30-day hospital readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2014;25(9):2079–2087. doi: 10.1681/ASN.2013080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall RK, Toles M, Massing M, et al. Utilization of acute care among patients with ESRD discharged home from skilled nursing facilities. Clin J Am Soc Nephrol. 2015;10(3):428–434. doi: 10.2215/CJN.03510414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 2005;68(2):818–825. doi: 10.1111/j.1523-1755.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 12.Parekh RS, Plantinga LC, Kao WH, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int. 2008;74(10):1335–1342. doi: 10.1038/ki.2008.449. [DOI] [PubMed] [Google Scholar]
- 13.Whitman IR, Feldman HI, Deo R. CKD and Sudden Cardiac Death: Epidemiology, Mechanisms, and Therapeutic Approaches. J Am Soc Nephrol. 2012;23(12):1929–1939. doi: 10.1681/ASN.2012010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6(11):2642–2649. doi: 10.2215/CJN.03680411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2(1):89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 16.Bansal N, Keane M, Delafontaine P, et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol. 2013;8(3):355–362. doi: 10.2215/CJN.06020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339(12):799–805. doi: 10.1056/NEJM199809173391203. [DOI] [PubMed] [Google Scholar]
- 18.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. 1996;49(5):1379–1385. doi: 10.1038/ki.1996.194. [DOI] [PubMed] [Google Scholar]
- 19.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11(7):1277–1285. [PubMed] [Google Scholar]
- 20.Foley RNPP, Harnett JD, Kent GM, Murray DC, Barre PE. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5:2024–2031. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- 21.Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36(2):286–290. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 22.Yamada S, Ishii H, Takahashi H, et al. Prognostic Value of Reduced Left Ventricular Ejection Fraction at Start of Hemodialysis Therapy on Cardiovascular and All-Cause Mortality in EndStage Renal Disease Patients. Clin J Am Soc Nephrol. 2010 doi: 10.2215/CJN.00050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu IW, Hung MJ, Chen YC, et al. Ventricular function and all-cause mortality in chronic kidney disease patients with angiographic coronary artery disease. J Nephrol. 2010;23(2):181–188. [PubMed] [Google Scholar]
- 24.Cai QZ, Lu XZ, Lu Y, Wang AY. Longitudinal changes of cardiac structure and function in CKD (CASCADE study) J Am Soc Nephrol. 2014;25(7):1599–1608. doi: 10.1681/ASN.2013080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganda A, Weiner SD, Chudasama NL, et al. Echocardiographic changes following hemodialysis initiation in patients with advanced chronic kidney disease and symptomatic heart failure with reduced ejection fraction. Clin Nephrol. 2012;77(5):366–375. doi: 10.5414/cn107169. [DOI] [PubMed] [Google Scholar]
- 26.Whalley GA, Marwick TH, Doughty RN, et al. Effect of early initiation of dialysis on cardiac structure and function: results from the echo substudy of the IDEAL trial. Am J Kidney Dis. 2013;61(2):262–270. doi: 10.1053/j.ajkd.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Kuzela DC, Huffer WE, Conger JD, Winter SD, Hammond WS. Soft tissue calcification in chronic dialysis patients. The American journal of pathology. 1977;86(2):403–424. [PMC free article] [PubMed] [Google Scholar]
- 28.Tuttle KR, Short RA. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin J Am Soc Nephrol. 2009;4(12):1968–1973. doi: 10.2215/CJN.01250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox CS, Larson MG, Keyes MJ, et al. Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: the Framingham Heart Study. Kidney Int. 2004;66(5):2017–2021. doi: 10.1111/j.1523-1755.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 30.Kramer H, Toto R, Peshock R, Cooper R, Victor R. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol. 2005;16(2):507–513. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 31.Budoff MJ, Rader DJ, Reilly MP, et al. Relationship of Estimated GFR and Coronary Artery Calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2011;58(4):519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J, Reilly M, Yang W, et al. Risk factors for coronary artery calcium among patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study) Am J Cardiol. 2012;110(12):1735–1741. doi: 10.1016/j.amjcard.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe R, Lemos MM, Manfredi SR, Draibe SA, Canziani ME. Impact of cardiovascular calcification in nondialyzed patients after 24 months of follow-up. Clin J Am Soc Nephrol. 2010;5(2):189–194. doi: 10.2215/CJN.06240909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malluche HH, Blomquist G, Monier-Faugere MC, Cantor TL, Davenport DL. High Parathyroid Hormone Level and Osteoporosis Predict Progression of Coronary Artery Calcification in Patients on Dialysis. J Am Soc Nephrol. 2015;26(10):2534–2544. doi: 10.1681/ASN.2014070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan AM, Chirinos JA, Litt H, Yang W, Rosas SE. FGF-23 and the progression of coronary arterial calcification in patients new to dialysis. Clin J Am Soc Nephrol. 2012;7(12):2017–2022. doi: 10.2215/CJN.02160212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sigrist MK, Taal MW, Bungay P, McIntyre CW. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol. 2007;2(6):1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 37.Ilyas B, Dhaun N, Markie D, et al. Renal function is associated with arterial stiffness and predicts outcome in patients with coronary artery disease. QJM. 2009;102(3):183–191. doi: 10.1093/qjmed/hcn171. [DOI] [PubMed] [Google Scholar]
- 38.Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45(3):494–501. doi: 10.1053/j.ajkd.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Kawamoto R, Kohara K, Tabara Y, et al. An association between decreased estimated glomerular filtration rate and arterial stiffness. Intern Med. 2008;47(7):593–598. doi: 10.2169/internalmedicine.47.0825. [DOI] [PubMed] [Google Scholar]
- 40.Townsend RR, Wimmer NJ, Chirinos JA, et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23(3):282–289. doi: 10.1038/ajh.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 42.Guerin AP, Pannier B, Marchais SJ, London GM. Cardiovascular disease in the dialysis population: prognostic significance of arterial disorders. Curr Opin Nephrol Hypertens. 2006;15(2):105–110. doi: 10.1097/01.mnh.0000203186.11772.21. [DOI] [PubMed] [Google Scholar]
- 43.Safar ME, Blacher J, Pannier B, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39(3):735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 44.Chirinos JA, Khan A, Bansal N, et al. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail. 2014;7(5):709–716. doi: 10.1161/CIRCHEARTFAILURE.113.001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeLoach SS, Townsend RR. Vascular stiffness: its measurement and significance for epidemiologic and outcome studies. Clin J Am Soc Nephrol. 2008;3(1):184–192. doi: 10.2215/CJN.03340807. [DOI] [PubMed] [Google Scholar]
- 46.Bansal N, McCulloch CE, Rahman M, et al. Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: the chronic renal insufficiency cohort study. Hypertension. 2015;65(1):93–100. doi: 10.1161/HYPERTENSIONAHA.114.04334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang TI, Zheng Y, Montez-Rath ME, Winkelmayer WC. Antihypertensive Medication Use in Older Patients Transitioning from Chronic Kidney Disease to End-Stage Renal Disease on Dialysis. Clin J Am Soc Nephrol. 2016 doi: 10.2215/CJN.10611015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molnar MZ, Gosmanova EO, Sumida K, et al. Predialysis Cardiovascular Disease Medication Adherence and Mortality After Transition to Dialysis. American Journal of Kidney Diseases. doi: 10.1053/j.ajkd.2016.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St Peter WL, Sozio SM, Shafi T, et al.
- 50.Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55(3):762–768. doi: 10.1161/HYPERTENSIONAHA.109.144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in u.s. Veterans with chronic kidney disease: a cohort study. Ann Intern Med. 2013;159(4):233–242. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63(3):793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 53.Zager PG, Nikolic J, Brown RH, et al. "U" curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998;54(2):561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 54.Robinson BM, Tong L, Zhang J, et al. Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2012;82(5):570–580. doi: 10.1038/ki.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3):507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 56.Inrig JK, Patel UD, Toto RD, Szczech LA. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis. 2009;54(5):881–890. doi: 10.1053/j.ajkd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45(4):811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 58.Li Z, Lacson E, Jr, Lowrie EG, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48(4):606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Mazzuchi N, Carbonell E, Fernandez-Cean J. Importance of blood pressure control in hemodialysis patient survival. Kidney Int. 2000;58(5):2147–2154. doi: 10.1111/j.1523-1755.2000.00388.x. [DOI] [PubMed] [Google Scholar]
- 60.Chang TI, Friedman GD, Cheung AK, Greene T, Desai M, Chertow GM. Systolic blood pressure and mortality in prevalent haemodialysis patients in the HEMO study. J Hum Hypertens. 2011;25(2):98–105. doi: 10.1038/jhh.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duranti E, Imperiali P, Sasdelli M. Is hypertension a mortality risk factor in dialysis? Kidney Int Suppl. 1996;55:S173–174. [PubMed] [Google Scholar]
- 62.Bansal N, McCulloch CE, Rahman M, et al. Blood Pressure and Risk of All-Cause Mortality in Advanced Chronic Kidney Disease and Hemodialysis: The Chronic Renal Insufficiency Cohort Study. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.04334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38(6):1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 64.A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 66.Heerspink HJ, Ninomiya T, Zoungas S, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373(9668):1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki H, Kanno Y, Sugahara S, et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52(3):501–506. doi: 10.1053/j.ajkd.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi A, Takase H, Toriyama T, et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis–a randomized study. Nephrol Dial Transplant. 2006;21(9):2507–2512. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 69.Tepel M, Hopfenmueller W, Scholze A, Maier A, Zidek W. Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant. 2008;23(11):3605–3612. doi: 10.1093/ndt/gfn304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70(7):1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 71.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41(9):1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 72.Cice G, Ferrara L, Di Benedetto A, et al. Dilated cardiomyopathy in dialysis patients–beneficial effects of carvedilol: a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2001;37(2):407–411. doi: 10.1016/s0735-1097(00)01158-x. [DOI] [PubMed] [Google Scholar]
- 73.Gul A, Miskulin D, Gassman J, et al. Design of the Blood Pressure in Dialysis Pilot Study. Am J Med Sci. 2013 doi: 10.1097/MAJ.0b013e31827daee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ku E, Glidden DV, Johansen KL, et al. Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney Int. 2015;87(5):1055–1060. doi: 10.1038/ki.2014.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 76.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 77.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.http://www.usrds.org/adr.htm.
- 79.Cannizzaro LA, Piccini JP, Patel UD, Hernandez AF. Device therapy in heart failure patients with chronic kidney disease. J Am Coll Cardiol. 2011;58(9):889–896. doi: 10.1016/j.jacc.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 80.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 81.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 82.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341(25):1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 83.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 84.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337(22):1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 85.Bansch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT) Circulation. 2002;105(12):1453–1458. doi: 10.1161/01.cir.0000012350.99718.ad. [DOI] [PubMed] [Google Scholar]
- 86.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 87.Pun PH, Al-Khatib SM, Han JY, et al. Implantable Cardioverter-Defibrillators for Primary Prevention of Sudden Cardiac Death in CKD: A Meta-analysis of Patient-Level Data From 3 Randomized Trials. Am J Kidney Dis. 2014 doi: 10.1053/j.ajkd.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldenberg I, Moss AJ, McNitt S, et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98(4):485–490. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 89.Singh SM, Wang X, Austin PC, Parekh RS, Lee DS. for the Ontario ICDDI Prophylactic Debrillators in Patients with Severe Chronic Kidney Disease. JAMA internal medicine. doi: 10.1001/jamainternmed.2014.1208. [DOI] [PubMed] [Google Scholar]
- 90.Charytan DM, Patrick AR, Liu J, et al. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis. 2011;58(3):409–417. doi: 10.1053/j.ajkd.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 91.Sakhuja R, Keebler M, Lai TS, McLaughlin Gavin C, Thakur R, Bhatt DL. Meta-analysis of mortality in dialysis patients with an implantable cardioverter defibrillator. Am J Cardiol. 2009;103(5):735–741. doi: 10.1016/j.amjcard.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 92.Aggarwal A, Wang Y, Rumsfeld JS, Curtis JP, Heidenreich PA, National Cardiovascular Data R Clinical characteristics and in-hospital outcome of patients with end-stage renal disease on dialysis referred for implantable cardioverter-defibrillator implantation. Heart rhythm : the official journal of the Heart Rhythm Society. 2009;6(11):1565–1571. doi: 10.1016/j.hrthm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 93.Tompkins C, McLean R, Cheng A, et al. End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol. 2011;22(10):1099–1104. doi: 10.1111/j.1540-8167.2011.02066.x. [DOI] [PubMed] [Google Scholar]
- 94.Cooper BA, Branley P, Bulfone L, et al. A Randomized, Controlled Trial of Early versus Late Initiation of Dialysis. New England Journal of Medicine. 2010;363(7):609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 95.Locatelli F, Marcelli D, Conte F, et al. Survival and development of cardiovascular disease by modality of treatment in patients with end-stage renal disease. J Am Soc Nephrol. 2001;12(11):2411–2417. doi: 10.1681/ASN.V12112411. [DOI] [PubMed] [Google Scholar]
- 96.Trespalacios FC, Taylor AJ, Agodoa LY, Bakris GL, Abbott KC. Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis. 2003;41(6):1267–1277. doi: 10.1016/s0272-6386(03)00359-7. [DOI] [PubMed] [Google Scholar]
- 97.Johnson DW, Dent H, Hawley CM, et al. Association of dialysis modality and cardiovascular mortality in incident dialysis patients. Clin J Am Soc Nephrol. 2009;4(10):1620–1628. doi: 10.2215/CJN.01750309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang IK, Lu CY, Lin CL, et al. Comparison of the risk of de novo cardiovascular disease between hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Int J Cardiol. 2016;218:219–224. doi: 10.1016/j.ijcard.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 99.Ganesh SK, Hulbert-Shearon T, Port FK, Eagle K, Stack AG. Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol. 2003;14(2):415–424. doi: 10.1097/01.asn.0000043140.23422.4f. [DOI] [PubMed] [Google Scholar]
- 100.Saito H, Kusano K, Kinosaki M, et al. Human fibroblast growth factor-23 mutants suppress Na+− dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278(4):2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 101.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117(12):4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 103.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5(2):286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91(8):3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 105.Bosworth CR, Levin G, Robinson-Cohen C, et al. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012;82(6):693–700. doi: 10.1038/ki.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Covic A, Voroneanu L, Goldsmith D. The effects of vitamin D therapy on left ventricular structure and function - are these the underlying explanations for improved CKD patient survival? Nephron Clin Pract. 2010;116(3):c187–195. doi: 10.1159/000317198. [DOI] [PubMed] [Google Scholar]
- 107.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 109.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 Associates with Death, Cardiovascular Events, and Initiation of Chronic Dialysis. Journal of the American Society of Nephrology : JASN. 2011;22(10):1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kendrick J, Cheung AK, Kaufman JS, et al. Associations of plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. Am J Kidney Dis. 2012;60(4):567–575. doi: 10.1053/j.ajkd.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jamal SA, Vandermeer B, Raggi P, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382(9900):1268–1277. doi: 10.1016/S0140-6736(13)60897-1. [DOI] [PubMed] [Google Scholar]
- 112.Tonelli M, Wiebe N, Culleton B, et al. Systematic review of the clinical efficacy and safety of sevelamer in dialysis patients. Nephrol Dial Transplant. 2007;22(10):2856–2866. doi: 10.1093/ndt/gfm421. [DOI] [PubMed] [Google Scholar]
- 113.Miskulin DC, Majchrzak K, Tighiouart H, et al. Ergocalciferol Supplementation in Hemodialysis Patients With Vitamin D Deficiency: A Randomized Clinical Trial. J Am Soc Nephrol. 2016;27(6):1801–1810. doi: 10.1681/ASN.2015040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. Jama. 2012;307(7):674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 115.Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 116.Imel EA, Zhang X, Ruppe MD, et al. Prolonged Correction of Serum Phosphorus in Adults With X-Linked Hypophosphatemia Using Monthly Doses of KRN23. J Clin Endocrinol Metab. 2015;100(7):2565–2573. doi: 10.1210/jc.2015-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carpenter TO, Imel EA, Ruppe MD, et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest. 2014;124(4):1587–1597. doi: 10.1172/JCI72829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ferreira SR, Moises VA, Tavares A, Pacheco-Silva A. Cardiovascular effects of successful renal transplantation: a 1-year sequential study of left ventricular morphology and function, and 24-hour blood pressure profile. Transplantation. 2002;74(11):1580–1587. doi: 10.1097/00007890-200212150-00016. [DOI] [PubMed] [Google Scholar]
- 119.Parfrey PS, Harnett JD, Foley RN, et al. Impact of renal transplantation on uremic cardiomyopathy. Transplantation. 1995;60(9):908–914. [PubMed] [Google Scholar]
- 120.Zolty R, Hynes PJ, Vittorio TJ. Severe left ventricular systolic dysfunction may reverse with renal transplantation: uremic cardiomyopathy and cardiorenal syndrome. Am J Transplant. 2008;8(11):2219–2224. doi: 10.1111/j.1600-6143.2008.02407.x. [DOI] [PubMed] [Google Scholar]
- 121.Melchor JL, Espinoza R, Gracida C. Kidney transplantation in patients with ventricular ejection fraction less than 50 percent: features and posttransplant outcome. Transplant Proc. 2002;34(7):2539–2540. doi: 10.1016/s0041-1345(02)03478-4. [DOI] [PubMed] [Google Scholar]
- 122.Wali RK, Wang GS, Gottlieb SS, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005;45(7):1051–1060. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 123.Oppert M, Schneider U, Bocksch W, et al. Improvement of left ventricular function and arterial blood pressure 1 year after simultaneous pancreas kidney transplantation. Transplant Proc. 2002;34(6):2251–2252. doi: 10.1016/s0041-1345(02)03223-2. [DOI] [PubMed] [Google Scholar]
- 124.Patel RK, Mark PB, Johnston N, McGregor E, Dargie HJ, Jardine AG. Renal transplantation is not associated with regression of left ventricular hypertrophy: a magnetic resonance study. Clin J Am Soc Nephrol. 2008;3(6):1807–1811. doi: 10.2215/CJN.01400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hawwa N, Shrestha K, Hammadah M, Yeo PS, Fatica R, Tang WH. Reverse Remodeling and Prognosis Following Kidney Transplantation in Contemporary Patients With Cardiac Dysfunction. J Am Coll Cardiol. 2015;66(16):1779–1787. doi: 10.1016/j.jacc.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim HS, Seung J, Lee JH, Chung BH, Yang CW. Clinical Significance of Pre-Transplant Arterial Stiffness and the Impact of Kidney Transplantation on Arterial Stiffness. PLoS One. 2015;10(9):e0139138. doi: 10.1371/journal.pone.0139138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abedi SA, Tarzamni MK, Nakhjavani MR, Bohlooli A. Effect of renal transplantation on coronary artery calcification in hemodialysis patients. Transplant Proc. 2009;41(7):2829–2831. doi: 10.1016/j.transproceed.2009.07.037. [DOI] [PubMed] [Google Scholar]


