Abstract
Background
Cystic fibrosis (CF) is a chronic catabolic disease often requiring hospitalization for acute episodes of worsening pulmonary exacerbations. Limited data suggest that vitamin D may have beneficial clinical effects, but the impact of vitamin D on systemic metabolism in this setting is unknown.
Objective
We used high-resolution metabolomics (HRM) to assess the impact of baseline vitamin D status and high-dose vitamin D3 administration on systemic metabolism in adults with CF with an acute pulmonary exacerbation.
Design
Twenty-five hospitalized adults with CF were enrolled in a randomized trial of high-dose vitamin D3 (250,000 IU vitamin D3 bolus) versus placebo. Age-matched healthy subjects served as a reference group for baseline comparisons. Plasma was analyzed with liquid chromatography/ultra-high resolution mass spectrometry. Using recent HRM bioinformatics and metabolic pathway enrichment methods, we examined associations with baseline vitamin D status (sufficient vs deficient per serum 25-hydroxyvitamin D concentrations) and the 7-day response to vitamin D3 supplementation.
Results
Several amino acids and lipid metabolites differed between CF and healthy control subjects, indicative of an overall catabolic state. In CF subjects, 343 metabolites differed (P<0.05) by baseline vitamin D status and were enriched within 7 metabolic pathways including fatty acid, amino acid, and carbohydrate metabolism. A total of 316 metabolites, which showed enrichment for 15 metabolic pathways--predominantly representing amino acid pathways-- differed between the vitamin D3- and placebo-treated CF subjects over time (P<0.05). In the placebo group, several tricarboxylic acid cycle intermediates increased while several amino acid-related metabolites decreased; in contrast, little change in these metabolites occurred with vitamin D3 treatment.
Conclusions
Numerous metabolic pathways detected by HRM varied in association with vitamin D status and high-dose vitamin D3 supplementation in adults with CF experiencing a pulmonary exacerbation. Overall, these pilot data suggest an anti-catabolic effect of high-dose vitamin D3 in this clinical setting.
Keywords: cystic fibrosis, vitamin D, cholecalciferol, metabolomics, pulmonary exacerbation, amino acids
1. INTRODUCTION
Cystic fibrosis (CF) is a genetic disease affecting function of the CF transmembrane conductance regulator protein, resulting in defective trans-epithelial ion transport and accumulation of viscous secretions in the respiratory, digestive, and other systems (1). Progressive decline in lung structural integrity and function, with repeat pulmonary exacerbations contributing to the rapid decline, is the primary cause of morbidity and mortality. Beyond its lung manifestation, CF is a complex disorder with numerous co-morbidities and metabolic alterations, including gut malabsorption and nutrient deficiencies, loss of lean body mass, glucose intolerance, inflammation, oxidative stress, and abnormalities in fatty acid metabolism (2,3). Detailed knowledge of the metabolic derangements of CF and episodes of pulmonary exacerbation is important, particularly as new therapies for CF are introduced.
Vitamin D is being tested as an adjunctive treatment strategy for pulmonary exacerbations in CF (4,5). Genome- and transcriptome-wide studies support broad effects of vitamin D on target tissues in health and disease, independent of classical effects on calcium absorption and bone biology (6–9). These vitamin D-related effects are relevant to CF because vitamin D deficiency is very common in these individuals (10). Adequate vitamin D status in CF, as determined by blood 25-hydroxyvitamin D [25(OH)D] concentrations, correlates with improved lung function (11,12), reduced pulmonary exacerbations (12) and hospitalizations (13), improved glucose tolerance (14), reduced inflammation (15) and decreased lung bacterial colonization (16). In a pilot clinical trial, we showed that high-dose vitamin D3, given to hospitalized CF patients during an acute pulmonary exacerbation, reduced inflammation and improved one-year survival (4,5); however, the impact of underlying vitamin D status and high-dose vitamin D administration on systemic metabolism in CF remains unknown. Two recent metabolomics studies in non-CF subjects explored the impact of vitamin D status in pregnant adolescents (17) and Finnish male smokers (18), respectively. These cross-sectional studies identified plasma metabolites spanning a wide-range of metabolic pathways associated with vitamin D status, including bile acid, fatty acid, eicosanoid and other lipid metabolism (17,18).
In the present study, we applied high-resolution metabolomics (HRM) methods to study metabolic changes in a subset of subjects from our recent double-blind, randomized pilot study of high-dose vitamin D3 administration in adult CF patients hospitalized for an acute pulmonary exacerbation (4,5). HRM methods use liquid chromatography coupled to ultra-high resolution mass spectrometry with advanced data extraction algorithms and biostatistics/bioinformatics and pathway enrichment analysis (19,20). Our aims were to 1) obtain an understanding of metabolic differences between acutely ill CF subjects and a healthy, age-matched reference group, 2) conduct a plasma metabolome-wide association study of vitamin D status in this adult CF population prior to vitamin D supplementation, and 3) compare high-dose vitamin D supplemented individuals to placebo-treated controls to identify metabolites and pathways impacted by this vitamin D treatment protocol.
2. SUBJECTS AND METHODS
2.1 Participants
Subjects were patients with CF who were hospitalized for an acute pulmonary exacerbation and enrolled in a double-blind, randomized, placebo-controlled, high-dose vitamin D3 supplementation trial, as previously described in detail (clinicatrials.gov NCT00788138) (4,5). In brief, within 48 hours of hospitalization, subjects with CF were sequentially screened for the following inclusion criteria: ≥18 yrs of age, diagnosis of acute pulmonary exacerbation by a pulmonologist, serum 25(OH)D < 75 ng/mL in the previous year, and current intake of supplemental vitamin D < 2,000 IU/day. Exclusion criteria were: history of disorder influencing vitamin D, calcium, or phosphorus metabolism; history of organ transplant; and current pregnancy or plans to become pregnant during trial. Diagnosis of pulmonary exacerbation by pulmonologists was based on standard clinical criteria including an aggregate of symptoms such as a decrease in lung function and/or increased sputum production, cough, crackles on auscultation, and/or weight loss. Following informed consent, subjects were randomly assigned to receive a single, oral bolus capsule containing 250,000 IU vitamin D3 (cholecalciferol) or a matching placebo capsule, dispensed by the Investigational Drug Service pharmacist in a double-blind manner using a computer-generated block randomization scheme (blocks of six). Clinical data were obtained from electronic medical records and a national CF patient registry maintained by the Cystic Fibrosis Foundation. All CF subjects underwent standard-of-care treatment for acute pulmonary exacerbations, as per their CF medical care team. Non-fasted plasma in the CF subjects was obtained for metabolomics analysis prior to study drug administration (baseline) and again one week following study drug administration. Only CF subjects with available plasma for metabolomics were included in the current study (see Supplemental Figure 1 for participant flow diagram). Serum 25(OH)D and parathyroid hormone (PTH) were measured with ELISA (Immunodiagnostic Systems, Ltd, Gaithersburg, MD and Immutopics, Inc, San Clemente, CA), as previously reported (4). Serum calcium was assessed using standard clinical chemistry methods in the Emory University Hospital laboratory. Based on current evidence-based recommendations from the Cystic Fibrosis Foundation, subjects were classified as vitamin D sufficient if their serum 25(OH)D concentration was ≥30 ng/mL, and as vitamin D insufficient if their serum 25(OH)D concentration was <30 ng/mL (21). Baseline data from CF subjects were compared to baseline data from non-fasted age-matched adults enrolled in a separate vitamin D supplementation trial (clinicaltrials.gov NCT01924910) (22). The studies were reviewed and approved by the Emory Institutional Review Board, and informed consent was obtained prior to any study procedures.
2.2 High-resolution metabolomics
Blood was collected in EDTA-containing tubes (BD Vacutainer®, Franklin Lakes, NJ, USA) and centrifuged at 1300 RCF for 10 min. Following separation, plasma was stored at −80°C until analysis. De-identified samples were randomized (by a computer-generated list) into blocks of 20 samples prior to transfer to the analytical laboratory where personnel were blinded to clinical and demographic data and treatment allocation. Samples were treated with acetonitrile (2:1, v/v), an internal standard mixture was added, and the samples centrifuged at 4°C, using established procedures (23,24). Each batch of 20 samples was preceded and followed by analysis of pooled reference samples to support quality control and quality assurance. Samples were analyzed in triplicate with high-performance liquid chromatography coupled to ultra-high resolution mass spectrometry (LC-MS; LTQ–Velos Orbitrap, Thermo Scientific, San Diego, CA, USA) using C18 chromatography and electrospray ionization in positive ion mode. Data were detected over 10 min using a formic acid/acetonitrile gradient with the mass spectrometer set to scan from 85 to 2000 m/z. For simplicity, “ions” are hereafter described as “metabolites”, recognizing that multiple ions can be derived from the same metabolite. Statistical and bioinformatics analyses (described below) were performed using m/z, associated retention time and intensity data to define metabolites, without requirement that measured ions have confirmed identity. The pooled reference plasma samples were calibrated to National Institute of Standards and Technology Standard Reference Material plasma (NIST SRM 1950) (25). Data extraction and peak alignment was completed using apLCMS (26) with data quality evaluation by xMSanalyzer (27). Default settings were used, and one CF subject was removed from metabolomics analyses due to poor reproducibility (correlation < 0.70 within technical replicates).
2.3 Quantification of Health Biomarkers
Identities of selected plasma amino acids, metabolic intermediates, nutrition and clinical health indicators were verified by ion dissociation tandem mass spectrometry (MS/MS) and co-elution of standards (28–30). Metabolite concentrations were determined using reference standardization relative to pooled reference plasma calibrated to NIST SRM1950 (28,30). Each metabolite concentration was calculated for each sample using the ion intensity and known concentration for the respective m/z in the pooled reference sample.
2.4 Statistical Analyses and Bioinformatics
Descriptive statistics of baseline demographic and clinical variables were performed for the CF and healthy reference groups and compared using a two-tailed t-test for continuous variables or Fisher’s exact test for categorical variables, as were vitamin D status and treatment groups within the CF subjects (JMP Pro 10.0.0, SAS Institute Inc., Cary, NC, USA).
Technical replicates were averaged, log2-transformed, quantile normalized, and filtered to include only metabolites that were present in ≥80% of subjects within at least one analytical group. Missing metabolomic data were imputed as half the value of the lowest observed intensity for each metabolite. Linear models for microarray data (LIMMA) (31) was used to assess metabolite differences between CF patients dichotomized by vitamin D status, i.e., vitamin D sufficient versus insufficient. Unsupervised principal component analysis (PCA) and two-way hierarchical cluster analysis (HCA) of differentially expressed metabolites were used to visualize metabolite and subject clusters co-regulated as a function of baseline vitamin D status. The effects of high-dose vitamin D3 supplementation on the plasma metabolome over 1 week were assessed relative to placebo-treated patients by linear mixed-effects models, adjusting for age and genotype (analyzed as a binary variable distinguishing patients homozygous for the most common mutation, F508Del, and others). Differences in plasma metabolites between vitamin D treatment groups over 1 week were examined with the treatment-by-time interaction effect. The false discovery rate (FDR) procedure of Benjamini and Hochberg (32) was applied to analyses to adjust for multiple comparisons. Because of the small number of subjects in this pilot study, some comparisons did not yield any FDR-significant metabolites. For these cases, metabolites with raw P<0.05 were used for subsequent analyses, with statistical tests for pathway enrichment. This approach provides a balance between type I and type II statistical error (33).
We used mummichog for pathway enrichment analyses (34). Mummichog annotates metabolites based on accurate mass m/z and tests significant pathway enrichment within a reference metabolic network using a Fisher’s exact test. To protect against incorrect pathway selection, redundant pathways or those enriched by less than four metabolites were excluded. Mummichog also includes metabolite module analysis based on provided inputs allowing for further exploration of the pathway enrichment results.
3. RESULTS
A total of 24 subjects with CF and 28 age-matched healthy subjects were included. Baseline demographic and clinical characteristics are detailed in Table 1. Compared to the healthy reference group, a higher proportion of CF subjects were male, had a lower BMI, and a lower prevalence of vitamin D insufficiency (all P<0.01). In CF subjects, the mean body mass index (BMI) was below CF recommendations (35) at 20.3 kg/m2. The majority of CF patients had pancreatic insufficiency and were homozygous for the F508Del allele, and 52% had CF- related diabetes. The mean percentage of predicted forced expiratory volume in 1 s (FEV1% predicted) was 43%, indicating severe lung disease. Demographic variables did not differ between the CF vitamin D status groups. As expected (36,37), serum PTH concentrations were higher in the vitamin D insufficient group at baseline (P = 0.04). Subjects in the vitamin D3 treatment subgroup were younger than the placebo-treated CF subgroup (25 versus 32 yr, P<0.05).
Table 1.
Baseline Characteristics of Participants with Cystic Fibrosis vs Healthy Reference Subjects
| Healthy Subjects (n=28) | All CF (N = 24) | Baseline vitamin D insufficient1 (n = 12) | Baseline vitamin D sufficient (n = 12) | Vitamin D (n = 12) | Placebo (n = 12) | |
|---|---|---|---|---|---|---|
| Age (yr) | 28± 6 | 29 ± 9 | 30 ± 9 | 27 ± 9 | 25 ± 54 | 32 ± 10 |
| Male (%) | 6 (21)b | 16 (67) | 10 (83) | 6 (50) | 8 (67) | 8 (67) |
| Caucasian (%) | 18 (64.3) | 21 (88) | 9 (75) | 12 (100) | 12 (100) | 9 (75) |
| BMI (kg/m2) | 22.9 ± 0.62 | 20.3 ± 3.8 | 20.9 ± 4.5 | 19.7 ± 3.2 | 19.5 ± 3.4 | 21.1 ± 4.3 |
| F508Del homozygous (%) | ------------- | 16 (64) | 8 (67) | 8 (67) | 6 (50) | 10 (83) |
| Pancreatic insufficient (%) | ------------- | 22 (92) | 11 (92) | 11 (92) | 10 (83) | 12 (100) |
| CF-related diabetes (%) | ------------- | 13 (52) | 5 (42) | 8 (67) | 5 (42) | 8 (67) |
| FEV1(% predicted) | ------------- | 43 ± 27 | 41 ± 23 | 45 ± 31 | 47 ± 34 | 39 ± 18 |
| Spring/Summer season of blood draw (%) | 0 (0)2 | 14 (58) | 6 (43) | 8 (57) | 8 (57) | 6 (43) |
| 25(OH)D (ng/mL) | 18 ± 62 | 28 ± 13 | 18 ± 73 | 38 ± 8 | 30 ± 12 | 26 ± 14 |
| Vitamin D insufficient1 | 26 (93)2 | 12 (50) | --------- | --------- | 4 (33) | 8 (67) |
| Calcium (mg/dL) | ------------- | 8.8 (0.6) | 8.6 (0.7) | 8.9 (0.4) | 8.9 (0.4) | 8.7 (0.7) |
| Parathyroid hormone (pg/mL) | ------------- | 64.8 (56.5) | 88.0 (63.4)3 | 41.5 (38.5) | 47.9 (38.9) | 81.6 (67.4) |
Defined as serum 25(OH)D < 30 ng/mL.
Significantly different from CF subjects at P<0.01.
Significantly different from vitamin D sufficient group at P<0.05.
Significantly different from placebo group at P<0.05.
3.1 Baseline Nutrition and Health Biomarkers
To gain an understanding of the general severity of metabolic disruption associated with acute pulmonary exacerbation and hospitalization in adult CF, 36 metabolites were quantified for comparison to healthy controls and existing reference human plasma data compiled in the Human Metabolomics Database (HMDB) (20) (Table 2). Seventeen out of 36 plasma metabolites were significantly different from the reference healthy population and 10 had mean values outside the reference value ranges available in HMDB. Several essential amino acids (lysine, methionine, tryptophan) and 5-oxoproline were significantly lower in CF subjects compared to the reference healthy subjects at baseline, independent of sex, BMI, and vitamin D sufficiency status (Table 2). The clinical health indicators, creatinine, creatine, and glucose, were higher in CF subjects. Likewise, markers of fatty acid and lipid metabolism (choline, several phosphatidylcholines, sphingosine, and sphinganine) were generally higher in CF subjects.
Table 2.
Quantified Nutrition and Health Biomarkers in Participants with Cystic Fibrosis and Healthy Reference Subjects
| Metabolite | m/z | Time (s) | CF Baseline (n=24) | Healthy Subjects (n=28) | Reference Range |
|---|---|---|---|---|---|
| Amino Acids & Related Metabolites (μM)1 | |||||
| Histidine | 156.0759 | 49 | 54 ± 18↓ | 81 ± 44 | 75–143 |
| Leucine/Isoleucine | 132.1011 | 48 | 160 ± 66 | 140 ± 59 | 155–355 |
| Lysine | 147.1120 | 44 | 182 ± 85↓ | 251 ± 102 | 178–456 |
| Methionine | 150.0575 | 77 | 6 ± 8↓ | 37 ± 22 | 22–46 |
| Phenylalanine | 210.0489 | 53 | 61 ± 17↓ | 138 ± 181 | 48–169 |
| Threonine | 120.0648 | 58 | 134 ± 75 | 114 ± 53 | 102–260 |
| Tryptophan | 205.0954 | 49 | 27 ± 9↓ | 41 ± 16 | 37–60 |
| Arginine | 175.1180 | 50 | 54 ± 30↓ | 176 ± 259 | 60–224 |
| Glutamate | 148.0595 | 67 | 104 ± 76 | 113 ± 187 | 24–151 |
| Glutamine | 147.0757 | 59 | 546 ± 473↑ | 266 ± 144 | 396–645 |
| Proline | 116.0698 | 55 | 217 ± 60 | 172 ± 98 | 168–239 |
| Tyrosine | 182.0791 | 49 | 59 ± 33 | 51 ± 20 | 54–144 |
| Hippurate | 180.0646 | 81 | 1.1 ± 1.4↑ | 0.5 ± 0.5 | 0–5 |
| Indoleacrylic acid | 188.0694 | 61 | 0.011 ± 0.006 | 0.012 ± 0.006 | NA |
| Oxoproline | 130.049 | 51 | 28 ±7↓ | 65 ± 27 | 13–161 |
| Clinical Health Indicators (μM)1 | |||||
| Creatinine | 114.0654 | 55 | 123 ± 44↑ | 80 ± 19 | 56.4–108.8 |
| Creatine | 132.076 | 54 | 63 ± 39↑ | 24 ± 16 | 8.4–65.0 |
| Glucose (mM) | 203.0514 | 52 | 13 ± 3↑ | 4 ± 1 | 3.9–6.1 |
| Vitamins & Related Metabolites (μM)1 | |||||
| Methylnicotinic acid | 138.0541 | 63 | 0.05 ± 0.06↑ | 0.02 ± 0.009 | NA |
| Pyridoxine | 152.0697 | 66 | 0.2 ± 0.4↑ | 0.005 ± 0.003 | 0.007–0.06 |
| Fatty Acid Metabolism (μM)1 | |||||
| Acetyl–carnitine | 204.1198 | 52 | 3.0 ±1.1 | 3.0 ± 2.7 | 3.2–7.6 |
| Carnitine | 162.1115 | 54 | 66 ±24↑ | 23 ± 13 | 26–79 |
| Oleic acid | 283.2615 | 177 | 2717± 4643↑ | 933 ± 454 | 1240–3380 |
| Lipid Metabolism (μM)1 | |||||
| Choline | 104.1062 | 56 | 1.2 ±0.4↑ | 0.6 ± 0.1 | 6.5–12.5 |
| Glycerophosphocholine | 258.1084 | 429 | 0.5 ±0.3↓ | 1.1 ± 0.6 | NA |
| Lyso-phosphatidylcholine (20:4) | 544.3359 | 398 | 5.6 ±2.3 | 6.3 ± 6.0 | NA |
| Lyso-phosphatidylcholine (16:1) | 494.3209 | 380 | 3.4 ±1.4↑ | 2.2 ± 1.2 | NA |
| Lyso-phosphatidylcholine (20:3) | 546.3512 | 429 | 4.0 ±2.9 | 5.1 ± 5.1 | NA |
| Phosphatidylcholine (34:2) | 758.5657 | 419 | 386 ± 100↑ | 151 ± 75 | NA |
| Phosphatidylcholine (36:4) | 782.5637 | 415 | 236 ± 54↑ | 164 ± 104 | NA |
| Phosphatidylcholine (36:3) | 784.5805 | 422 | 388 ± 101↑ | 144 ± 42 | NA |
| Phosphatidylcholine (36:2) | 786.5960 | 417 | 132 ± 47↓ | 291 ± 91 | NA |
| Phosphatidylcholine (38:4) | 810.5954 | 435 | 557 ± 272↑ | 173 ± 71 | NA |
| Sphingosine | 300.2879 | 333 | 0.11 ± 0.10↑ | 0.007 ± 0.01 | 0.05–0.51 |
| Sphinganine | 302.3036 | 336 | 0.07 ± 0.07↑ | 0.03 ± 0.04 | 0.0107–0.011 |
| Nucleotide-related metabolites (μM)1 | |||||
| Hypoxanthine | 137.0449 | 56 | 10 ± 6 | 10 ± 19 | 1.3–54.5 |
| Uric acid | 169.0346 | 56 | 194 ± 67 | 217 ± 70 | 238–506 |
All values are in μM concentrations unless otherwise noted. Reference values are derived from the Human Metabolome Database (HMBD)(20). Arrows indicate statistical significance prior to adjustments for confounders and directionality (higher or lower) of concentration relative to healthy controls. Values in bold indicate statistical significance after adjustment for sex, vitamin D sufficiency status, and BMI. NA, not available
3.2 Metabolome-Wide Association Study of Baseline Vitamin D Status in Subjects with CF
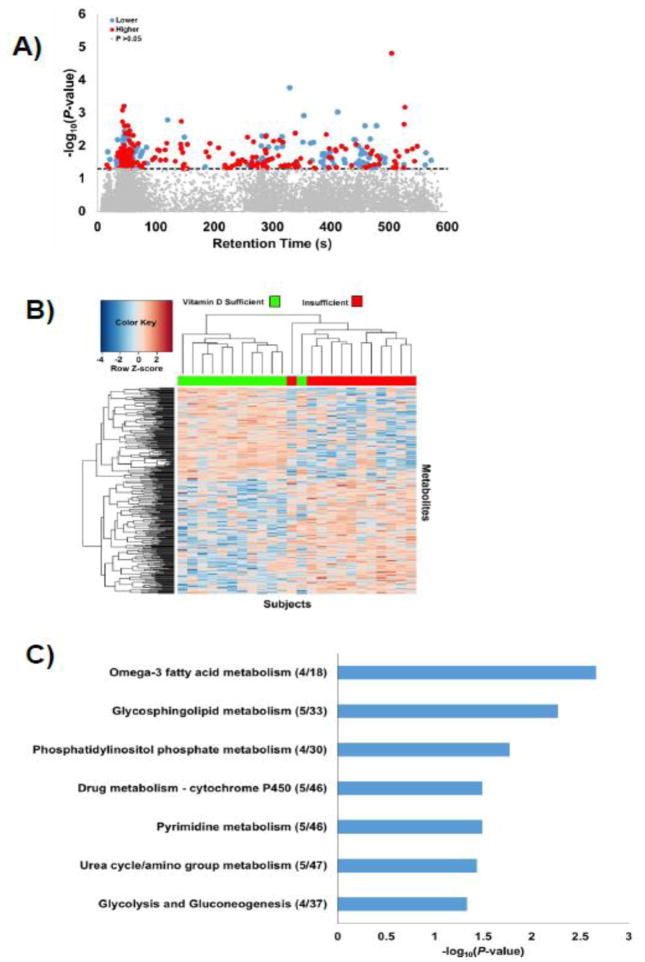
To evaluate differences between subjects with CF with sufficient versus insufficient vitamin D status, comparisons were performed baseline. We filtered data to obtain 9,828 metabolic features that were present in at least 80% of either the vitamin D sufficient or insufficient groups at baseline. A total of 343 metabolic features differed by baseline vitamin D status at raw P < 0.05 (LIMMA results, Figure 1A, Supplemental File 2); none of these differed after FDR adjustment, indicating that larger sample sizes will be needed for biomarker discovery. The retention times for elution of the metabolites varied over the entire chromatographic run, indicating that these features included a broad range of water-soluble (eluting early on the chromatographic column) and more hydrophobic chemicals (eluting later). Ten-fold cross-validation showed 100% accuracy of LIMMA results, and two-way HCA showed separation between the two CF vitamin D status groups (Figure 1B). PCA also resulted in a clear separation between vitamin D status groups, with principal components 1 and 2 explaining 95% of the total variance as well as high R2 and Q2 scores (all >0.90), reflecting goodness of fit and predictive ability, respectively. Although the extent of over-fitting of data with this small pilot study is unknown, the results show that the selected metabolic features with raw P < 0.05 discriminate the groups according to vitamin D status. Mummichog pathway enrichment analysis of differentially expressed metabolites showed that these were enriched (P < 0.05) in 7 pathways spanning a range of metabolic processes, including those related to fatty acid and glycosphingolipid metabolism, urea cycle metabolism, and glycolysis/gluconeogenesis (Figure 1C).
Figure 1. LIMMA analysis discriminates between vitamin D sufficient and insufficient adult subjects with CF.
Vitamin D sufficiency is defined as serum 25-hydroxyvitamin D [25(OH)D] ≥30 ng/mL. A total of 343 metabolites differed by vitamin D status at P < 0.05 using LIMMA. A) Type 2 Manhattan plot showing the negative log10P-value of metabolite comparisons as a function of the chromatographic retention time. Metabolites above the dotted horizontal line were significant at a P-value <0.05. Metabolites shaded in blue and red are lower and higher, respectively, in the vitamin D insufficient group relative to the sufficient group. B) Two-way hierarchical cluster analysis of significant metabolites illustrating separation of the CF subjects as a function of baseline vitamin D status. Vitamin D sufficient CF subjects are depicted in green along the upper X-axis, while vitamin D insufficient CF subjects are depicted in red. C) Mummichog pathway enrichment analysis. The number of significant metabolites over the number of possible metabolites in each pathway is provided in parentheses. Pathways with less than 4 matches were excluded. Horizontal bars depict the negative log of pathway enrichment p values.
3.3 Metabolic effects of high-dose vitamin D3 intervention
In CF subjects, serum 25(OH)D concentrations were similar between treatment groups at baseline and increased significantly in the vitamin D3 treatment group at 1 week (least squares mean ± SD: 30.2 ± 3.8 to 60.6 ± 3.8 ng/mL). In contrast, there was no change over time in serum 25(OH)D concentrations in the placebo group (least squares mean ± SD: 27.1 ± 3.7 ng/mL to 27.3 ± 3.8 ng/mL; P for group-by-time interaction <0.001). Treatment group changes in plasma PTH or calcium did not differ (P for group-by-time interaction = 0.10 and 0.71, respectively). With the exception of an increase in methionine concentrations (baseline 6.4 ± 8.1, 1 week 8.9 ± 5.8 μM), there were no significant changes in the quantified amino acid and metabolites after 1 week in the CF subjects, independent of age and study drug allocation (not shown).
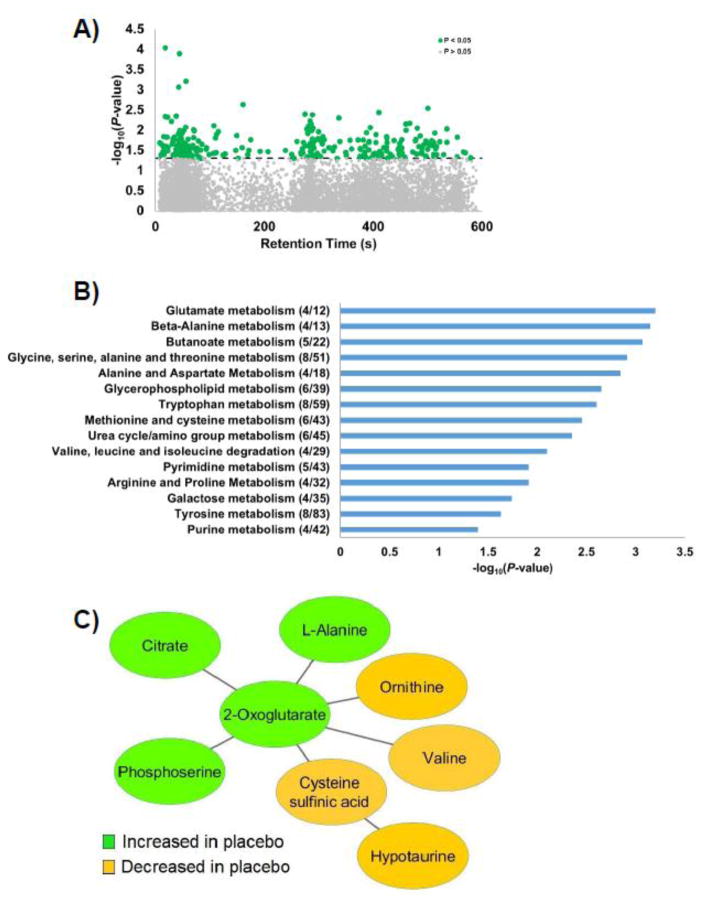
To examine metabolic effects of high-dose vitamin D3 in CF patients undergoing acute exacerbation, we filtered data to obtain 9,258 metabolites detected in at least 80% of either the vitamin D or placebo groups across both time points (baseline and 7 days). Linear mixed-effect model analyses were used to examine metabolites that differed by treatment over time. The results revealed a significant treatment group-by-time interaction for 316 metabolites (P<0.05, Figure 2A, Supplemental File 3); none were significant after FDR adjustment. Pathway enrichment analysis of these metabolites showed that 15 pathways (P<0.05) differed in the vitamin D3 treatment group relative to patients receiving the placebo (Figure 2B). These pathways spanned a variety of metabolic processes including amino acid, lipid, and carbohydrate metabolism. Pathways linked to amino acids comprised the largest proportion of pathways (67%) that differed between treatment groups over 1 week.
Figure 2. Metabolic effects of high-dose vitamin D3 treatment in hospitalized adults with CF.
Subjects were given 250,000 IU vitamin D3 or placebo within 48 h of hospital admission for pulmonary exacerbation. High-resolution metabolomics analysis was performed in plasma collected before study drug administration and 1 week later. Linear mixed-effects model analyses, adjusted for age and genotype, were conducted on all detected metabolites. Out of 9,258 metabolites present in at least 80% of either the vitamin D or placebo groups across both points, 316 metabolites demonstrated a significant group-by-time interaction (P<0.05). A) Type 2 Manhattan plot showing the negative log10P-value of the group-by-time interaction term of each metabolite as a function of the chromatographic retention time. Metabolites above dashed horizontal line were statistically significant. B) Mummichog pathway enrichment analysis of significant metabolites. The number of significant metabolites over the number of possible metabolites in each pathway are provided in parenthesis. Only pathways with 4 or more significant metabolites were included. The bar graphs indicate the negative log10P-value. C) Representative metabolites related to amino acid pathways and the TCA cycle based on mummichog module analysis (P<0.001).
Module analysis in mummichog linked several metabolites from amino acid pathways and the TCA cycle into an overarching metabolic module (P<0.001, Figure 2C). Inspection of individual metabolites indicated a pattern of intensity changes within the placebo group but with unchanged metabolite levels in the vitamin D group over time. Over the 1-week period, levels increased for 2-oxoglutarate, citrate, and phosphoserine, and decreased for L-alanine, ornithine, valine, cysteine sulfinic acid, and hypotaurine in the placebo group relative to the vitamin D3- treated group.
4. DISCUSSION
Given the pleiotropic effects of vitamin D and the increasing literature on the importance of vitamin D in cystic fibrosis (38), we sought to investigate the effects of vitamin D3 supplementation using a discovery-based, plasma HRM approach in adults with CF during an acute pulmonary exacerbation. Our data revealed alterations in multiple metabolic pathways, including those related to amino acid, carbohydrate, and lipid metabolism, which were associated with vitamin D status upon hospital admission and which changed with high-dose vitamin D3 intervention relative to placebo administration.
For an indication of how our CF study population compared to a healthy reference group before the study intervention, we used reference standardization (28–30) to quantify nutrition and health-related biomarkers detected using HRM. This approach allows for the measurement of multiple metabolites spanning a range of metabolic processes in a single plasma sample. In our CF cohort, essential amino acid concentrations were generally lower compared to healthy controls. Decreased plasma amino acids concentrations in CF patients with an acute pulmonary exacerbation is likely multifactorial, and may occur due to a decreased total body amino acid pool with skeletal muscle wasting, decreased protein intake associated with acute illness, decreased intestinal absorption, and/or increased amino acid utilization (3,39) Our results are consistent with a previous report on amino acid concentrations in CF patients aged 16 to 39 years during a pulmonary exacerbation (40). The lower concentration of oxoproline may reflect the known defects in glutathione redox balance (41) or arginine metabolism in CF (40). The elevations in creatinine, creatine, and glucose are consistent with the catabolic response and insulin resistance that occurs during infection and pulmonary exacerbation in CF (42,43), or may reflect renal changes or other dysregulation of metabolism.
Sphingosine and sphinganine were elevated in our CF subjects. Sphingolipids play an important role in the inflammatory response and are known to be dysregulated in CF (44). Phosphatidylcholine metabolites were also generally elevated compared to healthy controls, in contrast to previous studies indicating lower levels in clinically-stable CF (45,46), and may reflect the acutely ill state of our hospitalized subjects. Taken together, these HRM data provide the first detailed plasma metabolomics assessment of the catabolic and inflammatory response to acute pulmonary exacerbation in adults with CF in relation to a healthy reference group. As the CF patients were in the early phase of their pulmonary exacerbation and still hospitalized at the 1-week time point, longer-term studies are required to determine metabolic changes occurring upon partial or full recovery from a pulmonary exacerbation in adult CF subjects.
Few studies are available to compare the interactions between vitamin D and the metabolome, especially in a clinically, catabolic state such as CF. In a cohort of pregnant African American non-CF adolescents, Finklestein et al. (17) examined the metabolomic differences between subjects dichotomized by vitamin D status using a 25(OH)D concentration cut-point of 20 ng/mL. Differentiating pathways included glycolysis/gluconeogenesis, lipid metabolism (specifically bile acids, leukotriene, fatty acid dicarboxylates), and porphyrin metabolism (bilirubin) (17). In a recent study of adult smokers in Finland by Nelson et al. (18), serum 25(OH)D concentrations were linked (both positive and negative associations) with several lipid- and amino acid-related metabolic pathways (18). In the current study, we investigated metabolomic differences as a function of vitamin D status in CF subjects with a cut-point of 30 ng/mL (the recommended concentration to define vitamin D sufficiency by the CF Foundation (21)). Despite differences in study populations and analytic methods, our CF data were consistent with Finklestein et al. (17) and Nelson et al. (18) in showing that pathways associated with vitamin D status included fatty acid and glycosphingolipid metabolism, urea cycle metabolism, and glycolysis/gluconeogenesis.
The high-dose vitamin D3 intervention in our cohort of adult CF patients provides further evidence that vitamin D regulates numerous major metabolic pathways outside of the traditional skeletal effects, including those related to the metabolism of amino acids and membrane lipids. Only two previous studies have addressed effects of a vitamin D intervention on the metabolome (47,48). Stepien et al. (47) performed a targeted fatty acid profile comparison of peripheral blood mononuclear cells in 8 healthy adults who received 600 IU vitamin D3 daily for 4 weeks or placebo; differences in the measured metabolomic profile were not significant. In a similar intervention (600 IU daily x 4 weeks), O’Sullivan et al. (48) compared the pre- and post-intervention metabolome of 30 healthy subjects who had improvements in their metabolic health (as assessed by fasting insulin, insulin resistance, and C-reactive protein); highly discriminating metabolites included glycerophosphocholine, lipoproteins, glucose, taurine, and glutamine. Although the study populations and vitamin D dosing regimens were different than in our study, our data are more consistent with those of O’Sullivan et al. (48). However, the metabolic effects of vitamin D in healthy subjects may not reflect the same changes or magnitude of changes that occur in individuals with CF, particularly during a pulmonary exacerbation.
Several amino acid pathways were influenced by the vitamin D3 intervention in this study, and module analysis grouped many metabolites enriched within amino acid pathways involved in the TCA cycle. Two important intermediates within the TCA cycle, 2-oxoglutarate and citrate, increased acutely in the placebo group but remained stable over time in the vitamin D3-treated CF group. Other amino acids and related metabolites within the module generally decreased in the placebo group and remained stable in the vitamin D3 group. These included ornithine, a precursor of citrulline and arginine, as well as hypotaurine and its precursor, cysteine sulfinic acid. In CF, arginine bioavailability is low, possibly contributing to the reduced nitric oxide formation in CF airways and subsequent lung pathophysiology (40). Hypotaurine and cysteine sulfinic acid have known antioxidant effects (49), although they have not been specifically investigated in CF patients to date. These data may indicate a greater shift in amino acid utilization towards energy generation via the TCA cycle within the placebo group as compared to the vitamin D3-treated group. It is also possible that the TCA cycle metabolites in the plasma of the placebo group are accumulating as damage-associated molecular pattern (DAMP) molecules, intracellular molecules that are released into circulation following tissue injury or stress (50). Taken together, results from pathway and module analyses suggest a more catabolic state in the placebo group and metabolic stabilization within the vitamin D3 group. This is consistent with the vitamin D3-induced improvements in the pro-inflammatory state previously reported in this cohort and may have contributed to the clinical improvements observed (4,5). The effects of vitamin D on amino acid pathways during catabolic states have not been previously investigated and will require additional studies in CF and other populations.
This study used a HRM approach to examine the effects of vitamin D3 supplementation in adults with CF. This approach differs from more commonly used targeted analyses in that the workflow is inverted; analyses are performed to broadly capture ultra-high resolution mass spectral signals from plasma, select for those differing between populations, and then map to pathways using the novel pathway enrichment approach provided by mummichog. However, as a pilot discovery-oriented study, limitations include the small sample size, which, in combination with the large number of comparisons made, limit statistical power for biomarker discovery. Pathway enrichment analysis overcomes this limitation and is less susceptible to type II error. Thus, the results provide biological insight into human biochemical metabolic pathways despite lack of evidence for significant effects on individual metabolites. Although we corrected for age and genotype, the small sample size also limited our ability to correct for other potential confounders or conduct further subgroup analyses in their response to the vitamin D intervention, such as by pancreatic insufficiency or CF-related diabetes status. As this study was conducted during pulmonary exacerbation, it is possible that the metabolic changes induced by vitamin D3 were specific to this acute period and involved interactions with the conventional treatments that were administered during hospitalization. Further, because clinical monitoring for vitamin D deficiency and supplementation are routine in our CF clinical center, the mean serum 25(OH)D concentration in our study population was near the sufficient range (28.6 ± 12.9 ng/mL); thus, it is possible that systemic metabolic responses may differ following vitamin D3 intervention in patients with more severe vitamin D deficiency. Indeed, the results showed little overlap in metabolic pathways that differed as a function of underlying vitamin D status compared to the response to high-dose vitamin D3 intervention. Finally, although this discovery-based high-resolution plasma metabolomics study was not designed to target specific metabolites, our data enable power analysis of future targeted metabolomics studies in adult CF patients focusing on specific metabolites of interest.
4.1 Conclusions
In summary, this pilot study showed that adults with CF during a pulmonary exacerbation have different levels of amino acid, lipids and other metabolites compared to healthy, age-matched subjects, indicative of a catabolic state. Both baseline vitamin D status and high-dose vitamin D3 supplementation influenced numerous metabolic pathways in adult CF, many of which have previously been described as altered or dysregulated in CF patients. These data also suggest that the beneficial effects of high-dose vitamin D3 observed in this hospitalized adult CF population with acute pulmonary exacerbation may have occurred through an anti-catabolic effect. Additional targeted studies will be required to more clearly define the role of vitamin D on systemic metabolism in adults with CF.
Supplementary Material
Acknowledgments
This work was supported, in part, by the National Institutes of Health [grants K01 DK102851 (JAA), K24 DK096574 (TRZ), K01 GM109309 (REG), T32 DK007734 (EMS), R01 HL126603 (RT), and UL1 TR000454 (Atlanta Clinical and Translational Science Institute)]; and the Cystic Fibrosis Foundation [grants TANGPR11A0 (VT), CHANDL16F0 (JDC) and a Student Trainee Award (REG)]. The funding sources had no involvement in the study design; collection, analysis, or interpretation of data; the writing of the manuscript; or the decision to submit the article for publication.
Abbreviations uised
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CF
cystic fibrosis
- FEV1 %predicted
percentage of predicted forced expiratory volume in 1 s
- FDR
false discovery rate
- HCA
hierarchical cluster analysis
- Human Metabolomics Database
HMDB
- HRM
high-resolution metabolomics
- LIMMA
linear models for microarray data
- NIST SRM
National Institute of Standards and Technology Standard Reference Material
- PCA
principal component analysis
- tricarboxylic acid
TCA
Footnotes
Clinical Trials Registry: clinicatrials.gov NCT00788138
6. DISCLOSURE STATEMENT
None of the authors have a conflict of interest to declare.
7. AUTHOR CONTRIBUTIONS
Author contributions were as follows: conception and design of the study (JAA, VT, TRZ); acquisition of data (JAA, REG, VTT, DIW, KU, SL); analysis and interpretation of data (JAA, EYC, DIW, EMS, JKF, JDC, RT, DPJ, TRZ); drafting the article (JAA); and critical revisions for important intellectual content (EYC, DIW, JDC, EMS, REG, JKF, RT, VTT, KU, SL, VT, DPJ, TRZ). All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spoonhower KA, Davis PB. Epidemiology of Cystic Fibrosis. Clin Chest Med. 2016;37:1–8. doi: 10.1016/j.ccm.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Assis DN, Freedman SD. Gastrointestinal Disorders in Cystic Fibrosis. Clin Chest Med. 2016;37:109–18. doi: 10.1016/j.ccm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Solomon M, Bozic M, Mascarenhas MR. Nutritional Issues in Cystic Fibrosis. Clin Chest Med. 2016;37:97–107. doi: 10.1016/j.ccm.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Grossmann RE, Zughaier SM, Kumari M, Seydafkan S, Lyles RH, Liu S, Sueblinvong V, Schechter MS, Stecenko AA, Ziegler TR, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial. Dermatoendocrinol. 2012;4:191–7. doi: 10.4161/derm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. 2012;66:1072–4. doi: 10.1038/ejcn.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Molecular endocrinology. 2005;19:2685–95. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 7.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiological reviews. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front Physiol. 2014;5:167. doi: 10.3389/fphys.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seuter S, Neme A, Carlberg C. Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res. 2016;44:4090–104. doi: 10.1093/nar/gkv1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008;69:374–81. doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green D, Carson K, Leonard A, Davis JE, Rosenstein B, Zeitlin P, Mogayzel P., Jr Current Treatment Recommendations for Correcting Vitamin D Deficiency in Pediatric Patients with Cystic Fibrosis Are Inadequate. The Journal of Pediatrics. 2008;153:554–9. e2. doi: 10.1016/j.jpeds.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 12.McCauley LA, Thomas W, Laguna TA, Regelmann WE, Moran A, Polgreen LE. Vitamin D Deficiency Is Associated with Pulmonary Exacerbations in Children with Cystic Fibrosis. Annals of the American Thoracic Society. 2013;11:198–204. doi: 10.1513/AnnalsATS.201208-068OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcondes NA, Raimundo FV, Vanacor R, Corte BP, Ascoli AM, de Azambuja AZ, Scopel L, dos Santos PV, Dalcin PdTR, Rotta LN, et al. Hypovitaminosis D in patients with cystic fibrosis: a cross-section study in South Brazil. The Clinical Respiratory Journal. 2014;8:455–9. doi: 10.1111/crj.12097. [DOI] [PubMed] [Google Scholar]
- 14.Pincikova T, Nilsson K, Moen IE, Fluge G, Hollsing A, Knudsen PK, Lindblad A, Mared L, Pressler T, Hjelte L, et al. Vitamin D deficiency as a risk factor for cystic fibrosis-related diabetes in the Scandinavian Cystic Fibrosis Nutritional Study. Diabetologia. 2011;54:3007–15. doi: 10.1007/s00125-011-2287-1. [DOI] [PubMed] [Google Scholar]
- 15.Pincikova T, Nilsson K, Moen IE, Karpati F, Fluge G, Hollsing A, Knudsen PK, Lindblad A, Mared L, Pressler T, et al. Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J Clin Nutr. 2011;65:102–9. doi: 10.1038/ejcn.2010.194. [DOI] [PubMed] [Google Scholar]
- 16.Simoneau T, Bazzaz O, Sawicki GS, Gordon C. Vitamin D Status in Children with Cystic Fibrosis. Associations with Inflammation and Bacterial Colonization. Annals of the American Thoracic Society. 2014;11:205–10. doi: 10.1513/AnnalsATS.201306-171BC. [DOI] [PubMed] [Google Scholar]
- 17.Finkelstein JL, Pressman EK, Cooper EM, Kent TR, Bar HY, O'Brien KO. Vitamin D Status Affects Serum Metabolomic Profiles in Pregnant Adolescents. Reproductive sciences. 2015;22:685–95. doi: 10.1177/1933719114556477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson SM, Panagiotou OA, Anic GM, Mondul AM, Mannisto S, Weinstein SJ, Albanes D. Metabolomics analysis of serum 25-hydroxy-vitamin D in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study. Int J Epidemiol. 2016:1–11. doi: 10.1093/ije/dyw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones DP, Park Y, Ziegler TR. Nutritional metabolomics: progress in addressing complexity in diet and health. Annu Rev Nutr. 2012;32:183–202. doi: 10.1146/annurev-nutr-072610-145159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, et al. HMDB 3. 0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–7. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tangpricha V, Kelly A, Stephenson A, Maguiness K, Enders J, Robinson KA, Marshall BC, Borowitz D Committee CFFVDE-BR. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab. 2012;97:1082–93. doi: 10.1210/jc.2011-3050. [DOI] [PubMed] [Google Scholar]
- 22.Kearns MD, Binongo JN, Watson D, Alvarez JA, Lodin D, Ziegler TR, Tangpricha V. The effect of a single, large bolus of vitamin D in healthy adults over the winter and following year: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2015;69:193–7. doi: 10.1038/ejcn.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soltow QA, Strobel FH, Mansfield KG, Wachtman L, Park Y, Jones DP. High-performance metabolic profiling with dual chromatography-Fourier-transform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics. 2013;9:S132–S43. doi: 10.1007/s11306-011-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frediani JK, Jones DP, Tukvadze N, Uppal K, Sanikidze E, Kipiani M, Tran VT, Hebbar G, Walker DI, Kempker RR, et al. Plasma metabolomics in human pulmonary tuberculosis disease: a pilot study. PloS one. 2014;9:e108854. doi: 10.1371/journal.pone.0108854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phinney KW, Ballihaut G, Bedner M, Benford BS, Camara JE, Christopher SJ, Davis WC, Dodder NG, Eppe G, Lang BE, et al. Development of a Standard Reference Material for metabolomics research. Analytical chemistry. 2013;85:11732–8. doi: 10.1021/ac402689t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu T, Park Y, Johnson JM, Jones DP. apLCMS--adaptive processing of high-resolution LC/MS data. Bioinformatics. 2009;25:1930–6. doi: 10.1093/bioinformatics/btp291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC bioinformatics. 2013;14:15. doi: 10.1186/1471-2105-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Go YM, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, Strobel F, Quyyumi AA, Ziegler TR, Pennell KD, et al. Reference Standardization for Mass Spectrometry and High-resolution Metabolomics Applications to Exposome Research. Toxicol Sci. 2015;148:531–43. doi: 10.1093/toxsci/kfv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Go YM, Liang Y, Uppal K, Soltow QA, Promislow DE, Wachtman LM, Jones DP. Metabolic Characterization of the Common Marmoset (Callithrix jacchus) PloS one. 2015;10:e0142916. doi: 10.1371/journal.pone.0142916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Accardi CJ, Walker DI, Uppal K, Quyyumi AA, Rohrbeck P, Pennell KD, Mallon CT, Jones DP. High-Resolution Metabolomics for Nutrition and Health Assessment of Armed Forces Personnel. Journal of occupational and environmental medicine /American College of Occupational and Environmental Medicine. 2016;58:S80–8. doi: 10.1097/JOM.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 31.Smyth GK. limma: Linear Models for Microarray Data. In: Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 33.Uppal K, Walker DI, Liu K, Li S, Go YM, Jones DP. Computational metabolomics: a framework for the million metabolome. Chemical research in toxicology. 2016 doi: 10.1021/acs.chemrestox.6b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, Jones DP, Pulendran B. Predicting network activity from high throughput metabolomics. PLoS computational biology. 2013;9:e1003123. doi: 10.1371/journal.pcbi.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H Clinical Practice Guidelines on G, Nutrition S, Ad Hoc Working G. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108:832–9. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 36.West NE, Lechtzin N, Merlo CA, Turowski JB, Davis ME, Ramsay MZ, Watts SL, Stenner SP, Boyle MP. Appropriate goal level for 25-hydroxyvitamin D in cystic fibrosis. Chest. 2011;140:469–74. doi: 10.1378/chest.10-2114. [DOI] [PubMed] [Google Scholar]
- 37.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 38.Chesdachai S, Tangpricha V. Treatment of vitamin D deficiency in cystic fibrosis. The Journal of steroid biochemistry and molecular biology. 2015 doi: 10.1016/j.jsbmb.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelen MP, Com G, Deutz NE. Protein is an important but undervalued macronutrient in the nutritional care of patients with cystic fibrosis. Current opinion in clinical nutrition and metabolic care. 2014;17:515–20. doi: 10.1097/MCO.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grasemann H, Schwiertz R, Grasemann C, Vester U, Racke K, Ratjen F. Decreased systemic bioavailability of L-arginine in patients with cystic fibrosis. Respiratory research. 2006;7:87. doi: 10.1186/1465-9921-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleme ML, Levy E. Cystic fibrosis-related oxidative stress and intestinal lipid disorders. Antioxidants & redox signaling. 2015;22:614–31. doi: 10.1089/ars.2014.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell SC, Bowerman AM, Nixon LE, Macdonald IA, Elborn JS, Shale DJ. Metabolic and inflammatory responses to pulmonary exacerbation in adults with cystic fibrosis. European journal of clinical investigation. 2000;30:553–9. doi: 10.1046/j.1365-2362.2000.00667.x. [DOI] [PubMed] [Google Scholar]
- 43.Sc NN, Shoseyov D, Kerem E, Zangen DH. Patients with cystic fibrosis and normoglycemia exhibit diabetic glucose tolerance during pulmonary exacerbation. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2010;9:199–204. doi: 10.1016/j.jcf.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Ghidoni R, Caretti A, Signorelli P. Role of Sphingolipids in the Pathobiology of Lung Inflammation. Mediators of inflammation. 2015;2015:487508. doi: 10.1155/2015/487508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grothe J, Riethmuller J, Tschurtz SM, Raith M, Pynn CJ, Stoll D, Bernhard W. Plasma phosphatidylcholine alterations in cystic fibrosis patients: impaired metabolism and correlation with lung function and inflammation. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;35:1437–53. doi: 10.1159/000373964. [DOI] [PubMed] [Google Scholar]
- 46.Guerrera IC, Astarita G, Jais JP, Sands D, Nowakowska A, Colas J, Sermet-Gaudelus I, Schuerenberg M, Piomelli D, Edelman A, et al. A novel lipidomic strategy reveals plasma phospholipid signatures associated with respiratory disease severity in cystic fibrosis patients. PloS one. 2009;4:e7735. doi: 10.1371/journal.pone.0007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stepien M, Nugent AP, Brennan L. Metabolic profiling of human peripheral blood mononuclear cells: influence of vitamin d status and gender. Metabolites. 2014;4:248–59. doi: 10.3390/metabo4020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Sullivan A, Gibney MJ, Connor AO, Mion B, Kaluskar S, Cashman KD, Flynn A, Shanahan F, Brennan L. Biochemical and metabolomic phenotyping in the identification of a vitamin D responsive metabotype for markers of the metabolic syndrome. Molecular nutrition & food research. 2011;55:679–90. doi: 10.1002/mnfr.201000458. [DOI] [PubMed] [Google Scholar]
- 49.Fontana M, Giovannitti F, Pecci L. The protective effect of hypotaurine and cysteine sulphinic acid on peroxynitrite-mediated oxidative reactions. Free radical research. 2008;42:320–30. doi: 10.1080/10715760801999727. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Martinez I, Shaker ME, Mehal WZ. Therapeutic Opportunities in Damage-Associated Molecular Pattern-Driven Metabolic Diseases. Antioxidants & redox signaling. 2015;23:1305–15. doi: 10.1089/ars.2015.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.