Abstract
Oxytocin- and vasopressin-related systems are present in invertebrate and vertebrate bilaterian animals, including humans, and exhibit conserved neuroanatomical and functional properties. In vertebrates, these systems innervate conserved neural networks that regulate social learning and behavior, including conspecific recognition, social attachment, and parental behavior. Individual and species-level variation in central organization of oxytocin and vasopressin systems has been linked to individual and species variation in social learning and behavior. In humans, genetic polymorphisms in the genes encoding oxytocin and vasopressin peptides and/or their respective target receptors have been associated with individual variation in social recognition, social attachment phenotypes, parental behavior, and psychiatric phenotypes such as autism. Here we describe both conserved and variable features of central oxytocin and vasopressin systems in the context of social behavioral diversity, with a particular focus on neural networks that modulate social learning, behavior, and salience of sociosensory stimuli during species-typical social contexts.
Keywords: social behavior network, social decision-making network, salience, valence, social behavior, social cognition, neuropeptides, functional connectivity, functional coupling, autism spectrum disorders, social attachment, pair bonding, oxtr, avpr1a
Introduction
In nature, many forms of social perception, learning, and behavior vary at the individual and species level. Major research efforts have identified conserved neural systems that regulate these processes across species. For example, homologues of the mammalian oxytocin (OT) and arginine vasopressin (AVP) neuropeptides and their respective target receptors (for clarity, homologous neuropeptides and target receptors in invertebrates and non-mammalian vertebrates will hereafter be referred to as “OT/AVP-like”) modulate social and reproductive behaviors across bilaterian animals—including social behavior, social cognition, and psychiatric phenotypes such as autism spectrum disorder (ASD) in humans (Donaldson and Young, 2008). Here we review anatomical and functional properties of central OT/AVP-like networks and tie them to individual and species diversity in social learning and behavior.
OT/AVP-like neurons
OT/AVP-like neurons exhibit a conserved transcriptional architecture (Donaldson and Young, 2008). In annelid worms and zebrafishes, these neurons express common cell-type specific transcription factors, microRNAs, and opsin genes, suggesting common evolutionary origins (likely as neurosecretory cells) (Tessmar-Raible et al., 2007). In vertebrates, transcription of the genes encoding OT/AVP-like proneuropeptides is regulated by strongly conserved cis-regulatory mechanisms, such that transgenically integrated OT-like (isotocin) and AVP-like (vasotocin) gene regulatory constructs from puffer fish are faithfully transcribed in mammalian OT and AVP neurons, respectively (Gilligan et al., 2003; Murphy et al., 1998; Venkatesh et al., 1997). Despite these conserved transcriptional features, genetic variation in the OXT/AVP gene region may contribute to individual variation in social cognition and behavior. For example, polymorphisms in OXT (the gene encoding OT) have been associated with individual variation in maternal care, postpartum depression, attachment anxiety, and ASD phenotypes in humans (Jonas et al., 2013; Love et al., 2012; Mileva-Seitz et al., 2013).
In vertebrates, OT- and AVP-like peptides are predominantly synthesized in hypothalamic neurons and can be released centrally and peripherally through the posterior pituitary (Lee et al., 2009). It was long thought that magnocellular OT/AVP-like neurons project exclusively to the posterior pituitary, while smaller parvocellular neurons project widely throughout brain. However, magnocellular OT- and/or AVP-like neurons in fishes, birds, and mammals also project to forebrain targets (Godwin and Thompson, 2012; Goodson and Kabelik, 2009; Knobloch et al., 2012; Mahoney et al., 1990; Ross et al., 2009a; Saito et al., 2004), revealing a conserved organization by which peripheral and central OT/AVP-like release may be coupled in vertebrates. AVP-like neurons, although predominantly localized within the hypothalamus, often populate extrahypothalamic sites (e.g. medial amygdala, bed nucleus of stria terminals) in a species-, sex-, and experience-dependent manner (Albers, 2015). Although specific OT- and AVP-like neuronal populations have been implicated in a variety of social behaviors, their functional features are beyond the scope of this review and are described elsewhere (Kelly and Goodson, 2014).
OT/AVP-like peptide release
OT- and AVP-like peptides are stored in large dense core vesicles (LDCVs) distributed throughout the soma, dendrites, and axons of OT- and AVP-like neurons. These LDCVs are exocytosed in response to increased concentrations of intracellular Ca2+ (Stoop, 2012). A variety of osmotic, reproductive, and social stimuli have been shown to elicit OT and AVP release (Lee et al., 2009). For example, OT and AVP are released centrally during parturition, aggressive interaction, and social defeat in rats and/or sheep (Veenema and Neumann, 2008). OT is also released during mating and suckling (Veenema and Neumann, 2008), and in response to more subtle social stimuli including vocalizations (Seltzer et al., 2010), eye contact (Nagasawa et al., 2015), and touch (Okabe et al., 2015).
Somatodendritic exocytosis of LDCVs allows OT/AVP-like peptides to diffuse to hypothalamic and possibly extrahypothalamic sites (Ludwig et al., 2002). In mammals, like other vertebrate lineages, dendritic innervation of the third ventricle may also allow OT/AVP release directly into the ventricular circulation (Fig. 1) (Knobloch and Grinevich, 2014). In addition to dendritic release, OT/AVP-like peptides can be released focally from axons innervating extrahypothalamic brain regions, allowing for more rapid modulation of behavior (Knobloch et al., 2012; Oettl et al., 2016). In some instances, these multiple release mechanisms—volume diffusion through the extracellular space, widespread circulation through the ventricular system, and focal release into extrahypothalamic regions—are thought to occur simultaneously and interact to modulate neural networks in a multimodal fashion (Landgraf and Neumann, 2004; Ross and Young, 2009).
Figure 1. Morphology, neuroanatomical projections, and release mechanisms of OT neurons.

This schematic represents current views of OT neuronal structure and function. Magnocellular and parvocellular OT neurons project to distinct targets. In addition to projections to the posterior pituitary, magnocellular OT axons can innervate extrahypothalamic forebrain and midbrain regions, and dendrites can contact the third ventricle. In contrast, parvocellular OT neurons are thought to project predominantly to hindbrain and brainstem regions. Glutamatergic networks link magnocellular OT neurons and are thought to synchronize pulsatile release into the periphery. Though not pictured, axon collaterals from some parvocellular OT neurons can synapse onto, excite, and trigger OT release from magnocellular OT neurons in the SON. OT is stored in LDCVs that populate the soma, dendrites, and axons but are typically absent in terminals; thus en passant release is thought to be the primary form of axonal OT release. Axonal and somatodendritic OT release can be triggered without depolarization via activation of MC4Rs or the activity of transmembrane proteins such as CD38. Following somatodendritic release, OT can bind to autoreceptors and prime OT neurons for firing, and may also volume diffuse to other central targets. Abbreviations: BBB=blood brain barrier; 3V=3rd ventricle.
Importantly, OT/AVP release can be uncoupled from depolarization entirely. For example, α-MSH can activate melanocortin 4 receptors (MC4Rs) expressed on OT neurons, triggering mobilization of intracellular Ca2+ stores and somatodendritic OT release, thereby increasing local OT concentrations without depolarization or axonal release (Ludwig and Leng, 2006). MC4R agonists have been proposed as an alternative pharmacological strategy for targeting the OT system in psychiatric disorders such as ASD (Young and Barrett, 2015). Similarly, CD38 is a transmembrane protein expressed in OT neurons that contributes to mobilization of intracellular Ca2+ stores, triggering OT secretion without depolarization (Higashida et al., 2012; Young, 2007). In mice, CD38 regulates OT-dependent social memory and maternal behavior; in humans, genetic polymorphisms in the CD38 gene are associated with individual variation in parental behavior and ASD phenotypes, suggesting a conserved role for this pathway in social function across rodents and humans (Feldman et al., 2016; Feldman et al., 2012; Higashida, 2016; Higashida et al., 2012; Jin et al., 2007).
OT/AVP-like neurons co-express OT/AVP-like receptors in both invertebrates and mammals, suggesting autocrine regulation of these neurons has been strongly conserved (Berlove and Piekut, 1990; Gillard et al., 2007; van Kesteren et al., 1995). In mammals, OT/AVP binding to autoreceptors “primes” the neuron into a more excitable state for up to an hour, augmenting somatodendritic OT/AVP release in response to depolarization or osmotic stimuli (Brussaard, 1995; Dayanithi et al., 2000; Gillard et al., 2007; Ludwig and Leng, 2006; Ludwig et al., 2002; Sabatier et al., 2004). Other signaling mechanisms may also prime OT/AVP-like neurons. For example, melanotan II, a MC4R agonist, potentiates OT release in the striatum following a physiological stimulus and facilitates OT-dependent behaviors (Modi et al., 2015).
It is unclear whether OT/AVP-like neuropeptides act as synaptic neurotransmitters themselves. Electrophysiological and behavioral effects following evoked axonal OT release in forebrain regions occur relatively slowly (on the order of 2-20 seconds), suggesting that axonal OT release may occur non-synaptically through periaxonal or en passant exocytosis of LDCVs (Fig. 1) (Hokfelt, 1991; Knobloch and Grinevich, 2014). Further, OT terminals in the striatum originating from magnocellular neurons are typically devoid of LDCVs, consistent with en passant OT release from unmyelinated axons (Ross et al., 2009a).
In rodents, hypothalamic OT and AVP neurons predominantly express VGLUT2, suggesting that depolarization of these neurons is coupled with synaptic glutamate release (Dabrowska et al., 2011; Kawasaki et al., 2005). Consistent with this idea, OT-OT and AVP-AVP magnocellular neurons are linked by intrahypothalamic glutamatergic synapses and exhibit electrical coupling following electrophysiological stimulation (Fig. 1) (Boudaba and Tasker, 2006; Eliava et al., 2016; Israel et al., 2003; Jourdain et al., 1998; Saito and Urano, 2001). This autoexcitatory network structure is thought to synchronize pulsatile OT- or AVP-like release into the periphery in response to robust osmotic or reproductive stimuli (e.g. during parturition). This organization may also be relevant for subtler forms of social stimuli. For example, anatomical studies in mice have identified sensory neurons in the olfactory epithelium expressing the OR37 receptor, which selectively binds ligands secreted from the mammalian anal gland (Bader et al., 2012a; Bautze et al., 2014). These sensory neurons project to glomeruli in the ventral main olfactory bulb; which in turn project to the hypothalamus and synapse selectively onto AVP neurons (Bader et al., 2012b; Schaefer et al., 2002; Schaefer et al., 2001). This circuit illustrates how social stimuli, such as socially-relevant odorant molecules, may selectively access hypothalamic AVP networks and trigger AVP release.
OT/AVP-like receptors
Upon release, OT- and AVP-like neuropeptides exhibit high affinities for their respective target GPCRs and can exert their effects at low nanomolar concentrations, suggesting a capacity for high signaling efficacy following the release of a small number of LDCVs (Ludwig and Leng, 2006). Several OT/AVP-like receptors are expressed in the brain. In mammals, the majority of investigations have focused on three OT/AVP receptors expressed in nervous tissue: oxytocin receptor (OTR), vasopressin receptor 1a (V1aR), and vasopressin receptor 1b (V1bR) (Gimpl and Fahrenholz, 2001; Gruber et al., 2010). OTRs and V1aRs are abundantly expressed in the brain and modulate a variety of mammalian social behaviors, and therefore will be a primary focus of this review.
In contrast to the conserved transcriptional regulation of Oxt and Avp, Oxtr and Avpr1a regulation appears to be more phylogenetically labile. Polymorphisms in non-coding regions of Oxtr and Avpr1a likely influence behavioral diversity by modulating transcription levels and patterns of region-specific expression in the brain. Indeed, genetic polymorphisms in Oxtr and Avpr1a predict individual variation in region-specific receptor expression and social behavior in both prairie voles and humans (Hammock and Young, 2005; King et al., 2016; Knafo et al., 2008; Okhovat et al., 2015; Ophir et al., 2008; Reuter et al., 2016). For example, in male prairie voles, polymorphisms in Avpr1a predict differential V1aR expression in the retrosplenial cortex and individual variation in mating strategies in semi-natural field enclosures (Okhovat et al., 2015). Also in prairie voles, polymorphisms in Oxtr predict OTR expression in the NAc and individual variation in pair bonding (King et al., 2016). In primates, polymorphisms in OXTR and AVPR1A predict individual variation in social behavior and cognition in chimpanzees and humans (Hopkins et al., 2014; Skuse et al., 2014; Staes et al., 2015; Waller et al., 2016)—including social attachment behaviors in humans (Walum et al., 2012; Walum et al., 2008); neural activity during social contexts in humans (Feng et al., 2015; Loth et al., 2014; Tost et al., 2010; Tost et al., 2011); and ASD phenotypes in humans (LoParo and Waldman, 2015; Procyshyn et al., 2016).
Central distribution patterns of OT- and AVP-like receptors have been mapped in numerous species. These efforts have revealed several particularly striking themes: firstly, a high degree of promiscuous binding of OT-like ligands to AVP-like receptors and AVP-like ligands to OT-like receptors (Albers, 2015); secondly, a remarkable degree of complementarity between OT- and AVP-like receptor expression patterns in the brain (Dumais and Veenema, 2016; Stoop, 2012); thirdly, region-specific plasticity in expression depending on sex and social experience (Albers, 2015; Dumais and Veenema, 2016; Gil et al., 2013); and fourthly, an extraordinary degree of evolutionary plasticity in region-specific expression levels across neural networks that modulate social perception, learning, and behavior (Johnson and Young, 2015). Comprehensive discussion of each of these themes is beyond the scope of this review; however, it is important to note that variation in region-specific OT- and AVP-like receptor expression has been linked to individual and/or species diversity in social behaviors in multiple vertebrate lineages (Goodson et al., 2009; Okhovat et al., 2015; Ophir et al., 2012; Young and Wang, 2004). These topics will be reviewed further in following sections.
OT/AVP-like receptors and species-specific sociosensory processing
A remarkable degree of evolutionary plasticity is observed in OT/AVP-like receptor expression throughout sensory pathways, with patterns of receptor expression appearing to reflect the dominant sociosensory modalities utilized by the species. For example, teleost fishes rely on visual cues during reproductive and dominance interactions, and dense expression of OT/AVP-like receptors has been reported in visual processing nuclei (e.g. optic tectum) (Grosenick et al., 2007; Huffman et al., 2012; Kline et al., 2011; Moons et al., 1989). Chemosensory molecules are also critical mediators of social information in teleosts (Crapon de Caprona, 1980; Hubbard et al., 2014; Simoes et al., 2015), and OT/AVP-like receptors are densely expressed in olfactory and gustatory nuclei (e.g. olfactory bulb, tertiary gustatory nucleus) (Huffman et al., 2012; Kline et al., 2011). Interestingly, OT/AVP-like receptor expression has also been reported in specific vocal-acoustic and lateral line sensory processing circuits (Huffman et al., 2012; Kline et al., 2011); reflecting additional sociosensory domains that are important during teleost social interaction (Butler and Maruska, 2016; Goodson and Bass, 2000, 2002; Goodson et al., 2003). In tungara frogs, mating vocalizations elicit robust immediate early gene (IEG) expression in auditory midbrain nuclei (e.g. torus semicircularis) (Hoke et al., 2004). Although OT/AVP-like receptors have not yet been mapped in this species, dense receptor expression in this nucleus has been reported in the only frog species analyzed to date (Acharjee et al., 2004). Many frogs also rely on visual throat displays and posturing during dominance interactions (Wells, 1977), and OT/AVP-like receptors are densely expressed in visual processing nuclei (e.g. optic tectum) (Acharjee et al., 2004). Similar to frogs, patterns of OT/AVP-like receptor expression reflect patterns of vocalization-induced IEG expression in songbirds (Leung et al., 2011; Leung et al., 2009). Plumage and behavioral displays (e.g. copulation solicitation) also represent major sociosensory cues that are transmitted during avian social interactions (Candolin, 2003; O’Loghlen and Rothstein, 2010; Searcy, 1992), and investigations in songbirds have revealed dense expression of OT/AVP-like receptors in visual-processing nuclei (e.g. oculomotor nucleus, optic tectum) (Leung et al., 2011; Leung et al., 2009). In rodents, olfaction is the dominant mode of sociosensory processing. OTRs and V1aRs are densely expressed throughout olfactory pathways; and OTR signaling directly modulates processing of social olfactory stimuli in mice (Choe et al., 2015; Oettl et al., 2016). Interestingly, in postpartum mice, OTRs in auditory cortex have been shown to modulate excitatory-inhibitory balance and enhance processing of neonatal vocalizations (Marlin et al., 2015; Mitre et al., 2016). In contrast to rodents, vision and audition are the dominant modes of sociosensory processing in primates. OTRs and V1aRs are preferentially expressed in visual and auditory processing regions; and intranasal OT delivery modulates neural and behavioral responses to visual and acoustic social stimuli in multiple primate species, including humans (Blandon-Gitlin et al., 2014; Dal Monte et al., 2014; Freeman and Young, 2016; Liu et al., 2015b; Luo et al., 2015; Strathearn et al., 2009; Taylor and French, 2015). These and other data suggest that OT/AVP-like receptors preferentially modulate the dominant forms of sociosensory processing in a species-specific manner across vertebrates, perhaps to increase the salience of sociosensory cues during social contexts.
OT/AVP-like receptors contribute to social behavioral diversity
In addition to sensory systems, conserved limbic and mesolimbic networks are critical modulators of vertebrate social behavior (O’Connell and Hofmann, 2011, 2012), although the homologues (or lack thereof) of some brain regions across vertebrate classes are still debated (e.g. ventral tegmental area) (Goodson and Kingsbury, 2013; Tay et al., 2011). Experiments spanning a wide range species have revealed diverse expression patterns of OT- and AVP-like receptors across these neural networks (Johnson and Young, 2015). This evolutionary plasticity is thought to be a major neurobiological mechanism contributing to species-typical patterns of social information processing and behavior (Donaldson and Young, 2008).
Region-specific expression levels of OT- and AVP-like receptors have been associated with social behavioral divergence between species. For example, OT- and AVP-like receptor densities in the lateral septum are correlated with species differences in flocking behavior in estrildid finches (Goodson and Kabelik, 2009), and—in the nucleus accumbens-ventral pallidum (NAc-VP) circuit—with species differences in mating systems and parental behavior in rodents (Kalamatianos et al., 2010; Olazabal and Young, 2006; Young and Wang, 2004) and possibly primates (Freeman and Young, 2016; Johnson and Young, 2015). For example, socially monogamous and alloparental prairie voles exhibit higher OTR expression in the NAc and higher V1aR expression in the VP compared to non-monogamous meadow and montane voles (Young and Wang, 2004). Similarly, eusocial and alloparental naked mole rats exhibit dense OTR binding in the NAc compared to solitary cape mole rats (Kalamatianos et al., 2010). In primates, socially monogamous and alloparental marmosets exhibit dense OTR expression in the NAc, while socially monogamous and alloparental coppery titi monkeys exhibit dense V1aR expression in this region (Freeman and Young, 2016); in contrast, non-monogamous rhesus macaques exhibit low levels of OTR and V1aR binding in the NAc (Figure 2) (Freeman et al., 2014; Young et al., 1999). Humans also exhibit capacities for alloparental care and selective social attachments with mating partners; ligands promiscuous for both OTRs and V1aRs have revealed dense binding in the human VP (Loup et al., 1991). It may be that OTR/V1aR signaling in the NAc-VP circuit plays a critical role in attaching valence and incentive salience to the sociosensory cues of conspecific neonates and mating partners. Experiments in voles suggest such species differences in region-specific receptor expression have functional significance: selective blockade and RNAi knockdown of V1aR in the VP of prairie voles inhibits pair bond formation, while selective overexpression of V1aR in the VP of non-monogamous meadow voles increases monogamous-like behavior (Barrett et al., 2013; Lim et al., 2004). Interestingly, intranasal OT administration in pair-bonded men increases self-reported attraction to their partner and increases BOLD response in the NAc when viewing images of their partner, raising the possibility that OTRs directly modulate the NAc-VP circuit and selective social attachment in humans (Scheele et al., 2013).
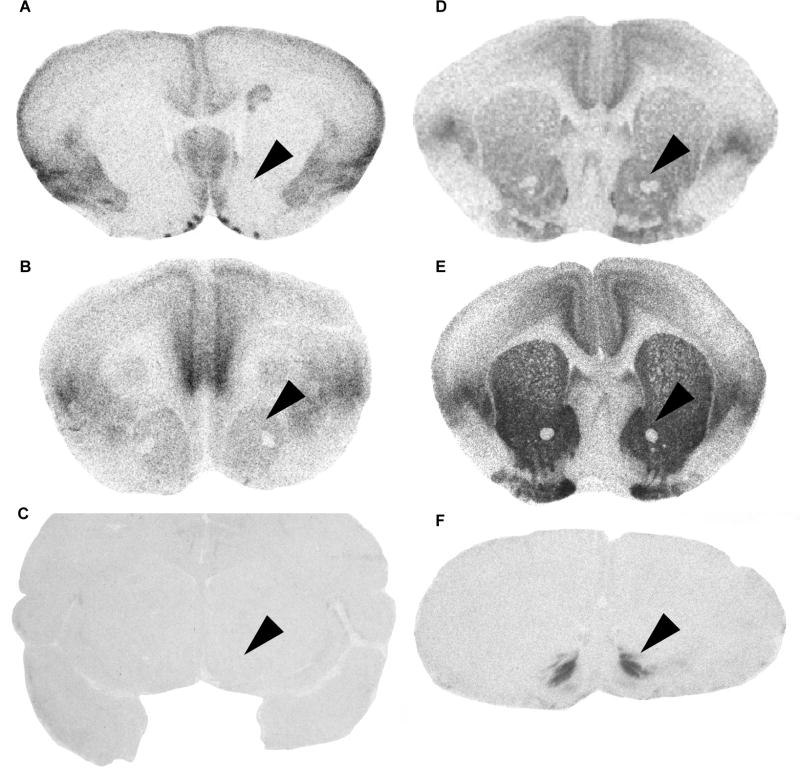
Figure 2. Diversity in region-specific OTR expression is associated with diversity in social behavior.
Representative autoradiograms illustrate accumbal OTR binding density (indicated by black labeling) in rodent and primate species exhibiting divergent social behaviors and mating strategies: (A) mouse, (B) montane vole, (C) rhesus macaque, (D) “low” OTR-expressing prairie vole, (E) “high” OTR-expressing prairie vole, and (F) marmoset. Black arrowheads indicate position of the NAc in each section. In contrast to mice, montane voles, and rhesus macaques, prairie voles and marmosets exhibit alloparental behavior, socially monogamous mating strategies, and dense OTR binding in the NAc (D-F versus A-C). This same pattern is observed across individual prairie voles, which vary in region-specific OTR densities (e.g. D versus E) and social behavior: OTR density in the NAc is positively correlated with individual variation in both alloparental care and socially monogamous behavior. Thus, region-specific OTR expression is associated with individual and species diversity in social behavior, although it is not sufficient to predict social behavioral phenotype in all mammals. OTR expression in the NAc represents one of several examples linking region-specific OT/AVP-like receptor expression to social behavioral phenotype in birds and mammals, a topic that is discussed in more depth in under “OT/AVP-like receptors contribute to social behavioral diversity.” Autoradiograms were adapted from (Burbach et al., 2006) and (Freeman and Young, 2016) with permission.
In some instances, the same patterns linking region-specific OT/AVP-like receptor densities to interspecies behavioral diversity are mirrored by patterns of individual variation within species. For example, individual prairie voles vary in monogamous mating behavior, and this behavioral variation is associated with individual variation in OTR and V1aR densities in the NAc and VP, respectively. Socially monogamous females exhibit denser V1aR expression in the VP compared to “wandering” females (Zheng et al., 2013); and in males, overexpression of V1aR in the VP accelerates pair bonding (Pitkow et al., 2001), while RNAi knockdown of V1aR in the VP inhibits pair bonding (Barrett et al., 2013). Similarly, pair bonded males exhibit denser OTR expression in the NAc compared to “wandering” males in semi-natural field enclosures (Ophir et al., 2012); overexpression of OTR in the NAc accelerates pair bond formation in females (Keebaugh and Young, 2011; Ross et al., 2009b); and genetic polymorphisms that predict high OTR expression in the NAc are associated with accelerated pair bond formation in males (King et al., 2016). OTR binding density in the NAc is also associated with individual variation in alloparental behavior in prairie voles (Olazabal and Young, 2006). Thus, variation between Microtine species is reflected by individual variation within prairie voles. It may be that a significant degree of regulatory diversity in Oxtr and Avpr1a contributing to species divergence in region-specific receptor expression persists following speciation, thus contributing to parallel patterns within and across species.
Recent experiments suggest central OT/AVP systems may play a conserved role in modulating additional dimensions of complex social cognition and behavior in rodents and humans. For example, activation of OTRs in the medial amygdala is essential for social recognition in mice (Ferguson et al., 2001). In humans, polymorphisms in OXTR are associated with individual variation in facial recognition, suggesting the OT system may play a conserved role in modulating conspecific recognition in rodents and humans (Skuse et al., 2014). Many mammalian species—including humans—exhibit anxiety-like, depressive-like, and stress-coping phenotypes following periods of social isolation, separation from familiar conspecifics, or partner loss (Amiri et al., 2015; Bosch et al., 2009; Lukkes et al., 2009; Stroebe et al., 2007). In prairie voles, separation from a bonded partner, but not from a sibling, induces depressive-like behavior in males, an effect that is mediated by activation of corticotropin-releasing factor receptor 2 in the NAc, and rescued by activation of OTRs in this region (Bosch et al., 2016; Bosch et al., 2009). In humans, polymorphisms in OXTR have been associated with attachment phenotypes, separation anxiety (both in patients with depression), sensitivity to social exclusion, and cortisol levels following social exclusion; suggesting the OT system may play a conserved role in modulating hormonal and behavioral responses to social separation in prairie voles and humans (Costa et al., 2009; McQuaid et al., 2015). In female prairie voles, OTR density in the NAc predicts resilience to the effects of neonatal social isolation on adult social attachment phenotypes, suggesting that individual variation in central OTR organization interacts with early life social environment to shape adult social behavior (Barrett et al., 2015). Similarly, in humans, polymorphisms in the OXT and AVPR1A genes predict how early life adversity is associated with maternal care, postpartum depression, social integration, and/or social attachment later in life (Jonas et al., 2013; Liu et al., 2015a; Mileva-Seitz et al., 2013). Recently, prairie voles were demonstrated to exhibit consoling behavior towards a partner following an unobserved stressor, an effect that is mediated by OTR activation in the anterior cingulate cortex (ACC) (Fig. 3) (Burkett et al., 2016). Intranasal OT, genetic variation in OXTR and AVPR1A, and the ACC have been implicated in human empathy and emotion recognition, raising the possibility of conserved neural mechanisms modulating empathic behavior in rodents and humans (Burkett et al., 2016; Domes et al., 2007; Hurlemann et al., 2010; Lamm et al., 2011; Laursen et al., 2014; Uzefovsky et al., 2015).
Figure 3. Oxytocin and vasopressin modulate distributed neural networks during social contexts.
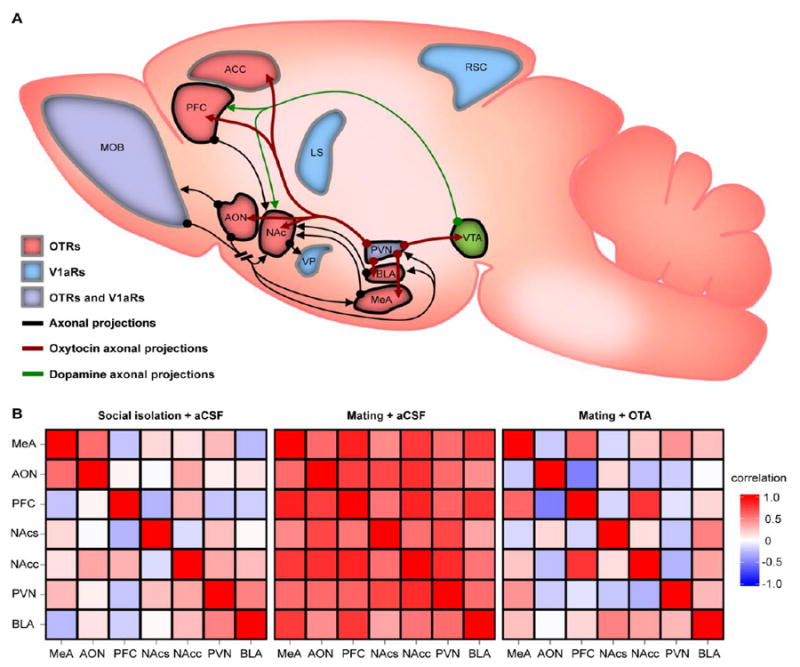
(A) Schematic highlighting brain regions in which OTR (red) and/or V1aR (blue; purple indicates both systems are important) activation is thought to modulate social information processing and/or behavior in prairie voles and other species. For example, regions outlined in black represent a simplified neural network model of OTR-expressing brain regions that are thought to distributively encode both valence and incentive salience of social stimuli (e.g. individual sociosensory profiles). Select dopamine (green), OT (maroon), and other (black) axonal projections are thought to be critical for these processes. AVP axonal projections are not depicted for clarity; however, V1aR activation in the LS and VP are also thought to modulate these processes. Additional systems that are not depicted (e.g. corticotrophin releasing factor, µ- and κ-opioid, etc.) are also important. Regions that are thought to modulate additional dimensions of social learning and behavior in prairie voles are included. For example, V1aRs in the retrosplenial cortex (RSC) are thought to modulate sociospatial memory, while OTRs in the anterior cingulate cortex (ACC) are critical for consoling behavior. Abbreviations: ACC=anterior cingulate cortex; AON=anterior olfactory nucleus; BLA=basolateral amygdala; LS=lateral septum; MeA=medial amygdala; MOB=main olfactory bulb; NAc=nucleus accumbens; PFC=prefrontal cortex; PVN=paraventricular nucleus of the hypothalamus; RSC=retrosplenial cortex; VP=ventral pallidum; VTA=ventral tegmental area.
(B) Central OTRs modulate correlated Fos expression across a hypothesized “social salience network” during sociosexual interaction and mating in male prairie voles. Heatmaps represent pairwise correlation coefficients of Fos expression between nodes of the network in three experimental treatment groups. Following central administration of artificial cerebral spinal fluid (aCSF), social isolation is associated with weakly correlated Fos expression across the network. In contrast, aCSF-treatment followed by sociosexual interaction and mating with a female is associated with strongly and positively correlated Fos expression across the network as a whole. This effect is disrupted by central administration of a selective OTR antagonist (OTA) prior to sociosexual interaction and mating, which is associated with a significant decrease in correlated Fos expression across nodes of the network. These and other data are consistent with hypotheses that central OT/AVP-like signaling modulates distributed network states during social contexts in vertebrates, and are reviewed under “OT/AVP-like neuromodulation of social information processing networks.” Heatmaps were adapted from (Johnson et al., 2016a) with permission. Abbreviations (see above): NAcc=nucleus accumbens core; NAcs=nucleus accumbens shell.
OT/AVP-like neuromodulation of social information processing networks
Although many experiments have linked region-specific OTR/V1aR populations to social behavior, it remains unclear how OT/AVP-like systems modulate neural function during social contexts. Emerging models in this domain have emphasized network level approaches toward understanding the social brain (Bethlehem et al., 2013). For example, experiments across vertebrates, including humans, have shown that social context rapidly shifts patterns of correlated activity-dependent signals (e.g. immediate early genes, neural plasticity markers, metabolic markers, BOLD responses, etc.) across nodes of conserved social information processing networks (Hoke et al., 2005; Kelly and Goodson, 2015; Liu et al., 2016; Sakata et al., 2000; Sato et al., 2016; Teles et al., 2015; Teles et al., 2016; Yang and Wilczynski, 2007). These data suggest that social contexts recruit unique “states” of interaction among network nodes.
The central projections and release properties of OT/AVP-like neurons, together with the widely distributed and variable expression profiles of central OT/AVP-like receptors, indicate that these systems are ideally organized to modulate widely distributed neural networks during social contexts. Indeed, emerging models propose that activation of OT/AVP-like receptors during social contexts modulates patterns of functional coupling across conserved social information processing networks to facilitate processing of sociosensory cues and generation of appropriate social behavioral responses (Goodson and Kabelik, 2009; Johnson and Young, 2015; Rose and Moore, 2002).
We recently tested these models by measuring how endogenous OTR activation during social contexts modulates expression of the IEG product Fos—a marker for transcriptional regulation and synaptic plasticity in neurons—across a neural network that is thought to attach valence and incentive salience to sociosensory cues in male prairie voles (Figure 3). We measured Fos expression across anatomically interconnected, OTR-expressing nodes of this “social salience network” (SSN) in a non-social context (social isolation in the home cage) and two social contexts (sociosexual interaction and mating with a female, and sociosexual interaction and mating with a female following central OTR blockade). Sociosexual interaction and mating was associated with a robust increase in Fos expression across the network compared to social isolation; but no brain region-specific differences in levels of Fos expression distinguished the two social treatment groups. In contrast, these groups were distinguished by robust differences in patterns of correlated Fos expression across the SSN. Specifically, in vehicle-treated males, sociosexual interaction and mating with a female was associated with strongly and positively correlated Fos expression across SSN nodes; and this pattern was disrupted in OTA-treated males (Johnson et al., 2016a). These data are consistent with the hypothesis that central OTR signaling during social contexts modulates coupling between nodes of widely distributed social information processing networks (Fig. 3). We then used the same paradigm to test how endogenous OTR signaling specifically within the NAc—a mesolimbic reward nucleus and network “hub” within the SSN—modulates Fos expression across the network. Consistent with our previous experiments, selective OTA administration into the NAc was not associated with differences in levels of region-specific Fos expression, but robustly modulated correlated Fos expression between the NAc shell and other SSN nodes. Specifically, Fos expression across non-accumbal nodes strongly predicted Fos expression in the NAc shell in vehicle-treated but not OTA-treated males (Johnson et al., 2016b). These data are consistent with the hypothesis that region-specific OTR activation modulates coupling of specific brain nuclei with other network nodes during species-typical social interactions.
Though different in important regards, these data are consistent with functional magnetic resonance imaging studies in humans and other primates, in which BOLD responses can be measured on a relatively rapid time scale. For example, intranasal OT administration modulates functional coupling between cortical, limbic, and/or striatal nuclei during processing of emotional facial expressions in both rhesus macaques and humans (Hu et al., 2015; Kirsch et al., 2005; Liu et al., 2015b). Intranasal AVP administration, polymorphisms in OXTR, and methylation of OXTR are also associated with differences in functional coupling between cortical, limbic, striatal, and/or brainstem nuclei during emotional face processing and other social contexts in humans (Brunnlieb et al., 2016; Puglia et al., 2015; Rilling et al., 2012; Tost et al., 2010; Tost et al., 2011; Zink et al., 2010). Thus, central OT and AVP signaling may play a conserved role in modulating network “states” during social contexts across species.
Several electrophysiological experiments reveal how OT/AVP-like signaling can modulate interregional circuitry at a cellular level. In the main olfactory bulb (MOB), cortically projecting mitral and tufted cells (M/TCs) facilitate odor processing through lateral inhibition of surrounding M/TCs, a process that is augmented by inhibitory interneuronal granule cells (GCs), increasing the “signal-to-noise” ratio of incoming odors (Brennan and Kendrick, 2006; Yokoi et al., 1995). GCs receive top-down glutamatergic inputs from OTR-expressing projection neurons in the anterior olfactory nucleus (AON). It was recently demonstrated that OTR-mediated excitation of these projection neurons increases signal-to-noise ratio of odorant-evoked responses in the MOB (increasing peak odorant responses of M/TCs while lowering background noise) (Oettl et al., 2016). Thus, OTR-mediated activity in the AON modulates odorant-evoked activity in the MOB, perhaps increasing salience of social olfactory cues. Other studies show that theta stimulation of the accessory olfactory bulb (AOB) induces LTD in the MeA, and this is thought to reflect a critical component of social memory formation; during theta stimulation of the AOB, OTR agonists augment—while OTR antagonists inhibit—LTD formation in the MeA (Gur et al., 2014). OTR activation also modulates Schaffer collateral projections from CA3 onto pyramidal neurons in CA1 of the hippocampus, a region that is important for social memory storage (Okuyama et al., 2016). During stimulation of these projections, selective OTR agonists increase signal-to-noise ratio by suppressing background firing and increasing fidelity of spike transmission and timing onto CA1 pyramidal neurons (Owen et al., 2013). Other experiments have shown that AVP and OT differentially regulate excitatory projections from the basolateral amygdala (BLA) to the medial central amygdala (CeM), a circuit that regulates fear learning and that is thought to be important for maternal behavior; AVP increases, while OT decreases, the probability that stimulation of these BLA projections will evoke excitation of CeM neurons (Campbell-Smith et al., 2015; Huber et al., 2005; Lubin et al., 2003; Numan et al., 2010). Lastly, in mice, OTRs modulate projections from the dorsal raphe to the NAc. Activation of presynaptic OTRs on serotonergic projections from the dorsal raphe modulates serotonin-dependent LTD of medium spiny neurons in the NAc, a process that is critical for social learning (Dolen et al., 2013). Taken together, these studies show that OTRs and V1aRs can exploit a variety of circuit mechanisms to augment and/or attenuate transmission across interregional projections that modulate social learning and behavior.
It may be useful to consider these data in the context of recent evidence suggesting that psychiatric phenotypes such as depression, schizophrenia, generalized social anxiety, posttraumatic stress disorder (PTSD), and ASD are associated with differences in functional connectivity across widely distributed networks in the brain (Guye et al., 2010; Kennedy and Adolphs, 2012; Kleinhans et al., 2008; Lynall et al., 2010; von dem Hagen et al., 2013). Individual variation in multiple parameters of OT and AVP systems (e.g. peripheral levels, polymorphisms in the ligand and receptor genes, etc.) have been repeatedly linked with these psychiatric phenotypes (Ebstein et al., 2009; Feldman et al., 2014; Heinrichs et al., 2009; Meyer-Lindenberg et al., 2011; Myers et al., 2014). Thus, organizing principles that describe how OT and AVP modulate widely-distributed network states will likely have important translational implications for human social cognition. A few recent neuroimaging studies have revealed interesting results in this regard. In patients with generalized social anxiety, intranasal OT administration increases functional connectivity between the amygdala and cortical regions during processing of fearful faces when compared to healthy controls (Gorka et al., 2015). Similarly, intranasal OT administration differentially modulates functional connectivity between amygdalar and cortical nuclei in patients with PTSD compared to controls (Frijling et al., 2016; Koch et al., 2016). In youth diagnosed with ASD, polymorphisms in OXTR are associated with differences in resting state functional coupling between the NAc and frontal cortex (Hernandez et al., 2016), and intranasal OT administration increases functional coupling between reward—nuclei including the NAc—and regions involved in socioemotional processing during exposure to multimodal sociosensory stimuli (Gordon et al., 2016; Hernandez et al., 2016).
In summary, multiple experimental approaches suggest that central OT/AVP-like signaling during social contexts modulates widely distributed networks that are critical for social learning and behavior. Data from our laboratory and others’ suggests that OT/AVP-like neuromodulation during social contexts may transiently promote “states” of network function that are more strongly linked to social context, learning, and behavior than other potential levels of analyses (e.g. univariate analyses of neural activity or plasticity in individual brain regions). While these data may not be surprising, they indicate that OT/AVP-like systems represent an excellent opportunity to understand diversity in social information processing at a network level, and urge future experimental designs and analyses that are capable of testing network level hypotheses. Within this framework, individual and species level variation in patterns of central OT/AVP-like receptor expression may reflect a diverse range of neuronal populations, circuit mechanisms, and network “states” that can be transiently recruited during social contexts. Identifying anatomical and functional organizing principles of OT/AVP-like systems at the network level should be a primary goal for the field in coming years, and will likely provide valuable insights into human social cognitive and behavioral diversity.
Conclusions
OT/AVP-like systems exhibit conserved anatomical and functional properties. In vertebrates, these systems innervate conserved and widely distributed neural networks that regulate social information processing and behavior. Individual and species variation in the neuroanatomical organization of OT/AVP-like receptors may reflect diversity in network states that are transiently recruited during social contexts. Identifying links between central OTR/V1aR distributions and distributed network states will likely have critical implications for understanding individual and species level diversity in social perception, learning, and behavior; and for developing novel therapeutic strategies that target social cognitive symptom domains in human psychiatric phenotypes such as ASD (Modi and Young, 2012; Young and Barrett, 2015).
Highlights.
Oxytocin and vasopressin systems exhibit conserved properties
Receptor expression patterns reflect species-specific sociosensory pathways
Diversity in receptors expression contributes to diversity in social behavior
Oxytocin and vasopressin modulate widely distributed brain networks
Oxytocin is a potential target for improving social cognition in autism
Acknowledgments
Preparation of this manuscript was supported by the National Institutes of Health R01MH096983 and 1P50MH100023. The authors would like to thank Sara M. Freeman for providing rhesus and marmoset autoradiograms in Figure 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharjee S, Do-Rego JL, Oh DY, Moon JS, Ahn RS, Lee K, Bai DG, Vaudry H, Kwon HB, Seong JY. Molecular cloning, pharmacological characterization, and histochemical distribution of frog vasotocin and mesotocin receptors. J Mol Endocrinol. 2004;33:293–313. doi: 10.1677/jme.0.0330293. [DOI] [PubMed] [Google Scholar]
- Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol. 2015;36:49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri S, Haj-Mirzaian A, Rahimi-Balaei M, Razmi A, Kordjazy N, Shirzadian A, Ejtemaei Mehr S, Sianati H, Dehpour AR. Co-occurrence of anxiety and depressive-like behaviors following adolescent social isolation in male mice; possible role of nitrergic system. Physiol Behav. 2015;145:38–44. doi: 10.1016/j.physbeh.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Bader A, Breer H, Strotmann J. Untypical connectivity from olfactory sensory neurons expressing OR37 into higher brain centers visualized by genetic tracing. Histochem Cell Biol. 2012a;137:615–628. doi: 10.1007/s00418-012-0919-2. [DOI] [PubMed] [Google Scholar]
- Bader A, Klein B, Breer H, Strotmann J. Connectivity from OR37 expressing olfactory sensory neurons to distinct cell types in the hypothalamus. Front Neural Circuits. 2012b;6:84. doi: 10.3389/fncir.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry. 2015;5:e606. doi: 10.1038/tp.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav. 2013;63:518–526. doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautze V, Schwack W, Breer H, Strotmann J. Identification of a natural source for the OR37B ligand. Chem Senses. 2014;39:27–38. doi: 10.1093/chemse/bjt051. [DOI] [PubMed] [Google Scholar]
- Berlove DJ, Piekut DT. Co-localization of putative vasopressin receptors and vasopressinergic neurons in rat hypothalamus. Histochemistry. 1990;94:653–657. doi: 10.1007/BF00271993. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38:962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Blandon-Gitlin I, Pezdek K, Saldivar S, Steelman E. Oxytocin eliminates the own-race bias in face recognition memory. Brain Res. 2014;1580:180–187. doi: 10.1016/j.brainres.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology. 2016;64:66–78. doi: 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudaba C, Tasker JG. Intranuclear coupling of hypothalamic magnocellular nuclei by glutamate synaptic circuits. Am J Physiol Regul Integr Comp Physiol. 2006;291:R102–111. doi: 10.1152/ajpregu.00795.2005. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Philos Trans R Soc Lond B Biol Sci. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnlieb C, Nave G, Camerer CF, Schosser S, Vogt B, Munte TF, Heldmann M. Vasopressin increases human risky cooperative behavior. Proc Natl Acad Sci U S A. 2016;113:2051–2056. doi: 10.1073/pnas.1518825113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB. Oxytocin suppresses the GABAergic synaptic input in supraoptic neurones from the rat. Adv Exp Med Biol. 1995;395:105–115. [PubMed] [Google Scholar]
- Burbach JP, Young LJ, Russell J. Oxytocin: synthesis, secretion, and reproductive functions. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Third Edition. Elsevier Academic Press; New York City: 2006. pp. 3055–3127. [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Maruska KP. The Mechanosensory Lateral Line System Mediates Activation of Socially-Relevant Brain Regions during Territorial Interactions. Frontiers in Behavioral Neuroscience. 2016;10 doi: 10.3389/fnbeh.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Smith EJ, Holmes NM, Lingawi NW, Panayi MC, Westbrook RF. Oxytocin signaling in basolateral and central amygdala nuclei differentially regulates the acquisition, expression, and extinction of context-conditioned fear in rats. Learn Mem. 2015;22:247–257. doi: 10.1101/lm.036962.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. [DOI] [PubMed] [Google Scholar]
- Choe HK, Reed MD, Benavidez N, Montgomery D, Soares N, Yim YS, Choi GB. Oxytocin Mediates Entrainment of Sensory Stimuli to Social Cues of Opposing Valence. Neuron. 2015;87:152–163. doi: 10.1016/j.neuron.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Pini S, Gabelloni P, Abelli M, Lari L, Cardini A, Muti M, Gesi C, Landi S, Galderisi S, Mucci A, Lucacchini A, Cassano GB, Martini C. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34:1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Crapon de Caprona MD. Olfactory communication in a Cichlid fish, Haplochromis burtoni. Z Tierpsychol. 1980;52:113–134. doi: 10.1111/j.1439-0310.1980.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Noble PL, Costa VD, Averbeck BB. Oxytocin enhances attention to the eye region in rhesus monkeys. Front Neurosci. 2014;8:41. doi: 10.3389/fnins.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanithi G, Sabatier N, Widmer H. Intracellular calcium signalling in magnocellular neurones of the rat supraoptic nucleus: understanding the autoregulatory mechanisms. Exp Physiol. 2000;85(Spec No):75S–84S. doi: 10.1111/j.1469-445x.2000.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, Gritsenko I, Riebold M, Salomon S, Yirmiya N. Arginine vasopressin and oxytocin modulate human social behavior. Ann N Y Acad Sci. 2009;1167:87–102. doi: 10.1111/j.1749-6632.2009.04541.x. [DOI] [PubMed] [Google Scholar]
- Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, Ciobanu AC, Triana Del Rio R, Roth LC, Althammer F, Chavant V, Goumon Y, Gruber T, Petit-Demouliere N, Busnelli M, Chini B, Tan LL, Mitre M, Froemke RC, Chao MV, Giese G, Sprengel R, Kuner R, Poisbeau P, Seeburg PH, Stoop R, Charlet A, Grinevich V. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron. 2016;89:1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin Pathway Genes: Evolutionary Ancient System Impacting on Human Affiliation, Sociality, and Psychopathology. Biol Psychiatry. 2016;79:174–184. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Feldman R, Vengrober A, Ebstein RP. Affiliation buffers stress: cumulative genetic risk in oxytocin-vasopressin genes combines with early caregiving to predict PTSD in war-exposed young children. Transl Psychiatry. 2014;4:e370. doi: 10.1038/tp.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Shalev I, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Feng C, Lori A, Waldman ID, Binder EB, Haroon E, Rilling JK. A common oxytocin receptor gene (OXTR) polymorphism modulates intranasal oxytocin effects on the neural response to social cooperation in humans. Genes Brain and Behavior. 2015;14:516–525. doi: 10.1111/gbb.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative Perspectives on Oxytocin and Vasopressin Receptor Research in Rodents and Primates: Translational Implications. J Neuroendocrinol. 2016;28 doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling JL, van Zuiden M, Koch SB, Nawijn L, Veltman DJ, Olff M. Intranasal Oxytocin Affects Amygdala Functional Connectivity after Trauma Script-Driven Imagery in Distressed Recently Trauma-Exposed Individuals. Neuropsychopharmacology. 2016;41:1286–1296. doi: 10.1038/npp.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M, Bhatt R, Picotte KB, Hull EM. Sexual experience increases oxytocin receptor gene expression and protein in the medial preoptic area of the male rat. Psychoneuroendocrinology. 2013;38:1688–1697. doi: 10.1016/j.psyneuen.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard ER, Coburn CG, de Leon A, Snissarenko EP, Bauce LG, Pittman QJ, Hou B, Curras-Collazo MC. Vasopressin autoreceptors and nitric oxide-dependent glutamate release are required for somatodendritic vasopressin release from rat magnocellular neuroendocrine cells responding to osmotic stimuli. Endocrinology. 2007;148:479–489. doi: 10.1210/en.2006-0995. [DOI] [PubMed] [Google Scholar]
- Gilligan P, Brenner S, Venkatesh B. Neurone-specific expression and regulation of the pufferfish isotocin and vasotocin genes in transgenic mice. J Neuroendocrinol. 2003;15:1027–1036. doi: 10.1046/j.1365-2826.2003.01090.x. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Godwin J, Thompson R. Nonapeptides and social behavior in fishes. Horm Behav. 2012;61:230–238. doi: 10.1016/j.yhbeh.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. Journal of Comparative Neurology. 2000;422:363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. Journal of Comparative Neurology. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: Relation to vasotocin and vocal-acoustic circuitry. Journal of Comparative Neurology. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front Neuroendocrinol. 2009;30:429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA. What’s in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm Behav. 2013;64:103–112. doi: 10.1016/j.yhbeh.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Jack A, Pretzsch CM, Vander Wyk B, Leckman JF, Feldman R, Pelphrey KA. Intranasal Oxytocin Enhances Connectivity in the Neural Circuitry Supporting Social Motivation and Social Perception in Children with Autism. Sci Rep. 2016;6:35054. doi: 10.1038/srep35054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, Labuschagne I, Hosanagar A, Wood AG, Nathan PJ, Phan KL. Oxytocin modulation of amygdala functional connectivity to fearful faces in generalized social anxiety disorder. Neuropsychopharmacology. 2015;40:278–286. doi: 10.1038/npp.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Gruber CW, Muttenthaler M, Freissmuth M. Ligand-based peptide design and combinatorial peptide libraries to target G protein-coupled receptors. Curr Pharm Des. 2010;16:3071–3088. doi: 10.2174/138161210793292474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R, Tendler A, Wagner S. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol Psychiatry. 2014;76:377–386. doi: 10.1016/j.biopsych.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Guye M, Bettus G, Bartolomei F, Cozzone PJ. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. MAGMA. 2010;23:409–421. doi: 10.1007/s10334-010-0205-z. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hernandez LM, Krasileva K, Green SA, Sherman LE, Ponting C, McCarron R, Lowe JK, Geschwind DH, Bookheimer SY, Dapretto M. Additive effects of oxytocin receptor gene polymorphisms on reward circuitry in youth with autism. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H. Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J Physiol Sci. 2016;66:275–282. doi: 10.1007/s12576-015-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Kikuchi M, Munesue T. CD38 and its role in oxytocin secretion and social behavior. Horm Behav. 2012;61:351–358. doi: 10.1016/j.yhbeh.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Hoke KL, Burmeister SS, Fernald RD, Rand AS, Ryan MJ, Wilczynski W. Functional mapping of the auditory midbrain during mate call reception. J Neurosci. 2004;24:11264–11272. doi: 10.1523/JNEUROSCI.2079-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc Natl Acad Sci U S A. 2005;102:10712–10717. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Keebaugh AC, Reamer LA, Schaeffer J, Schapiro SJ, Young LJ. Genetic influences on receptive joint attention in chimpanzees (Pan troglodytes) Sci Rep. 2014;4:3774. doi: 10.1038/srep03774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Qi S, Becker B, Luo L, Gao S, Gong Q, Hurlemann R, Kendrick KM. Oxytocin selectively facilitates learning with social feedback and increases activity and functional connectivity in emotional memory and reward processing regions. Hum Brain Mapp. 2015;36:2132–2146. doi: 10.1002/hbm.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard PC, Mota VC, Keller-Costa T, da Silva JP, Canario AV. Chemical communication in tilapia: a comparison of Oreochromis mossambicus with O. niloticus. Gen Comp Endocrinol. 2014;207:13–20. doi: 10.1016/j.ygcen.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Huffman LS, O’Connell LA, Kenkel CD, Kline RJ, Khan IA, Hofmann HA. Distribution of nonapeptide systems in the forebrain of an African cichlid fish, Astatotilapia burtoni. J Chem Neuroanat. 2012;44:86–97. doi: 10.1016/j.jchemneu.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel JM, Le Masson G, Theodosis DT, Poulain DA. Glutamatergic input governs periodicity and synchronization of bursting activity in oxytocin neurons in hypothalamic organotypic cultures. European Journal of Neuroscience. 2003;17:2619–2629. doi: 10.1046/j.1460-9568.2003.02705.x. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, Young LJ. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm Behav. 2016a;79:8–17. doi: 10.1016/j.yhbeh.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Xiao Y, Riefkohl PC, Young LJ. Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm Behav. 2016b;87:16–24. doi: 10.1016/j.yhbeh.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Current Opinion in Behavioral Sciences. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas W, Mileva-Seitz V, Girard AW, Bisceglia R, Kennedy JL, Sokolowski M, Meaney MJ, Fleming AS, Steiner M, Team MR. Genetic variation in oxytocin rs2740210 and early adversity associated with postpartum depression and breastfeeding duration. Genes Brain Behav. 2013;12:681–694. doi: 10.1111/gbb.12069. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Israel JM, Dupouy B, Oliet SH, Allard M, Vitiello S, Theodosis DT, Poulain DA. Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro. J Neurosci. 1998;18:6641–6649. doi: 10.1523/JNEUROSCI.18-17-06641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamatianos T, Faulkes CG, Oosthuizen MK, Poorun R, Bennett NC, Coen CW. Telencephalic binding sites for oxytocin and social organization: a comparative study of eusocial naked mole-rats and solitary cape mole-rats. J Comp Neurol. 2010;518:1792–1813. doi: 10.1002/cne.22302. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Hoshi K, Kawano M, Nogami H, Yoshikawa H, Hisano S. Up-regulation of VGLUT2 expression in hypothalamic-neurohypophysial neurons of the rat following osmotic challenge. Eur J Neurosci. 2005;22:672–680. doi: 10.1111/j.1460-9568.2005.04240.x. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60:498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front Neuroendocrinol. 2014;35:512–529. doi: 10.1016/j.yfrne.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Functional interactions of dopamine cell groups reflect personality, sex, and social context in highly social finches. Behav Brain Res. 2015;280:101–112. doi: 10.1016/j.bbr.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ. Variation in the Oxytocin Receptor Gene Predicts Brain Region-Specific Expression and Social Attachment. Biol Psychiatry. 2016;80:160–169. doi: 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Kline RJ, O’Connell LA, Hofmann HA, Holt GJ, Khan IA. The distribution of an AVT V1a receptor in the brain of a sex changing fish, Epinephelus adscensionis. J Chem Neuroanat. 2011;42:72–88. doi: 10.1016/j.jchemneu.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Knafo A, Israel S, Darvasi A, Bachner-Melman R, Uzefovsky F, Cohen L, Feldman E, Lerer E, Laiba E, Raz Y, Nemanov L, Gritsenko I, Dina C, Agam G, Dean B, Bornstein G, Ebstein RP. Individual differences in allocation of funds in the dictator game associated with length of the arginine vasopressin 1a receptor RS3 promoter region and correlation between RS3 length and hippocampal mRNA. Genes Brain Behav. 2008;7:266–275. doi: 10.1111/j.1601-183X.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front Behav Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal Oxytocin Normalizes Amygdala Functional Connectivity in Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41:2041–2051. doi: 10.1038/npp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Laursen HR, Siebner HR, Haren T, Madsen K, Gronlund R, Hulme O, Henningsson S. Variation in the oxytocin receptor gene is associated with behavioral and neural correlates of empathic accuracy. Front Behav Neurosci. 2014;8:423. doi: 10.3389/fnbeh.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CH, Abebe DF, Earp SE, Goode CT, Grozhik AV, Mididoddi P, Maney DL. Neural Distribution of Vasotocin Receptor mRNA in Two Species of Songbird. Endocrinology. 2011;152:4865–4881. doi: 10.1210/en.2011-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CH, Goode CT, Young LJ, Maney DL. Neural Distribution of Nonapeptide Binding Sites in Two Species of Songbird. Journal of Comparative Neurology. 2009;513:197–208. doi: 10.1002/cne.21947. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Lou F, Lavebratt C, Forsell Y. Impact of Childhood Adversity and Vasopressin receptor 1a Variation on Social Interaction in Adulthood: A Cross-Sectional Study. PLoS One. 2015a;10:e0136436. doi: 10.1371/journal.pone.0136436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Hadj-Bouziane F, Jones KB, Turchi JN, Averbeck BB, Ungerleider LG. Oxytocin modulates fMRI responses to facial expression in macaques. Proc Natl Acad Sci U S A. 2015b;112:E3123–3130. doi: 10.1073/pnas.1508097112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Hadj-Bouziane F, Moran R, Ungerleider LG, Ishai A. Facial Expressions Evoke Differential Neural Coupling in Macaques. Cereb Cortex. 2016 doi: 10.1093/cercor/bhv345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- Loth E, Poline JB, Thyreau B, Jia T, Tao C, Lourdusamy A, Stacey D, Cattrell A, Desrivieres S, Ruggeri B, Fritsch V, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Carvalho FM, Conrod PJ, Fauth-Buehler M, Flor H, Gallinat J, Garavan H, Heinz A, Bruehl R, Lawrence C, Mann K, Martinot JL, Nees F, Paus T, Pausova Z, Poustka L, Rietschel M, Smolka M, Struve M, Feng J, Schumann G, Consortium I. Oxytocin receptor genotype modulates ventral striatal activity to social cues and response to stressful life events. Biol Psychiatry. 2014;76:367–376. doi: 10.1016/j.biopsych.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Love TM, Enoch MA, Hodgkinson CA, Pecina M, Mickey B, Koeppe RA, Stohler CS, Goldman D, Zubieta JK. Oxytocin gene polymorphisms influence human dopaminergic function in a sex-dependent manner. Biol Psychiatry. 2012;72:198–206. doi: 10.1016/j.biopsych.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Luo L, Ma X, Zheng X, Zhao W, Xu L, Becker B, Kendrick KM. Neural systems and hormones mediating attraction to infant and child faces. Front Psychol. 2015;6:970. doi: 10.3389/fpsyg.2015.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney PD, Koh ET, Irvin RW, Ferris CF. Computer-Aided Mapping of Vasopressin Neurons in the Hypothalamus of the Male Golden Hamster: Evidence of Magnocellular Neurons that do not Project to the Neurohypophysis. J Neuroendocrinol. 1990;2:113–122. doi: 10.1111/j.1365-2826.1990.tb00840.x. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Matheson K, Anisman H. Distress of ostracism: oxytocin receptor gene polymorphism confers sensitivity to social exclusion. Soc Cogn Affect Neurosci. 2015;10:1153–1159. doi: 10.1093/scan/nsu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, Kennedy JL, Sokolowski MB, Fleming AS. Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS One. 2013;8:e61443. doi: 10.1371/journal.pone.0061443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, Froemke RC. A Distributed Network for Social Cognition Enriched for Oxytocin Receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:2517–2535. doi: 10.1523/JNEUROSCI.2409-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Inoue K, Barrett CE, Kittelberger KA, Smith DG, Landgraf R, Young LJ. Melanocortin Receptor Agonists Facilitate Oxytocin-Dependent Partner Preference Formation in the Prairie Vole. Neuropsychopharmacology. 2015;40:1856–1865. doi: 10.1038/npp.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61:340–350. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons L, Cambre M, Batten TF, Vandesande F. Autoradiographic localization of binding sites for vasotocin in the brain and pituitary of the sea bass (Dicentrarchus labrax) Neurosci Lett. 1989;100:11–16. doi: 10.1016/0304-3940(89)90652-6. [DOI] [PubMed] [Google Scholar]
- Murphy D, Si-Hoe SL, Brenner S, Venkatesh B. Something fishy in the rat brain: molecular genetics of the hypothalamo-neurohypophysial system. Bioessays. 1998;20:741–749. doi: 10.1002/(SICI)1521-1878(199809)20:9<741::AID-BIES7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Myers AJ, Williams L, Gatt JM, McAuley-Clark EZ, Dobson-Stone C, Schofield PR, Nemeroff CB. Variation in the oxytocin receptor gene is associated with increased risk for anxiety, stress and depression in individuals with a history of exposure to early life stress. J Psychiatr Res. 2014;59:93–100. doi: 10.1016/j.jpsychires.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, Onaka T, Mogi K, Kikusui T. Social evolution. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science. 2015;348:333–336. doi: 10.1126/science.1261022. [DOI] [PubMed] [Google Scholar]
- Numan M, Bress JA, Ranker LR, Gary AJ, Denicola AL, Bettis JK, Knapp SE. The importance of the basolateral/basomedial amygdala for goal-directed maternal responses in postpartum rats. Behav Brain Res. 2010;214:368–376. doi: 10.1016/j.bbr.2010.06.006. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336:1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- O’Loghlen AL, Rothstein SI. It’s Not Just the Song: Male Visual Displays Enhance Female Sexual Responses to Song in Brown-Headed Cowbirds. Condor. 2010;112:615–621. [Google Scholar]
- Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, Meyer-Lindenberg A, Grinevich V, Shusterman R, Kelsch W. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron. 2016;90:609–621. doi: 10.1016/j.neuron.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Yoshida M, Takayanagi Y, Onaka T. Activation of hypothalamic oxytocin neurons following tactile stimuli in rats. Neurosci Lett. 2015;600:22–27. doi: 10.1016/j.neulet.2015.05.055. [DOI] [PubMed] [Google Scholar]
- Okhovat M, Berrio A, Wallace G, Ophir AG, Phelps SM. Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science. 2015;350:1371–1374. doi: 10.1126/science.aac5791. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–1541. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Campbell P, Hanna K, Phelps SM. Field tests of cis-regulatory variation at the prairie vole avpr1a locus: association with V1aR abundance but not sexual or social fidelity. Horm Behav. 2008;54:694–702. doi: 10.1016/j.yhbeh.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm Behav. 2012;61:445–453. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyshyn TL, Hurd PL, Crespi BJ. Association testing of vasopressin receptor 1a microsatellite polymorphisms in non-clinical autism spectrum phenotypes. Autism Res. 2016 doi: 10.1002/aur.1716. [DOI] [PubMed] [Google Scholar]
- Puglia MH, Lillard TS, Morris JP, Connelly JJ. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc Natl Acad Sci U S A. 2015;112:3308–3313. doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Montag C, Altmann S, Bendlow F, Elger C, Kirsch P, Becker A, Schoch-McGovern S, Simon M, Weber B, Felten A. Functional characterization of an oxytocin receptor gene variant (rs2268498) previously associated with social cognition by expression analysis in vitro and in human brain biopsy. Soc Neurosci. 2016:1–8. doi: 10.1080/17470919.2016.1214174. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JD, Moore FL. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front Neuroendocrinol. 2002;23:317–341. doi: 10.1016/s0091-3022(02)00004-3. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009a;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009b;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Shibuya I, Dayanithi G. Intracellular calcium increase and somatodendritic vasopressin release by vasopressin receptor agonists in the rat supraoptic nucleus: involvement of multiple intracellular transduction signals. J Neuroendocrinol. 2004;16:221–236. doi: 10.1111/j.0953-8194.2004.01155.x. [DOI] [PubMed] [Google Scholar]
- Saito D, Komatsuda M, Urano A. Functional organization of preoptic vasotocin and isotocin neurons in the brain of rainbow trout: central and neurohypophysial projections of single neurons. Neuroscience. 2004;124:973–984. doi: 10.1016/j.neuroscience.2003.12.038. [DOI] [PubMed] [Google Scholar]
- Saito D, Urano A. Synchronized periodic Ca2+ pulses define neurosecretory activities in magnocellular vasotocin and isotocin neurons. J Neurosci. 2001;21:RC178. doi: 10.1523/JNEUROSCI.21-21-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Coomber P, Gonzalez-Lima F, Crews D. Functional connectivity among limbic brain areas: differential effects of incubation temperature and gonadal sex in the leopard gecko, Eublepharis macularius. Brain Behav Evol. 2000;55:139–151. doi: 10.1159/000006648. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S, Toichi M. Direction of Amygdala-Neocortex Interaction During Dynamic Facial Expression Processing. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw036. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci U S A. 2013;110:20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy WA. Song Repertoire and Mate Choice in Birds. Am Zool. 1992;32:71–80. [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc Biol Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes JM, Barata EN, Harris RM, O’Connell LA, Hofmann HA, Oliveira RF. Social odors conveying dominance and reproductive information induce rapid physiological and neuromolecular changes in a cichlid fish. BMC Genomics. 2015;16:114. doi: 10.1186/s12864-015-1255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, Lehtimaki T, Binder EB, Young LJ. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci U S A. 2014;111:1987–1992. doi: 10.1073/pnas.1302985111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staes N, Koski SE, Helsen P, Fransen E, Eens M, Stevens JM. Chimpanzee sociability is associated with vasopressin (Avpr1a) but not oxytocin receptor gene (OXTR) variation. Horm Behav. 2015;75:84–90. doi: 10.1016/j.yhbeh.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370:1960–1973. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- Tay TL, Ronneberger O, Ryu S, Nitschke R, Driever W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat Commun. 2011;2:171. doi: 10.1038/ncomms1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, French JA. Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Horm Behav. 2015;75:154–159. doi: 10.1016/j.yhbeh.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles MC, Almeida O, Lopes JS, Oliveira RF. Social interactions elicit rapid shifts in functional connectivity in the social decision-making network of zebrafish. Proc Biol Sci. 2015;282 doi: 10.1098/rspb.2015.1099. 20151099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles MC, Cardoso SD, Oliveira RF. Social Plasticity Relies on Different Neuroplasticity Mechanisms across the Brain Social Decision-Making Network in Zebrafish. Front Behav Neurosci. 2016;10:16. doi: 10.3389/fnbeh.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, Hausen H, Arendt D. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci U S A. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Verchinski BA, Bilek E, Goldman AL, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Neurogenetic effects of OXTR rs2254298 in the extended limbic system of healthy Caucasian adults. Biol Psychiatry. 2011;70:e37–39. doi: 10.1016/j.biopsych.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Uzefovsky F, Shalev I, Israel S, Edelman S, Raz Y, Mankuta D, Knafo-Noam A, Ebstein RP. Oxytocin receptor and vasopressin receptor 1a genes are respectively associated with emotional and cognitive empathy. Horm Behav. 2015;67:60–65. doi: 10.1016/j.yhbeh.2014.11.007. [DOI] [PubMed] [Google Scholar]
- van Kesteren RE, Tensen CP, Smit AB, van Minnen J, van Soest PF, Kits KS, Meyerhof W, Richter D, van Heerikhuizen H, Vreugdenhil E, et al. A novel G protein-coupled receptor mediating both vasopressin- and oxytocin-like functions of Lys-conopressin in Lymnaea stagnalis. Neuron. 1995;15:897–908. doi: 10.1016/0896-6273(95)90180-9. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Si-Hoe SL, Murphy D, Brenner S. Transgenic rats reveal functional conservation of regulatory controls between the Fugu isotocin and rat oxytocin genes. Proc Natl Acad Sci U S A. 1997;94:12462–12466. doi: 10.1073/pnas.94.23.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. 2013;8:694–701. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Corral-Frias NS, Vannucci B, Bogdan R, Knodt AR, Hariri AR, Hyde LW. An oxytocin receptor polymorphism predicts amygdala reactivity and antisocial behavior in men. Soc Cogn Affect Neurosci. 2016 doi: 10.1093/scan/nsw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, Pedersen NL, Anckarsater H, Larsson H, Westberg L. Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biol Psychiatry. 2012;71:419–426. doi: 10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci U S A. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KD. Social-Behavior of Anuran Amphibians. Animal Behaviour. 1977;25:666–693. [Google Scholar]
- Yang EJ, Wilczynski W. Social experience organizes parallel networks in sensory and limbic forebrain. Dev Neurobiol. 2007;67:285–303. doi: 10.1002/dneu.20347. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ. Regulating the social brain: a new role for CD38. Neuron. 2007;54:353–356. doi: 10.1016/j.neuron.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Young LJ, Barrett CE. Neuroscience. Can oxytocin treat autism? Science. 2015;347:825–826. doi: 10.1126/science.aaa8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol. 1999;11:291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zheng DJ, Larsson B, Phelps SM, Ophir AG. Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behav Brain Res. 2013;246:139–147. doi: 10.1016/j.bbr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J Neurosci. 2010;30:7017–7022. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]