Abstract
Several laboratories recently identified that astrocytes are critical regulators of addiction machinery. It is now known that astrocyte pathology is a common feature of ethanol exposure in both humans and animal models, as even brief ethanol exposure is sufficient to elicit long-lasting perturbations in astrocyte gene expression, activity, and proliferation. Astrocytes were also recently shown to modulate the motivational properties of ethanol and other strongly reinforcing stimuli. Given the role of astrocytes in regulating glutamate homeostasis, a crucial component of alcohol use disorder, astrocytes might be an important target for the development of next generation alcoholism treatments. This review will outline some of the more prominent features displayed by astrocytes, how these properties are influenced by acute and long term ethanol exposure, and future directions that may help to disentangle astrocytic from neuronal functions in the etiology of alcohol use disorder.
Keywords: Astrocyte, gliotransmission, glutamate, alcohol use disorder
Introduction
Alcohol use disorder (AUD) is a complex and chronically relapsing disorder that has a great impact on societies worldwide. Recent research has identified astrocytes as critical regulators of alcohol-intake, and ethanol-induced effects on astrocyte physiology appear to cause pronounced downstream effects on excitability, neurotransmission and neuronal health. This review begins by summarizing basic astrocyte functions prior to discussing the role of astrocytes in maintaining neuronal function during physiological conditions. The objective of the second part of this review is to highlight the impact of acute or long-term ethanol exposure on astrocyte function, astrocyte-neuron crosstalk, and behavior. The last part of the review describes future research directions that could be pursued to more precisely define the role of astrocytes in the etiology of AUD. While we predominately focus on excitatory mechanisms, we briefly cover the role of astrocytes in modulating non-glutamatergic neurotransmission.
Astrocytes maintain extracellular homeostasis and support neurotransmission
Glial cells (astrocytes, oligodendrocytes, and microglia) represent the most abundant cell group in the central nervous system (Sherwood et al., 2006). The star-shaped astrocyte, often characterized by expression of the cytoskeletal protein Glial Fibrillary Acidic Protein (GFAP) or the astrocyte-specific marker ALDH1L1, is the most numerous cell type of the glia family (Figure 1) (Yang et al., 2011). Astrocytes give structural and metabolic support while maintaining homeostasis of the extracellular milieu through clearance of potassium and neurotransmitters. Astrocytes play critical roles in glutamatergic neurotransmission. Astrocytes can regulate excitability through the clearance of glutamate and glycine as well as by the release of gliotransmitters such as glutamate and D-serine (Figure 2) (Hansson et al., 2000, Malarkey and Parpura, 2008). Clearance of extracellular glutamate is primarily mediated via the astrocytic glutamate transporters GLAST/EAAT1 and GLT-1/EAAT2, while glycine is taken up through glycine transporters GlyT1 and GlyT2 (Zhou and Danbolt, 2013, Aroeira et al., 2014). Glutamate and glycine are co-transported with Na+, which activates the Na+/K+-ATPase, thereby promoting astrocytic metabolism while simultaneously reducing neuronal excitability via clearing K+ from the extracellular space (Kirischuk et al., 2015). Following astrocytic uptake, glutamate is converted to its non-excitable precursor glutamine by glutamine synthetase; glutamine is subsequently released to the extracellular space and taken up by neurons to be converted back into glutamate (Figure 2) (Yudkoff et al., 1993). This astrocyte-dependent glutamate-glutamine cycle is required to maintain active neurotransmission at excitatory terminals, and may also play a vital role in GABAergic neurotransmission since glutamate can be converted into GABA via glutamate decarboxylase (Kaczor et al., 2015, Tani et al., 2014). The importance of astrocytes in maintaining glutamatergic tone are highlighted in mice lacking the glutamate transporter GLAST. These mice show reduced synaptic plasticity and phenotypic abnormalities thought to model the negative and cognitive symptoms of schizophrenia (Karlsson et al., 2012, Karlsson et al., 2008, Karlsson et al., 2009).
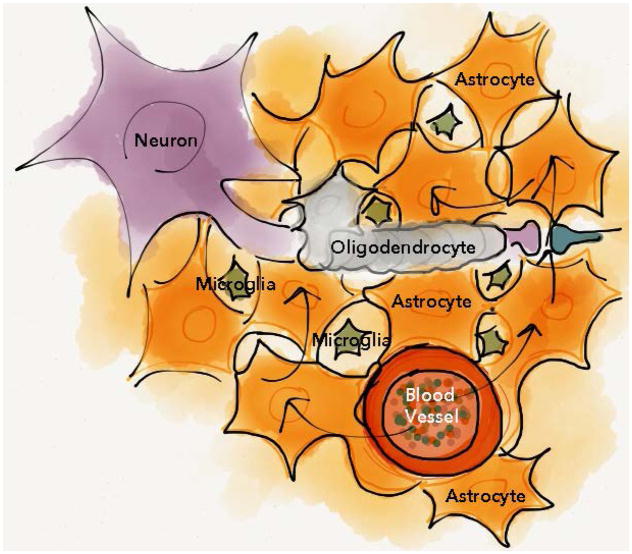
Figure 1. Glial cells provide metabolic support while buffering the extracellular milieu.
The glia cell family is pivotal for neuronal function, and astrocytes are the most numerous glial cells in mammalian brain. By enseathing blood vessels, astrocytes can serve as a conduit for delivery of energetic metabolites to distal neurons during energy consuming processes in addition to buffering excess central nervous system ions and small molecules. In the mature brain, oligodendrocytes increase conduction velocity of larger nerve fibers by enseathing axons between nodes of Ranvier. Microglia, and to a limited extent astrocytes, provide innate immune responses. Arrows represents the flow of nutrients that is taken up by astrocytes from the blood vessels, and transported to surrounding cells.
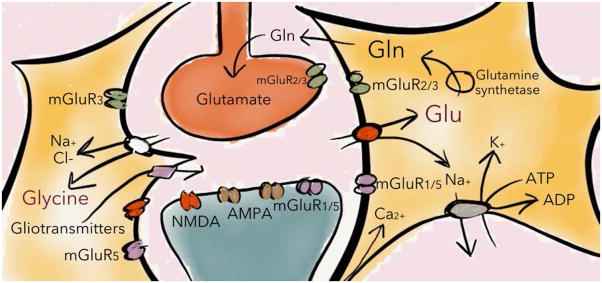
Figure 2. Astrocytes control excitatory neurotransmission.
Astrocytes regulate glutamatergic signaling on multiple levels. Not only are astrocytes primarily responsible for clearing glutamate in the brain, they also take up glycine, which is a co-agonist for the NMDA receptor. In addition, astrocytes release gliotransmitters such as D-serine and glutamate, which could further influence excitatory transmission especially at ionotropic NMDA receptors. See text for further details. Glu=glutamate, Gln=glutamine, mGluR=metabotropic glutamate receptors, AMPA/NMDA=ionotropic glutamate receptors, ADP=adenosine diphosphate.
The end feet of astrocytic processes encapsulate capillaries, allowing exchange of small molecules including ions, glucose, and amino acids between smaller blood vessels and the brain tissue. This anatomical arrangement allows astrocytes to provide biochemical, metabolic, and structural support to surrounding neurons. For example, the intercellular trafficking of glucose through astroglial networks is increased during energy consuming processes (Magistretti and Pellerin, 1999, Magistretti et al., 1999, Rouach et al., 2008). Astrocytic glycogen breakdown into lactate and subsequent transport of lactate into neurons is required for long-term memory formation (Suzuki et al., 2011), and astrocytes may also support synaptic plasticity mechanisms through the release of thrombospondins and gliotransmitters (Crawford et al., 2012, Henneberger et al., 2010). Astrocytes can also modulate neurotransmission via specialized extracellular matrix components known as perineuronal nets that can be found near glial processes. Perineuronal nets are a form of molecular scaffold deposited at the interface between the astrocyte and neuronal synapse (Dzyubenko et al., 2016). Thus, astrocytes appear to play a key role in regulating the formation, maturation, stability, and maintenance of neuronal synapses (Slezak and Pfrieger, 2003). Malfunctioning of astrocytes may thus not only cause instability of neuronal networks, but also impinge upon the intrinsic ability of the central nervous system to appropriately adapt to external influences due to impaired synaptic plasticity.
Calcium signaling through the astrocytic network
Electrophysiological recordings and functional analyses have demonstrated that astrocytes are extensively interconnected through gap junction channels, built up by connexins (Giaume et al., 1991, Adermark and Lovinger, 2008). Several different connexins have been identified, but connexin-43 is the primary subtype interconnecting astrocytes. Because gap junction channels allow diffusion of small molecules, such as Ca2+ and inositol (1,4,5) trisphosphate (IP3), these channels represent a direct pathway for bidirectional electrical coupling and metabolic signaling (Blomstrand et al., 1999a) while also promoting coordinated activation of neuronal networks and synaptic plasticity (Pannasch et al., 2011, Chever et al., 2014, Chever et al., 2016). Connexin-43 is highly expressed by astrocytes located at the blood-brain barrier (BBB) interface (Nagy and Rash, 2000) where they play critical roles in the clearance of glutamate and potassium. Gating of gap junction channels is influenced by neuronal activity as well as ion concentrations within the astrocyte, indirectly resulting in neuronal control of intercellular signal propagation through the astrocytic syncytium (Blomstrand et al., 1999b).
A functional astrocytic network may thus be essential for precise information transfer between neurons, and reduced function of astrocyte connexins 30 and 43 has been associated with severe psychiatric conditions such as increased risk of suicide (Ernst et al., 2011, Nevin, 2012). Connexins, and the closely related pannexins, can also form hemichannels, which are gap junction channel-like pores that open to the extracellular space instead of directly coupling neighboring cells (Iglesias et al., 2009). Hemichannels can release gliotransmitters such as adenosine, glutamate, and D-serine, and these channels are tightly regulated during physiological conditions. However, dysregulation of hemichannel properties may occur in response to injury or inflammatory factors, resulting in relatively nonselective opening of large membrane pores (Montero and Orellana, 2015, Orellana and Stehberg, 2014, Kim et al., 2016). It should be additionally noted that gliotransmitters can also be released through the two-pore domain potassium channel TREK-1, and the Ca2+-activated anion channel Best1; these channels can differentially influence neighboring neurons due to discrete channel distribution in distinct astrocytic subdomains (Woo et al., 2012).
Although astrocytes do not generate action potentials, they show intrinsic cellular excitability based on intracellular Ca2+ transients (Figure 3). These Ca2+ variations may arise spontaneously or as a consequence of different stimuli including depolarization of neighboring neurons and/or neurotransmitter binding. Astrocytic Ca2+ transients produce a nonlinear input–output response that may tune neuronal activity via gliotransmitter release and changes in metabolic support (Navarrete et al., 2012, Schummers et al., 2008, Takata et al., 2011). Transient astrocytic Ca2+ signals are primarily mediated through Gαq-coupled G-protein receptor-mediated activation of phospholipase C (PLC) and subsequent formation of IP3, which leads to release of Ca2+ from internal stores and, to a lesser extent, Ca2+ influx through voltage-dependent and in-dependent Ca2+ channels (Verkhratsky and Kettenmann, 1996) (Figure 3). The amplitude and intracellular propagation of Ca2+ signals are partially modulated by activity of different synaptic inputs, but astrocytes also display cell-specific intrinsic properties that regulate excitability (Perea and Araque, 2005). Elevated levels of intracellular IP3 or [Ca2+]i induce the release of gliotransmitters, and neuronal activity has been shown to mediate gliotransmitter-release in several brain areas (Nedergaard, 1994, Wang et al., 2013, Zorec et al., 2012), including in human brain preparations (Navarrete et al., 2013). In addition to calcium signaling, neuronal activity may also evoke astrocytic sodium transients that can spread through the astrocytic syncytium (Rose and Chatton, 2015). Increased [Na+]i can influence the driving force for GABA and glutamate uptake, as well as increase the release of gliotransmitters, thereby modulating the spatio-temporal profiles of transmitter levels in the extracellular space (Kirischuk et al., 2015). Gliotransmitters act on pre-, post-, and extrasynaptic targets, thereby producing a complex reciprocal communication between astrocytes and neurons. Interfering with astrocytic Ca2+ homeostasis, or IP3 signaling, disrupts gliotransmitter-release, and perturbs synaptic transmission and plasticity, supporting the hypothesis that astrocytes play crucial roles in activity-dependent adaptations of neural circuits (Henneberger et al., 2010, Takata et al., 2011).
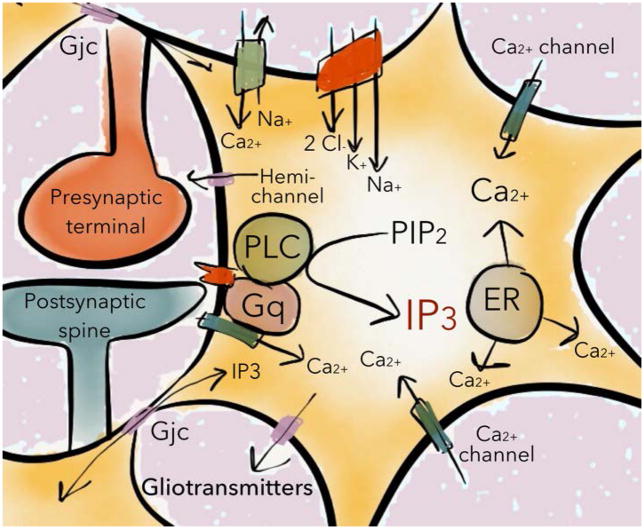
Figure 3. Receptor activated calcium signaling in astrocytes.
Astrocytic calcium transients are primarily mediated through the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol (1,4,5) trisphosphate (IP3) following activation of G-protein coupled receptors. IP3 promotes the release of Ca2+ predominately from intracellular pools (endoplasmatic reticulum; ER), into the cytoplasm, and influx of calcium through calcium channels primarily activated by changes in membrane potential. Following formation/release of IP3 or Ca2+, these signaling molecules may spread to surrounding astrocytes through gap junction channels (Gjc) and lead to coordinated release of gliotransmitters.
Astrocyte-neuron crosstalk occurs in a pathway specific manner
A single astrocyte can make contact with thousands of synapses. This enables the astrocyte to serve as an active processing bridge for synaptic interaction and crosstalk between synapses that have no direct neuronal connectivity (Bushong et al., 2002, Covelo and Araque, 2015) (Figure 4). Moreover, astrocytes can interact with neurons at multiple levels including through direct communication at the synapse (Perea et al., 2014). Subtypes of astrocytes can form direct synaptic contacts with axons (Bergles et al., 2010), while others ensheathe pre- and postsynaptic terminals, thereby constituting an integral element of the neuronal synapse (Rossi, 2015, Halassa et al., 2007). Astrocyte process motility often correlates with movement in associated dendritic protrusions, and this structural relationship undergoes activity-dependent changes with consequences on synaptic regulation and metaplasticity (Perez-Alvarez et al., 2014). At the site of synaptic contact on stratum radiatum, hippocampal, and cerebellar parallel fiber neurons, astrocyte processes can preferentially appose postsynaptic spines. This structural arrangement is hypothesized to favor fast presynaptic autoreceptor feedback while preserving specificity of the postsynaptic input (Lehre and Rusakov, 2002).
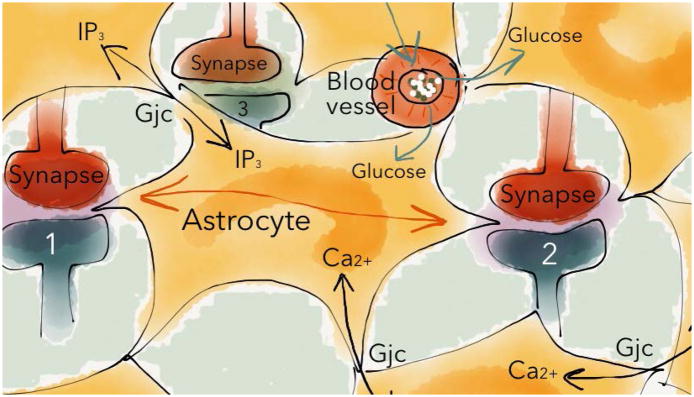
Figure 4. Pathway specific integration of synaptic information.
Astrocytes are important information processors in the neural network, and astrocytes may support and regulate neurotransmission in a pathway specific manner (for instance interacting with synapse 1 and 2 but not 3). Neuronal activity evokes complex intrinsic changes in intracellular Ca2+ that may spread through the astrocytic network, and provoke the release of gliotransmitters at distal synapses. One astrocyte may be in contact with thousands of synapses, enabling synaptic interaction without direct neuronal connectivity, as shown between synapse 1 and 2. See text for further details. Gjc=gap junction coupling.
Astrocytes have been shown to discriminate activity of synaptic terminals belonging to specific neural circuits (Perea and Araque, 2005). This study found that astrocytes specifically respond to activation of discrete axon pathways that release different neurotransmitters, and that astrocytes distinguish between synapses belonging to different axon pathways that use the same neurotransmitter. In addition, because of intrinsic cellular properties, the astrocytic Ca2+ signal is bidirectionally modulated through interaction of different synaptic inputs. Together, these data indicate that astrocytes can integrate synaptic information in a pathway-specific manner (Perea and Araque, 2005). Thus, despite anatomically apposed neurons participating in dissimilar networks, the neighboring astrocytes can help coordinate activity of neurons within the same network (Martin et al., 2015, Perea and Araque, 2005)).
Modulation of astrocyte number by ethanol
Astrocytes are a major target of ethanol in the human brain (de la Monte and Kril, 2014, Butterworth, 1995). Astrocyte adaptations are commonly measured by immunoreactivity of their major structural filament GFAP (Sofroniew and Vinters, 2010). For example, density (cells per region volume) of GFAP-expressing (GFAP+) astrocytes is lower in the prelimbic prefrontal cortex of ethanol-naive rats bred for high ethanol preference over water (Miguel-Hidalgo, 2005). Moreover, a reduction in cell number has been reported for several glial cell types in animal models of Fetal Alcohol Syndrome (FAS) (Wilhelm and Guizzetti, 2015). In contrast, increased GFAP+ astrocyte number is observed throughout the rat cerebral cortex following repeated gavage of a high ethanol dose or ethanol-containing diet (Udomuksorn et al., 2011, Dalcik et al., 2009). Astrocyte density also increases in the prelimbic cortex of ethanol-preferring rats during ethanol abstinence (Miguel-Hidalgo, 2006). Importantly, in standard outbred rats, GFAP+ astrocyte density changes are dependent on time, region, and method of ethanol administration (Bull et al., 2015). For example, after 3 weeks abstinence, GFAP+ astrocyte density decreases in prelimbic and orbitofrontal prefrontal cortex of rats that had continuous access to ethanol in the home cage, whereas anterior cingulate and orbitofrontal cortex astrocyte density decreases in rats abstinent from operant ethanol self-administration. No change in astrocyte density was seen in the infralimbic cortex or after intermittent access to ethanol in the home cage. Lower astrocyte density is also seen in the dorsolateral and orbitofrontal prefrontal cortex (Miguel-Hidalgo et al., 2002, Miguel-Hidalgo et al., 2006) and hippocampus (Korbo, 1999) of human alcoholics. Thus, astrocyte number is sensitive to ethanol in a region and exposure-dependent manner, and the importance of these changes is only beginning to be unraveled. For example, nucleus accumbens astrocytes also display time-, region-, and treatment-dependent changes in astrocyte density that can correlate with ethanol-seeking behavior (Bull et al., 2014). More specifically, GFAP+ astrocyte density in the nucleus accumbens shell increases after either 24 hours or 3 weeks abstinence from intermittent access to ethanol in the home cage. In the nucleus accumbens core, astrocyte density changes were only seen after 3 weeks abstinence, and this increase positively correlated with the motivation to resume ethanol self-administration after abstinence (Bull et al., 2014). Interestingly, astrocyte density increases in nucleus accumbens core after ethanol self-administration, yet decreases after sucrose self-administration (Bull et al., 2014). Ethanol might thus exert long-lasting and widespread perturbations in neurotransmission given these region-specific changes in astrocyte number during protracted abstinence. It should be noted, however, that reactive astrocytes are characterized by upregulated GFAP, and the increase in GFAP+ astrocyte number could in this respect also reflect a long-lasting inflammatory response to ethanol (Ben Haim et al., 2015). Thus, while it is currently unknown if changes in astrocyte density reflect general inflammatory processes or alterations in innate immunity, it is clear that astrocyte density can be related to the motivation to resume ethanol self-administration after abstinence.
Ethanol induced inflammation and glia
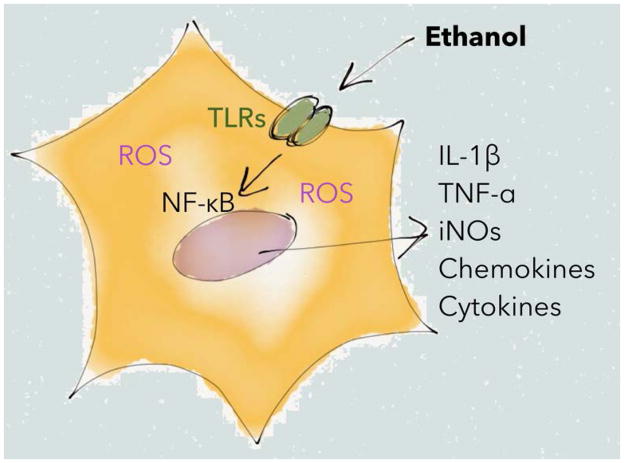
Ethanol intoxication is associated with oxidative stress, pro-inflammatory mediators and cytokine production, which may contribute to neuronal injury (He and Crews, 2008). Unlike neurons, microglia and to a lesser extent astrocytes mediate innate immunity, and both microglia and astrocytes express Toll-like receptors (TLRs). The TLRs are activated by a wide variety of microbial components and elicit innate immune responses (Kawai and Akira, 2007, Beutler et al., 2006). Distinct inflammatory signaling appears to occur in response to ethanol intoxication, causing the secretion of pro-inflammatory molecules from glial cells, predominately the cytokines interleukin-1 β (IL-1β) and tumor necrosis factor α (TNF-α) as well as chemokines (Blanco et al., 2004) (Figure 5). This is followed by release of inflammatory mediators such as cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS) via activation of nuclear factor kappa B (NF-κB), mitogen-activated protein kinase, and activator protein 1 (Akira and Takeda, 2004). The TLRs, which belong to the IL-1 receptor family, appear to be a key receptor mediating this signaling cascade. TLRs promote secretion of pro-inflammatory molecules in response to ethanol in a biphasic manner (Blanco et al., 2004, Blanco et al., 2005), and some of the acute effects of ethanol on GABAergic signaling are mediated by TLR4 (Bajo et al., 2014). Moreover, chronic ethanol consumption has been shown to impair proteolytic pathways, and the immune response mediated by TLR4 receptors (Pla et al., 2016). Since inflammatory processes mediated through TLRs also have been implicated in the development of neurological disorders such as Alzheimer’s disease (Walter et al., 2007), TLR4 recruitment in response to ethanol abuse could play a key role in ethanol-mediated neurodegeneration (Floreani et al., 2010). Further supporting a role for immune signaling in AUD are data from adolescent TLR4 knockout mice, which in contrast to wild type littermates, do not escalate ethanol preference over time (Montesinos et al., 2016). These mice also appear protected from ethanol-induced elevation of BDNF and FosB in mPFC, which might play a role in epigenetic transformation (Montesinos et al., 2016). Taken together, ethanol appears to enhance glial inflammatory signaling, which in turn may further promote the escalation of ethanol intake and subsequent neurodegeneration. At the same time, ethanol may also increase in the release of glutathione from astrocytes, which protects neurons from oxidative stress and apoptosis (Rathinam et al., 2006). Importantly, these processes have the potential for therapeutic intervention. For example, the dietary supplement N-acetylcysteine is essentially a prodrug that reduces oxidative stress by facilitating astrocytic glutathione production. In short, N-acetylcysteine is taken up by the astrocytic cysteine-glutamate antiporter prior to enzymatic conversion into glutathione. Thus, N-acetylcysteine increases extracellular glutamate while reducing oxidative stress. Increasing extracellular glutamate with N-acetylcyesteine has been shown to reduce relapse-like behavior for a number of substances (Baker et al., 2003, Bowers et al., 2016, Ramirez-Nino et al., 2013, Reissner et al., 2015). However, N-acetylcysteine failed to block cue-primed reinstatement of ethanol-seeking behavior in rats (Weiland et al., 2015). Interestingly, N-acetylcysteine restores GLT-1 expression following ethanol exposure (Roberts-Wolfe and Kalivas, 2015). Thus, N-acetylcysteine, and other reagents such as ceftriaxone that restore GLT-1, may exert a therapeutic effect for AUD via a number of mechanisms (Ferreira Seiva et al., 2009, Roberts-Wolfe and Kalivas, 2015).
Figure 5. Immune signaling during ethanol exposure.
Simplified schematic showing how ethanol exposure can promote signaling via Toll like receptors (TLRs), leading to activation of nuclear factor kappa B (NF-κB), secretion of pro-inflammatory cytokines and chemokines, and elevated levels of Reactive Oxygen Species (ROS). See text for further details.
Selective effects of ethanol on astrocytic calcium signaling
Increased [Ca2+]i is both necessary and sufficient for most forms of astrocytic gliotransmitter release (Parpura et al., 1994). Thus, it is significant that physiologically relevant ethanol concentrations increase astrocytic [Ca2+]i in a manner that parallels release of several gliotransmitters including glutamate, glutamine, and taurine (Salazar et al., 2008, Kimelberg et al., 1990, Kimelberg et al., 1993, Allen et al., 2002, Fonseca et al., 2001). Although ethanol does not enhance overall IP3 formation (Simonsson et al., 1989), ethanol can induce intracellular calcium transients in a subset of astrocytes (Allansson et al., 2001). In addition, ethanol pre-treatment appears to affect calcium responses in a neurotransmitter-dependent manner, where ethanol stimulates serotonin induced inositol metabolism, while suppressing muscarinic receptor-mediated calcium responses (Catlin et al., 2000, Simonsson et al., 1989). Ethanol might thereby indirectly shift the balance between different signaling pathways. Importantly, ethanol acutely suppresses gap junction permeability in defined brain regions, and may thus further impinge upon signal processing in a circuit specific manner (Adermark and Lovinger, 2006, Adermark et al., 2004). Additionally, immunoreactive puncta of the gap junction protein connexin-43 is reduced in post mortem samples from subjects with AUD, supporting a role for altered connectivity or hemichannel-based communication in the pathophysiology of alcoholism (Miguel-Hidalgo et al., 2014). Thus, it is interesting that subpopulations of astrocytes are known to have sexually dimorphic roles (Collado et al., 1995, Mong and McCarthy, 2002), which can include regulation of the gap junction protein connexin 43 (Gulinello and Etgen, 2005), and other gene expression changes in response to ethanol that are hypothesized to underlie increased vulnerability to ethanol-mediated neurotoxicity in females (Wilhelm et al., 2015). Ethanol can also modulate the biophysics of other ion channels expressed in astrocytes that are related to the connexins. For example, unlike the connexins, pannexin-containing hemichannels are opened by both ethanol and increased [Ca2+]i (Locovei et al., 2006, Scemes et al., 2007, Dahl and Harris, 2008). Thus, ethanol can modulate the ability of astrocytes to communicate by regulating both calcium transients and channel permeability.
Ethanol affects astrocytes in a manner that can disrupt the balance between excitation and inhibition
Alteration of glutamatergic neurotransmission is a hallmark of alcohol dependence, and ethanol can cause glutamatergic dysfunction by impinging upon important astrocytic functions (Smith, 1997, Ayers-Ringler et al., 2016, Dahchour et al., 2000, Ding et al., 2013, Mulholland et al., 2009). As noted earlier, a primary function of astrocytes is glutamate clearance via the glial excitatory amino acid transporters GLAST/EAAT1 and GLT-1/EAAT2. Because greater than 70% of glial processes immunolabeled for GLAST or GLT-1 were found to ensheathe dendritic spines or excitatory synapses (Cholet et al., 2002), it is important to note that ethanol can modulate expression of these transporters. For example, both GLAST and GLT-1 expression increased in organotypic cortical cultures chronically treated with ethanol (Zink et al., 2004). This upregulation may explain why glutamate uptake is increased following chronic treatment of organotypic cortical cultures with ethanol (Smith, 1997). Upregulation of GLT-1, but not GLAST, has also been found in the rat nucleus accumbens following voluntary ethanol consumption (Alhaddad et al., 2014, Das et al., 2015, Sari et al., 2016). Importantly, modulating expression and/or function of glial excitatory amino acid transporters affects AUD-associated phenotypes. For example, mice with a homozygous GLAST deletion do not exhibit a conditioned place preference for ethanol, and these mice also show reduced voluntary ethanol intake compared to wild type littermates (Karlsson et al., 2012). A similar effect was seen after inhibition of glial excitatory amino acid transporters via intracerebral ventricular infusion of the GLT-1 blocker dihydrokainic acid (Smith et al., 2014). On the other hand, mice with a genetic deletion of the circadian clock gene Per2, show reduced GLAST expression, elevated extracellular levels of glutamate in the nucleus accumbens, and increased ethanol intake (Spanagel et al., 2005). However, interpretation of this finding is complicated by the fact that, when compared to wild type mice, GLAST levels were only suppressed at one specific time point over the day.
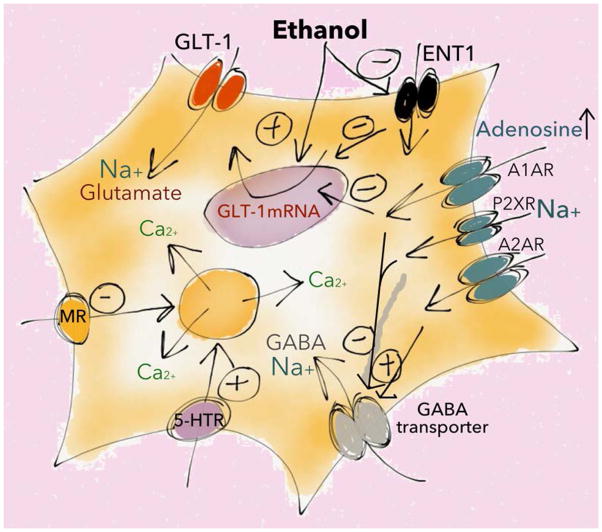
Glutamate uptake can also be regulated by other transmitters that shape ethanol-responsive behaviors, such as the gliotransmitter adenosine. Adenosine and purinergic signaling regulate numerous vital biological processes in the central nervous system, such as circadian rhythmicity, sleep, and energy homeostasis (Lindberg et al., 2015). Ethanol increases extracellular adenosine levels, and purinergic receptors have been proposed as a novel target for the development of therapeutics to treat AUD (Franklin et al., 2014). Activation of the adenosine A1 receptor (Wu et al., 2011) or the astrocytic equilibrative nucleoside transporter 1 (ENT1) (Wu et al., 2010) decreases GLT-1 mRNA expression and glutamate uptake (Figure 6). At the same time, acute ethanol inhibits adenosine uptake via ENT1 (Nam et al., 2012), and knockdown of ENT1 expression has been shown to prevent ethanol-induced elevation of GLT-1 (Wu et al., 2010). The acute increase in adenosine seen following ethanol exposure partly mediates the ataxic and hypnotic effects of acute ethanol, but adenosine is also associated with cellular tolerance and dependence (Diamond et al., 1991), and insomnia can develop as adenosine levels decrease following chronic ethanol (Ruby et al., 2014). Importantly, adenosine also exerts a complex effect on GABAergic neurotransmission by enhancing astrocyte GABA uptake via adenosine A2A receptors, while inhibiting uptake via A1 receptors (Cristovao-Ferreira et al., 2013). In addition, purinergic receptor activation may lead to disruption of the Na+ gradient, thereby suppressing high-affinity uptake of both GABA and glutamate (Barros-Barbosa et al., 2015) (Figure 6).
Figure 6. Ethanol-mediated disruption of the balance between excitation and inhibition.
Ethanol can perturb excitatory balance by altering astrocyte calcium signaling and the clearance of neurotransmitters. Ethanol modulates receptor-mediated responses in a pathway specific manner, and may directly increase Ca2+ signaling in a subset of astrocytes. Ethanol also suppresses adenosine-uptake via the astrocytic equilibrative nucleoside transporter 1 (ENT1), leading to increased levels of adenosine and activation of purinergic receptors (A1AR, A2AR, P2XR) resulting in indirect modulation of glutamate and GABA clearence. MR=muscarinic receptor, 5-HTR=serotonin receptor. See text for further details.
Besides changes in glutamatergic neurotransmission, ethanol also has pronounced effects on GABAergic neurotransmission. In vivo electrophysiology has shown that ethanol acutely suppresses GABAergic neuron firing frequency in the nucleus accumbens (Burkhardt and Adermark, 2014), and that voluntary ethanol intake leads to a long-lasting decrease in GABAergic neurotransmission in the striatum (Adermark et al., 2013, Wilcox et al., 2014). In the ventral tegmental area, ethanol exerts subregion-specific effects on GABAergic neuron activity (Guan et al., 2012). Research aimed at elucidating the role of astrocytes in ethanol-induced modulation of GABAergic neurotransmission is sparse. But, astrocytes express GABA receptors, and astrocytes may also release GABA, which could be sensitive to ethanol exposure (Lee et al., 2011, Yoon et al., 2014, Itzhak et al., 1993). In fact, the metabolic inhibitor fluorocitrate, which is preferably taken up by astrocytes, has been shown to enhance glutamatergic neurotransmission in the supraoptic nucleus by reducing the release of taurine from astrocytes (Choe et al., 2012). In addition, unpublished observations from our laboratory suggest that fluorocitrate increases excitation in the striatum by decreasing the firing frequency of inhibitory neurons, indicating that astrocytes also exert a powerful control over GABAergic neurotransmission. These data may be significant given that microinjection of fluorocitrate into the rat prefrontal cortex increased ethanol preference (Miguel-Hidalgo et al., 2009). Taken together, astrocytes appear to modulate the conditioned positive reinforcing effects of ethanol via complex regulation of uptake and release of a variety of neurotransmitters (Lee et al., 2013, Ruby et al., 2014) (Figure 6).
Ethanol-mediated cell swelling in astrocytes
Extrasynaptic volume transmission critically depends on the morphological and functional features of astrocytes, which can swell and shrink during physiological and pathological activity as a result of changes in osmolarity, or intensified neuronal activity (Fuxe et al., 2012). Changes in astrocytic cell volume may not only affect extrasynaptic homeostasis, but also size and geometry of the extracellular space, which can alter diffusion of neuroactive substances (Hansson and Ronnback, 2003, Vargova and Sykova, 2014). Glutamate has been shown to increase astrocytic cell volume in a complex manner that depends on the transport of K+, Na+ and Ca2+ over astrocytic cell membranes (Figure 7) (Bender et al., 1998, Schneider et al., 1992). Acute administration of ethanol can also induce astrocyte swelling in a manner that is sensitive to inhibition of the Na+/K+/2Cl−-co-transporter or the Na+/K+-ATPase, suggesting that similar mechanisms might apply for both glutamate- and ethanol-induced swelling (Adermark et al., 2011c, Allansson et al., 2001, Aschner et al., 2001, Vargova and Sykova, 2014). Interestingly, connexin-43 containing gap junction channels appear to regulate astrocyte cell volume, and ethanol induced suppression of gap junction permeability also involves changes in sodium homeostasis (Chever et al., 2014). Maintaining intracellular sodium homeostasis might thus be crucial for sustained astrocytic function during intense neuronal activity and during ethanol exposure.
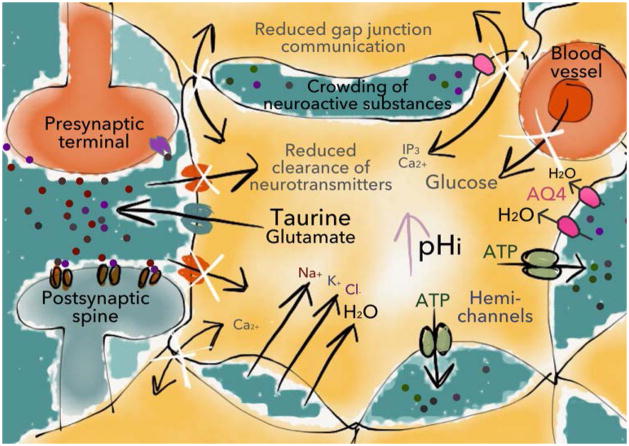
Figure 7. Ethanol-induced cell swelling.
A subset of astrocytes responds to ethanol exposure with an increase in cell volume, which can lead to crowding of molecules in the extracellular space, changes in volume transmission and possibly neurotoxicity. In addition, ethanol-induced cell swelling may lead to impaired communication through gap junction channels, reduced clearance of neuroactive substances and decreased glucose transport (illustrated with white x-marks) (Abdul Muneer et al., 2011), thereby indirectly affecting neurotransmission. Importantly, cell swelling can also promote the release of taurine, which appears to play a key role in the dopamine-elevating properties of ethanol. See text for further details. AQ4=aquaporin-4.
Rapid swelling and regulatory volume decrease is facilitated by astrocytic water channels containing aquaporin-4 (Figure 7) (Kuppers et al., 2008, Risher et al., 2009). Aquaporin-4 has been linked to reward-related motivational processes including alcohol consumption (Lee et al., 2013) and extracellular dopamine concentration. Specifically, the expression of aquaporin-4 correlates with dopamine levels in the nucleus accumbens (Kuppers et al., 2008), and inhibition of cell swelling suppresses ethanol induced dopamine release (Adermark et al., 2011a). Another important osmoregulator is the gliotransmitter taurine, which can be released from astrocytes during cell swelling and subsequently activate ligand gated ion channels such as NMDA, glycine, or GABAA receptors (Adermark et al., 2011b, Ericson et al., 2011, Ericson et al., 2006). In fact, this release of taurine appears to be vital for ethanol-induced dopamine release in the nucleus accumbens of rat (Ericson et al., 2011). Although better known for its role in modulating glutamatergic neurotransmission, acamprosate (Campral®), is a homotaurine that can increase nucleus accumbens dopamine by glycine receptor activation (Chau et al., 2010a, Chau et al., 2010b). Thus, the effectiveness of acamprosate in treating AUD may stem from both glutamatergic and dopaminergic mechanisms. Interestingly, pharmacological inhibition of either the dopamine D1 or D2 receptor facilitates gap junction permeability and astrocyte calcium excitability (Bosson et al., 2015), indicating that dopamine, by itself, may further promote taurine release. Thus, astrocytes appear to play a key role in supporting the dopamine elevating property of ethanol, which is believed to be important for the conditioned reinforcing properties of commonly abused substances.
Disentangling the role of astrocytes in alcohol use disorder
Because neurons and astrocytes express an overlapping array of receptors and channels, current approaches do not facilitate disentangling the role of astrocytes in mediating complex phenomena with traditional pharmacological approaches. However, through the use of optogenetic and chemogenetic approaches, unexpected roles of astrocytes have been recently uncovered. For example, mimicking pH-evoked Ca2+ responses, by optogenetic stimulation of astrocytes expressing channelrhodopsin-2, has been shown to trigger robust respiratory responses in vivo via an ATP-dependent mechanism (Gourine et al., 2010). An alternative approach to define the role of specific cell populations and circuits in complex phenomena are G-protein coupled receptors that have been modified such that they are activated by an otherwise physiologically inert ligand (Designer Receptors Exclusively Activated by Synthetic Ligand; DREADD) (Armbruster et al., 2007). Responding for intracranial self-stimulation (ICSS) has been shown to be facilitated by activation of astrocytes expressing DREADDs coupled to Gαq, revealing that astrocytes in the nucleus accumbens can regulate ethanol-seeking behavior (Bull et al., 2014). Specifically, Gαq-DREADD activation allows astrocyte-selective increases in [Ca2+]i, which is both necessary and sufficient for gliotransmitter release from astrocytes. Stimulation of nucleus accumbens core astrocytes in this manner decreases the motivation of abstinent rats to resume ethanol self-administration. Intriguingly, because Gαq-DREADD-mediated astrocyte stimulation left-shifted the frequency-effect curve for intracranial self-stimulation, astrocyte stimulation may have reduced the motivation to self-administer ethanol by facilitating reward-associated phenomena (Bull et al., 2014). Subsequently, stimulation of nucleus accumbens astrocytes via Gq-DREADD activation was found to increase extracellular glutamate and reduce the reinstatement of cue-induced cocaine-seeking behavior (Scofield et al., 2015). Thus, selective astrocyte stimulation could be used to abate behaviors associated with drug abuse and these approaches will likely be facilitated by the identification of glia specific genes (Nwaobi et al., 2016) that could be exploited for therapeutic benefit.
Conclusion and future directions
Here, we reviewed a rapidly expanding literature uncovering the critical roles that astrocytes play in modulating neurotransmission with relevance to AUD. Ethanol is well known to interrupt blood brain barrier integrity and to impart long-lasting perturbations in neuronal function. More recently, increasing attention is being given to the ability of glial cells, in particular astrocytes, to actively respond to and modulate neuronal communication and ultimately impact behavior. We now know that astrocyte number is altered following ethanol administration and that density of the astrocyte population positively correlates with the relapse-like motivation to self-administer ethanol. While the precise mechanisms underlying this relationship remain unknown, it may stem from altered diffusion or uptake of neuroactive substances or by altered bidirectional communication between astrocytes and neurons. Moreover, we know that several AUD-associated phenotypes can be influenced by manipulating astrocyte function including ethanol preference, self-administration, and conditioned reinforcement. Because astrocytes play major roles in regulating extracellular glutamate and ethanol can regulate many forms of astrocytic glutamate uptake, release, and metabolism, astrocytes likely exert many of their effects on AUD-associated phenotypes via glutamate. AUD-associated phenotypes are also critically regulated via the astrocytic role in modulating inflammation and neurotrophic factors. Moreover, an elegant series of reports established that the ethanol-mediated release of the astrocytic osmoregulator taurine regulates ethanol-mediated increases in nucleus accumbens dopamine. Thus, akin to the role of astrocytes in responding to and modulating neurotransmission, astrocytes respond to ethanol and mediate many ethanol effects at the molecular, cellular, and behavioral levels.
A focus of future astrocyte addiction research will undoubtedly center around capitalizing upon therapeutic potential. Given that astrocytes and neurons express a similar complement of receptors and channels as neurons, targeting astrocytes with traditional pharmaceutics is unlikely, but capitalizing on astrocyte-specific gene expression (Nwaobi et al., 2016) will likely facilitate these efforts. Studies outlined above indicate that AUD-like behaviors can be reduced by employing innovative approaches that selectively manipulate astrocytes. Indeed, novel strategies using engineered receptors activated by synthetic ligands (DREADDS) or by light (optogenetics) have significantly contributed to a new era of brain research that allows for precise experimental manipulation of cellular and circuitry activity, and increasingly these techniques are being applied to non-neuronal cells.
Future research directions will likely include novel approaches that identify and target astrocyte subtypes, as it is not uncommon for astrocytes to exhibit functional heterogeneity even within brain region (Adermark and Lovinger, 2008, Isokawa and McKhann, 2005). Identifying these subpopulations will probably involve imaging using a combination of genetically encoded calcium or voltage sensors such as glial specific gcAMP or ArcLight-like imaging (Jackson and Robinson, 2015, Lee et al., 2011), via either implanted fiber optics or cranial windows, in awake behaving animals. Other novel approaches may interface with brain energetics via modulating neurovascular coupling (Robinson and Jackson, 2016), or cell-type-specific gene silencing (Merienne et al., 2015).
In conclusion, it is clear that astrocytes, quiescent cells that until fairly recently were relegated to performing purely homeostatic functions, are actively shaping the pathophysiology of ethanol abuse and relapse. Precisely defining the molecular milieu of these astrocytes will lead to targeted and highly efficacious therapeutics to combat AUD.
Acknowledgments
This work was supported by Stiftelsen Psykiatriska Forskningsfonden (LA), the Swedish Medical Research Council (LA), the Swedish Brain foundation (LA), the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly (LA), Åke Wibergs stiftelse (LA), ABMRF/The foundation for Alcohol Research (MSB), and NIH/NIAAA P50 AA022537 (MSB).
Footnotes
The authors have no competing or conflicting interests to declare.
References
- Adermark L, Clarke RB, Olsson T, Hansson E, Soderpalm B, Ericson M. Implications for glycine receptors and astrocytes in ethanol-induced elevation of dopamine levels in the nucleus accumbens. Addict Biol. 2011a;16:43–54. doi: 10.1111/j.1369-1600.2010.00206.x. [DOI] [PubMed] [Google Scholar]
- Adermark L, Clarke RB, Soderpalm B, Ericson M. Ethanol-induced modulation of synaptic output from the dorsolateral striatum in rat is regulated by cholinergic interneurons. Neurochem Int. 2011b;58:693–699. doi: 10.1016/j.neuint.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Adermark L, Jonsson S, Ericson M, Soderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011c;61:1160–1165. doi: 10.1016/j.neuropharm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Adermark L, Jonsson S, Soderpalm B, Ericson M. Region-specific depression of striatal activity in Wistar rat by modest ethanol consumption over a ten-month period. Alcohol. 2013;47:289–298. doi: 10.1016/j.alcohol.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Ethanol effects on electrophysiological properties of astrocytes in striatal brain slices. Neuropharmacology. 2006;51:1099–1108. doi: 10.1016/j.neuropharm.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Electrophysiological properties and gap junction coupling of striatal astrocytes. Neurochemistry international. 2008;52:1365–1372. doi: 10.1016/j.neuint.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Olsson T, Hansson E. Ethanol acutely decreases astroglial gap junction permeability in primary cultures from defined brain regions. Neurochemistry international. 2004;45:971–978. doi: 10.1016/j.neuint.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, Wei Y, Sari Y. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Frontiers in behavioral neuroscience. 2014;8:366. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allansson L, Khatibi S, Olsson T, Hansson E. Acute ethanol exposure induces [Ca2+]i transients, cell swelling and transformation of actin cytoskeleton in astroglial primary cultures. Journal of neurochemistry. 2001;76:472–479. doi: 10.1046/j.1471-4159.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- Allen JW, Mutkus LA, Aschner M. Chronic ethanol produces increased taurine transport and efflux in cultured astrocytes. Neurotoxicology. 2002;23:693–700. doi: 10.1016/S0161-813X(02)00027-X. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeira RI, Sebastiao AM, Valente CA. GlyT1 and GlyT2 in brain astrocytes: expression, distribution and function. Brain Struct Funct. 2014;219:817–830. doi: 10.1007/s00429-013-0537-3. [DOI] [PubMed] [Google Scholar]
- Aschner M, Allen JW, Mutkus LA, Cao C. Ethanol-induced swelling in neonatal rat primary astrocyte cultures. Brain research. 2001;900:219–226. doi: 10.1016/s0006-8993(01)02314-9. [DOI] [PubMed] [Google Scholar]
- Ayers-Ringler JR, Jia YF, Qiu YY, Choi DS. Role of astrocytic glutamate transporter in alcohol use disorder. World J Psychiatry. 2016;6:31–42. doi: 10.5498/wjp.v6.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Madamba SG, Roberto M, Blednov YA, Sagi VN, Roberts E, Rice KC, Harris RA, Siggins GR. Innate immune factors modulate ethanol interaction with GABAergic transmission in mouse central amygdala. Brain Behav Immun. 2014;40:191–202. doi: 10.1016/j.bbi.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Barros-Barbosa AR, Lobo MG, Ferreirinha F, Correia-de-Sa P, Cordeiro JM. P2X7 receptor activation downmodulates Na(+)-dependent high-affinity GABA and glutamate transport into rat brain cortex synaptosomes. Neuroscience. 2015;306:74–90. doi: 10.1016/j.neuroscience.2015.08.026. [DOI] [PubMed] [Google Scholar]
- Ben Haim L, Carrillo-de Sauvage MA, Ceyzeriat K, Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015;9:278. doi: 10.3389/fncel.2015.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AS, Schousboe A, Reichelt W, Norenberg MD. Ionic mechanisms in glutamate-induced astrocyte swelling: role of K+ influx. J Neurosci Res. 1998;52:307–321. doi: 10.1002/(SICI)1097-4547(19980501)52:3<307::AID-JNR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jabs R, Steinhauser C. Neuron-glia synapses in the brain. Brain Res Rev. 2010;63:130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Pascual M, Valles SL, Guerri C. Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-kappa B. Neuroreport. 2004;15:681–685. doi: 10.1097/00001756-200403220-00021. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- Blomstrand F, Aberg ND, Eriksson PS, Hansson E, Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999a;92:255–265. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- Blomstrand F, Khatibi S, Muyderman H, Hansson E, Olsson T, Ronnback L. 5-Hydroxytryptamine and glutamate modulate velocity and extent of intercellular calcium signalling in hippocampal astroglial cells in primary cultures. Neuroscience. 1999b;88:1241–1253. doi: 10.1016/s0306-4522(98)00351-0. [DOI] [PubMed] [Google Scholar]
- Bosson A, Boisseau S, Buisson A, Savasta M, Albrieux M. Disruption of dopaminergic transmission remodels tripartite synapse morphology and astrocytic calcium activity within substantia nigra pars reticulata. Glia. 2015;63:673–683. doi: 10.1002/glia.22777. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Jackson A, Maldoon PP, Damaj MI. N-acetylcysteine decreased nicotine reward-like properties and withdrawal in mice. Psychopharmacology (Berl) 2016;233:995–1003. doi: 10.1007/s00213-015-4179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology. 2014;39:2835–2845. doi: 10.1038/npp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Syed WA, Minter SC, Bowers MS. Differential response of glial fibrillary acidic protein-positive astrocytes in the rat prefrontal cortex following ethanol self-administration. Alcohol Clin Exp Res. 2015;39:650–658. doi: 10.1111/acer.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt JM, Adermark L. Locus of onset and subpopulation specificity of in vivo ethanol effect in the reciprocal ventral tegmental area-nucleus accumbens circuit. Neurochemistry international. 2014;76:122–130. doi: 10.1016/j.neuint.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth RF. Pathophysiology of alcoholic brain damage: synergistic effects of ethanol, thiamine deficiency and alcoholic liver disease. Metab Brain Dis. 1995;10:1–8. doi: 10.1007/BF01991777. [DOI] [PubMed] [Google Scholar]
- Catlin MC, Guizzetti M, Costa LG. Effect of ethanol on muscarinic receptor-induced calcium responses in astroglia. J Neurosci Res. 2000;60:345–355. doi: 10.1002/(SICI)1097-4547(20000501)60:3<345::AID-JNR9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Chau P, Hoifodt-Lido H, Lof E, Soderpalm B, Ericson M. Glycine receptors in the nucleus accumbens involved in the ethanol intake-reducing effect of acamprosate. Alcoholism, clinical and experimental research. 2010a;34:39–45. doi: 10.1111/j.1530-0277.2009.01063.x. [DOI] [PubMed] [Google Scholar]
- Chau P, Stomberg R, Fagerberg A, Soderpalm B, Ericson M. Glycine receptors involved in acamprosate’s modulation of accumbal dopamine levels: an in vivo microdialysis study. Alcoholism, clinical and experimental research. 2010b;34:32–38. doi: 10.1111/j.1530-0277.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- Chever O, Dossi E, Pannasch U, Derangeon M, Rouach N. Astroglial networks promote neuronal coordination. Sci Signal. 2016;9:ra6. doi: 10.1126/scisignal.aad3066. [DOI] [PubMed] [Google Scholar]
- Chever O, Pannasch U, Ezan P, Rouach N. Astroglial connexin 43 sustains glutamatergic synaptic efficacy. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130596. doi: 10.1098/rstb.2013.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KY, Olson JE, Bourque CW. Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:12518–12527. doi: 10.1523/JNEUROSCI.1380-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+,K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex. 2002;12:515–525. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- Collado P, Beyer C, Hutchison JB, Holman SD. Hypothalamic distribution of astrocytes is gender-related in Mongolian gerbils. Neurosci Lett. 1995;184:86–89. doi: 10.1016/0304-3940(94)11175-i. [DOI] [PubMed] [Google Scholar]
- Covelo A, Araque A. Lateral regulation of synaptic transmission by astrocytes. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.02.036. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Jiang X, Taylor A, Mennerick S. Astrocyte-derived thrombospondins mediate the development of hippocampal presynaptic plasticity in vitro. J Neurosci. 2012;32:13100–13110. doi: 10.1523/JNEUROSCI.2604-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristovao-Ferreira S, Navarro G, Brugarolas M, Perez-Capote K, Vaz SH, Fattorini G, Conti F, Lluis C, Ribeiro JA, McCormick PJ, Casado V, Franco R, Sebastiao AM. A1R-A2AR heteromers coupled to Gs and G i/0 proteins modulate GABA transport into astrocytes. Purinergic Signal. 2013;9:433–449. doi: 10.1007/s11302-013-9364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, Hoffman A, Deitrich R, de Witte P. Effects of ethanol on extracellular amino acid levels in high-and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol. 2000;35:548–553. doi: 10.1093/alcalc/35.6.548. [DOI] [PubMed] [Google Scholar]
- Dahl G, Harris A. In: Pannexins or Connexins?, in Connexins: a guide, Connexins: a guide. HARRIS A, LOCKE D, editors. Humana Press; New York: 2008. pp. 287–301. [Google Scholar]
- Dalcik H, Yardimoglu M, Filiz S, Gonca S, Dalcik C, Erden BF. Chronic ethanol-induced glial fibrillary acidic protein (GFAP) immunoreactivity: an immunocytochemical observation in various regions of adult rat brain. Int J Neurosci. 2009;119:1303–1318. doi: 10.1080/00207450802333672. [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127:71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Nagy L, Mochly-Rosen D, Gordon A. The role of adenosine and adenosine transport in ethanol-induced cellular tolerance and dependence. Possible biologic and genetic markers of alcoholism. Ann N Y Acad Sci. 1991;625:473–487. doi: 10.1111/j.1749-6632.1991.tb33878.x. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzyubenko E, Gottschling C, Faissner A. Neuron-Glia Interactions in Neural Plasticity: Contributions of Neural Extracellular Matrix and Perineuronal Nets. Neural Plast. 2016;2016:5214961. doi: 10.1155/2016/5214961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Chau P, Clarke RB, Adermark L, Soderpalm B. Rising taurine and ethanol concentrations in nucleus accumbens interact to produce dopamine release after ethanol administration. Addict Biol. 2011;16:377–385. doi: 10.1111/j.1369-1600.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Stomberg R, Soderpalm B. Taurine elevates dopamine levels in the rat nucleus accumbens; antagonism by strychnine. The European journal of neuroscience. 2006;23:3225–3229. doi: 10.1111/j.1460-9568.2006.04868.x. [DOI] [PubMed] [Google Scholar]
- Ernst C, Nagy C, Kim S, Yang JP, Deng X, Hellstrom IC, Choi KH, Gershenfeld H, Meaney MJ, Turecki G. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry. 2011;70:312–319. doi: 10.1016/j.biopsych.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Ferreira Seiva FR, Amauchi JF, Ribeiro Rocha KK, Souza GA, Ebaid GX, Burneiko RM, Novelli EL. Effects of N-acetylcysteine on alcohol abstinence and alcohol-induced adverse effects in rats. Alcohol. 2009;43:127–135. doi: 10.1016/j.alcohol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Floreani NA, Rump TJ, Abdul Muneer PM, Alikunju S, Morsey BM, Brodie MR, Persidsky Y, Haorah J. Alcohol-induced interactive phosphorylation of Src and toll-like receptor regulates the secretion of inflammatory mediators by human astrocytes. J Neuroimmune Pharmacol. 2010;5:533–545. doi: 10.1007/s11481-010-9213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca LL, Alves PM, Carrondo MJ, Santos H. Effect of ethanol on the metabolism of primary astrocytes studied by (13)C- and (31)P-NMR spectroscopy. J Neurosci Res. 2001;66:803–811. doi: 10.1002/jnr.10039. [DOI] [PubMed] [Google Scholar]
- Franklin KM, Asatryan L, Jakowec MW, Trudell JR, Bell RL, Davies DL. P2X4 receptors (P2X4Rs) represent a novel target for the development of drugs to prevent and/or treat alcohol use disorders. Front Neurosci. 2014;8:176. doi: 10.3389/fnins.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Diaz-Cabiale Z, Rivera A, Ferraro L, Tanganelli S, Tarakanov AO, Garriga P, Narvaez JA, Ciruela F, Guescini M, Agnati LF. Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal-glial networks. Front Physiol. 2012;3:136. doi: 10.3389/fphys.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Fromaget C, el Aoumari A, Cordier J, Glowinski J, Gros D. Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron. 1991;6:133–143. doi: 10.1016/0896-6273(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Xiao C, Krnjevic K, Xie G, Zuo W, Ye JH. GABAergic actions mediate opposite ethanol effects on dopaminergic neurons in the anterior and posterior ventral tegmental area. J Pharmacol Exp Ther. 2012;341:33–42. doi: 10.1124/jpet.111.187963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Etgen AM. Sexually dimorphic hormonal regulation of the gap junction protein, CX43, in rats and altered female reproductive function in CX43+/− mice. Brain Res. 2005;1045:107–115. doi: 10.1016/j.brainres.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E, Muyderman H, Leonova J, Allansson L, Sinclair J, Blomstrand F, Thorlin T, Nilsson M, Ronnback L. Astroglia and glutamate in physiology and pathology: aspects on glutamate transport, glutamate-induced cell swelling and gap-junction communication. Neurochemistry international. 2000;37:317–329. doi: 10.1016/s0197-0186(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isokawa M, McKhann GM., 2nd Electrophysiological and morphological characterization of dentate astrocytes in the hippocampus. J Neurobiol. 2005;65:125–134. doi: 10.1002/neu.20186. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Baker L, Norenberg MD. Characterization of the peripheral-type benzodiazepine receptors in cultured astrocytes: evidence for multiplicity. Glia. 1993;9:211–218. doi: 10.1002/glia.440090306. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Robinson MB. Reciprocal Regulation of Mitochondrial Dynamics and Calcium Signaling in Astrocyte Processes. J Neurosci. 2015;35:15199–15213. doi: 10.1523/JNEUROSCI.2049-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczor P, Rakus D, Mozrzymas JW. Neuron-astrocyte interaction enhance GABAergic synaptic transmission in a manner dependent on key metabolic enzymes. Frontiers in cellular neuroscience. 2015;9:120. doi: 10.3389/fncel.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Adermark L, Molander A, Perreau-Lenz S, Singley E, Solomon M, Holmes A, Tanaka K, Lovinger DM, Spanagel R, Heilig M. Reduced alcohol intake and reward associated with impaired endocannabinoid signaling in mice with a deletion of the glutamate transporter GLAST. Neuropharmacology. 2012;63:181–189. doi: 10.1016/j.neuropharm.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Heilig M, Holmes A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biological psychiatry. 2008;64:810–814. doi: 10.1016/j.biopsych.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kim Y, Davidson JO, Gunn KC, Phillips AR, Green CR, Gunn AJ. Role of Hemichannels in CNS Inflammation and the Inflammasome Pathway. Adv Protein Chem Struct Biol. 2016;104:1–37. doi: 10.1016/bs.apcsb.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Cheema M, O’Connor ER, Tong H, Goderie SK, Rossman PA. Ethanol-induced aspartate and taurine release from primary astrocyte cultures. J Neurochem. 1993;60:1682–1689. doi: 10.1111/j.1471-4159.1993.tb13391.x. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Heja L, Kardos J, Billups B. Astrocyte sodium signaling and the regulation of neurotransmission. Glia. 2015 doi: 10.1002/glia.22943. [DOI] [PubMed] [Google Scholar]
- Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res. 1999;23:164–168. [PubMed] [Google Scholar]
- Kuppers E, Gleiser C, Brito V, Wachter B, Pauly T, Hirt B, Grissmer S. AQP4 expression in striatal primary cultures is regulated by dopamine--implications for proliferation of astrocytes. The European journal of neuroscience. 2008;28:2173–2182. doi: 10.1111/j.1460-9568.2008.06531.x. [DOI] [PubMed] [Google Scholar]
- Lee M, Schwab C, McGeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59:152–165. doi: 10.1002/glia.21087. [DOI] [PubMed] [Google Scholar]
- Lee MR, Ruby CL, Hinton DJ, Choi S, Adams CA, Young Kang N, Choi DS. Striatal adenosine signaling regulates EAAT2 and astrocytic AQP4 expression and alcohol drinking in mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:437–445. doi: 10.1038/npp.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Rusakov DA. Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys J. 2002;83:125–134. doi: 10.1016/S0006-3495(02)75154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg D, Shan D, Ayers-Ringler J, Oliveros A, Benitez J, Prieto M, McCullumsmith R, Choi DS. Purinergic signaling and energy homeostasis in psychiatric disorders. Curr Mol Med. 2015;15:275–295. doi: 10.2174/1566524015666150330163724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Astrocytes Couple Synaptic Activity to Glucose Utilization in the Brain. News Physiol Sci. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science. 2015;349:730–734. doi: 10.1126/science.aaa7945. [DOI] [PubMed] [Google Scholar]
- Merienne N, Delzor A, Viret A, Dufour N, Rey M, Hantraye P, Deglon N. Gene transfer engineering for astrocyte-specific silencing in the CNS. Gene Ther. 2015;22:830–839. doi: 10.1038/gt.2015.54. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo J, Shoyama Y, Wanzo V. Infusion of gliotoxins or a gap junction blocker in the prelimbic cortex increases alcohol preference in Wistar rats. J Psychopharmacol. 2009;23:550–557. doi: 10.1177/0269881108091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Lower packing density of glial fibrillary acidic protein-immunoreactive astrocytes in the prelimbic cortex of alcohol-naive and alcohol-drinking alcohol-preferring rats as compared with alcohol-nonpreferring and Wistar rats. Alcohol Clin Exp Res. 2005;29:766–772. doi: 10.1097/01.alc.0000164378.92680.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol Alcohol. 2006;41:379–385. doi: 10.1093/alcalc/agl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–1855. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wilson BA, Hussain S, Meshram A, Rajkowska G, Stockmeier CA. Reduced connexin 43 immunolabeling in the orbitofrontal cortex in alcohol dependence and depression. Journal of psychiatric research. 2014;55:101–109. doi: 10.1016/j.jpsychires.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res Dev Brain Res. 2002;139:151–158. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- Montero TD, Orellana JA. Hemichannels: new pathways for gliotransmitter release. Neuroscience. 2015;286:45–59. doi: 10.1016/j.neuroscience.2014.11.048. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Rodriguez-Arias M, Minarro J, Guerri C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav Immun. 2016;53:159–171. doi: 10.1016/j.bbi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Ethanol disrupts NMDA receptor and astroglial EAAT2 modulation of Kv2.1 potassium channels in hippocampus. Alcohol. 2009;43:45–50. doi: 10.1016/j.alcohol.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Nam HW, McIver SR, Hinton DJ, Thakkar MM, Sari Y, Parkinson FE, Haydon PG, Choi DS. Adenosine and glutamate signaling in neuron-glial interactions: implications in alcoholism and sleep disorders. Alcohol Clin Exp Res. 2012;36:1117–1125. doi: 10.1111/j.1530-0277.2011.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Fernandez de Sevilla D, Gomez-Gonzalo M, Nunez A, Martin ED, Araque A. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 2012;10:e1001259. doi: 10.1371/journal.pbio.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Maglio L, Pastor J, Garcia de Sola R, Araque A. Astrocyte calcium signal and gliotransmission in human brain tissue. Cereb Cortex. 2013;23:1240–1246. doi: 10.1093/cercor/bhs122. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Nevin RL. Mefloquine blockade of connexin 36 and connexin 43 gap junctions and risk of suicide. Biol Psychiatry. 2012;71:e1–2. doi: 10.1016/j.biopsych.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 2016;132:1–21. doi: 10.1007/s00401-016-1553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Stehberg J. Hemichannels: new roles in astroglial function. Frontiers in physiology. 2014;5:193. doi: 10.3389/fphys.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannasch U, Vargova L, Reingruber J, Ezan P, Holcman D, Giaume C, Sykova E, Rouach N. Astroglial networks scale synaptic activity and plasticity. Proc Natl Acad Sci U S A. 2011;108:8467–8472. doi: 10.1073/pnas.1016650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Sur M, Araque A. Neuron-glia networks: integral gear of brain function. Front Cell Neurosci. 2014;8:378. doi: 10.3389/fncel.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci. 2014;34:12738–12744. doi: 10.1523/JNEUROSCI.2401-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla A, Pascual M, Guerri C. Autophagy Constitutes a Protective Mechanism against Ethanol Toxicity in Mouse Astrocytes and Neurons. PLoS One. 2016;11:e0153097. doi: 10.1371/journal.pone.0153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Nino AM, D’Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl) 2013;225:473–482. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam ML, Watts LT, Stark AA, Mahimainathan L, Stewart J, Schenker S, Henderson GI. Astrocyte control of fetal cortical neuron glutathione homeostasis: up-regulation by ethanol. J Neurochem. 2006;96:1289–1300. doi: 10.1111/j.1471-4159.2006.03674.x. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 2015;20:316–323. doi: 10.1111/adb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Andrew RD, Kirov SA. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia. 2009;57:207–221. doi: 10.1002/glia.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Kalivas PW. Glutamate Transporter GLT-1 as a Therapeutic Target for Substance Use Disorders. CNS Neurol Disord Drug Targets. 2015;14:745–756. doi: 10.2174/1871527314666150529144655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MB, Jackson JG. Astroglial glutamate transporters coordinate excitatory signaling and brain energetics. Neurochem Int. 2016 doi: 10.1016/j.neuint.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Chatton JY. Astrocyte sodium signaling and neuro-metabolic coupling in the brain. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Rossi D. Astrocyte physiopathology: At the crossroads of intercellular networking, inflammation and cell death. Progress in neurobiology. 2015;130:86–120. doi: 10.1016/j.pneurobio.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Ruby CL, O’Connor KM, Ayers-Ringler J, Choi DS. Adenosine and glutamate in neuroglial interaction: implications for circadian disorders and alcoholism. Adv Neurobiol. 2014;11:103–119. doi: 10.1007/978-3-319-08894-5_6. [DOI] [PubMed] [Google Scholar]
- Salazar M, Pariente JA, Salido GM, Gonzalez A. Ethanol induces glutamate secretion by Ca2+ mobilization and ROS generation in rat hippocampal astrocytes. Neurochem Int. 2008;52:1061–1067. doi: 10.1016/j.neuint.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Sari Y, Toalston JE, Rao PS, Bell RL. Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience. 2016;326:117–125. doi: 10.1016/j.neuroscience.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider GH, Baethmann A, Kempski O. Mechanisms of glial swelling induced by glutamate. Can J Physiol Pharmacol. 1992;70(Suppl):S334–343. doi: 10.1139/y92-280. [DOI] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking. Biol Psychiatry. 2015;78:441–451. doi: 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci U S A. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsson P, Hansson E, Alling C. Ethanol potentiates serotonin stimulated inositol lipid metabolism in primary astroglial cell cultures. Biochemical pharmacology. 1989;38:2801–2805. doi: 10.1016/0006-2952(89)90434-6. [DOI] [PubMed] [Google Scholar]
- Slezak M, Pfrieger FW. New roles for astrocytes: regulation of CNS synaptogenesis. Trends Neurosci. 2003;26:531–535. doi: 10.1016/j.tins.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Smith KL, John CS, Sypek EI, Ongur D, Cohen BM, Barry SM, Bechtholt AJ. Exploring the role of central astrocytic glutamate uptake in ethanol reward in mice. Alcoholism, clinical and experimental research. 2014;38:1307–1314. doi: 10.1111/acer.12361. [DOI] [PubMed] [Google Scholar]
- Smith TL. Regulation of glutamate uptake in astrocytes continuously exposed to ethanol. Life Sci. 1997;61:2499–2505. doi: 10.1016/s0024-3205(97)00985-5. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, Reimer RJ. A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron. 2014;81:888–900. doi: 10.1016/j.neuron.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udomuksorn W, Mukem S, Kumarnsit E, Vongvatcharanon S, Vongvatcharanon U. Effects of alcohol administration during adulthood on parvalbumin and glial fibrillary acidic protein immunoreactivity in the rat cerebral cortex. Acta Histochem. 2011;113:283–289. doi: 10.1016/j.acthis.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Vargova L, Sykova E. Astrocytes and extracellular matrix in extrasynaptic volume transmission. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130608. doi: 10.1098/rstb.2013.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends in neurosciences. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang F, Smith NA, Xu Q, Goldman S, Peng W, Huang JH, Takano T, Nedergaard M. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:17404–17412. doi: 10.1523/JNEUROSCI.2178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland A, Garcia S, Knackstedt LA. Ceftriaxone and cefazolin attenuate the cue-primed reinstatement of alcohol-seeking. Front Pharmacol. 2015;6:44. doi: 10.3389/fphar.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology. 2014;39:579–594. doi: 10.1038/npp.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Guizzetti M. Fetal Alcohol Spectrum Disorders: An Overview from the Glia Perspective. Front Integr Neurosci. 2015;9:65. doi: 10.3389/fnint.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Andrew MR, Wiren KM. Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain Pathol. 2015 doi: 10.1111/bpa.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY, Oh SJ, Hwang EM, Marmorstein AD, Bae YC, Park JY, Lee CJ. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151:25–40. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Wu J, Lee MR, Choi S, Kim T, Choi DS. ENT1 regulates ethanol-sensitive EAAT2 expression and function in astrocytes. Alcohol Clin Exp Res. 2010;34:1110–1117. doi: 10.1111/j.1530-0277.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lee MR, Kim T, Johng S, Rohrback S, Kang N, Choi DS. Regulation of ethanol-sensitive EAAT2 expression through adenosine A1 receptor in astrocytes. Biochem Biophys Res Commun. 2011;406:47–52. doi: 10.1016/j.bbrc.2011.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Frankl M, Rothstein JD. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia. 2011;59:200–207. doi: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BE, Woo J, Chun YE, Chun H, Jo S, Bae JY, An H, Min JO, Oh SJ, Han KS, Kim HY, Kim T, Kim YS, Bae YC, Lee CJ. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol. 2014;592:4951–4968. doi: 10.1113/jphysiol.2014.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Nissim I, Daikhin Y, Lin ZP, Nelson D, Pleasure D, Erecinska M. Brain glutamate metabolism: neuronal-astroglial relationships. Developmental neuroscience. 1993;15:343–350. doi: 10.1159/000111354. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC. GABA and Glutamate Transporters in Brain. Front Endocrinol (Lausanne) 2013;4:165. doi: 10.3389/fendo.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M, Schmitt A, Vengeliene V, Henn FA, Spanagel R. Ethanol induces expression of the glutamate transporters EAAT1 and EAAT2 in organotypic cortical slice cultures. Alcohol Clin Exp Res. 2004;28:1752–1757. doi: 10.1097/01.alc.0000145810.12545.b3. [DOI] [PubMed] [Google Scholar]
- Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 2012:4. doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]