Considerable investigation is currently directed towards identifying pathways that may render donor-specific T cells permanently unresponsive to obviate the need for ongoing immunosuppression. Antigen-specific T cell unresponsiveness observed during chronic viral infection may provide fundamental insights into the cellular and molecular mechanisms that regulate tolerance. PD-1, a CD28 family member that transduces coinhibitory signals to T cells, is highly up-regulated in the setting of chronic viral infection and plays a critical role in T cell exhaustion. Indeed, blockade of this pathway can rescue T cell effector function and prevent apoptosis (1–3). However, mechanisms by which PD-1 blockade restores T cell survival and functionality have not been precisely characterized. Moreover, it has not been known whether inhibition of PD-1 signaling could permanently alter T cell programming, or, if anti-PD-1 could rescue the differentiation of long-lived memory T cells.
Two recent manuscripts in Science (Pauken et al, and Sen et al, 4, 5) addressed these questions. The authors tackle several key aspects of the effect of PD-1 signaling on programmed CD8+ T cell differentiation including the characterization of CD8+ T cell exhaustion following chronic viral (LCMV) infection as a unique differentiation program resulting from PD-1-directed genetic remodeling.
In detail, Sen and colleagues interrogated chromatin accessibility patterns in virus-specific CD8+ exhausted T cells (TEX) using an assay to detect transposase-accessible chromatin followed by high-throughput sequencing of the accessible regions (ATAC-seq). Their results demonstrate that differentiation of naïve cells is accompanied by alterations in chromatin accessible regions (chARs), patterns that differ between functional effector T cells (TEFF) as compared to TEX. For example, TEX exhibited ChARs adjacent to the genes encoding PD-1, TIM-3, and BATF, all upregulated in exhausted T cells.
Pauken et al, used a similar approach to show that TEX displayed ~6000 unique chAR sites as compared to TEFF and memory T cells (TMEM). Importantly, the unique epigenetic profiles identified in exhausted murine T cells were also detectable in human patients with HIV.
The work by Pauken et al, further addressed the question of whether blockade of the PD-1 pathway could improve long-term T cell functionality, while inducing the generation of long-lived memory cells. Intriguingly, they found that PD-L1 blockade altered TEX gene expression patterns only transiently, increasing the expression of genes associated with leukocyte activation and cell cycle pathways that peaked at ~3 weeks postblockade. Importantly, these changes in gene expression were lost by 11 weeks postblockade, when number or function of control vs. anti-PD-L1 treated TEX cells did not differ. Further, of the ~6000 chAR sites that were unique to TEX as compared to TEFF or TMEM, only ~650 were reversed following PD-L1 blockade.
Together, these findings indicate that TEX are only partially and temporarily reinvigorated after PD-L1 blockade and that they become “re-exhausted” upon cessation of treatment.
Overall, findings from both reports suggest that conditions under which CD8+ T cells are primed epigenetically imprint their differentiation programs. The programs can be transiently altered by perturbations in T cell co-signaling pathways, but changes in chromatin structure irrevocably commit T cells to an exhausted vs. memory lineage. These data are consistent with a study in a murine transplant model which demonstrated that tolerant animals experiencing infection-induced abrogation of tolerance were capable of spontaneously accepting a second donor-matched allograft once the infection was cleared, indicating a “memory” of tolerance (6). Similarly, Schietinger and colleagues (7) reported that self-tolerant T cells could be transiently reactivated, but reverted back to their tolerant state as a result of distinct epigenetic changes that occurred during tolerogenic programming.
These results have important implications for transplantation, as they suggest that chromatin remodeling increases chAR sites upstream of exhaustion-associated genes while decreasing chAR sites upstream of effector/memory-associated genes. Those findings provide a novel approach to inducing robust and durable transplant tolerance. Moreover, the field may be uniquely poised to capitalize on this approach because unlike in instances of chronic viral infection, cancer, and autoimmunity, the time of the priming event following transplantation is precisely known (ie, at the time of surgery). This situation affords the opportunity to intervene at the most critical stage in T cell differentiation and reinforces the concept that establishing tolerizing conditions at the time of engraftment will be paramount to the success of any tolerance induction strategy.
Of course, the optimal tolerance regimen will need to be defined. Drawing from studies of chronic viral infection, it seems likely that high and prolonged antigen exposure is of critical relevance to induce T cell exhaustion. Indeed, almost all successful experimental tolerance induction protocols depend on the use of donor-specific transfusion or hematopoietic chimerism, providing a large source of donor antigen to which T cells are exposed. Identifying additional key signals required to further promote T cell exhaustion and/or deletion in these settings remains an important goal.
Moreover, in some situations donor-reactive memory T cells may exist, precluding the opportunity to induce a TEX differentiation program at the time of priming. Based on the data of Pauken and Sen et al, one might predict that T cells undergoing an effector differentiation program at priming may be relatively insensitive to exhaustion-inducing signals during a secondary alloantigen challenge. Nevertheless, the epigenetic programs of TEFF placed into exhaustion-inducing conditions remain to be elucidated. Investigations along those lines will have important implications to design tolerance protocols in sensitized recipients. Further, identification of the epigenetic landscape of exhausted T cells may provide a blueprint by which to evaluate the permanence of transplant tolerance strategies. Thus, from both a diagnostic and therapeutic perspective, analysis and manipulation of epigenetic remodeling has the potential to profoundly influence assessment and activity of alloreactive T cell populations following transplantation.
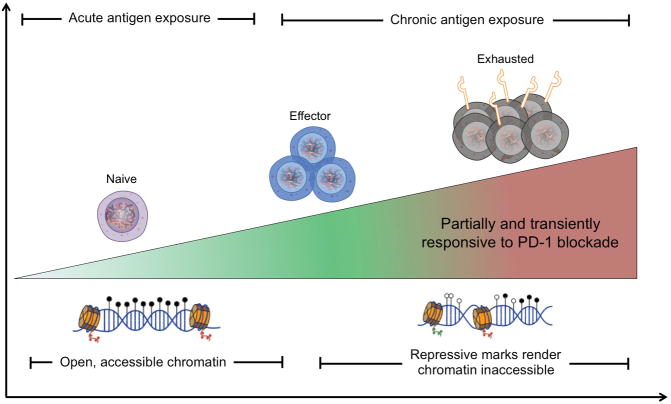
Figure 1.
Chronic antigen exposure installs enduring and irreversible epigenetic changes with a permanent exhaustion of antigen-specific CD8+ T cells that renders them only partially and temporarily responsive to PD-1 blockade. Following activation, effector T cells express permissive histone marks making their chromatin accessible for remodeling. As high levels of antigen persist, T cells upregulate PD-1 and repressive methylation that accumulates during chronic antigen exposure preventing exhausted cells from responding to checkpoint blockade. Thus, while blockade of PD-1 signaling can transiently restore T cell functionality, the underlying epigenetic landscape of exhausted T cells is not malleable and cannot be altered.
Adapted from Hazem E. Ghoneim, H. E., Zamora, A. E., Thomas, P. G., and Youngblood, B. A. Trends in Molecular Medicine, Vol. 22, Issue 12, p1000–1011
Footnotes
Disclosure: The author declares no conflicts of interest.
References
- 1.Yang J, Riella LV, Chock S, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011;187(3):1113–1119. doi: 10.4049/jimmunol.1100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koehn BH, Ford ML, Ferrer IR, et al. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol. 2008;181(8):5313–5322. doi: 10.4049/jimmunol.181.8.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. A J Transplant. 2012;12(10):2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauken KE, Sammons MA, Odorizzi PM, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354(6316):1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen DR, Kaminski J, Barnitz RA, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller ML, Daniels MD, Wang T, et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat Commun. 2015;6:7566. doi: 10.1038/ncomms8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]