Abstract
The aryl hydrocarbon receptor (AhR) is overexpressed in some patients with different tumor types, and the receptor can be a negative or positive prognostic factor. There is also evidence from both in vivo and in vitro cell culture models that the AhR can exhibit tumor-specific pro-oncogenic and tumor suppressor-like functions and therefore can be treated with AhR antagonists or agonists, respectively. Successful clinical applications of AhR ligands will require the synthesis and development of selective AhR modulators (SAhRMs) with tumor-specific AhR agonist or antagonist activity, and some currently available compounds such as indole-3-carbinol and diindolylmethane-(DIM) and synthetic AhR antagonists are potential drug candidates. There is also evidence that some AhR-active pharmaceuticals, including tranilast, flutamide, hydroxytamoxifen and omeprazole or their derivatives, may be effective AhR-dependent anticancer agents for single or combination cancer chemotherapies for treatment of breast and pancreatic cancers.
Keywords: Ah receptor ligands, anticancer activities, tumor specific
INTRODUCTION
The aryl hydrocarbon receptor (AhR), members of the nuclear receptor superfamily, and some G-protein-coupled receptors, such as the cannabinoid receptor, exhibit some unique and common properties, namely they all bind small molecules. Moreover, most these receptors interact not only with low molecular weight endogenous ligands but also an array of synthetic ligands which may exhibit tissue-specific agonist or antagonist activities [1–3]. These ligand-activated receptors play important roles in normal physiology and pathophysiology. With the exception of the AhR, the pharmaceutical industry has extensively focused on developing drugs targeting receptors for treatment of multiple diseases, including cancer [4]. For example, the estrogen receptor (ER) ligand tamoxifen has been successfully used by millions of women worldwide for treatment of early stage breast cancer and for chemoprevention in high risk women [5,6]. In contrast, clinical applications of drugs targeting AhR have been sadly lacking and this is primarily due to the fact that among all receptors, the AhR was initially identified as the receptor that bound and mediated the toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related halogenated aromatic (HA) environmental contaminants [7,8]. The stigma of being the receptor that mediated the toxicity of TCDD and HAs in the environment and in several human poisonings, including those in Italy (Seveso), Japan (Yusho) and Taiwan (Kyushu) [9–13], has significantly hindered the objective development of drugs targeting the AhR. This stigma has only been overcome by more recent discoveries of the critical role of the AhR in many disease processes, including cancer, and by the identification of structurally-diverse AhR ligands that include industrial compounds, health-promoting phytochemicals and microbial metabolites, and pharmaceuticals [1–3]. Nevertheless, only a few AhR-active drugs, including laquinimod and aminoflavone (NSC686288), have been in clinical trials for treating multiple sclerosis and breast cancer, respectively [14–16].
ROLE OF THE AhR IN CARCINOGENESIS
Effects of TCDD and dioxin-like compounds
Early studies on the role of the AhR in carcinogenesis primarily focused on AhR ligands, particularly TCDD and its effect in humans and animal models. Epidemiologic studies on exposures to TCDD and related compounds in the workplace do not consistently show an increased incidence of specific tumors in all exposure groups. However, the International Agency For Research on Cancer (IARC) has classified TCDD as a Group 1 carcinogen based on results from selected exposure industrial cohorts [17] and this conclusion has received support and criticism [18,19]. The carcinogenicity of other dioxin-like compounds in human studies is not well established; moreover, the role of the AhR alone and effects of endogenous ligands such as 6-formylindolo[3,2-b]carbazole (FICZ) in human cancers is also not known.
The carcinogenicity of TCDD has been well established in animal models; however, carcinogenic effects of this compound are species-, strain- and sex-dependent and also dependent on the timing of exposure. For example, the first long term cancer feeding study by Kociba and coworkers was carried out in male and female Sprague-Dawley rats and hepatocellular carcinomas were observed in female but not male rats [20]. Moreover, in female rats, there was a TCDD-dependent decrease in formation of uterine and mammary tumors which spontaneously form in aged Sprague-Dawley but not in other rat strains, and this indicated that the ligand-activated AhR inhibited estrogen-induced responses (i.e. uterine and mammary cancer). The inhibitory AhR-ER crosstalk in rodent models and breast cancer cells has been extensively investigated [21,22]. Interestingly, other studies in female Sprague-Dawley rats showed similarities but also some differences in the site-specific carcinogenesis of TCDD and other dioxin-like compounds [23].
Endogenous role of the AhR in human cancer
Two recent papers have reviewed the role of the AhR and its ligands in carcinogenesis and showed that the AhR is a bona fide drug target [24,25]. This article will primarily focus on subsequent publications that complement these reviews. Differential AhR expression has been observed in tumor vs. normal tissues for multiple tumors, and the levels and prognostic significance of the AhR are tumor-specific [26–37]. For example, a recent survey of tumor array database showed that AhR mRNA expression in breast, lung, prostate and cervical cancers was similar to non-tumor tissue, whereas expression was higher in stomach, thyroid, colon and pancreatic tumors compared to non-tumor tissue [34]. Moreover, high expression of the AhR in pancreatic tumors was a prognostic factor for increased patient survival [34]. In contrast, high expression of the nuclear AhR was a negative prognostic factor for urothelial cancer patients [30]. It was also reported that AhR mRNA overexpression in oral squamous cell carcinomas was due to elevated levels of nuclear AhR, whereas in adjacent normal tissue, the receptor was primarily cytosolic [35]. A similar distribution of the receptor protein was also observed in kidney tumors (nuclear) vs. normal renal tissue (cytosolic) and the AhR was a negative prognostic factor but was only expressed in 14/102 of the kidney tumors examined [36]. It is possible that increased nuclear localized of the receptor in some tumors may have some functional significance, thus the future application of selective AhR modulators (SAhRMs) that target specific tumors will require a personalized medicine approach and require prior knowledge of endogenous AhR expression in the tumor of interest and its intracellular location (nuclear vs cytosolic).
AhR function in rodent models and cancer cell line
The functions of the AhR in rodent models (primarily mice) and in cancer cell lines have been extensively investigated primarily in the AhR knockout (AhR−/−) mice or by knockdown of the AhR in cells. The APCmin+ mouse model has been used in studies on colon cancer, and mice expressing mutations in the adenomas polyposis coli (APC) gene spontaneously develop colonic and intestinal tumors and these are significantly enhanced in APCmin+/AhR+/− (crossed) mice [38]. Diethylnitrosamine-induced liver cancer is also enhanced in AhR−/− mice compared to wild-type mice, suggesting that endogenous expression of the AhR protects against colon and liver cancer [38,39]. The role of the AhR in other mouse models that develop cancer has not been determined and the contributions (if any) of endogenous AhR ligands are also unknown. There is evidence that the AhR plays a role in polycyclic aromatic hydrocarbon (PAH)-induced skin carcinogenesis; however, this is most likely due to AhR-dependent induction of CYP1A/CYP1B enzymes required for metabolic activation of PAHs [40,41].
The effect of AhR knockdown in cancer cell lines is tissue specific and exhibits pro-oncogenic or tumor suppressor-like activity or no apparent function and these responses have previously been summarized [24,25]. Cancer cell lines can be highly variable with respect to cell passage number, the type of serum used, other growth conditions, and contamination with other cell lines, and the reliability of determining functional effects of the AhR knockdown may be questionable. For example, stable knockdown of AhR by RNAi in MDA-MB-231 cells decreased proliferation, anchorage-independent growth and migration and induced apoptosis, suggesting a pro-oncogenic function for the AhR in the cell line [42]. In contrast, another report showed that knockdown of AhR (by RNAi) in MDA-MB-231 cells resulted in increased invasion [43], and results of comparable studies in both ER-positive and ER-negative breast cancer cells gave contrasting results [44–47].
AhR ligands and their activity as anticancer agents
Depending on the tumor type, overexpression of the AhR may be a positive or negative prognostic factor for cancer patient prognosis, and AhR may also play a functional “endogenous” role in some tumors. Despite the differing prognostic/functional role of the receptor, expression of the AhR can provide patients with a unique option, namely treatment with AhR-active compounds (Fig. 1) [24,25]. For example, in oral squamous cell carcinoma-derived cell lines, prototypic AhR agonists exhibit pro-oncogenic activity and, in mouse xenograft experiments, a novel AhR antagonist CB7993113 [48] inhibited tumor growth and overall survival [5]. Similar results were observed for head and neck cancer where AhR antagonists were effective as anticancer agents [49]. The growth and invasion of breast and other cancers are inhibited by AhR agonists, and there has been development of relatively non-toxic SAhRMs which include aminoflavone and NKI150460, a novel selective anticancer agent and AhR agonist, for treatment of breast cancer [14,50]. Among the more classical AhR ligands, indole-3-carbinol (from cruciferous vegetables) and β-naphthoflavone and its dimer, diindolylmethane (DIM), exhibit promising anticancer activity and these compounds and synthetic analogs should be further investigated for their potential clinical applications.
Figure 1.
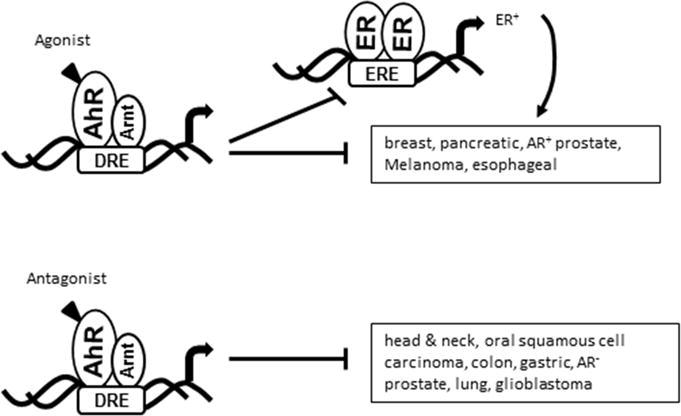
AhR signaling inhibits crosstalk with ERα in breast cancer cells and AhR agonists and antagonists are tumor-specific anticancer agents (24, 25). In pancreatic cancer, there is evident for a non-genomic pathway for inhibition of migration (34).
REPOSITIONING AhR-ACTIVE PHARMACEUTICALS
A promising and productive approach for developing anticancer agents has been the repositioning of pharmaceuticals used for other purposes. The antidiabetic drug metformin is one of the best examples of drug repositioning; diabetics treated with metformin exhibit a lower incidence of several cancers, and metformin is now being used in clinical trials for cancer chemotherapy [51,52]. Several studies show that a wide range of pharmaceuticals exhibit AhR activity in one or more assays, and these compounds are now being investigated as anticancer agents [24,53]. Using induction of CYP1A1 as a marker of Ah-responsiveness, it was reported that several selective ER modulators (SERMs) were AhR agonists and this included 4-hydroxytamoxifen, the active metabolite of tamoxifen used extensively as an antiestrogen for treating early stage breast cancer in women [54]. 4-Hydroxytamoxifen induces expression of AhR-regulated genes in the absence of ER and there is evidence that some of the therapeutic efficacy of tamoxifen, particularly in the suppression of osteoclast differentiation, may be due, in part, to activation of the AhR [54]. Several other reports demonstrate the anticancer activities of leflunomide (an antiarthritic drug), raloxifene (an antiestrogen), tranilast (an anti-allergic drug), and the anti-androgenic drug flutamide (Fig. 2) [55–58]. Flutamide, but not TCDD, induced TGFβ in liver cancer cells and this response was AhR-dependent [56]. Studies in this laboratory screened a panel of AhR-active pharmaceuticals, including 4-hydroxytamoxifen, flutamide, leflunomide, mexiletine, nimodiphine, omeprazole, sulindac and tranilast, in cell migration. The results and mechanism of action were ligand-, tumor type-, response- and cell context-dependent. For example, in BT474 and MDA-MD-231 breast cancer cell lines, these compounds differentially induced CYP1A1 and CYP1B1 gene expression and, in ER-negative MDA-MB-468 cells, all of the AhR-active compounds except mexiletine and also TCDD inhibited migration [43,56,57]. In ER-negative MDA-MD-231 cells, TCDD and omeprazole, but not 4-hydroxytamoxifen, flutamide, leflunomide, mexiletine, nimodipine, sulindac and tranilast, inhibited migration and this was due, in part, to AhR-dependent downregulation of the pro-migration gene CXCR4 [43]. We also carried out a similar migration inhibition study in pancreatic cancer cells and among the pharmaceuticals, only omeprazole and tranilast (but not TCDD) were active and mechanistic studies showed that this inhibitory response was CXCR4-independent and due to a non-genomic pathway [34]. The effects of omeprazole as an inhibitor of breast and pancreatic cancer cell invasion and metastasis in vivo and in vitro suggest that benzimidazole analogs of omeprazole may be useful scaffolds for developing AhR mediated anticancer agents, and these studies are currently in progress. The a priori effectiveness of an AhR-active pharmaceutical cannot be predicted and can only be identified using an appropriate screening assay. The advantages in identifying and repositioning AhR-active pharmaceuticals as anticancer agents are that these compounds and their analogs have been identified and clinically approved during their initial development.
Figure 2.
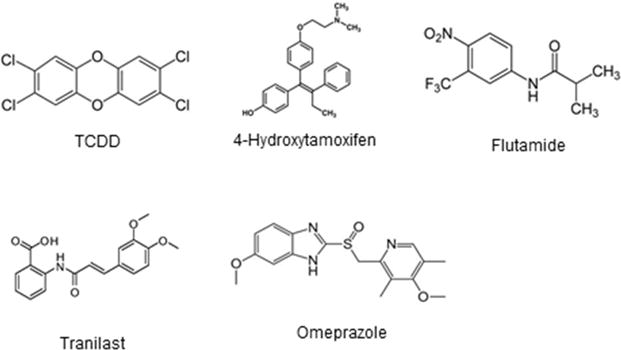
TCDD and AhR active pharmaceuticals.
SUMMARY
The AhR has emerged as an important new drug target for cancer chemotherapy. Moreover, depending on the tumor type and endogenous role of the receptor, clinical treatment with AhR agonists or antagonists alone or in combination with other drugs constitutes an approach for cancer chemotherapy. The repositioning of AhR-active pharmaceuticals is also feasible and their structure can serve as scaffolds for the synthesis and testing of structural analogs.
HIGHLIGHTS.
The AhR plays an important tumor-specific role in enhancing or inhibiting carcinogenesis.
AhR agonists and antagonists represent a novel class of anticancer agents.
AhR-active pharmaceuticals are compounds that can be repositioned for cancer therapy.
Acknowledgments
The financial support of Texas AgriLife, the Syd Kyle endowment, and the National Institutes of Health (R01-CA142697, R01-CA202697 and P30-ES023512) is gratefully appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124(1):1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safe S, Chadalapaka G, Jutooru I. AHR-reactive compounds in the human diet. In: Pohjanvirta R, editor. The Ah Receptor in Biology and Toxicology. John Wiler & Sons; Hoboken, NJ: 2012. pp. 331–342. [Google Scholar]
- 3.Soshilov AA, Denison MS. Ligand promiscuity of aryl hydrocarbon receptor agonists and antagonists revealed by site-directed mutagenesis. Mol Cell Biol. 2014;34(9):1707–1719. doi: 10.1128/MCB.01183-13. This is a key reference on structurally-diverse ligands that bind the AhR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006;5(10):821–834. doi: 10.1038/nrd2132. [DOI] [PubMed] [Google Scholar]
- 5.Jordan VC. SERMs: meeting the promise of multifunctional medicines. J Natl Cancer Inst. 2007;99(5):350–356. doi: 10.1093/jnci/djk062. [DOI] [PubMed] [Google Scholar]
- 6.Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69(4):1243–1254. doi: 10.1158/0008-5472.CAN-09-0029. [DOI] [PubMed] [Google Scholar]
- 7.Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol: evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936–4946. A classical reference on the pioneering work by Poland and coworkers who first purified the mouse AhR. [PubMed] [Google Scholar]
- 8.Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons. Examinations of the mechanism of toxicity. Ann Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 9.Kuratsune M, Yoshimura T, Matsuzaka J, Yamaguchi A. Epidemiologic study on Yusho, a poisoning caused by ingestion of rice oil contaminated with a commercial brand of polychlorinated biphenyls. Environ Health Perspect. 1972;1:119–128. doi: 10.1289/ehp.7201119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters–what we have learned from Yusho disease. Environ Res. 2001;86(1):2–11. doi: 10.1006/enrs.2001.4244. [DOI] [PubMed] [Google Scholar]
- 11.Yao Y, Takasuga T, Masunaga S, Nakanishi J. Detailed study on the levels of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans and polychlorinated biphenyls in Yusho rice oil. Chemosphere. 2002;46(9–10):1461–1469. doi: 10.1016/s0045-6535(01)00254-5. [DOI] [PubMed] [Google Scholar]
- 12.Masuda Y. Health status of Japanese and Taiwanese after exposure to contaminated rice oil. Environ Health Perspect. 1985;60:321–325. doi: 10.1289/ehp.8560321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertazzi PA, Bernucci I, Brambilla G, Consonni D, Pesatori AC. The Seveso studies on early and long-term effects of dioxin exposure: a review. Environ Health Perspect. 1998;106(Suppl 2):625–633. doi: 10.1289/ehp.98106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loaiza-Perez AI, Kenney S, Boswell J, Hollingshead M, Alley MC, Hose C, Ciolino HP, Yeh GC, Trepel JB, Vistica DT, Sausville EA. Aryl hydrocarbon receptor activation of an antitumor aminoflavone: basis of selective toxicity for MCF-7 breast tumor cells. Mol Cancer Ther. 2004;3(6):715–725. [PubMed] [Google Scholar]
- 15.Haggiag S, Ruggieri S, Gasperini C. Efficacy and safety of laquinimod in multiple sclerosis: current status. Ther Adv Neurol Disord. 2013;6(6):343–352. doi: 10.1177/1756285613499424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preiningerova J. Oral laquinimod therapy in relapsing multiple sclerosis. Expert Opin Investig Drugs. 2009;18(7):985–989. doi: 10.1517/13543780903044944. [DOI] [PubMed] [Google Scholar]
- 17.IARC IAfRoC. Polychlorinated Dibenzo-para-dioxins and Polychlorinated Dibenzofurans. Vol. 69. World Health Organization; IARC Press, Lyon, France: 1997. [Google Scholar]
- 18.Steenland K, Bertazzi P, Baccarelli A, Kogevinas M. Dioxin revisited: developments since the 1997 IARC classification of dioxin as a human carcinogen. Environ Health Perspect. 2004;112(13):1265–1268. doi: 10.1289/ehp.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole P, Trichopoulos D, Pastides H, Starr T, Mandel JS. Dioxin and cancer: a critical review. Regul Toxicol Pharmacol. 2003;38(3):378–388. doi: 10.1016/j.yrtph.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Kociba RJ, Keyes DG, Beger JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CL, Barnard SD, Hummel RA, et al. Results of a 2-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in rats. Toxicol Appl Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. A classical study on long term carcinogenic and anticarcinogenic effects of TCDD in Sprague-Dawley rats. [DOI] [PubMed] [Google Scholar]
- 21.Safe S, Walker K, Zhang S. Dioxin as an environmental pollutant and its role in breast cancer. In: Russo J, editor. Environment and Breast Cancer. Spring-Verlag; New York: 2011. pp. 127–146. [Google Scholar]
- 22.Safe S, Wormke M. Inhibitory aryl hydrocarbon-estrogen receptor α crosstalk and mechanisms of action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 23.Walker NJ, Wyde ME, Fischer LJ, Nyska A, Bucher JR. Comparison of chronic toxicity and carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in 2-year bioassays in female Sprague-Dawley rats. Mol Nutr Food Res. 2006;50(10):934–944. doi: 10.1002/mnfr.200600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safe S, Lee SO, Jin UH. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci. 2013;135(1):1–16. doi: 10.1093/toxsci/kft128. A major review on the AhR and cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nature Reviews Cancer. 2014;14(12):801–814. doi: 10.1038/nrc3846. Another major review on the AhR and cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin P, Chang H, Tsai WT, Wu MH, Liao YS, Chen JT, Su JM. Overexpression of aryl hydrocarbon receptor in human lung carcinomas. Toxicol Pathol. 2003;31(1):22–30. doi: 10.1080/01926230390173824. [DOI] [PubMed] [Google Scholar]
- 27.Portal-Nunez S, Shankavaram UT, Rao M, Datrice N, Atay S, Aparicio M, Camphausen KA, Fernandez-Salguero PM, Chang H, Lin P, Schrump DS, et al. Aryl hydrocarbon receptor-induced adrenomedullin mediates cigarette smoke carcinogenicity in humans and mice. Cancer Res. 2012;72(22):5790–5800. doi: 10.1158/0008-5472.CAN-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gramatzki D, Pantazis G, Schittenhelm J, Tabatabai G, Kohle C, Wick W, Schwarz M, Weller M, Tritschler I. Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene. 2009;28(28):2593–2605. doi: 10.1038/onc.2009.104. [DOI] [PubMed] [Google Scholar]
- 29.Jaffrain-Rea ML, Angelini M, Gargano D, Tichomirowa MA, Daly AF, Vanbellinghen JF, D’Innocenzo E, Barlier A, Giangaspero F, Esposito V, Ventura L, et al. Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications. Endocr Relat Cancer. 2009;16(3):1029–1043. doi: 10.1677/ERC-09-0094. [DOI] [PubMed] [Google Scholar]
- 30.Ishida M, Mikami S, Kikuchi E, Kosaka T, Miyajima A, Nakagawa K, Mukai M, Okada Y, Oya M. Activation of the aryl hydrocarbon receptor pathway enhances cancer cell invasion by upregulating the MMP expression and is associated with poor prognosis in upper urinary tract urothelial cancer. Carcinogenesis. 2010;31(2):287–295. doi: 10.1093/carcin/bgp222. [DOI] [PubMed] [Google Scholar]
- 31.Gluschnaider U, Hidas G, Cojocaru G, Yutkin V, Ben-Neriah Y, Pikarsky E. beta-TrCP inhibition reduces prostate cancer cell growth via upregulation of the aryl hydrocarbon receptor. PLoS One. 2010;5(2):e9060. doi: 10.1371/journal.pone.0009060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koliopanus A, Kleeff J, Xiao Y, Safe S, Zimmerman A, Buchler MW, Friess H. Increased aryl hydrocarbon receptor expression offers a potential therapeutic target in pancreatic cancer. Oncogene. 2002;21:6059–6070. doi: 10.1038/sj.onc.1205633. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Zong H, Li S, Zhang D, Zhang L, Xia Q. Activation of aryl hydrocarbon receptor suppresses invasion of esophageal squamous cell carcinoma cell lines. Tumori. 2012;98(1):152–157. doi: 10.1177/030089161209800121. [DOI] [PubMed] [Google Scholar]
- 34.Jin UH, Kim SB, Safe S. Omeprazole inhibits pancreatic cancer cell invasion through a nongenomic aryl hydrocarbon receptor pathway. Chem Res Toxicol. 2015;28(5):907–918. doi: 10.1021/tx5005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanford EA, Ramirez-Cardenas A, Wang Z, Novikov O, Alamoud K, Koutrakis P, Mizgerd JP, Genco CA, Kukuruzinska M, Monti S, Bais MV, et al. Role for the aryl hydrocarbon receptor and diverse ligands in oral squamous cell carcinoma migration and tumorigenesis. Mol Cancer Res. 2016;14(8):696–706. doi: 10.1158/1541-7786.MCR-16-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishida M, Mikami S, Shinojima T, Kosaka T, Mizuno R, Kikuchi E, Miyajima A, Okada Y, Oya M. Activation of aryl hydrocarbon receptor promotes invasion of clear cell renal cell carcinoma and is associated with poor prognosis and cigarette smoke. Int J Cancer. 2015;137(2):299–310. doi: 10.1002/ijc.29398. [DOI] [PubMed] [Google Scholar]
- 37.Su JM, Lin P, Chang H. Prognostic value of nuclear translocation of aryl hydrocarbon receptor for non-small cell lung cancer. Anticancer Res. 2013;33(9):3953–3961. [PubMed] [Google Scholar]
- 38.Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, Hirokawa T, Akiyama T, et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci USA. 2009;106(32):13481–13486. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y, Puga A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010;70(1):212–220. doi: 10.1158/0008-5472.CAN-09-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ide F, Suka N, Kitada M, Sakashita H, Kusama K, Ishikawa T. Skin and salivary gland carcinogenicity of 7,12-dimethylbenz[a]anthracene is equivalent in the presence or absence of aryl hydrocarbon receptor. Cancer Lett. 2004;214(1):35–41. doi: 10.1016/j.canlet.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2000;97(2):779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goode GD, Ballard BR, Manning HC, Freeman ML, Kang Y, Eltom SE. Knockdown of aberrantly upregulated aryl hydrocarbon receptor reduces tumor growth and metastasis of MDA-MB-231 human breast cancer cell line. Int J Cancer. 2013;133(12):2769–2780. doi: 10.1002/ijc.28297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin UH, Lee SO, Pfent C, Safe S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer. 2014;14:498. doi: 10.1186/1471-2407-14-498. The in vivo and in vitro anticancer activity of omeprazole, an AhR-active pharmaceutical, is described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, Lei P, Liu X, Li X, Walker K, Kotha L, Rowlands C, Safe S. The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr Relat Cancer. 2009;16(3):835–844. doi: 10.1677/ERC-09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore M, Wang X, Lu YF, Wormke M, Craig A, Gerlach JH, Burghardt R, Barhoumi R, Safe S. Benzo[a]pyrene-resistant MCF-7 human breast cancer cells. A unique aryl hydrocarbon-nonresponsive clone. J Biol Chem. 1994;269(16):11751–11759. [PubMed] [Google Scholar]
- 46.Moore M, Ruh M, Steinberg M, Safe S. Isolation and characterization of variant benzo[a]pyrene-resistant T47D human breast-cancer cells. Int J Cancer. 1996;66:117–123. doi: 10.1002/(SICI)1097-0215(19960328)66:1<117::AID-IJC20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 47.O’Donnell EF, Koch DC, Bisson WH, Jang HS, Kolluri SK. The aryl hydrocarbon receptor mediates raloxifene-induced apoptosis in estrogen receptor-negative hepatoma and breast cancer cells. Cell Death Dis. 2014;5:e1038. doi: 10.1038/cddis.2013.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parks AJ, Pollastri MP, Hahn ME, Stanford EA, Novikov O, Franks DG, Haigh SE, Narasimhan S, Ashton TD, Hopper TG, Kozakov D, et al. In silico identification of an aryl hydrocarbon receptor antagonist with biological activity in vitro and in vivo. Mol Pharmacol. 2014;86(5):593–608. doi: 10.1124/mol.114.093369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiNatale BC, Smith K, John K, Krishnegowda G, Amin SG, Perdew GH. Ah receptor antagonism represses head and neck tumor cell aggressive phenotype. Mol Cancer Res. 2012;10(10):1369–1379. doi: 10.1158/1541-7786.MCR-12-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukasawa K, Kagaya S, Maruyama S, Kuroiwa S, Masuda K, Kameyama Y, Satoh Y, Akatsu Y, Tomura A, Nishikawa K, Horie S, et al. A novel compound, NK150460, exhibits selective antitumor activity against breast cancer cell lines through activation of aryl hydrocarbon receptor. Mol Cancer Ther. 2015;14(2):343–354. doi: 10.1158/1535-7163.MCT-14-0158. [DOI] [PubMed] [Google Scholar]
- 51.Kasznicki J, Sliwinska A, Drzewoski J. Metformin in cancer prevention and therapy. Ann Transl Med. 2014;2(6):57. doi: 10.3978/j.issn.2305-5839.2014.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khawaja MR, Nick AM, Madhusudanannair V, Fu S, Hong D, McQuinn LM, Ng CS, Piha-Paul SA, Janku F, Subbiah V, Tsimberidou A, et al. Phase I dose escalation study of temsirolimus in combination with metformin in patients with advanced/refractory cancers. Cancer Chemother Pharmacol. 2016;77(5):973–977. doi: 10.1007/s00280-016-3009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu W, Sorrentino C, Denison MS, Kolaja K, Fielden MR. Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol Pharmacol. 2007;71(6):1475–1486. doi: 10.1124/mol.106.032748. A large scale screening assay identified several AhR-active pharmaceuticals. [DOI] [PubMed] [Google Scholar]
- 54.DuSell CD, Nelson ER, Wittmann BM, Fretz JA, Kazmin D, Thomas RS, Pike JW, McDonnell DP. Regulation of aryl hydrocarbon receptor function by selective estrogen receptor modulators. Mol Endocrinol. 2010;24(1):33–46. doi: 10.1210/me.2009-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Donnell EF, Kopparapu PR, Koch DC, Jang HS, Phillips JL, Tanguay RL, Kerkvliet NI, Kolluri SK. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PLoS One. 2012;7(7):e40926. doi: 10.1371/journal.pone.0040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koch DC, Jang HS, O’Donnell EF, Punj S, Kopparapu PR, Bisson WH, Kerkvliet NI, Kolluri SK. Anti-androgen flutamide suppresses hepatocellular carcinoma cell proliferation via the aryl hydrocarbon receptor mediated induction of transforming growth factor-beta1. Oncogene. 2015;34(50):6092–6104. doi: 10.1038/onc.2015.55. [DOI] [PubMed] [Google Scholar]
- 57.Subramaniam V, Ace O, Prud’homme GJ, Jothy S. Tranilast treatment decreases cell growth, migration and inhibits colony formation of human breast cancer cells. Exp Mol Pathol. 2011;90(1):116–122. doi: 10.1016/j.yexmp.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 58.Prud’homme GJ, Glinka Y, Toulina A, Ace O, Subramaniam V, Jothy S. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS One. 2010;5(11):e13831. doi: 10.1371/journal.pone.0013831. [DOI] [PMC free article] [PubMed] [Google Scholar]


