Abstract
Selinexor, a Selective Inhibitor of Nuclear Export (SINE) compound targeting exportin-1, has previously been shown to inhibit melanoma cell growth in vivo. We hypothesized that combining selinexor with antibodies that block or disrupt T cell checkpoint molecule signaling would exert superior anti-melanoma activity. In vitro, selinexor increased PD-1 and CTLA4 gene expression in leukocytes and induced PD-L1 gene expression in human melanoma cell lines. Mice bearing syngeneic B16F10 melanoma tumors demonstrated a significant reduction in tumor growth rate in response to the combination of selinexor and anti-PD-1 or anti-PD-L1 antibodies (p<0.05). Similar results were obtained in B16F10-bearing mice treated with selinexor combined with anti-CTLA4 antibody. Immunophenotypic analysis of splenocytes by flow cytometry revealed that selinexor alone or in combination with anti-PD-L1 antibody significantly increased the frequency of both natural killer cells (p≤0.050) and CD4+ T cells with a TH1 phenotype (p≤0.050). Further experiments indicated that the anti-tumor effect of selinexor in combination with anti-PD-1 therapy persisted under an alternative dosing schedule but was lost when selinexor was administered daily. These data indicate that the efficacy of selinexor against melanoma may be enhanced by disrupting immune checkpoint activity.
Introduction
Antibodies targeting the immune checkpoint molecules PD-1 (nivolumab, pembrolizumab) and CTLA4 (ipilimumab) have advanced clinical care for a number of malignancies including melanoma, non-small cell lung cancer, and renal cell carcinoma (1). Even as monotherapy, these agents often elicit durable responses, albeit only in a subset of patients (2). In an effort to improve clinical efficacy, pre-clinical and clinical studies are rapidly emerging to test the impact of checkpoint inhibition in combination with other chemotherapeutic, targeted, or immunologic agents.
The rationale for combining checkpoint blockade with other therapies are numerous (2). First, clinical responses to immunotherapy often require several months to achieve (2), and it is thought that combining them with targeted agents could achieve both rapid and sustained tumor regression (2). Second, induction of apoptosis or other mechanisms of cell death induced by targeted agents may induce antigen release and provide additional stimuli to the immune system, thereby augmenting and sustaining the initial response to therapy. Finally, combined blockade of multiple checkpoint molecules has potential to overcome redundant immunosuppressive mechanisms, unleashing cytotoxic immune effector activity. This approach has recently shown clinical promise in the setting of melanoma upon concurrent inhibition of CTLA4 and PD-1 (3).
Combining immune checkpoint blockade with other therapies, particularly therapies that target mutated or aberrantly regulated pathways within tumors, is another promising approach to increase the proportion of patients who undergo durable complete responses (2). This approach is particularly relevant in melanoma, in which a number of pathways are frequently mutated or dysregulated (e.g. BRAFV600 mutations, NRASQ61 mutations) (4). One novel target is the nuclear export protein, Exportin-1 (XPO1). XPO1 is one of seven nuclear export proteins, and is the primary mediator of nuclear export for many key tumor suppressor and cell-cycle regulatory proteins, including p53 and cyclin-dependent kinase 1A (CDKN1A/p21)(5). We have previously reported that XPO1 is significantly elevated over the course of melanoma progression with highest expression in metastatic lesions (6). XPO1 is inhibited by selinexor, an orally bioavailable Selective Inhibitor of Nuclear Export (SINE) compound, currently in advanced phase 2 and 3 clinical trials against solid and hematological cancers (7). Selinexor exerted significant anti-tumor activity against human melanoma xenograft tumors in nude mice. One particular strength of selinexor is the apparent sensitivity of melanoma cells to this drug, regardless of genotype (6).
In addition to suppressing the nuclear export of tumor suppressor and cell-cycle regulatory proteins, selinexor blocks the nuclear export of STAT3 (8) and NFATc1(9), signal transduction molecules with potent immunomodulatory activities. These transcription factors are heavily involved in immune function and implicated in the regulation of T cell checkpoint molecules in other models (10,11). Because of this, we hypothesized that selinexor treatment might increase T cell checkpoint molecule expression on tumor and immune cells, thus counter-acting the in vivo anti-tumor activity of this drug. In the present study, we tested whether combining selinexor with antibodies that block/disrupt T cell checkpoint molecule signaling would exert superior anti-melanoma activity.
Here we report that combined therapy with selinexor and PD-1 blocking antibodies significantly limited tumor growth rate in the aggressive, immune competent B16F10 murine model of melanoma. Similar efficacy was observed when selinexor was combined with PD-L1 or CTLA4 blockade in the B16F10 melanoma model. This reduction in tumor growth was accompanied by systemic changes in natural killer (NK) cells, myeloid derived suppressor cells (MDSC), T cell differentiation, and increased infiltration of T cells in the tumor microenvironment. Further experiments revealed that scheduling of selinexor with anti-PD-1 antibodies impacted the in vivo efficacy of this treatment. Together, this pre-clinical work demonstrates that selinexor can elicit potent anti-tumor activity against melanoma tumors when administered in combination with immune checkpoint inhibitors.
Methods
Experimental agents
Selinexor was provided by Karyopharm Therapeutics, Inc. (Newton, MA) and was dissolved in DMSO at a stock concentration of 18.05 mM. For in vivo studies, selinexor was diluted to 1.5 mg/mL in water with 0.6% w/v Pluronic® F-68 and 0.6% w/v PVP K-29/32. Antibodies specific for murine PD-1 (clone RMP1-14), murine PD-L1 (clone 10F.9G2) and murine CTLA4 (clone 9D9) or isotype matched control antibodies were purchased from BioXCel, Inc. (West Lebanon, NH).
Cells and cell culture
Murine (B16F10) melanoma cell lines were obtained from the American Type Culture Collection (ATCC). At the time of their use in these studies, B16F10 cells had been cultured for ≥ 4 passages since receipt from ATCC. Briefly, all cell lines cells were maintained in complete media as indicated by ATCC. For in vitro culture/treatment of primary cells, cells were maintained in RPMI-1640 (Gibco) + 10% fetal bovine serum (Gibco) + 1% Anti-Anti (Gibco). Cells were cultured at 37°C in 5% CO2. Human melanoma (A375, CHL-1), breast cancer (MDA-MB-468), and prostate cancer (PC3) cell lines were obtained from ATCC. The alveolar soft part sarcoma cell line (ASPS-KY) was a gift to YL by Dr. Akira Ogose (12). The identity of these cell lines were not tested or verified prior to use in this study. Human donor blood was collected in Vacutainer® EDTA tubes (BD Biosciences, San Jose, CA) by BioreclamationIVT (Westbury, NY) and peripheral blood leukocytes were isolated using the Buffer EL™ kit (Qiagen, Hilden, Germany). Human leukocytes and human and murine melanoma cell lines were cultured in selinexor (30–1000nM) for 24 hours, as indicated.
Quantitative real-time polymerase chain reaction
Following selinexor treatment, RNA was isolated from human leukocytes using the QIAmp RNA blood mini kit (Qiagen) and from melanoma cells using TRIzol (ThermoFischer, Waltham MA) following the manufacturer’s specifications. Reverse transcription of isolated RNA was performed using high capacity cDNA reverse transcription kit (Life Technologies, Carlsbad, CA) and Real Time PCR was performed using Taqman fast advanced master mix (Life Technologies) and the following TaqMan probes: XPO1 (cat# Hs00418963_m1), PD-1 (Hs01550088_m1), PD-L1 (Hs01125301_m1), CTLA4 (Hs03044418_m1), with and GAPDH (cat#4326317) as the loading control using a Viia7 instrument (Life Technologies).
In vivo experiments
All animal studies were conducted under a protocol approved by The Ohio State University Institutional Animal Care and Use Committee (IACUC). Female, immune competent, C57BL/6 mice were injected subcutaneously in the flank with 5×105 murine B16F10 melanoma cells (Day 0). All studies utilized n = 5–6 mice/group at 6–8 weeks of age, purchased from The Jackson Laboratory (Bar Harbor, ME). Once tumors were palpable (day 6), mice were randomized to treatment groups. Selinexor treatments were administered at multiple dose schedules via oral gavage in a volume of 200 µL, at a dose of 15 mg/kg (Mondays and Thursdays or Tuesdays and Fridays), 10 mg/kg (on Mondays and Tuesdays) or 5 mg/kg (Monday-Friday). Control mice were given an equivalent volume of vehicle via the same route. Antibodies were administered via intraperitoneal (i.p.) injection at 100–200 µg/mouse (in a volume of 50 uL) as indicated (Mondays and Thursdays, Tuesdays and Fridays, or Wednesdays and Fridays). Bi-dimensional tumor measurements were obtained three times weekly using microcalipers. Tumor volume was calculated as: (0.5) × (length [long dimension]) × (width [short dimension])2. Mice were euthanized and tumors collected from all animals once vehicle-treated tumors reached a volume of 1500 mm3. Animal weight was monitored for dosing and to assess toxicity throughout the study. Standard mouse chow was supplemented with wet food for all treatment groups.
Flow cytometry
Phenotypic analysis of splenocytes was carried out by flow cytometry as previously described (13). Briefly, cryopreserved splenocytes were thawed at 37°C, washed, and resuspended in FACS buffer (PBS supplemented with 5% FBS). Cells were stained with appropriate antibodies in the dark for 45 minutes at 4°C, washed, fixed in PBS + 1% formalin, and batch analyzed with an LSRII or FACS Canto II (both from BD Biosciences), or Attune (Applied Biosystems, Carlsbad, CA) flow cytometer. Data were analyzed using FlowJo software version 7.6.4 (FlowJo; Ashland, OR). Flow cytometry antibodies used in this study were purchased from BD Biosciences (anti-CD3-AlexaFluor647 [Clone 17A2], anti-CD11b-AlexaFluor647 [M1/70], anti-CD11c-FITC [HL3], anti-CD49b-FITC [DX5], anti-Gr1-FITC [RB6-8C5], anti-Ly6C-FITC [AL-21], anti-Ly6G-PE [1A8], anti-CD8α-PE/Cy7 [53–6.7], anti-CCR6-AlexaFluor647 [104706], anti-CD62L-PE [MEL-14]) and from Biolegend (San Diego CA)(anti-PD-L1-APC [10F.9G2], anti-hPD-L1-APC [29E.2A3], anti-CD4-FITC [GK 1.5], anti-CXCR3-PE [CXCR3-173], anti-CCR4-PE/Cy7 [2G12], anti-CD44-APC [IM7]). Unless otherwise noted, all flow cytometry antibodies were murine specific.
Statistical Analysis
Tumor growth rates were analyzed using a mixed-effects regression approach assuming exponential growth over time. A random intercept and slope were included for each animal. All other comparisons were carried out by one-way ANOVA followed by Turkey HSD post-hoc test for between group comparisons (14). Differences were considered significant when p<0.05.
Results
Selinexor induces immune checkpoint expression by leukocytes and tumor cells in vitro
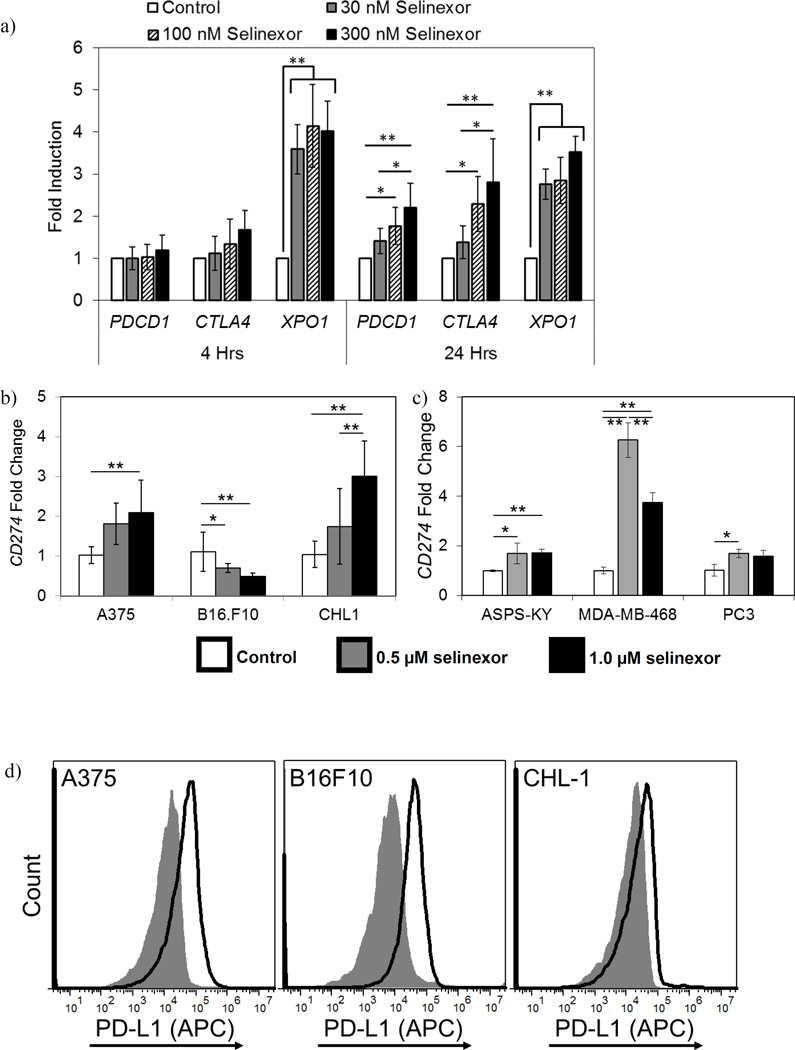
Prior studies have shown that selinexor has potent pro-apoptotic effects on tumor cells (6); however, the immunological effects of selinexor have not been carefully examined. To understand how XPO1 inhibition affects the expression of checkpoint molecules on immune cells, leukocytes from healthy donors were cultured in vitro with selinexor and changes in gene expression of the clinically relevant T cell checkpoint molecules PDCD1 (encoding PD-1), CD274 (encoding PD-L1), and CTLA4 (encoding CTLA4) were measured by qPCR. At 24 hours, selinexor significantly increased the expression of both PDCD1 and CTLA4 (2.2 fold and 2.8 fold, respectively, p<0.01) (Fig. 1a). As previously reported, selinexor induced a compensatory increase in XPO1 transcript (P<0.01) (Fig. 1a.) (7).
Figure 1. Expression of immune checkpoint molecules on leukocytes and tumor cells at baseline and in response to selinexor.
(a and b) Selinexor alters the expression of PDCD1, CTLA4, and XPO1 on immune and tumor cells. (a) Peripheral blood leukocytes were cultured in selinexor (30–300 nM) for up to 24 hours and mRNA transcript levels of PDCD1, CTLA4, and XPO1 assessed via qPCR, relative to the level in untreated cells. n=6 donors. (b) Melanoma cells or (c) human breast cancer (MDA-MB-468), prostate cancer (PC3), and sarcoma cells (ASPS-KY) were cultured in vehicle or up to 1 µM selinexor for 24 hours and CD274 mRNA transcript levels assessed via qPCR, relative to the untreated cells. n=9 (b) or 3 (c) independent biological replicates. Mean ± S.D. (d) Melanoma cells express PD-L1 on the cell surface. Surface expression of PD-L1 on human and murine melanoma cells was assessed by flow cytometry. Grey histogram = isotype control, open histogram = anti-PD-L1. Representative of 3 independent experiments. *, p<0.05; **, p<0.01. Mean ± S.D.
The impact of XPO1 inhibition on the expression of CD274 was also evaluated in human (A375, CHL-1) and murine (B16F10) melanoma cell lines. These three cell lines were treated with 1 µM selinexor for 24 hours and CD274 transcript levels quantified by qPCR. Selinexor treatment induced a significant increase in CD274 expression in human melanoma cell lines (A375, CHL-1) (p=0.0015 and p<0.0001, respectively Fig. 1b). However, 24 hours of selinexor treatment resulted in a modest though significant decrease in Cd274 mRNA expression in the murine B16F10 cell line (p=0.0006, Fig. 1b). Selinexor also significantly upregulated CD274 transcript in other human cancer cell lines, including breast (MDA-MB-468), sarcoma (ASPS-KY), and prostate (PC3) (MDA-MB-231 p<0.0001, ASPS-KY and PC3 p<0.05, Fig. 1c), indicating this effect was not exclusive to melanoma. At the protein level, and consistent with prior reports (15), expression of PD-L1 on the surface of the human and murine melanoma cell lines was evident at baseline (Fig. 1d.). While CD274 mRNA levels were altered following selinexor treatment, the high basal levels of surface protein expression of PD-L1 were not decreased in any of these cell lines by in vitro selinexor treatment at up to 1 µM (data not shown).
Addition of anti-PD-1, anti-PD-L1, or anti-CTLA4 to selinexor treatment increases anti-tumor activity
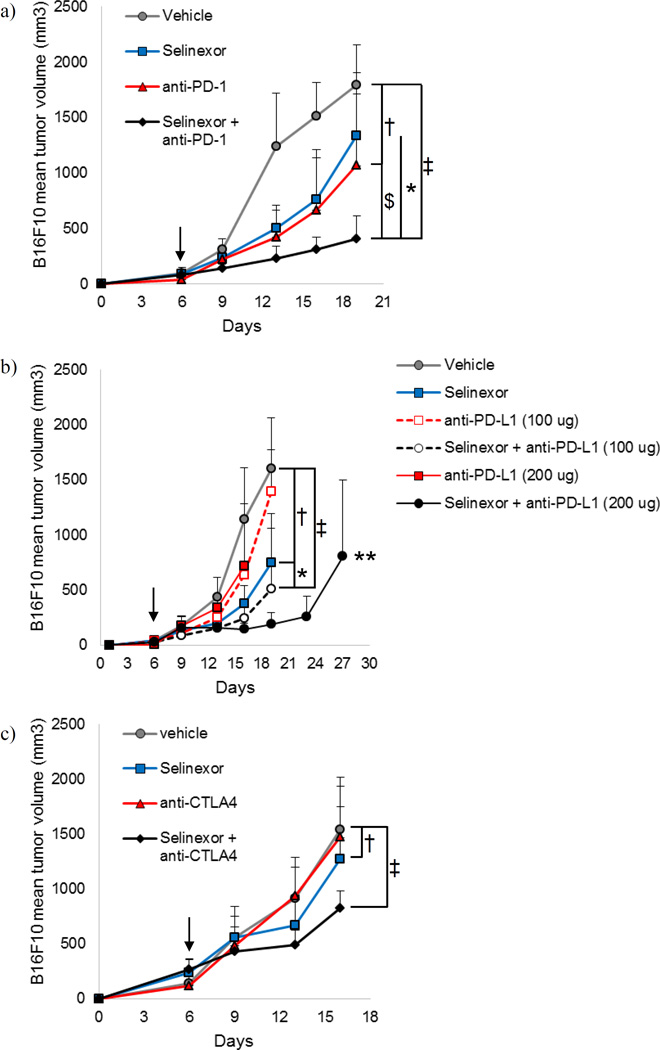
In an effort to improve upon the efficacy of single agent checkpoint blockade immunotherapy in melanoma, we evaluated combination therapy with selinexor and immune checkpoint blockade in immune competent mice bearing subcutaneous B16F10 melanoma tumors. Treatment commenced after tumors were established and had become palpable (6 days following injection). Monotherapy with anti-PD-1 had little effect on the rate of tumor growth (p=0.31; Fig. 2a), while monotherapy with selinexor exerted a modest but significantly reduced tumor growth rate (p=0.0166; Fig. 2a). The combination of selinexor and anti-PD-1 antibody administered concurrently led to a significant reduction in tumor growth versus either monotherapy (p=0.0337 vs. selinexor, p=0.0001 vs. anti-PD-1) and compared to vehicle/isotype control treated animals (p<0.0001) (Fig. 2a).
Figure 2. Selinexor combines with immune checkpoint blockade to slow B16F10 melanoma tumor growth.
C57BL/6 mice were injected subcutaneously with B16F10 cells on day 0 and were treated twice per week (Tuesdays and Fridays) with selinexor and immune checkpoint blockade (or appropriate vehicle/isotype control) beginning when tumors became palpable. (a) Selinexor + anti-PD-1. (b) Selinexor + anti-PD-L1. (c) Selinexor + anti-CTLA4. n=6 mice per group. Mean + S.D. *, p<0.05 between selinexor + checkpoint blockade antibody (anti-PD-1, anti-CTLA4, or anti-PD-L1 (100 µg)) and selinexor + isotype control; $, p<0.05 between selinexor + checkpoint blockade antibody (anti-PD-1, anti-CTLA4, or anti-PD-L1 (100 µg)) and vehicle + checkpoint blockade antibody; †, p<0.05 between selinexor + isotype control and vehicle/isotype control; ‡, p<0.05 between selinexor + checkpoint blockade antibody (anti-PD-1, anti-CTLA4, or anti-PD-L1 (100 µg)) and vehicle/isotype control; **, p<0.05 between selinexor + anti-PD-L1 (200 µg) and vehicle + isotype control. Arrows indicate when treatment was initiated.
We further explored whether selinexor enhanced the efficacy of antibodies targeting PD-L1 in the B16F10 model of melanoma. We observed that selinexor monotherapy significantly slowed the rate of tumor growth compared to isotype/vehicle control treated animals (p=0.0150; Fig. 2b). Treatment with single agent anti-PD-L1 at lower, suboptimal (100 µg) or higher (200 µg) concentrations showed minimal effect on the rate of tumor growth compared to vehicle/isotype control treated animals (p=0.53 and p=0.95, respectively). Similar to results with PD-1 blockade, combined therapy with selinexor and anti-PD-L1 significantly slowed tumor growth compared to vehicle/isotype control treated animals at both the 100 µg and 200 µg doses (p=0.0026 and p<0.0001, respectively; Fig. 2b). Due to the clear growth inhibitory effects when higher doses of anti-PD-L1 antibody were administered with selinexor, mice were continuously treated until their tumors displayed characteristics (excessive size or ulceration) requiring pre-specified removal from the study. All selinexor plus anti-PD-L1 (200 µg) treated mice remained on study for 34 days following tumor injection. Interestingly, one animal displayed a complete tumor regression. This animal also rejected subsequent re-challenge with B16F10 melanoma in the opposite flank, demonstrating durable immunological control of this tumor.
In vivo studies using CTLA4 blockade in combination with selinexor were also conducted. This experiment demonstrated that single agent selinexor significantly reduced the rate of tumor growth in mice with B16 melanoma (p=0.0229; Fig 2c). However, treatment with single-agent anti-CTLA4 had no impact on the rate of tumor growth as compared to control treated animals (p=0.75). Addition of CTLA4 blockade to selinexor reduced the tumor growth rate as compared to control mice (p=0.0065) and mice treated with single-agent anti-CTLA4 Ab (p=0.0126).
Combined treatment with selinexor and anti-PD-L1 alters systemic immune cell populations
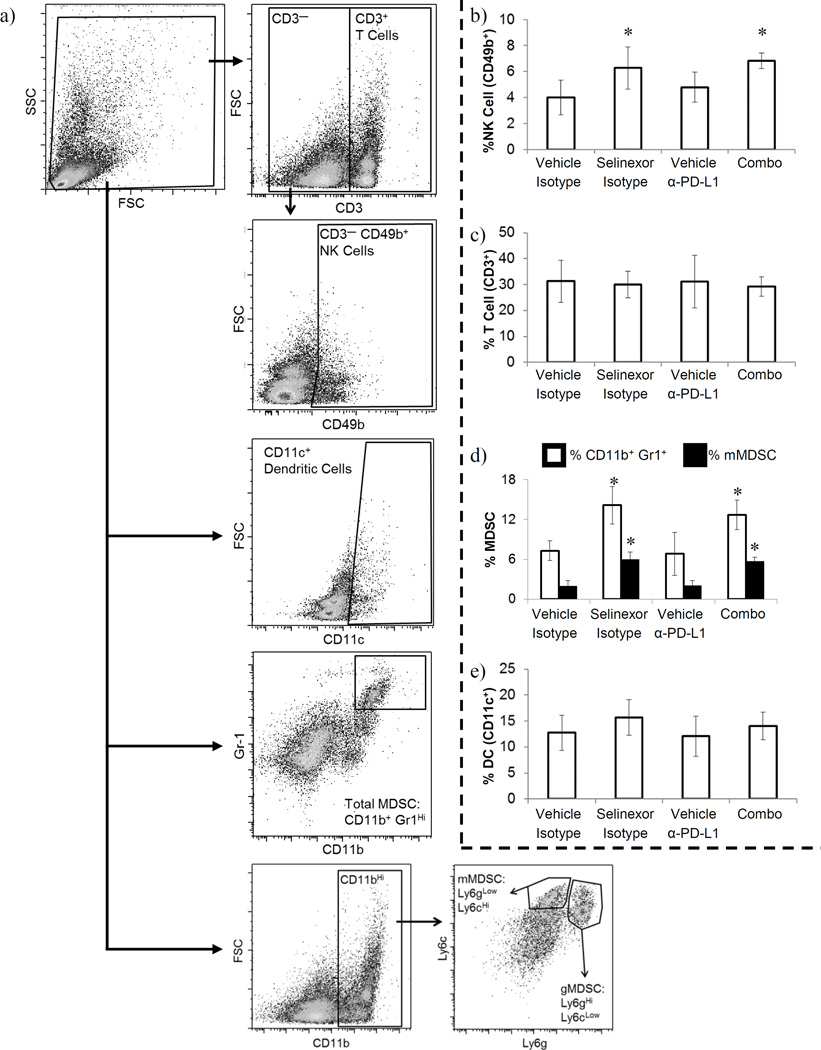
To determine potential mechanisms by which combined XPO1 and PD-L1 inhibition improved efficacy, we focused on the identification of systemic phenotypic changes in distinct immune cell subsets by conducting extensive immune phenotyping of splenocytes collected at the study endpoint. We used specimens from mice treated with 100 µg anti-PD-L1 ± selinexor and appropriate controls for extensive immune response analysis. Gating for populations of interest including NK cells, MDSCs, dendritic cells (DCs), and T cells are depicted in figure 3.a. We observed that combined treatment of B16F10-tumor bearing mice with selinexor and anti-PD-L1 antibody significantly increased the frequency of NK cells (CD3− CD49b+) in the spleen (p=0.0063; Fig. 3.b.). This activity appeared to be largely a function of selinexor treatment, as animals treated with selinexor monotherapy also showed elevated CD49b+ splenic NK cells (p<0.05; Fig. 3.b.), while treatment with anti-PD-L1 alone did not alter NK cell frequency (p=0.35). In contrast, the frequency of T cells (total CD3+ as well as CD4+ and CD8+) was unchanged in all treatment groups (Fig. 3c and data not shown, p=0.946 (CD3+), p=0.734 (CD4+), p=0.519 (CD8+)).
Figure 3. Selinexor plus anti-PD-L1 antibody alters immune cell frequencies in melanoma bearing animals.
The frequency of splenic immune cell subsets was determined by flow cytometry. (a) Gating strategy. Cells were initially gated for viable populations, on the basis of forward and side scatter. Subsequently, T cells were identified by gating on CD3+ cells and NK cells identified by gating on CD3− CD49b+ cells. DCs were identified by gating on CD11c+ cells. MDSCs were identified both by gating on CD11b+ Gr1+ double positive cells (white bars) or by first gating on CD11b+ cells and then gating on Ly6CHi Ly6GLow (monocytic MDSC, black bars) or Ly6GHi Ly6CLow (granulocytic MDSC, data not shown) cells. Representative FACS plots. (b) NK cell frequency, (c) T cell frequency, (d) MDSC frequency, (e) DC frequency. (b–e) n=5–6 mice per group; Mean ± S.D.; *, p<0.05 as compared to vehicle + isotype control.
Analysis of other splenic immune cell populations in B16F10-tumor bearing mice following treatment with selinexor and anti-PD-L1 antibody revealed an increased frequency of splenocytes with an MDSC phenotype (defined as CD11b+ Gr1Hi cells; p=0.0002; Fig. 3.d.), a cell type that is typically considered pro-tumorigenic. As with the increase in NK cells, changes in MDSC frequency were largely due to selinexor. Both treatment groups containing selinexor (selinexor + isotype, selinexor + anti-PD-L1) exhibited increased MDSC frequency compared to the other two groups (vehicle control + isotype control, vehicle control + anti-PD-L1) (p<0.05; Fig. 3.d.). In contrast, single agent anti-PD-L1 led to no appreciable change in MDSC frequency (p>0.55). When MDSCs were further subdivided into monocytic Ly6CHi Ly5GMed/Low and granulocytic Ly6GHi Ly6CMed/Low subsets, it was apparent that the increase in splenic MDSCs occurred almost entirely within the monocytic subset, whereas the granulocytic MDSC subset was largely unchanged by any of these treatments (monocytic: p<0.0001, granulocytic: p=0.87; Fig. 3.d. and data not shown, respectively). Finally, there was no difference in the frequency of DCs in any treatment group (Fig. 3.e., p=0.37).
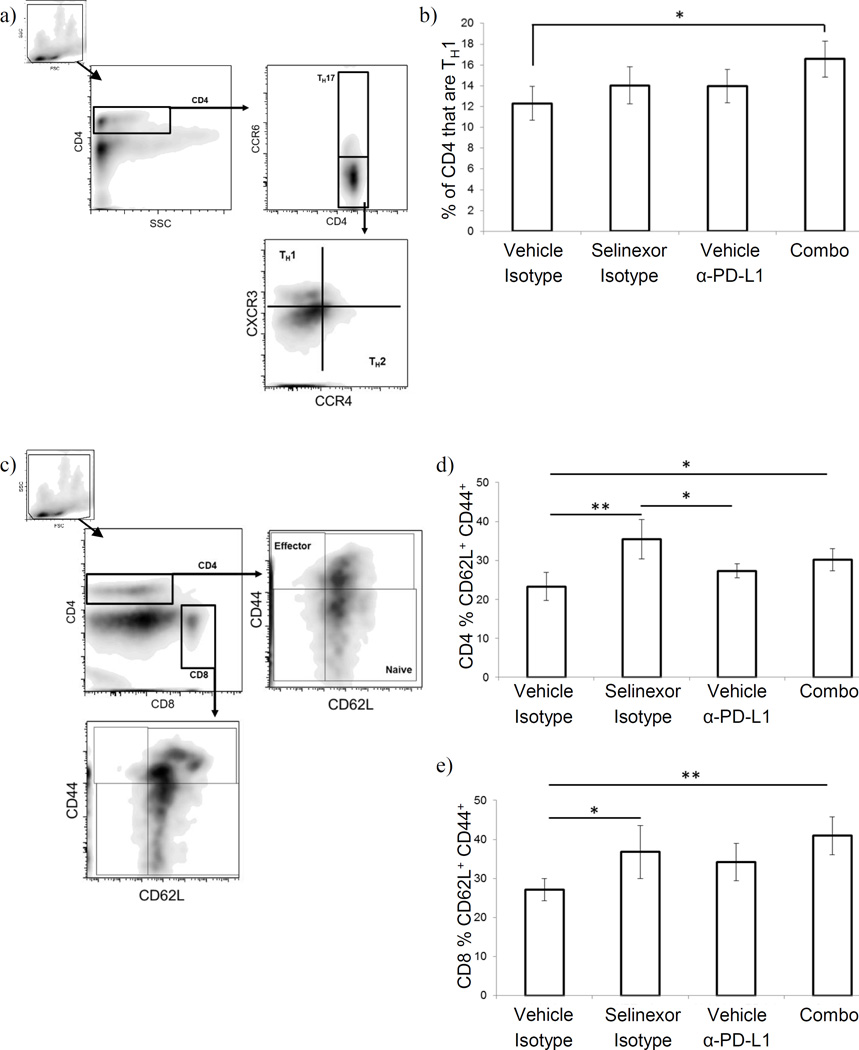
In addition to assessing the frequency of the above immune cell types, we also examined the effect that treatment with selinexor ± anti-PD-L1 had on helper T cell differentiation and T cell activation status among splenic T cells. The assessment of these characteristics was based on cellular phenotype via flow cytometry. In assessing helper T cell differentiation, we focused on characterizing the relative abundance of TH1 T cells which are typically considered beneficial in controlling tumor growth due to their production of IFNγ and support of CD8+ T cell cytotoxic activity (16). T-helper cells were classified phenotypically, on the basis of their pattern of chemokine receptor expression (TH1: CD4+ CCR4− CCR6− CXCR3+; TH2: CD4+ CCR4+ CCR6− CXCR3−; TH17: CD4+ CCR6+) (Fig. 4.a.), in agreement with published reports (17–19). As shown in Fig. 4.b., combination treatment with selinexor + anti-PD-L1 antibody was associated with an increased proportion of CD4+ T cells expressing the TH1 phenotype (p<0.05). Monotherapy with either agent resulted in a trend towards increased proportions of TH1 T cells, though this effect was not statistically significant. T cell activation status was likewise assessed by flow cytometry, using the classic CD62L and CD44 markers (Fig. 4.c.). Treatment of B16F10 tumor bearing mice with selinexor + anti-PD-L1 was associated with significantly increased proportions of both CD4+ and CD8+ CD62L+ CD44+ T cells (early activation and central memory-like phenotype) (p<0.05 vs. vehicle/isotype control treated animals, Fig. 4.d. (CD4+) and e. (CD8+)), with corresponding decreases in the proportion of naïve (CD62L+ CD44−) T cells (data not shown). This effect appeared to be largely a result of selinexor treatment, as mice treated with selinexor monotherapy also exhibited greater proportions of CD62L+CD44+ T cells (both CD4+ and CD8+).
Figure 4. Selinexor + anti-PD-L1 antibodies induce T cell activation and TH1 differentiation in melanoma bearing animals.
(a–b) Helper T cells were quantified on the basis of their pattern of chemokine receptor expression. (a) gating strategy: CD4+ viable cells (based on forward/side scatter and anti-CD4) were gated based on CCR6 expression: CCR6+ cells were defined as TH17, while CC6− cells were further gated based on expression of CXCR3 (TH1 cells) and CCR4 (TH2 cells). (b) Proportion of TH1 cells among splenic CD4+ T cells. (c–e) T cell activation status was assessed on the basis of cell surface phenotype. (c) gating strategy: singly positive CD4+ or CD8+ viable cells (based on forward/side scatter and anti-CD4 and anti-CD8) were selected and identified as a naïve, early activated/central memory, or effector phenotype based on staining for CD44 and CD62L. (d & e) Proportion of CD4+ T cells (d) or CD8+ T cells (e) with an early activated/central memory phenotype (CD62L+ CD44+). n=5–6 mice per group; Mean ± S.D.; *, p<0.05 between indicated groups.
Examination of alternative dosing schedules for selinexor and anti-PD-1
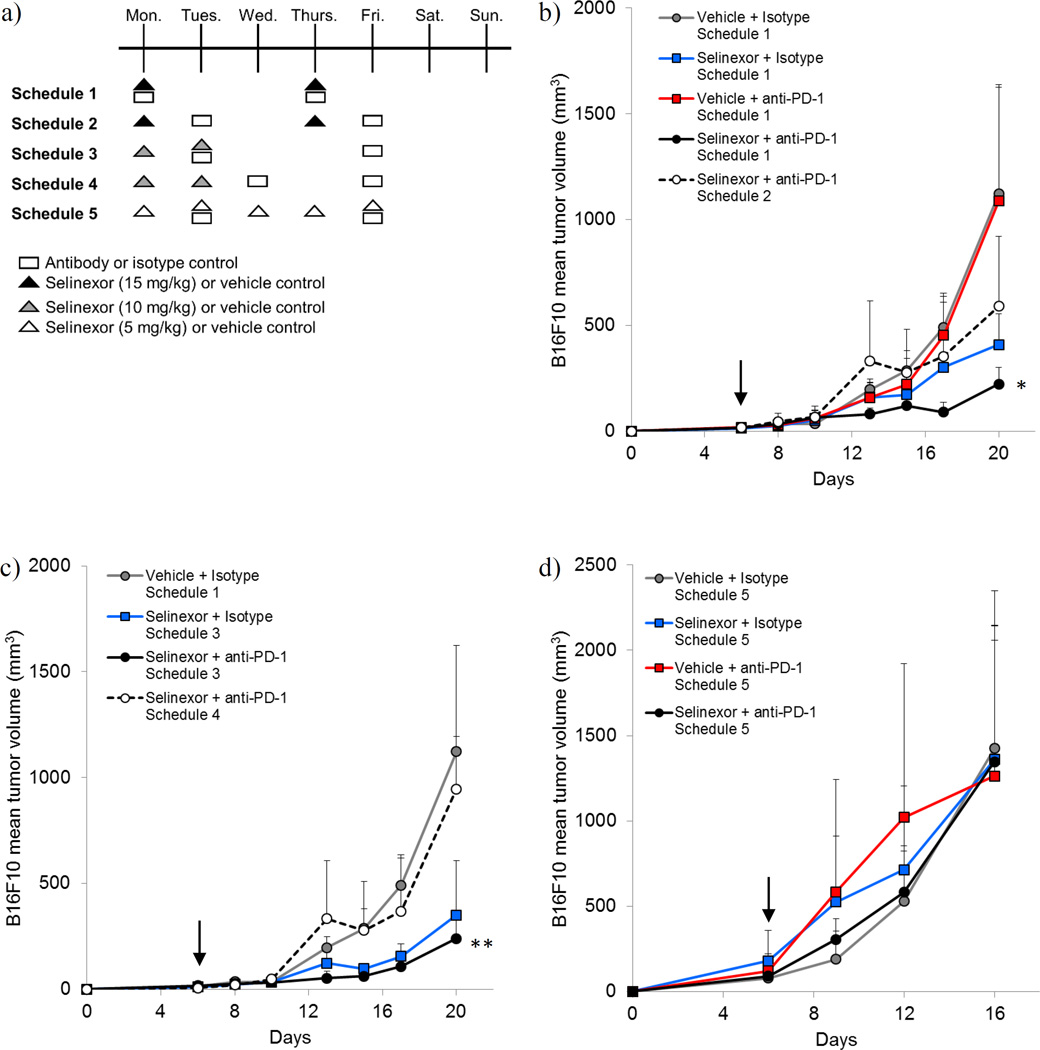
To gain further insight into the optimal dosing of this drug combination and guide future translation of these agents to clinical trials, we compared the initial dosing strategy (selinexor and anti-PD-1 administered concurrently on Mondays and Thursdays, schedule 1) with three alternatives wherein the agents were administered as depicted in Figure 5.a. These experiments determined that clinically recommended twice weekly regimens (7) including schedule 1 (15 mg/kg selinexor Monday/Thursday, anti-PD-1 Monday/Thursday) and schedule 3 (10 mg/kg selinexor Monday/Tuesday, anti-PD-1 Tuesday/Friday) were both more efficacious than schedules 2 and 4 (all p-values p<0.0330). However, there was little difference in tumor growth rate between schedule 1 and 3, indicating that these two treatment schedules were similarly effective. To study the immunologic effects of these alternative dosing schedules for selinexor and anti-PD-1, we examined systemic immune populations in animals treated on schedules 1–4 using the same flow cytometry strategy as described in Figs. 3a, 4a, and 4c (supplemental materials Fig. S1 and S2). Similar effects of selinexor and anti-PD-1 on immune cell phenotypes were observed in mice treated with 15 mg/kg selinexor according to schedules 1 and 2. Specifically, we observed a trend toward increased NK cells, MDSC, TH1 T cells, and CD62L+ CD44+ CD4+ and CD8+ T cells in animals treated with selinexor or selinexor + anti-PD-1. However, it is important to note that due to a higher degree of experimental variability in these studies, not all of these changes reached statistical significance (Fig. S1). Phenotypic changes in immune cell populations from mice in treatment groups using 10 mg/kg selinexor (schedules 3–4) were less prominent as compared to the 15 mg/kg treatment groups. These data suggest a dose-response relationship between selinexor and the changes in these immune populations (Fig. S2). Indeed, MDSCs were the only population observed to undergo significant changes in frequency among the various 10 mg/kg treatment groups (compared to control treated animals), though changes in the frequency of TH1-phenotype T cells approached significance (p=0.07; Fig. S2e). A fifth dosing schedule in which a lower dose of selinexor (5 mg/kg) was administered five times a week (Monday-Friday) while anti-PD-1 was administered on Tuesdays and Fridays (Schedule 5, Fig. 5.a.) had little effect on tumor growth when compared to vehicle/isotype control treated animals. While these data may indicate that a minimal threshold dose of selinexor is needed to demonstrate enhancement of anti-tumor immune responses when combined with anti-checkpoint blockade in vivo, a report in this issue by Tyler et al. suggests that daily selinexor dosing may inhibit T cell receptor function.
Figure 5. Evaluation of alternative dosing schedules for selinexor and anti-PD-1.
Animals were injected subcutaneously with B16F10 on day 0 and were treated twice per week with selinexor and immune checkpoint blockade (or appropriate vehicle/isotype control) beginning when tumors became palpable using the alternative treatment schedules depicted in (a). (b) Growth curves of B16F10 tumors administered Selinexor (15 mg/kg) + anti-PD-1 on schedules 1 and 2. (c) Growth curves of B16F10 tumors administered Selinexor (10 mg/kg) + anti-PD-1 on schedules 3 and 4. (d) Growth curves of B16F10 tumors administered Selinexor (5 mg/kg) + anti-PD-1 on schedule 5. n=5 mice per group. Arrow indicates initiation of treatment.
Note: The vehicle/isotype control group in 5.b. and 5.c. consisted of the same animals. This line is present on both graphs for ease of reading. *, p=0.0332 between Schedule 1 and Schedule 2; **, p<0.0001 between Schedule 3 and Schedule 4.
Discussion
Combination therapy with tumor-directed small molecules and immunotherapy is emerging as a prominent approach in early phase clinical trials for advanced malignancy (2). This strategy has the potential of eliciting rapid and durable responses by concurrently targeting the tumor and stimulating the host immune system. However, prioritizing amongst the many treatment combinations that could be tested clinically will be a challenge, underscoring the importance of careful pre-clinical studies and mechanistic data to support particular combinations, as well as informing their scheduling. This study demonstrated that selinexor, an oral Selective Inhibitor of Nuclear Export (SINE) drug targeting XPO1, showed efficacy in preclinical models when administered in combination with immune checkpoint blockade. Selinexor efficacy was consistently enhanced using multiple antibodies including those targeting PD-1, PD-L1, and CTLA4. From a mechanistic standpoint, selinexor has been well-characterized as a potent pro-apoptotic stimulus for melanoma cells (6), and our in vitro data demonstrate its ability to modulate PD-1 and CTLA4 expression on both tumor and immune cells. These results were complimented by several systemic changes that may favor immune-mediated recognition of melanoma following in vivo administration. Together, these observations suggest that therapeutic combinations of selinexor and immune checkpoint inhibitors elicit productive anti-tumor activity in vivo and should be considered beyond these pre-clinical studies.
Selinexor represents a unique targeted therapy that is being evaluated as a monotherapy in clinical trials for patients with advanced melanoma (NCT02120222) as well as in many other mid-late stage clinical trials in patients with relapsed and/or refractory hematological and solid tumor malignancies (clinicaltrials.gov). This drug has several features that bolster its potential as an emerging therapy. First, XPO1, the target of selinexor, is upregulated in melanoma metastases compared to primary tumors or dysplastic nevi, and melanoma cells treated with selinexor experience increased p53 accumulation and apoptosis (6). Second, selinexor treatment resulted in significant in vivo growth inhibition against human melanoma xenografts in athymic mice. Importantly, these effects are independent of BRAF or NRAS mutational status (6). In addition to these tumor-intrinsic properties, selinexor has other attributes that speak to its suitability for studies in combination with immune therapy. Indeed, data from the present study indicate that it may modulate the expression of immune checkpoint receptors and ligands that are already actionable therapeutic targets. Although the effect of selinexor was somewhat variable on tumor cell lines, consistent upregulation of transcripts for PDCD1 and CTLA4 in immune cells was observed. In other studies, XPO1 inhibition by selinexor resulted in modulation of multiple transcription factors that regulate immune function (e.g. STAT3—a transcription factor that mediates immunosuppressive cellular functions, and NFATc1—a key transcription factor involved in T cell activation and differentiation) (8,9,20,21). Finally, the potent induction of melanoma cell apoptosis in response to selinexor could promote the release of tumor antigens, amplifying the diversity or magnitude of anti-tumor immune responses. For these reasons, we conducted further investigation of selinexor with immunotherapy as a combination treatment for melanoma.
An important consideration of any new treatment combination is the potential for added toxicity. Throughout the course of this study there was no obvious impact of any treatment regimen on the overall body weight or general disposition of the mice. In broad terms, this is consistent with the manageable toxicity profile that has been reported in a recent selinexor clinical trial (7). Among the adverse events observed in selinexor-treated patients with solid tumors in the phase I clinical trial were fatigue, nausea, anorexia, thrombocytopenia, and vomiting (7). Selinexor is currently being evaluated in combination with other agents (targeted therapies and chemo-/radio-therapy). As these clinical trials proceed it will be important to carefully assess both the added efficacy and possible adverse events resulting from these combinations to better inform potential trials combining selinexor with immune checkpoint blockade.
Animals bearing B16F10 tumors that were treated with selinexor and anti-PD-L1, exhibited evidence of increased immune activation and anti-tumor immune polarization. Specifically, the presence of selinexor was associated with increased frequencies of NK cells and this was not further enhanced with the addition of anti-PD-L1. While selinexor monotherapy significantly reduced tumor growth compared to the vehicle/isotype control, combination therapy with anti-PD-L1 + selinexor exhibited enhanced anti-tumor activity, significantly slowing the rate of tumor growth compared to selinexor monotherapy. This suggests that while the increased frequency of NK cells associated with selinexor treatment may play a role in slowing tumor growth, it is not by itself optimal. A potential approach to enhance efficacy of this therapy regimen would be to combine it with agents that (further) enhance NK cell frequency and/or target-killing such as rIL-2 or rIL-15 (22,23), representing an intriguing future direction for this research. When combined with anti-PD-L1 antibody, a higher proportion of CD4+ CXCR3+ CCR4− (TH1 phenotype) and early activated/central memory T cells (CD4+ and CD8+) was observed. As discussed at some length in Groom et al., CXCR3+ CD4+ T cells are TH1 T cells and as such are prone to produce IFNγ.(18). Combined with the increased frequency of CD4+ and CD8+ early activated/central memory T cells in these mice, these observations suggest that the treatment combination may work in part by generating T cells with enhanced functional properties, without necessarily altering their numbers systemically. The presence of selinexor also, and somewhat paradoxically, increased the frequency of MDSCs while simultaneously acting to slow tumor growth, though it is not yet clear whether these cells, defined phenotypically, were functionally immunosuppressive following selinexor treatment. Indeed, the selinexor plus anti-PD-L1 treatment regimen elicited complete rejection of one animal’s established tumor. This animal was subsequently re-challenged with the same tumor type (B16F10) in the opposite flank in the absence of any additional therapy, and the tumor was likewise rejected. This demonstrates the development of effective and durable anti-tumor immunity as a result of this therapy combination; though it must be noted that the other animals treated with this combination had to eventually be euthanized due to tumor growth. These data suggest that late-developing selinexor resistance or immune escape—either as a result of antigen loss or alternative immunosuppressive mechanisms—remain barriers to complete tumor eradication.
A number of caveats regarding this study must be noted. Particularly, while anti-PD-1 proved largely ineffective as a single agent therapy in murine melanoma, it has induced robust responses in patients with metastatic melanoma (24). While an important difference to note, this raises the possibility that responses in (human) patients to this treatment combination might be more robust than those observed here. Further, splenocytes were collected at study endpoint, 2 weeks following the initiation of treatment. While this allowed an assessment of immune populations that could be directly correlated to the treatment outcome/final tumor growth, it may not capture early events in the anti-tumor immune response (e.g. early T cell activation, peak infiltration of tumors, production of relevant cytokines). Likewise, tumors were collected at the study endpoint for immunohistochemistry, when tumors were growing progressively in all treatment groups. At this time point no differences in tumor architecture or immune infiltrate were evident among groups. As with the analysis of systemic immune populations, this timepoint was too late to capture the likely critical early anti-tumor immune events elicited by these treatment combinations.
Throughout the earlier portions of this study, selinexor and antibodies blocking immune checkpoint molecules had both been given on Mondays and Thursdays. To determine if minor differences in the timing of selinexor/antibody administration could improve treatment efficacy or limit toxicity, we assessed alternative dosing strategies in the final phase of this study. We observed the greatest degree of tumor growth control under twice weekly treatment regimens schedule 1 (selinexor at 15 mg/kg and anti-PD-1 on Mondays and Thursdays) and schedule 3 (selinexor at 10 mg/kg + anti-PD-1 on Tuesdays and Fridays). Ultimately, the similar efficacy of these two different treatment schedules indicates that the benefits of combining selinexor and anti-PD-1 are robust and allow for some degree of flexibility in both the scheduling and dose employed in clinical practice. Of particular note, a report in this issue by Tyler et al. illustrates that selinexor transiently impairs T cell receptor signaling, thus implying that scheduling of selinexor dosing may be important. Indeed, we found that daily dosing of selinexor was ineffective, consistent with a transient block in T cell function.
This study demonstrates that selinexor and immune checkpoint blockade combine to elicit a considerable degree of immune control in an aggressive model of melanoma. In a murine setting, this treatment was well tolerated, without evidence of excessive toxicity. In all, this data suggests that this therapeutic approach should remain under consideration for testing in subsequent melanoma clinical trials.
Supplementary Material
Acknowledgments
We acknowledge the OSU CCC Analytical Cytometry, Biostatistics Shared Resources, Target Validation Shared Resource, and Raleigh Kladney in the OSU Solid Tumor Biology Program for assistance with these studies.
Financial Information
Funding for this work was provided by Karyopharm Therapeutics, Inc., The Siegle Fund for Melanoma Research, The Gill Fund for Melanoma Research, and via National Institutes of Health Cancer Center Support Grant P30CA16058 (P.I.: M.A. Caligiuri). M.R. Farren received salary support via NIH training grant T32CA090223-13 (P.I.: W.E. Carson) and the Pelotonia Fellowship Program. Any opinions, findings and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the Pelotonia Fellowship Program or any other funding source. Y. Landesman, S. Elloul, B. Klebanov, T. Kashyap, and M. Crochiere are current or former employees of Karyopharm Therapeutics, Inc., and receive/received compensation and hold equity in the company.
References
- 1.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wargo JA, Cooper ZA, Flaherty KT. Universes collide: combining immunotherapy with targeted therapy for cancer. Cancer Discov. 2014;4:1377–1386. doi: 10.1158/2159-8290.CD-14-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haluska FG, Tsao H, Wu H, Haluska FS, Lazar A, Goel V. Genetic alterations in signaling pathways in melanoma. Clin Cancer Res. 2006;12:2301s–2307s. doi: 10.1158/1078-0432.CCR-05-2518. [DOI] [PubMed] [Google Scholar]
- 5.Gravina GL, Senapedis W, McCauley D, Baloglu E, Shacham S, Festuccia C. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol. 2014;7:85. doi: 10.1186/s13045-014-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Bill MA, Young GS, La Perle K, Landesman Y, Shacham S, et al. Novel small molecule XPO1/CRM1 inhibitors induce nuclear accumulation of TP53, phosphorylated MAPK and apoptosis in human melanoma cells. PLoS One. 2014;9:e102983. doi: 10.1371/journal.pone.0102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdul Razak AR, Mau-Soerensen M, Gabrail NY, Gerecitano JF, Shields AF, Unger TJ, et al. First-in-Class, First-in-Human Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Patients With Advanced Solid Tumors. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.65.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y, Holloway MP, Nguyen K, McCauley D, Landesman Y, Kauffman MG, et al. XPO1 (CRM1) inhibition represses STAT3 activation to drive a survivin-dependent oncogenic switch in triple-negative breast cancer. Mol Cancer Ther. 2014;13:675–686. doi: 10.1158/1535-7163.MCT-13-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28:155–165. doi: 10.1038/leu.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin JW, Lu P, Majumder P, Ahmed R, Boss JM. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J Immunol. 2014;192:4876–4886. doi: 10.4049/jimmunol.1302750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoshino M, Ogose A, Kawashima H, Izumi T, Hotta T, Hatano H, et al. Molecular analyses of cell origin and detection of circulating tumor cells in the peripheral blood in alveolar soft part sarcoma. Cancer Genet Cytogenet. 2009;190:75–80. doi: 10.1016/j.cancergencyto.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Farren MR, Mace TA, Geyer S, Mikhail S, Wu C, Ciombor K, et al. Systemic Immune Activity Predicts Overall Survival in Treatment-Naïve Patients with Metastatic Pancreatic Cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowry R. VassarStats: Statistical Computation Web Site. 1998 < vassarstats.net>. [Google Scholar]
- 15.Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(Suppl):S52–S61. doi: 10.1007/pl00014051. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto Y, Bian Y, Gao P, Yashiro-Ohtani Y, Zhou XY, Ono S, et al. Induction of surface CCR4 and its functionality in mouse Th2 cells is regulated differently during Th2 development. J Leukoc Biol. 2005;78:753–761. doi: 10.1189/jlb.0305139. [DOI] [PubMed] [Google Scholar]
- 18.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317:620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 22.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basse PH, Whiteside TL, Herberman RB. Cancer immunotherapy with interleukin-2-activated natural killer cells. Mol Biotechnol. 2002;21:161–170. doi: 10.1385/MB:21:2:161. [DOI] [PubMed] [Google Scholar]
- 24.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.