Abstract
Nivolumab is a humanized immunoglobulin gamma-4 kappa anti-programmed cell death 1 monoclonal antibody that is currently approved in the treatment of several solid tumors and recently gained accelerated approval in classical Hodgkin lymphoma (cHL) that has relapsed or progressed following autologous hematopoietic stem-cell transplantation and post-transplantation brentuximab vedotin. The purpose of this article is to review the immunophysiologic basis, clinical efficacy, and toxicity of nivolumab in the treatment of cHL. In addition, we will review ongoing clinical trials and potential future directions of checkpoint inhibition in the treatment of cHL.
Keywords: checkpoint inhibitor, Hodgkin lymphoma, immunotherapy, nivolumab, programmed cell death 1, programmed cell death-ligand 1, programmed cell death-ligand 2
Introduction
Classical Hodgkin lymphoma (cHL) has become a highly curable malignancy. Even advanced disease has a long-term cure rate above 70% with risk-adapted combination chemotherapy with or without radiation [Gordon et al. 2013]. In patients with relapsed or refractory disease, sustained remissions can be achieved with high-dose chemotherapy and autologous stem-cell transplantation (ASCT) in nearly 50% of cases [Majhail et al. 2006; Sirohi et al. 2008]. The addition of the anti-CD30 antibody drug conjugate, brentuximab vedotin, to pretransplantation salvage and post-transplantation consolidation and maintenance regimens also appears to improve outcomes and is being investigated in combination with chemotherapy in the frontline setting [Moskowitz et al. 2015; Younes et al. 2013; Chen et al. 2015; Sweetenham et al. 2015]. However, a significant minority of patients relapse following ASCT with poor outcomes and a median overall survival (OS) of 1–2 years [Kewalramani et al. 2003]. Various third- or fourth-line regimens including single agent and combination chemotherapies, immunomodulatory agents, histone deacetylase inhibitors, mammalian target of rapamycin inhibitors, and radiation have been used with response rates between 20% and 40%, and typically of short duration [Younes et al. 2012; Fehniger et al. 2011; Johnston et al. 2010; Josting et al. 2005; Gopal et al. 2010].
Salvage allogeneic transplantation is a remaining curative option, but limitations to this approach include donor availability and significant treatment-related morbidity and mortality with 3-year OS rates ranging from 45% to 66% [Thomson et al. 2008]. The 50% of patients who relapse after ASCT present a major treatment challenge and there is an unmet need for new and less toxic salvage therapies [Majhail et al. 2006; Sirohi et al. 2008]. There are recent phase II trial data that indicate that single agent brentuximab vedotin may also provide significant chance of long-term remission after failed ASCT with 5-year OS rates of 41%. Notably, median OS and progression-free survival (PFS) were unreached in the subset of patients who achieved complete response (CR) [Chen et al. 2016].
Immunobiology of cHL
Over the past several years, a greater understanding of human immune system regulatory pathways and the immunophysiologic interactions that allow for tumor immune evasion have led to an explosion of new immunotherapeutic options in the treatment of several solid tumors.
Immune surveillance and antitumor immune activity can play a significant role in the control of hematologic malignancies, as demonstrated by the efficacy of allogeneic transplantation, immunomodulatory drugs (IMiDs), and adoptive cellular therapies [Tai et al. 2005; Maus et al. 2014]. Cancer suppression of T-cell function, through the augmentation of T-cell receptor co-inhibitory and co-stimulatory checkpoint molecules including programmed cell death 1 (PD-1) and its ligands, programmed cell death-ligand 1 (PD-L1) and programmed cell death 2 (PD-L2), is now known to play a role in immune evasion by several hematologic malignancies [Bryan and Gordon, 2015].
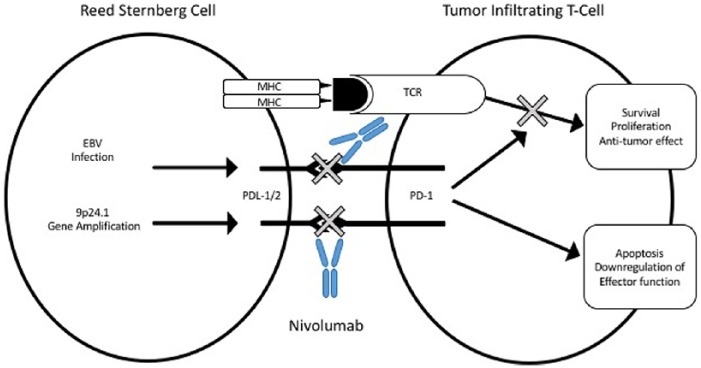
The histology of cHL demonstrates rare large CD30+ tumor-infiltrating Reed–Sternberg (HRS) cells surrounded by a dense inflammatory/immune micro-environment with mixed cellularity [Kuppers, 2009]. Despite this extensive polymorphous inflammatory infiltrate there is poor antitumor immune response to neoplastic HRS cells, which demonstrate a high expression of the immunoregulatory glycan-binding protein and galectin-1 resulting in a type 2 T-helper cell and T-regulatory cell skewed tumor micro-environment [Green et al. 2010]. Furthermore, immunoregulatory gene alterations are an important defining feature of cHL. Amplification at the 9p24.1 locus, which contains genes for PD-L1 and PD-L2, is commonly found in cHL and results in high expression of PD-L1 and PD-L2 on the tumor cell surface. In addition, latent Epstein–Barr virus infection is present in around 40% of cHL cases and is thought to contribute to high expression of PD-L1 and PD-L2 [Paydas et al. 2015]. Tumor immune evasion through PD-1 augmentation appears to play a major role in the oncogenesis of cHL and has consequently led to great interest in immune checkpoint inhibition using anti-PD-1 and anti-PD-L1 immunotherapy (Figure 1).
Figure 1.
Gene amplification and/or Epstein–Barr virus (EBV) infection induces overexpression of programmed cell death-ligand 1 (PD-L1) and programmed cell death-ligand 2 (PD-L2) on malignant Reed–Stemberg cells. This leads to inhibition of T-cell receptor (TCR) signaling-mediated survival, proliferation, and antitumor effect. Nivolumab disrupts programmed cell death 1 (PD-1) interaction with PD-L1 and PD-L2 causing restoration of effector T-cell function and antitumor activity. MHC, major histocompatibility complex.
Nivolumab in Hodgkin lymphoma
Nivolumab is a humanized immunoglobulin gamma-4 kappa anti-PD-1 monoclonal antibody that is currently approved for use in advanced melanoma, non-small cell lung cancer, and advanced renal cell carcinoma [Borghaei et al. 2015; Brahmer et al. 2015; Larkin et al. 2015; Motzer et al. 2015].
Checkmate 039 is a multicohort phase I dose escalation study that included a cohort of 23 heavily pretreated patients (87% had received three or more prior systemic therapies) with relapsed/refractory cHL treated with nivolumab at 3 mg/kg every 2 weeks [Ansell et al. 2015]. Nivolumab treatment resulted in an impressive overall response rate (ORR) of 87% (n = 20) with 17% (n = 4) achieving a CR at a median follow up of 86 weeks. The PFS at 24 weeks was 86%. Median duration of response was not reached with ongoing responses in 48% of patients (n = 11) at the time of analysis. Drug-related adverse events were seen in 78% of all patients, most of which (56%) were grade 1–2, the most common being rash (22%) and thrombocytopenia (17%). Grade 3 or higher drug-related adverse events were seen in 22% of patients and included lymphopenia, pancreatitis, elevated lipase, stomatitis, and myelodysplastic syndrome.
Checkmate 205 is a multicohort phase II study of 80 patients with cHL who relapsed after both ASCT and brentuximab vedotin and were treated with nivolumab 3 mg/kg every 2 weeks. The ORR was 66%, with an ORR of 72% in brentuximab refractory disease [Younes et al. 2016]. Median duration of response was 7.8 months. Treatment-related adverse events were seen in 89% of patients with 64% experiencing grade 1–2 events, most commonly fatigue, rash, and arthralgia; 25% of patients experienced at least one grade 3–4 event, most commonly increased lipase and neutropenia. Based on the results of checkmate 039 and 205, nivolumab was given accelerated approval in May 2016 by the US Food and Drug Administration (FDA) for the treatment of cHL that has relapsed or progressed after ASCT and post-transplantation brentuximab vedotin.
Anti-PD-1 with nivolumab before and after allogeneic transplantation
Allogeneic stem-cell transplantation remains the only known potentially curative therapy in patients with relapsed or refractory disease after ASCT and brentuximab vedotin. The safety and efficacy of nivolumab, both as a bridge to transplantation and as salvage therapy after allogeneic transplantation, are therefore of particular interest.
In checkmate 205 and 039, the combined safety analysis of the 17 patients who received an allogeneic transplant after nivolumab showed that 35% (6/17) died from complications of the allogeneic transplant of which five patients died from severe or refractory graft versus host disease (GvHD). Immune-related adverse events in allogeneic recipients included 29% (5/17) grade 3 or higher GvHD, 12% (2/17) hyper-acute GvHD, 12% (2/17) grade 3 encephalitis, and one case of hepatic veno-occlusive disease [Bristol-Myers Squibb, 2016; Younes et al. 2016]. These data prompted a warning from the FDA at the time of nivolumab approval to closely follow patients after allogeneic transplantation for toxicities including hyper-acute GvHD, steroid-requiring febrile syndrome, hepatic veno-occlusive disease, and immune-mediated adverse reactions [Food and Drug Administration, 2016]. Whether these immune events would have occurred in the absence of PD-1 inhibition is not clear as these are known potential complications of allogeneic transplantation.
For now, it seems reasonable for responders to nivolumab after failure of ASCT and brentuximab to be considered for potentially curative allogeneic transplantation as the durability of response to nivolumab is uncertain. More data are needed to quantify benefits and risks of immune toxicity with checkpoint inhibition in relation to transplantation.
There are recent data to suggest that nivolumab salvage therapy after allogeneic transplant failure may be effective and reasonably safe. In a 2015 American Society of Hematology abstract, retrospective data on 12 patients treated in France with nivolumab monotherapy after allogeneic stem-cell transplantation were presented. Seven out of eight evaluable patients achieved a response with four partial responses and three CRs by Cheson 2007 criteria. Toxicities included grade 3 acute skin GvHD in one patient after one injection of nivolumab and grade IV acute skin GvHD after two injections, which resolved with steroids and extracorporeal photopheresis, respectively. Both of these patients had a history of grade 2 GvHD. Grade 4 neutropenia occurred in one patient and grade 3 thrombocytopenia occurred in one patient. At a median follow up 60 days after initiation of nivolumab nine patients remained on drug, two discontinued due to GvHD, and one discontinued due to progressive disease [Herbaux et al. 2015]. It is interesting that checkpoint inhibition with ipilimumab induced remission in several patients with relapsed acute myeloid leukemia after allogeneic transplantation [Davids et al. 2016]. Although prospective data are needed, early results suggest that patients with persistent disease after allogeneic transplantation may be candidates for checkpoint inhibition including nivolumab in the future.
Although not discussed in detail here, it should be noted that early phase data on the treatment of refractory/relapsed cHL after ASCT and brentuximab with the PD-1 inhibitor pembrolizumab has also demonstrated significant clinical activity with an acceptable safety profile. In the phase Ib Keynote 013 study, an ORR was seen in 65% of patients (CR = 16%) with 70% of responses lasting longer than 24 weeks. The toxicity profile was favorable and similar to that seen with PD-1 inhibition in other studies [Armand et al. 2016]. These data have led to breakthrough designation and priority review by the FDA for pembrolizumab in relapsed/refractory cHL.
Conclusion and future directions
There is ample immunophysiologic evidence to suggest the oncogenesis of cHL may be particularly sensitive to immunotherapy with checkpoint blockade by disrupting oncogenic immune evasion. Recent early phase studies have shown impressive response rates to nivolumab monotherapy in patients with persistent disease after ASCT and brentuximab vedotin forming the basis for recent accelerated FDA approval. Toxicity in checkmate 205 and 039 was acceptable and included grade 3 or higher treatment-related events in 22% and 25% of patients, respectively. Notable events included lymphopenia, neutropenia, pancreatitis, elevated lipase, stomatitis, and myelodysplastic syndrome [Younes et al. 2016; Ansell et al. 2015].
It is unclear if nivolumab, when used as ‘bridge-to-allogeneic’ transplantation or post-transplantion salvage, increases transplant immune-related adverse events. Potential toxicity and transplant compromise should be taken into consideration prior to choosing nivolumab, however early data suggest that side-effect profiles may be reasonable in the setting of multiply relapsed/refractory disease. Several trials are actively recruiting and investigating the combination of nivolumab with brentuximab vedotin, chemotherapy, and additional checkpoint blockade with the CTLA-4 inhibitor ipilimumab in both the relapsed/refractory setting as well as frontline setting in patients unable to tolerate standard first-line therapy (Table 1). The role of nivolumab with respect to other immunotherapies including the IMiDs, allogeneic stem-cell transplantation, and adoptive cellular therapy will continue to be clarified with more trial data and use of these therapies should take place in the setting of a clinical trial.
Table 1.
Clinical trials with nivolumab in classical Hodgkin lymphoma.
| ClinicalTrials.gov identifier | Phase | Population | Regimen | Status |
|---|---|---|---|---|
| NCT02758717 | II | Patients with untreated Hodgkin lymphoma over the age of 60 years or unable to receive standard doxorubicin + bleomycin + vinblastine + dacarbazine (ABVD) | Nivolumab and brentuximab vedotin | Recruiting |
| NCT01896999 | I | Relapsed or refractory Hodgkin lymphoma | Ipilimumab + nivolumab/nivolumab + brentuximab vedotin/ipilimumab + nivolumab + brentuximab vedotin | Recruiting |
| NCT02181738 | II | Previously treated or newly diagnosed classical Hodgkin lymphoma | Nivolumab/nivolumab + doxorubicin + vinblastine + dacarbazine | Recruiting |
| NCT02572167 | I, II | Relapsed or refractory Hodgkin lymphoma | Brentuximab vedotin + nivolumab | Recruiting |
| NCT01822509 | I, Ib | Relapsed hematologic malignancies (including classical Hodgkin lymphoma) after donor stem-cell transplantation | Ipilimumab/nivolumab | Recruiting |
| NCT02304458 | I, II | Children, adolescents, and young adults with recurrent or refractory solid tumors | Nivolumab +/− ipilimumab | Recruiting |
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: AIC has served on advisory boards for Genentech and Seattle Genetics and has received research funding from Genentech and Seattle Genetics. ECS has served on advisory boards for Amgen, Takeda, Novartis, and Incyte and has received research funding from Amgen and Takeda. CYO and NDG have no conflicts of interest to disclose.
Contributor Information
Nathan D. Gay, Knight Cancer Institute, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA.
Craig Y. Okada, Knight Cancer Institute, Oregon Health and Science University, Portland, OR, USA
Andy I. Chen, Knight Cancer Institute, Oregon Health and Science University, Portland, OR, USA
Emma C. Scott, Knight Cancer Institute, Oregon Health and Science University, Portland, OR, USA
References
- Ansell S., Lesokhin A., Borrello I., Halwani A., Scott E., Gutierrez M., et al. (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand P., Shipp M., Ribrag V., Michot J., Zinzani P., Kuruvilla J., et al. (2016) Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol pii: JCO673467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D., Steins M., Ready N., et al. (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K., Baas P., Crino L., Eberhardt W., Poddubskaya E., et al. (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol-Myers Squibb B. (2016) Opdivo® (Nivolumab) Granted First Approval of a PD-1 Inhibitor in Hematology for the Treatment of Classical Hodgkin Lymphoma Patients Who Have Relapsed or Progressed after Auto-HSCT and Post-transplantation Brentuximab Vedotin by the FDA1. New York: Bristol-Myers Squibb; [http://investor.bms.com/investors/news-and-events/press-releases/press-release-details/2016/Opdivo-nivolumab-Granted-First-Approval-of-a-PD-1-Inhibitor-in-Hematology-for-the-Treatment-of-Classical-Hodgkin-Lymphoma-Patients-Who-Have-Relapsed-or-Progressed-After-Auto-HSCT-and-Post-transplantation-Brentuximab-Vedotin-by-the-FDA1/default.aspx] [Google Scholar]
- Bryan L., Gordon L. (2015) Blocking tumor escape in hematologic malignancies: the anti-PD-1 strategy. Blood Rev 29: 25–32. [DOI] [PubMed] [Google Scholar]
- Chen R., Gopal A., Smith S., Ansell S., Rosenblatt J., Savage K., et al. (2016) Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 128: 1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Palmer J., Martin P., Tsai N., Kim Y., Chen B., et al. (2015) Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 21: 2136–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M., Kim H., Bachireddy P., Costello C., Liguori R., Savell A., et al. (2016) Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med 375: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T., Larson S., Trinkaus K., Siegel M., Cashen A., Blum K., et al. (2011) A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood 118: 5119–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. (2016) Nivolumab (Opdivo) for Hodgkin Lymphoma. Silver Spring, MD: Food and Drug Administration; [http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm501412.htm] [Google Scholar]
- Gopal A., Press O., Shustov A., Petersdorf S., Gooley T., Daniels J., et al. (2010) Efficacy and safety of gemcitabine, carboplatin, dexamethasone, and rituximab in patients with relapsed/refractory lymphoma: a prospective multi-center phase II study by the Puget Sound Oncology Consortium. Leuk Lymphoma 51: 1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L., Hong F., Fisher R., Bartlett N., Connors J., Gascoyne R., et al. (2013) Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol 31: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Monti S., Rodig S., Juszczynski P., Currie T., O’Donnell E., et al. (2010) Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116: 3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbaux C., Gauthier J., Brice P., Fornecker L., Bouabdallah K., Manson G., et al. (2015) Nivolumab is effective and reasonably safe in relapsed or refractory Hodgkin’s lymphoma after allogeneic hematopoietic cell transplantation: a study from the Lysa and SFGM-TC. Blood 126: 3979. [Google Scholar]
- Johnston P., Inwards D., Colgan J., Laplant B., Kabat B., Habermann T., et al. (2010) A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol 85: 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josting A., Nogova L., Franklin J., Glossmann J., Eich H., Sieber M., et al. (2005) Salvage radiotherapy in patients with relapsed and refractory Hodgkin’s lymphoma: a retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol 23: 1522–1529. [DOI] [PubMed] [Google Scholar]
- Kewalramani T., Nimer S., Zelenetz A., Malhotra S., Qin J., Yahalom J., et al. (2003) Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin’s disease or aggressive non-Hodgkin’s lymphoma. Bone Marrow Transplant 32: 673–679. [DOI] [PubMed] [Google Scholar]
- Kuppers R. (2009) The biology of Hodgkin’s lymphoma. Nat Rev Cancer 9: 15–27. [DOI] [PubMed] [Google Scholar]
- Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J., Cowey C., Lao C, et al. (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majhail N., Weisdorf D., Defor T., Miller J., Mcglave P., Slungaard A., et al. (2006) Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant 12: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Maus M., Grupp S., Porter D., June C. (2014) Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 123: 2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz C., Nademanee A., Masszi T., Agura E., Holowiecki J., Abidi M., et al. (2015) Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 385: 1853–1862. [DOI] [PubMed] [Google Scholar]
- Motzer R., Escudier B., McDermott D., George S., Hammers H., Srinivas S., et al. (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paydas S., Bagir E., Seydaoglu G., Ercolak V., Ergin M. (2015) Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol 94: 1545–1552. [DOI] [PubMed] [Google Scholar]
- Sirohi B., Cunningham D., Powles R., Murphy F., Arkenau T., Norman A., et al. (2008) Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma. Ann Oncol 19: 1312–1319. [DOI] [PubMed] [Google Scholar]
- Sweetenham J., Walewski J., Nademanee A., Masszi T., Agura E., Holowiecki J., et al. (2015) Updated efficacy and safety data from the AETHERA trial of consolidation with brentuximab vedotin after autologous stem cell transplant (ASCT) in Hodgkin lymphoma patients at high risk of relapse. Blood 126: 3172. [PubMed] [Google Scholar]
- Tai Y., Li X., Catley L., Coffey R., Breitkreutz I., Bae J., et al. (2005) Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer Res 65: 11712–11720. [DOI] [PubMed] [Google Scholar]
- Thomson K., Peggs K., Smith P., Cavet J., Hunter A., Parker A., et al. (2008) Superiority of reduced-intensity allogeneic transplantation over conventional treatment for relapse of Hodgkin’s lymphoma following autologous stem cell transplantation. Bone Marrow Transplant 41: 765–770. [DOI] [PubMed] [Google Scholar]
- Younes A., Connors J., Park S., Fanale M., O’Meara M., Hunder N., et al. (2013) Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol 14: 1348–1356. [DOI] [PubMed] [Google Scholar]
- Younes A., Santoro A., Shipp M., Zinzani P., Timmerman J., Ansell S., et al. (2016) Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 17: 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes A., Sureda A., Ben-Yehuda D., Zinzani P., Ong T., Prince H., et al. (2012) Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol 30: 2197–2203. [DOI] [PubMed] [Google Scholar]