Abstract
Accumulating studies have revealed the hypoxic condition and its crucial role in the distinctive progression of infantile hemangioma (IH), the most common benign tumor in infancy. Activating transcription factor 4 (ATF4), an important gene mediating cellular adaptation to various stress signals, could confer a survival advantage for tumor cells under hypoxia and regulate tumor progression. However, the potential role of ATF4 in IH was still unknown. In this study, the expression of hypoxia inducible factor (HIF)-1α, ATF4, and macrophage colony-stimulating factor (M-CSF) in 27 specimens of IH was measured by immunochemistry and double-labeling immunofluorescence, followed by the Spearman rank correlation test. Our results showed that the expression of HIF-1α, ATF4, and M-CSF was significantly upregulated in proliferating IH compared with involuting IH. Meanwhile, HIF-1α and ATF4, in parallel with ATF4 and M-CSF, exhibited positive correlation and synchronous expression. In addition, our in vitro studies demonstrated that hypoxia obviously upregulated the expression of HIF-1α, ATF4, and M-CSF in hemangioma stem cells. Most importantly, their expression was uniformly correlated with the percentage of M2-polarized macrophages in IH. All those results and established evidence indicated that hypoxia-induced ATF4 expression may promote progression of proliferating IH through M-CSF-induced M2-polarized macrophages infiltration.
Keywords: ATF4, HIF-1α, infantile hemangioma, macrophage, M-CSF
Introduction
Infantile hemangioma (IH) is the most common benign vascular tumor in infancy, with an estimated prevalence ranging from 1% to 10% on account of sample size and racial difference.1,2 It often develops intractable complications, including disfigurement, function destruction, and even life-threatening symptoms.3 Unlike other vascular tumors, IH has a distinctive life cycle that progresses from a phase of rapid proliferation followed by spontaneous involution. During the proliferative phase, IH is composed of disorganized blood vessels and full of multitudinous immature endothelial cells (ECs)4 and hemangioma stem cells (HemSCs).5 In contrast, the involuting phase of IH is characterized by vascular tissue replacement by fat and connective tissue.4 Undoubtedly, exploring the underlying mechanisms of this unique course is of great value and can help improve the treatment of IH.
As a common environmental stress involved in numerous pathophysiological conditions, hypoxia has been proposed as a critical driving force for the pathogenesis of vascular proliferation.6 Hypoxia is also closely related to vascular remodeling in various pathological situations.7 Both previous studies and physical manifestations have indicated the presence of hypoxic condition and its promotive role in IH.8,9 However, the precise mechanism accounting for hypoxia-mediated IH progression has not been fully clarified. Therefore, it is essential to discover novel molecular events underlying this process, which may provide better insights into the biological course of IH.
Activating transcription factor 4 (ATF4), a member of ATF family of basic region-leucine zipper transcription factors, is induced by stress signals including anoxia/hypoxia, endoplasmic reticulum (ER) stress, and oxidative stress.10 ATF4 is capable of regulating expression of a wide range of genes implicated in oxidative stress, amino acid synthesis, differentiation, metastasis, and angiogenesis.10 ATF4 has been reported to be overexpressed in multiple cancers.11 More importantly, ATF4 confers a survival advantage for tumor cells under hypoxia and regulates processes relevant to cancer progression.12 However, the expression status and functional significance of ATF4 in IH is not clear and remains to be determined.
Previous studies, including those from our laboratory, have revealed that macrophages, particularly M2-polarized macrophages, play a vital role in IH progression.13–15 M2-polarized macrophages can not only promote the proliferation and endothelial differentiation but also suppress the adipogenesis of HemSCs.15 Macrophage colony-stimulating factor (M-CSF) is a well-known key regulator for the differentiation, polarization, and survival of macrophages among a broad spectrum of pathologies.16–18 However, whether hypoxia, a well-established driving force in IH progression, may affect or regulate macrophages in IH remains largely unknown. Besides, how the infiltration of M2-polarized macrophages in proliferating IH is modulated is still far from clear. In the present study, we hypothesized that hypoxia-induced ATF4 may promote progression of proliferating IH, which possibly involves M2-polarized macrophages. Thus, we sought to explore the potential role of ATF4 in IH, and attempted to unmask its pathophysiological link to M2-polarized macrophages and IH progression.
Materials and Methods
Clinical Samples
Twenty-seven IH samples without any treatment before surgery were obtained from the Hospital of Stomatology, Wuhan University. The detailed information of each sample was shown in Table 1. The procedures were performed according to the National Institutes of Health guidelines regarding the use of human tissues. This study was approved by the review board of the Ethics Committee of Hospital of Stomatology, Wuhan University. The diagnosis and classification of each IH case was established according to clinical presentation and histopathological features, as we described previously.15
Table 1.
Summary of Clinical Features of IH Patients.
| Patient No. | Gender | Age (Months) | Location | Therapy | Stage of IH |
|---|---|---|---|---|---|
| 1 | F | 6 | Cheek | NO | Proliferative |
| 2 | M | 22 | Head | NO | Involuting |
| 3 | M | 9 | Tongue | NO | Involuting |
| 4 | M | 16 | Head | NO | Involuting |
| 5 | F | 4 | Neck | NO | Proliferative |
| 6 | F | 3 | Cheek | NO | Proliferative |
| 7 | M | 1.5 | Head | NO | Proliferative |
| 8 | M | 7 | Tongue | NO | Involuting |
| 9 | F | 2 | Forearm | NO | Proliferative |
| 10 | M | 8 | Shoulder | NO | Involuting |
| 11 | F | 2 | Tempus | NO | Proliferative |
| 12 | M | 8 | Tongue | NO | Involuting |
| 13 | F | 12 | Tempus | NO | Involuting |
| 14 | F | 4 | Neck | NO | Proliferative |
| 15 | M | 13 | Left arm | NO | Involuting |
| 16 | M | 18 | Head | NO | Involuting |
| 17 | M | 2 | Tongue | NO | Proliferative |
| 18 | F | 9 | Upper lip | NO | Involuting |
| 19 | F | 3 | Ear | NO | Proliferative |
| 20 | M | 4 | Tongue | NO | Proliferative |
| 21 | F | 7 | Parotid | NO | Involuting |
| 22 | M | 4.5 | Head | NO | Proliferative |
| 23 | M | 7 | Neck | NO | Involuting |
| 24 | F | 9 | Tongue | NO | Involuting |
| 25 | M | 2 | Head | NO | Proliferative |
| 26 | M | 3 | Upper lip | NO | Proliferative |
| 27 | F | 9 | Cheek | NO | Involuting |
Abbreviations: M, male; F, female; IH, infantile hemangioma; NO, no treatment before surgery.
Immunohistochemistry
The immunohistochemical analysis was performed as we described previously.19 In brief, the 4-µm tissue sections of paraformaldehyde-fixed and paraffin-embedded specimens were dewaxed in xylene, rehydrated in a graded series of ethanol, and antigen retrieved by high pressure. After incubation within 3% hydrogen peroxide and goat serum for 20 min at 37C sequentially, the sections were incubated overnight at 4C with primary antibodies hypoxia inducible factor (HIF)-1α (1:200; Abcam, Cambridge, UK), ATF4 (1:300; Proteintech, Wuhan, China), and M-CSF (1:200; Epitomics, Burlingame, CA). Then, the sections were incubated with horseradish peroxidase–conjugated secondary antibody, followed by detection of the antibody binding with the diaminobenzidine substrate kit (Dako, Carpinteria, CA). For analysis of immunohistochemical staining, five representative fields were randomly selected at a 200× magnification under a light microscope (Leica, Heidelberg, Germany) for each sample, and mean optical density was analyzed using Image-Pro Plus 6.0 (Media Cybernetics) by two independent researchers. All the antibodies used here have been validated according to the manufacturer-suggested positive control and negative control (antibody diluent), and those results were shown in Supplementary Fig. 1.
Immunofluorescence
The immunofluorescence was conducted as similar to our previous report.19 Briefly, the tissue sections were dewaxed in xylene and rehydrated in a graded series of ethanol. Antigen retrieval was performed using high pressure. The sections were then blocked with BSA (Sigma, St. Louis, MO) for 1 hr at 37C, followed by overnight incubation at 4C with primary antibodies (1:150, mouse anti-human ATF4 [Proteintech]; 1:100, rabbit anti-human HIF-1α [Abcam]; 1:100, rabbit anti-human M-CSF [Epitomics]; 1:150, rabbit anti-human CD133 [Proteintech]; 1:150, rabbit anti-human CD31 [Epitomics]; 1:100, rabbit anti-human α-SMA [Epitomics]). After washing with PBS, the sections were incubated with specific fluorescent secondary antibodies (1:200, DyLight 488 goat anti-mouse antibody, DyLight 488 goat anti-rabbit antibody, DyLight 594 goat anti-mouse antibody, and DyLight 594 goat anti-rabbit antibody; Abbkine, Redlands, CA) for 1 hr at 37C. In the end, after counterstain of the nuclei with 4′,6-diamino-2-phenylindole (DAPI), the sections were observed and photographed under a fluorescence microscope (Leica).
Double-Labeling Immunohistochemistry
For the detection of M2-polarized macrophages in IH, we performed the double-labeling immunohistochemistry as we described previously.14 After tissue section preparation following above procedures, the sections were incubated with anti-CD163 antibody (1:50, Proteintech) overnight at 4C, followed by a streptavidin alkaline phosphatase–labeled mouse EnVision Plus detection system (MXB, Fuzhou, China). Positive expression was visualized by using 5-bromo-4-chloro-3-indolyl phosphate and exhibited a dark purple cytoplasm staining. Sections were then incubated with anti-CD68 antibody (1:50, ZSGB-BIO, Beijing, China) at 37C for 1 hr, followed by incubation with secondary goat anti-mouse biotinylated antibody and hydrogen peroxidase–labeled detection system sequentially. The positive CD68 expression exhibited an overwhelming red staining of the cytoplasm. For quantitative analysis of the M2-polarized macrophages, five representative fields were randomly selected at a 200× magnification under a light microscope (Leica) for each sample, and the percentage of M2-polarized macrophage in total cells was calculated by two observers blindly. CD68 and CD163 have been validated according to the manufacturer-suggested positive control and negative control (antibody diluent), respectively (Supplementary Fig. 1).
Cell Culture
The isolation and culture of human IH stem cells (HemSCs) and human umbilical vein endothelial cells (HUVECs) were as described in our previous studies.15,20 When grown to 70% confluence, HUVECs and HemSCs were cultured in Anoxomat chambers (Mart Microbiology, Lichtenvoorde, The Netherlands) for physiological hypoxia (5% O2) or normoxia (21% O2). After 36 hr, the cells were collected for total RNA or protein extraction to analyze HIF-1α, ATF4, and M-CSF expression.
Real-Time PCR
Briefly, total RNA was extracted by using the E.Z.N.A. Total RNA Kit (Omega Bio-Tek, Norcross, GA) according to the manufacturer’s protocol, and 1 µg of RNA was reverse transcribed to cDNA by using the First-Strand cDNA Synthesis kit (Thermo Scientific, Pittsburgh, PA). After that, obtained cDNA was amplified with FastStart Universal SYBR Green Master (Roche, Branchburg, NJ) on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA) according to standard conditions. β-Actin was selected as internal control, and all the primer nucleotide sequences for real-time polymerase chain reaction (PCR) were shown in Table 2.
Table 2.
Primer Sequences Used for Real-Time PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-Actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT |
| HIF-1α | ATCCATGTGACCATGAGGAAATG | TCGGCTAGTTAGGGTACACTTC |
| ATF4 | CTCCGGGACAGATTGGATGTT | GGCTGCTTATTAGTCTCCTGGAC |
| M-CSF | AGACCTCGTGCCAAATTACATT | AGGTGTCTCATAGAAAGTTCGGA |
Abbreviations: PCR, polymerase chain reaction; HIF-1α, hypoxia inducible factor-1α; ATF4, activating transcription factor 4; M-CSF, macrophage colony-stimulating factor.
Western Blot Analysis
The cell protein was extracted and measured the concentration of total protein by using bicinchoninic acid assay (Pierce, Rockford, IL). Western blot analysis was performed according to our previous studies.19 Briefly, 20 µg of total protein extracted from each sample was separated on 10% SDS-polyacrylamide gels and electroblotted on polyvinylidene fluoride membranes (Roche Applied Science, Branchburg, NJ). After blocked with 5% skimmed milk, the membranes were incubated with primary antibodies (1:800, mouse anti-human ATF4 [Proteintech]; 1:1000, rabbit anti-human HIF-1α [Abcam]) overnight at 4C. Immunoblots were visualized by using a chemiluminescence kit (Pierce) and recorded by using the scanner (Canon, Tokyo, Japan).
Statistical Analysis
Student’s t-test and Spearman rank correlation test were used for statistical analysis; p<0.05 was considered to be significantly different.
Results
Overexpression of ATF4 in Proliferating IH and Its Possible Association With Tissue Hypoxia
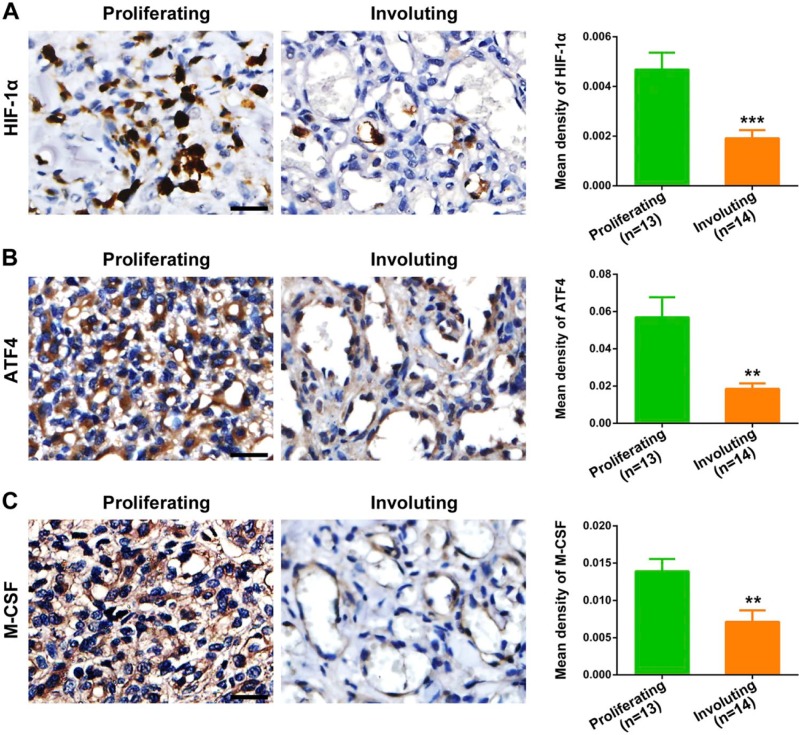
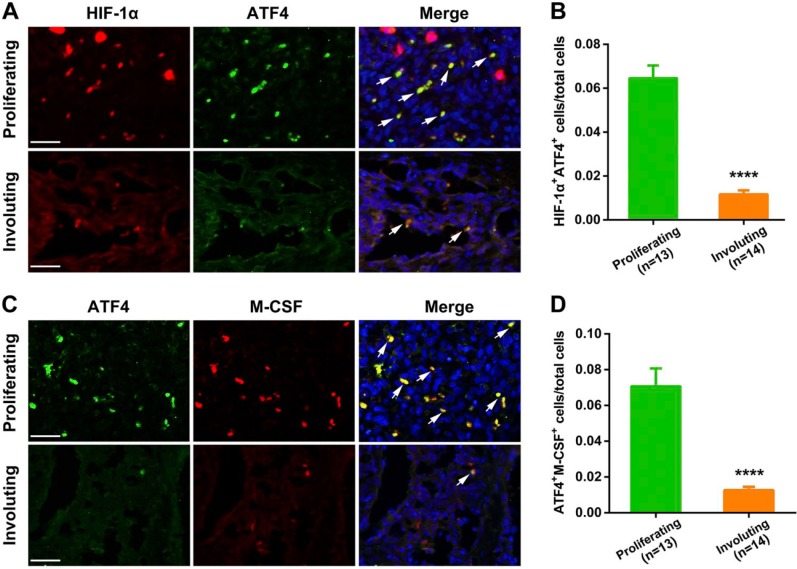
We first evaluated the hypoxic status in different IH phases by assessing the expression of HIF-1α. As shown in Fig. 1A, proliferating IH exhibited strong and intensive HIF-1α-positive staining, whereas its expression level was significantly downregulated in the involuting phase (p=0.0009), indicating the presence of hypoxia in proliferating IH. Next, we detected the expression of ATF4 and analyzed its association with hypoxia. We found that ATF4 expression was markedly increased in proliferating IH compared with the involuting phase (Fig. 1B). Importantly, ATF4 expression showed close correlation with HIF-1α in IH specimens (p<0.0001), as evidenced by Spearman’s rank correlation test (Fig. 2A). Furthermore, double-labeling immunofluorescence analyses revealed prominent colocalization of ATF4 with the HIF-1α signals in proliferating IH (Fig. 3). In contrast, very weak co-expression of ATF4 and HIF-1α was observed in the involuting phase. These results suggested activation of ATF4 in proliferating IH and its potential association with tissue hypoxia.
Figure 1.
Detection and quantification of HIF-1α, ATF4, and M-CSF expression in IH tissues. HIF-1α (A), ATF4 (B), and M-CSF (C) expression in different IH phases was detected by immunohistochemistry and quantitatively analyzed by mean optical density. Proliferating IH (n=13); involuting IH (n=14). Data are expressed as mean ± SEM. Abbreviations: HIF-1α, hypoxia inducible factor-1α; ATF4, activating transcription factor 4; M-CSF, macrophage colony-stimulating factor; IH, infantile hemangioma. **p<0.01, ***p<0.001. Scale bars: A–C = 30 µm.
Figure 2.
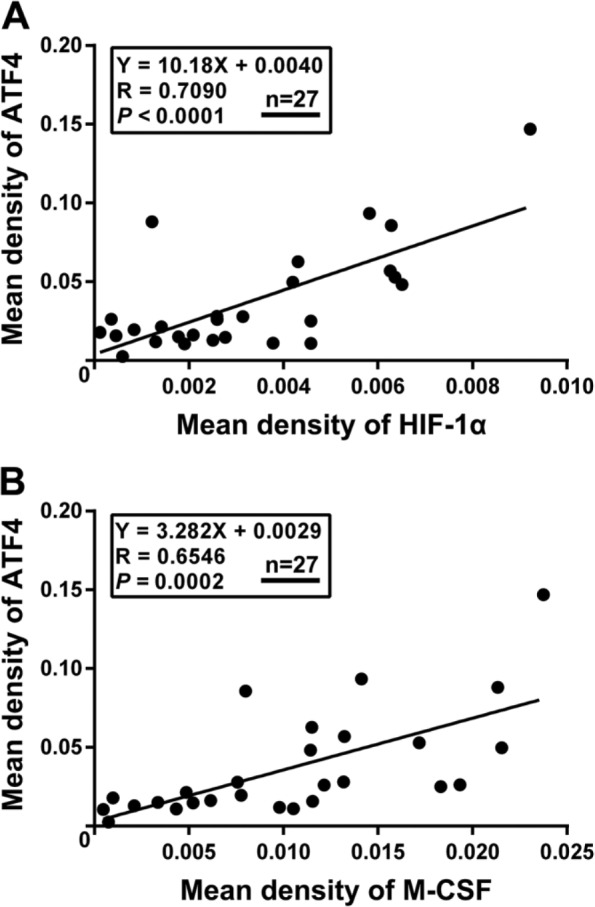
Spearman rank test analysis for HIF-1α, ATF4, and M-CSF expression in IH tissues. (A) HIF-1α expression was positively correlated with ATF4 in IH tissues. (B) M-CSF expression showed a close correlation with ATF4 expression in IH tissues (N=27). Abbreviations: HIF-1α, hypoxia inducible factor-1α; ATF4, activating transcription factor 4; M-CSF, macrophage colony-stimulating factor; IH, infantile hemangioma.
Figure 3.
Double-labeling immunofluorescence staining for HIF-1α and ATF4 or ATF4 and M-CSF in IH tissues. (A) The results showed that the expression of HIF-1α and ATF4 was pronouncedly colocalized in proliferating IH, and concurrently markedly decreased in the involuting IH tissues. (B) Quantification of co-expression of ATF4 and HIF-1α in proliferating and involuting IH tissues. The white arrows indicated the colocalization of tested proteins’ positive signals. (C) The results showed that the expression of ATF4 and M-CSF was pronouncedly colocalized in proliferating IH, and concurrently markedly decreased in the involuting IH tissues. (D) Quantification of co-expression of ATF4 and M-CSF in proliferating and involuting IH tissues. Proliferating IH (n=13); involuting IH (n=14). Abbreviations: HIF-1α, hypoxia inducible factor-1α; ATF4, activating transcription factor 4; M-CSF, macrophage colony-stimulating factor; IH, infantile hemangioma. ****p<0.0001. Scale bars: A, C = 30 µm.
Elevated Expression of M-CSF in Proliferating IH and Its Close Correlation With ATF4
We then examined the expression of M-CSF in different IH phases. As shown in Fig. 1C, the proliferating IH specimens exhibited obviously higher expression of M-CSF compared with involuting IH specimens (p=0.0060). Moreover, the results from immunochemical analysis also revealed a close correlation between M-CSF and ATF4 (Fig. 2B), suggesting that M-CSF secretion in IH is possibly associated with ATF4 expression. Most importantly, synchronous distribution and expression of ATF4 and M-CSF could be further observed in both proliferating and involuting phases of IH (Fig. 3). All these findings indicated a possible link between elevated expression of M-CSF and overexpression of ATF4 in IH.
Induction of ATF4 and M-CSF by Hypoxia in HemSCs In Vitro
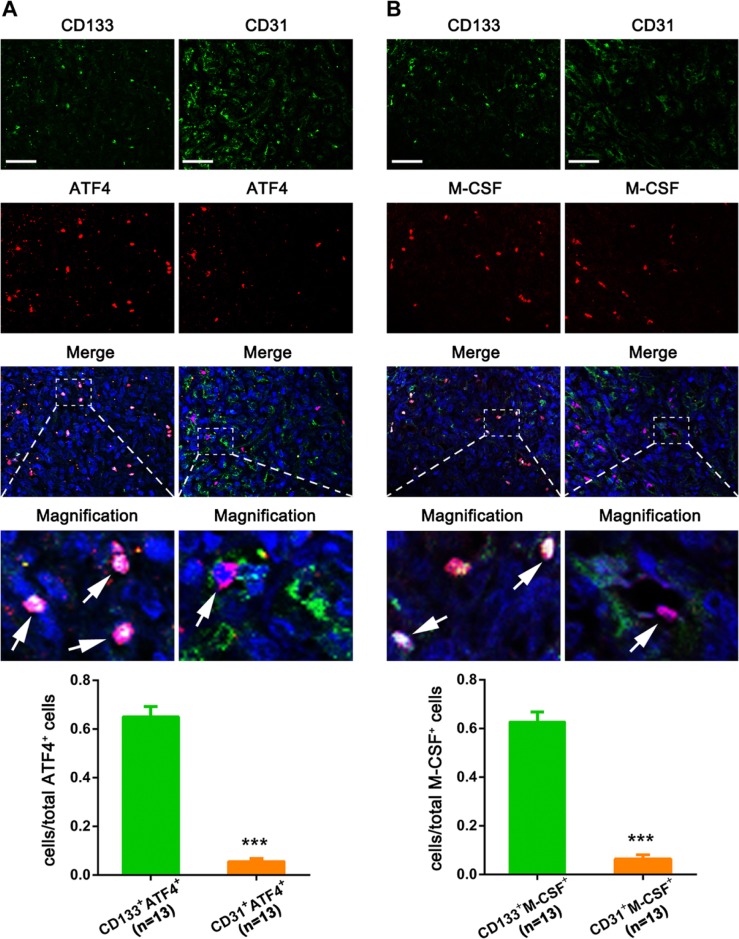
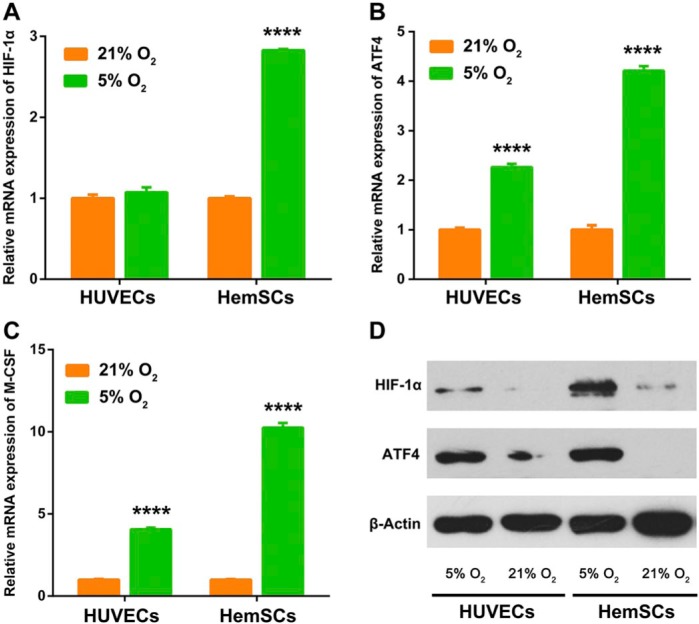
We next tried to explore which cells are producing ATF4 and M-CSF by performing immunofluorescence co-staining with CD133 (HemSC) and CD31 (EC) in IH tissues. As shown in Fig. 4, both ATF4 and M-CSF were more obviously colocalized with CD133 than CD31. To confirm above findings, we further performed the in vitro experiments by using HemSCs and HUVECs. As shown in Fig. 5, hypoxia (5% O2) obviously upregulated the expression of HIF-1α, ATF4, and M-CSF in HemSCs. By contrast, only the expression level of ATF4 and M-CSF was increased in HUVECs after hypoxia treatment, and the increasing trend was less significant than that in HemSCs. All the results indicated that HemSCs may be the most important candidate for the ATF4-induced M-CSF upregulation in hypoxia-related proliferating IH.
Figure 4.
Immunofluorescence co-staining with cell type–specific markers in IH tissues. ATF4 and M-CSF were co-stained with CD133 and CD31, and the quantitative analysis about the ratio of CD133+/ATF4+ cells and CD31+/ATF4+ cells to total ATF4+ cells (A) or CD133+/M-CSF+ cells and CD31+/M-CSF+ cells to total M-CSF+ cells (B) showed that both ATF4 and M-CSF were more obviously colocalized with CD133 than CD31 (n=13). The white arrows indicated the colocalization of tested proteins’ positive signals. Abbreviations: IH, infantile hemangioma; ATF4, activating transcription factor 4; M-CSF, macrophage colony-stimulating factor. ***p<0.001. Scale bars: A, B = 30 µm.
Figure 5.
Induction of ATF4 and M-CSF by hypoxia in HemSCs in vitro. (A-C) Hypoxia (5% O2) upregulated the mRNA expression of HIF-1α, ATF4, and M-CSF in HemSCs more significantly compared with HUVECs in vitro. (D) Hypoxia (5% O2) upregulated the protein expression of HIF-1α and ATF4 in HemSCs more significantly compared with HUVECs in vitro. Abbreviations: ATF4, activating transcription factor 4; M-CSF, macrophage colony-stimulating factor; HemSCs, hemangioma stem cells; HIF-1α, hypoxia inducible factor-1α; HUVECs, human umbilical vein endothelial cells. ****p<0.0001.
Close Correlation Among HIF-1α, ATF4, M-CSF, and M2-Polarized Macrophages Infiltration
Finally, we explored whether this regulatory network, which involves hypoxia-associated ATF4 activation and ATF4-associated M-CSF upregulation in HemSCs, has a role in M2-polarized macrophages infiltration of IH. The Spearman rank correlation test results revealed that the expression levels of HIF-1α, ATF4, and M-CSF were uniformly correlated with the M2-polarized macrophages (CD68+/CD163+) in IH (p<0.0001; Fig. 6), implying a potential novel mechanistic link between HemSCs activation and M2-polarized macrophages infiltration in proliferating IH.
Figure 6.
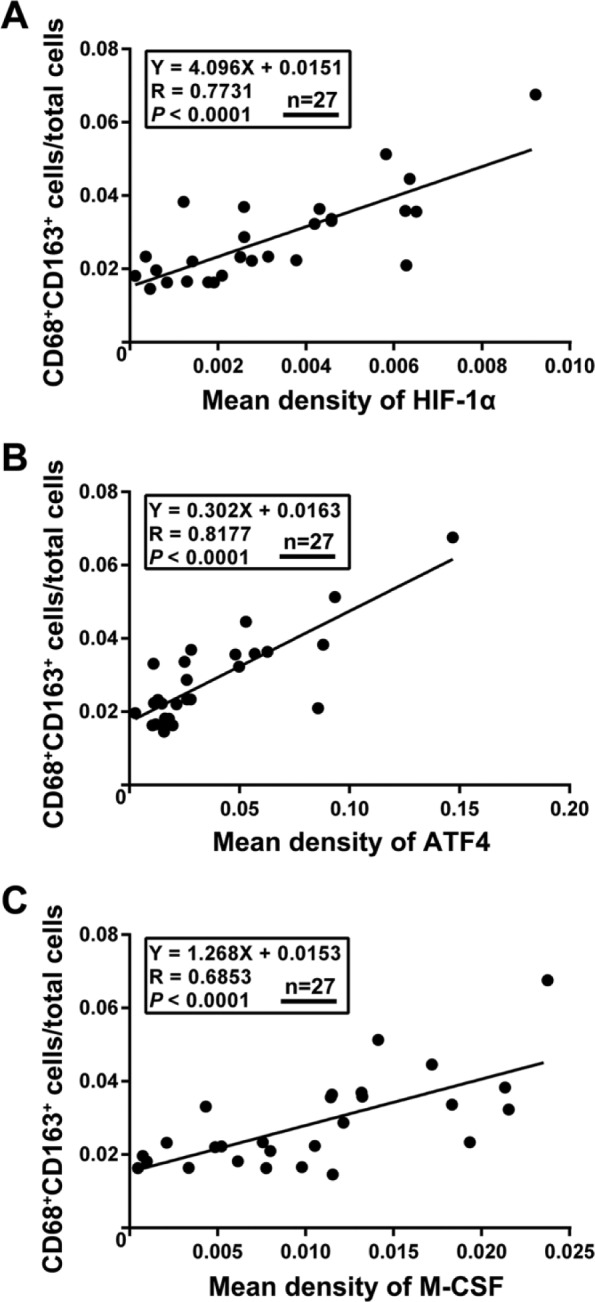
Spearman rank test analysis for the correlation between HIF-1α, ATF4, and M-CSF expression with M2-polarized macrophage infiltration in IH tissues. The expression of HIF-1α (A), ATF4 (B), and M-CSF (C) was positively correlated with the percentage of M2-polarized macrophages (CD68+/CD163+) in IH tissues (N=27). Abbreviations: HIF-1α, hypoxia inducible factor-1α; ATF4, activating transcription factor 4; M-CSF, macrophage colony-stimulating factor; IH, infantile hemangioma.
Discussion
Despite recent progress in unraveling the pathogenesis of IH, the pathophysiology associated with the unique natural history of this lesion has not been fully elucidated. Among various microenvironment factors which are tightly implicated in IH, hypoxia has attracted tremendous attention and has been considered to play a crucial role in IH progression. Several lines of evidence support that tissue hypoxia is not only a common event in IH but may contribute to explosive growth in proliferating IH. However, the exact alternations and the precise mechanisms of IH in response to hypoxia at the cellular and molecular level remain to be explored. Previous studies have revealed that HIF-1α is the central mediator of the cellular response to hypoxia.21 However, recent reports highlight that ATF4, a stress responsive gene from ATF family, is also induced by oxygen deprivation (hypoxia/anoxia) and functions as a pivotal transcriptional regulator of the cellular hypoxic response to the unfolded protein response following ER stress.10,22 ATF4 is believed to be a protective gene mediating cellular adaptation to stress factors by activating genes that promote restoration of normal ER function and survival under hypoxia.10 In the present study, we initially found that HIF-1α expression was markedly elevated in proliferating IH compared with the involuting phase as similar to previous reports,20,23 suggesting hypoxic status in proliferating IH. Meanwhile, we observed significant upregulation of ATF4 in proliferating IH in comparison with the involuting phase. Moreover, the Spearman rank correlation showed that ATF4 was positively correlated with HIF-1α expression in IH specimens. The close correlation between ATF4 and HIF-1α was further corroborated by double-labeling immunofluorescence that revealed a synchronous distribution for ATF4 with HIF-1α in proliferating IH. Thus, it is plausible that ATF4 expression is likely linked to hypoxic status of IH, and ATF4 may be implicated in hypoxia-induced cellular response in proliferating IH.
Macrophages, an active component in immune response, have been well characterized as a key player in tumor progression.24 Our recent studies have unveiled that macrophages contribute to IH progression via promoting the angiogenic process.14,15 Notably, a recent study provides evidence that hypoxia could upregulate ATF4 expression in macrophages.25 On the other hand, there is evidence showing that ATF4 in breast cancer cells is capable of promoting macrophage recruitment via secretion of M-CSF, thereby facilitating tumor angiogenesis.26 In addition, M-CSF, a secreted cytokine derived from various cells, is known to be a crucial regulator for the differentiation, recruitment, and polarization of macrophages.17,18 Therefore, we were intrigued to speculate that hypoxia-induced ATF4 may promote progression of proliferating IH through recruitment of macrophages into tumor tissues via induction of M-CSF. To test this hypothesis, we first evaluated the expression of M-CSF and its association with ATF4 at different stages of IHs. Consistent with prior report, the expression of M-CSF was dramatically upregulated in proliferative IH than in involuting IH and meanwhile closely correlated with infiltration of M2-polarized macrophages in IH. Importantly, we found that M-CSF expression was tightly correlated with ATF4, evidenced by both Spearman rank test and immunofluorescence co-expression in proliferative IH.
HemSCs own robust proliferative and clonogenic capacity and is thought to be the origin of multiple hemangioma-related cell lineages.5 In this article, we demonstrated the observably colocalization of ATF4 or M-CSF with CD133 and the induction of ATF4 and M-CSF by hypoxia in HemSCs in vitro. Considering the characteristic capacity for both self-renewal and pluripotency of stem cells, it is no wonder to speculate that HemSCs may be more sensitive to environmental stimulus and more active on interacting with surrounding cells. Lots of evidence has demonstrated the effects of hypoxia on stem cells27,28; here, we indicated the hypothesis that HemSCs may promote the explosive growth in proliferating IH through the elevated M-CSF secretion which was induced by hypoxia-upregulated ATF4 activation. Moreover, our hypothesis was also supported by close correlation between the expression of ATF4 as well as HIF-1α and the amount of M2-polarized macrophages in IH specimens, implying a positive regulation mechanism involving hypoxia-stimulated ATF4 activation and subsequent M-CSF-based M2-polarized macrophages recruitment for driving explosive growth in proliferating IH.
In summary, the present study revealed the elevated expression of ATF4 in proliferating IH, and its possible association with tissue hypoxia and M2-polarized macrophages infiltration. In addition, our study indicated the outstanding role of HemSCs in the above regulatory network and may shed new light on the mechanisms underlying hypoxia-elicited IH progression. Even though the precious mechanisms in this regulation remain to be clarified, the ATF4-centered regulation network may possess great value for elucidation of the pathogenesis in IH.
Supplementary Material
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: H-FX, J-YZ, and J-NW performed the immunohistochemistry. J-GR, YC, and F-QW carried out the molecular genetic studies. J-YZ, WZ, and GC analyzed the data. H-FX, Y-FZ, and J-HZ designed the study and drafted the manuscript. All authors have read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grants from National Natural Science Foundation of China to J-HZ (81671008), Y-FZ (81570994), and J-GR (81600385).
Compliance With Ethical Standards: The use of human tissues was in accordance with the National Institutes of Health guidelines. This study was approved by the review board of the Ethics Committee of Hospital of Stomatology, Wuhan University.
Literature Cited
- 1. Dickison P, Christou E, Wargon O. A prospective study of infantile hemangiomas with a focus on incidence and risk factors. Pediatr Dermatol. 2011;28:663–9. [DOI] [PubMed] [Google Scholar]
- 2. Munden A, Butschek R, Tom WL, Marshall JS, Poeltler DM, Krohne SE, Alio AB, Ritter M, Friedlander DF, Catanzarite V, Mendoza A, Smith L, Friedlander M, Friedlander SF. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoeger PH, Harper JI, Baselga E, Bonnet D, Boon LM, Ciofi Degli Atti M, El Hachem M, Oranje AP, Rubin AT, Weibel L, Leaute-Labreze C. Treatment of infantile haemangiomas: recommendations of a European expert group. Eur J Pediatr. 2015;174:855–65. [DOI] [PubMed] [Google Scholar]
- 4. Greenberger S, Bischoff J. Pathogenesis of infantile haemangioma. Br J Dermatol. 2013;169:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 2002;16:771–80. [DOI] [PubMed] [Google Scholar]
- 7. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bota M, Popa G, Blag C, Tataru A. Infantile hemangioma: a brief review. Clujul Med. 2015;88:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Jong S, Itinteang T, Withers AH, Davis PF, Tan ST. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219–27. [DOI] [PubMed] [Google Scholar]
- 10. Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40:14–21. [DOI] [PubMed] [Google Scholar]
- 11. Singleton DC, Harris AL. Targeting the ATF4 pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:1189–202. [DOI] [PubMed] [Google Scholar]
- 12. Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–8. [DOI] [PubMed] [Google Scholar]
- 13. Jia J, Bai Y, Fu K, Sun ZJ, Chen XM, Zhao YF. Expression of allograft inflammatory factor-1 and CD68 in haemangioma: implication in the progression of haemangioma. Br J Dermatol. 2008;159:811–9. [DOI] [PubMed] [Google Scholar]
- 14. Wang FQ, Chen G, Zhu JY, Zhang W, Ren JG, Liu H, Sun ZJ, Jia J, Zhao YF. M2-polarised macrophages in infantile haemangiomas: correlation with promoted angiogenesis. J Clin Pathol. 2013;66:1058–64. [DOI] [PubMed] [Google Scholar]
- 15. Zhang W, Chen G, Wang FQ, Ren JG, Zhu JY, Cai Y, Zhao JH, Jia J, Zhao YF. Macrophages contribute to the progression of infantile hemangioma by regulating the proliferation and differentiation of hemangioma stem cells. J Invest Dermatol. 2015;135:3163–72. [DOI] [PubMed] [Google Scholar]
- 16. Hamilton TA, Zhao C, Pavicic PG, Jr, Datta S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Front Immunol. 2014;5:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Y, Qin J, Lan L, Li N, Wang C, He P, Liu F, Ni H, Wang Y. M-CSF cooperating with NFκB induces macrophage transformation from M1 to M2 by upregulating c-Jun. Cancer Biol Ther. 2014;15:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Overmeire E, Stijlemans B, Heymann F, Keirsse J, Morias Y, Elkrim Y, Brys L, Abels C, Lahmar Q, Ergen C, Vereecke L, Tacke F, De Baetselier P, Van Ginderachter JA, Laoui D. M-CSF and GM-CSF receptor signaling differentially regulate monocyte maturation and macrophage polarization in the tumor microenvironment. Cancer Res. 2016;76:35–42. [DOI] [PubMed] [Google Scholar]
- 19. Ren JG, Chen G, Zhu JY, Zhang W, Sun YF, Jia J, Zhang J, Zhao YF. Downregulation of the transforming growth factor-β/connective tissue growth factor 2 signalling pathway in venous malformations: its target potential for sclerotherapy. Br J Dermatol. 2014;171:242–51. [DOI] [PubMed] [Google Scholar]
- 20. Chen G, Zhang W, Li YP, Ren JG, Xu N, Liu H, Wang FQ, Sun ZJ, Jia J, Zhao YF. Hypoxia-induced autophagy in endothelial cells: a double-edged sword in the progression of infantile haemangioma? Cardiovasc Res. 2013;98:437–48. [DOI] [PubMed] [Google Scholar]
- 21. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985). 2000;88:1474–80. [DOI] [PubMed] [Google Scholar]
- 22. Rzymski T, Milani M, Singleton DC, Harris AL. Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia. Cell Cycle. 2009;8:3838–47. [DOI] [PubMed] [Google Scholar]
- 23. Kleinman ME, Greives MR, Churgin SS, Blechman KM, Chang EI, Ceradini DJ, Tepper OM, Gurtner GC. Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27:2664–70. [DOI] [PubMed] [Google Scholar]
- 24. Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–15. [DOI] [PubMed] [Google Scholar]
- 25. Elbarghati L, Murdoch C, Lewis CE. Effects of hypoxia on transcription factor expression in human monocytes and macrophages. Immunobiology. 2008;213:899–908. [DOI] [PubMed] [Google Scholar]
- 26. Liu C, Li Z, Wang L, Tong L, He N, Chen Y, Liu Y, Wu Z, Sun P, Xiang R, Ren G, Su W. Activating transcription factor 4 promotes angiogenesis of breast cancer through enhanced macrophage recruitment. Biomed Res Int. 2015;2015:974615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee HJ, Ryu JM, Jung YH, Oh SY, Lee SJ, Han HJ. Novel pathway for hypoxia-induced proliferation and migration in human mesenchymal stem cells: involvement of HIF-1α, FASN, and mTORC1. Stem Cells. 2015;33:2182–95. [DOI] [PubMed] [Google Scholar]
- 28. Semenza GL. Dynamic regulation of stem cell specification and maintenance by hypoxia-inducible factors. Mol Aspects Med. 2016;47–48:15–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.