Abstract
Calprotectin is a heterodimer formed by two proteins, S100A8 and S100A9, which are mainly produced by activated monocytes and neutrophils in the circulation and in inflamed tissues. The implication of calprotectin in the inflammatory process has already been demonstrated, but its role in the pathogenesis, diagnosis, and monitoring of rheumatic diseases has gained great attention in recent years. Calprotectin, being stable at room temperature, is a candidate biomarker for the follow-up of disease activity in many autoimmune disorders, where it can predict response to treatment or disease relapse. There is evidence that a number of immunomodulators, including TNF-α inhibitors, may reduce calprotectin expression. S100A8 and S100A9 have a potential role as a target of treatment in murine models of autoimmune disorders, since the direct or indirect blockade of these proteins results in amelioration of the disease process. In this review, we will go over the biologic functions of calprotectin which might be involved in the etiology of rheumatic disorders. We will also report evidence of its potential use as a disease biomarker.
Impact statement
Calprotectin is an acute-phase protein produced by monocytes and neutrophils in the circulation and inflamed tissues. Calprotectin seems to be more sensitive than CRP, being able to detect minimal residual inflammation and is a candidate biomarker in inflammatory diseases. High serum levels are associated with some severe manifestations of rheumatic diseases, such as glomerulonephritis and lung fibrosis. Calprotectin levels in other fluids, such as saliva and synovial fluid, might be helpful in the diagnosis of rheumatic diseases. Of interest is also the potential role of calprotectin as a target of treatment.
Keywords: Calprotectin, S100A8/A9, rheumatic diseases, inflammation, biomarker, rheumatoid arthritis
Introduction
Calprotectin (CLP) is a heterodimer formed by two proteins, S100A8 and S100A9. These proteins represent half of the cytosolic protein content of monocytes and neutrophils. They are constitutively expressed as an anti-microbial agent by these cells. CLP was initially named major leukocyte protein L1 or 27E10.1,2 It was later identified as the combination of S100A8 and S100A9, which also have different synonyms: myeloid-related proteins-8 and -9 (MRP-8 and MRP-9); migration inhibitory factor-related protein of 8 and 14 kDa (MRP-8 and MRP-14); calgranulin A and B; alarmins.1,2
The implication of S100A8 and S100A9 in the inflammatory process was shown almost 20 years ago.3,4 However, its role in the pathogenesis of rheumatic diseases has only gained great attention in recent years. Upcoming evidence shows that CLP might also be involved in cancer development, obesity, progression of dementia, and formation of the atherosclerotic plaque.5–10 CLP, being stable at room temperature, is a candidate biomarker in inflammatory diseases and its levels in stools are routinely measured in the follow-up of inflammatory bowel diseases.9 Serum levels are usually reported below 1 µg/ml in healthy subjects, but during inflammation they may increase by 100 times.10
The use of CLP in pediatric rheumatic diseases, such as juvenile idiopathic arthritis, Henoch-Schönlein purpura, Kawasaki disease, cryopyrin-associated periodic syndromes and familiar Mediterranean fever, has already been reviewed.11 Here, we will go over the biologic functions of CLP which might be involved in the pathogenesis of rheumatic diseases. We will also report evidence of CLP use as a biomarker in the diagnosis and follow-up of adult rheumatic diseases (Tables 1 and 2).
Table 1.
Evidence supporting the use of serum calprotectin as a biomarker in rheumatic diseases
| Diagnosis | Disease assessment | Treatment assessment | Association with specific disease features | Prognosis | |
|---|---|---|---|---|---|
| Rheumatoid arthritis | Higher levels are found compared with HC and with OA, SpA, SLE, JIA, CPPD | Correlation with laboratory markers of inflammation Correlation with disease activity measures Correlation with ultrasound and radiographic damage scores | Reduction following effective treatment | High levels are associated with positive rheumatoid factor and ACPA | High levels are predictive of disease relapse High levels are predictive of structural damage |
| Spondyloarthritis | Controversial results are available of higher levels compared with HC | Correlation with laboratory markers of inflammation Modest correlation with disease activity measures | Reduction following effective treatment | High levels are associated with peripheral arthritis | NA |
| Psoriatic arthritis | Higher levels are found compared with HC | Correlation with laboratory markers of inflammation Correlation with disease activity measures Correlation with the extent of skin involvement Correlation with radiographic damage scores | Reduction following effective treatment | NA | NA |
| Adult-onset Still’s disease | Higher levels are found compared with HC and RA, OA, SLE, SS | Controversial correlation with laboratory markers of inflammation and ferritin Correlation with disease activity measures | Reduction following effective treatment | High levels are associated with sore throat | NA |
| Gout | Higher levels are found compared with HC | Correlation with laboratory markers of inflammation | Reduction following effective treatment | NA | NA |
| Osteoarthritis | Higher levels are found in case of synovial inflammation | Correlation with the extent of structural damage at the histological level | NA | NA | High levels are predictive of structural damage |
| Systemic lupus erythematosus | Higher levels are found compared with HC | Correlation with disease activity scores and damage scores | NA | High levels associated with cerebro-vascular events, acute myocardial infarction, proliferative glomerulonephritis, positive anti-dsDNA antibodies | NA |
| Sjögren syndrome | Higher levels are found compared with HC | No correlation with laboratory markers of inflammation No correlation with the extent of inflammation at the histological level | NA | NA | NA |
| Systemic sclerosis | Higher levels are found in circulating polymorphonucleates and monocytes compared with HC | No correlation with laboratory markers of inflammation | NA | High levels are associated with lung fibrosis, arthritis, gastrointestinal involvement, presence of anti-Scl70, anti-hystone, or anti-U1RNP antibodies High S100A8 levels are associated with kidney involvement High S100A9 are associated with myositis | High levels are predictive of reduced survival |
| Behçet’s disease | Higher levels are found compared with HC | No correlation with disease activity scores or quality of life indices | NA | NA | High levels are predictive of disease relapse |
| Antineutrophil cytoplasm antibody–associated vasculitis | Higher levels are found compared with HC | Correlation with disease activity scores No correlation with laboratory markers of inflammation or ANCA levels | Reduction following effective treatment | Associated with proliferative glomerulonephritis | High levels are predictive of disease relapse |
| Polymyalgia rheumatica and giant cell arteritis | Higher levels are found compared with HC | Correlation with laboratory markers of inflammation | Reduction following effective treatment | NA | NA |
Note: No data as a clinical biomarker were available for idiopathic inflammatory myopathies.
HC: healthy controls; OA: osteoarthritis; SpA: spondyloarthritis; SLE: systemic lupus erythematosus; JIA: juvenile idiopathic arthritis; CPPD: calcium pyrophosphate dehydrate deposition disease; ACPA: anti-citrullinated peptide antibodies; SS: Sjögren syndrome; ANCA: antineutrophil cytoplasm antibody; NA: not available.
Table 2.
Evidence supporting the use of calprotectin as a biomarker in fluids/faeces other than serum in rheumatic diseases
| Bronchoalveolar lavage | Systemic sclerosis High levels are associated with lung fibrosis. |
| Faeces | Rheumatoid arthritis, spondyloarthritis and psoriatic arthritis High levels are associated with bowel inflammation. Sjögren syndrome Higher levels are found compared with HC; high levels are associated with bowel inflammation Systemic sclerosis Higher levels are found compared with HC, SS, RA; high levels are associated with gastrointestinal involvement and micronutrient deficiency. |
| Saliva | Sjögren syndrome Higher levels are found compared with HC. Systemic sclerosis Higher levels of S100A8 and S100A9 are found compared with HC. |
| Synovial fluid | Rheumatoid arthritis Higher levels are found compared with the serum; higher levels are found compared with OA, SpA, PSA; controversial results are available compared with SLE, CPPD. Gout Higher levels are found compared with the serum; higher levels are found compared with OA, RA, SpA, PSA; levels are similar to CPPD. Osteoarthritis Controversial results are available compared to HC; higher levels are found in the case of synovial inflammation, lower levels are found compared with gout and RA. |
SSc: systemic sclerosis; RA: rheumatoid arthritis; SpA: spondyloarthritis; PSA: psoriatic arthritis; SS: Sjögren syndrome; HC: healthy controls; OA: osteoarthritis; SLE: systemic lupus erythematosus; CPPD: calcium pyrophosphate dehydrate deposition disease.
S100A8/9 Structure
S100A8 and S100A9 proteins belong to the S100 protein family12 and share a common helix-loop-helix motif structure consisting of two α-helices bound by a central hinge region. Every monomer can bind two Ca2+ ions and other divalent metal ions such as Zn2+. The ion-binding properties of the S100 protein family modulate oligomerisation and consequently their function.13–15 S100A8 and S100A9 can be found in the form of homodimers, heterodimers (S100A8/A9)2, or heterotetramers (S100A8/A9)4. The heterodimer is the most stable form and is responsible for the majority of the protein biologic interactions.16 S100A8 and S100A9 are expressed separately but S100A8 has a turnover higher than S100A9. In the absence of its binding partner, as in S100A9-knockout mice, S100A8 serum levels are almost undetectable.17
Biology of CLP
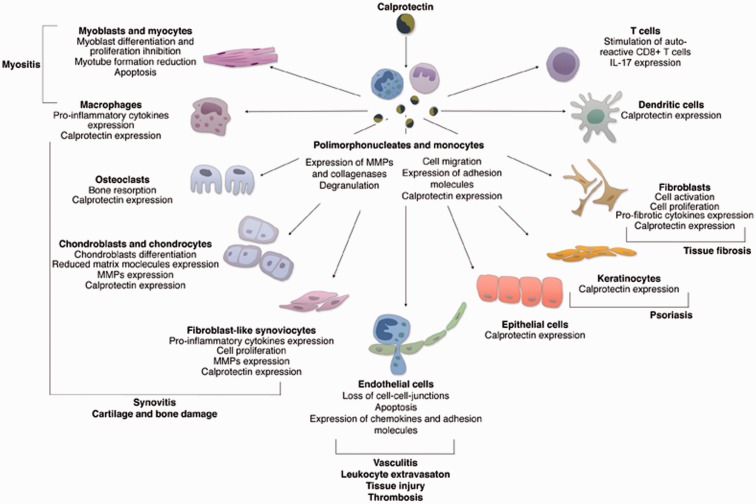
S100A8 and S100A9 proteins are classically expressed by granulocytes, monocytes, and macrophages in an early differentiation stage, after their activation via-pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs).18,19 Under specific conditions, other cell lines can express and secrete CLP such as endothelial cells, keratinocytes, osteoclasts, chondrocytes, and fibroblast-like synoviocytes.20–24 (Figure 1).
Figure 1.
Effects of calprotectin on the cells implicated in the pathogenesis of rheumatic diseases. CLP is mainly produced by activated monocytes and granulocytes and mediates the production of pro-inflammatory cytokines and chemokines, cell activation, and apoptosis in targeted cells. CLP induces the expression of further CLP with a consequent positive autocrine and paracrine feedback loop. (A color version of this figure is available in the online journal.)
The precise function of CLP in the immune process has not been unraveled yet. Some major functions have been acknowledged, such as the regulation of the cytoskeleton, leukocyte migration and trafficking, and amplification of inflammation, which are pivotal in the host response to infections. CLP also has a direct anti-microbial effect due to the chelation of Mn2+ and Zn2+, which are metal nutrients for bacteria.25
CLP is part of the innate immune response. It is recognized as a DAMP itself being an endogenous ligand of toll-like receptor (TLR) 4.17 TLR4 belongs to the pattern recognition receptor family and it transduces the danger signal after interacting with PAMPs and DAMPs. CLP mediates the response to pathogen-derived factors, such as lipopolysaccharide (LPS), and contributes to the inflammatory process occurring in infections and sepsis.7 It is involved in the inflammatory pathway upstream of tumor necrosis factor (TNF)-α and, like TNF-α, is crucial for LPS toxicity.26 Like all DAMP molecules, CLP has a double role in the homeostasis of phagocytes. In a steady condition, it contributes to the regulation of the cytoskeleton, and it is released as a danger signal when phagocytes are activated.
Intracellular functions of CLP
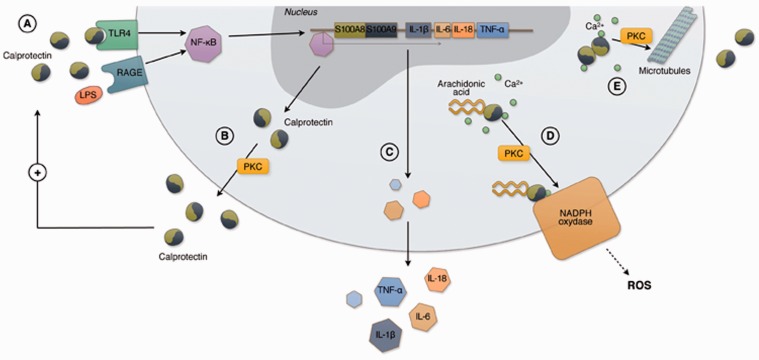
In polimorphonucleates (PMNs), CLP is implicated in the rapid rearrangement of tubulin-dependent cytoskeleton, which allows cell migration1,13,14,19,27 (Figure 2). In the presence of calcium, S100A8 and S100A9 form heterotetramers and translocate to the cell membrane allowing tubulin polymerisation.13,14 The evidence that S100A9-knockout mice have reduced granulocyte migration supports the idea that CLP has a role in cytoskeleton rearrangement.14
Figure 2.
Intracellular functions of calprotectin in polimorphonucleates and monocytes. CLP is a heterodimer composed of two proteins, S100A8 and S100A9. A danger signal molecule, such as LPS and CLP itself, can bind TLR4 and RAGE directly or through carboxylated glycans triggering inflammation via-NF-ĸB which translocates into the nucleus (a). In the nucleus NF-ĸB induces the expression of further S100A8 and S100A9. CLP is secreted through an energy dependent process, which requires PKC activation (b) and/or the interaction with microtubules (e). TLR4 or RAGE binding by CLP can induce the expression of proinflammatory cytokines and adhesion molecules, such as CD11b and CD18, contributing to the amplification of the inflammatory response and leading to leukocyte adhesion to the endothelium (c). In the presence of calcium, S100A9 subunit binds arachidonic acid and transports it to the NADPH oxidase complex expressed in the plasma membrane with a PKC-dependent mechanism. S100A9 transfers arachidonic acid to gp91phox subunit of the NADPH complex while S100A8 binds to p67phox and rac-2 subunits. Activated NADPH oxidase produces reactive oxygen species which are crucial for the inflammatory activity of granulocytes (d). In the presence of calcium, S100A8 and S100A9 also form heterotetrameters which translocate to the cell membrane and allow tubulin polymerization, microtubules bundling and stabilization of tubulin filaments. CLP regulates the cytoskeleton cell migration (e). (A color version of this figure is available in the online journal.)
CLP is implicated in the activation of the respiratory burst. In the presence of calcium, S100A9 binds to arachidonic acid in the cytosol and transports it to the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex in the neutrophil plasma membrane through a PKC-dependent mechanism. S100A8 and S100A9 have multiple effects on NADPH oxidase. By interacting with gp91phox, p67phox, and rac-2 subunits of the NADPH oxidase complex, S100A8 and S100A9 induce the production of reactive-oxygen species, which are necessary for PMNs activity.28
CLP is secreted by granulocytes and other cells after a danger signal by PAMPs or DAMPs. It appears to be mostly released through a non Golgi-associated pathway with an active non-classical secretion, which requires again PKC activation.14,19,29 An additional more recently discovered mechanism is the release of CLP bound to chromatin in neutrophil extracellular traps (NETs).30,31 The protein can also be passively secreted from necrotic cells after tissue insult has occurred.14,19
Extracellular functions of CLP
S100A8 and S100A9 bind their receptors with different affinity according to the protein conformation.32 CLP heterodimer binds different cell-surface proteins like heparan sulphate proteoglycan, carboxylated N-glycan, and, directly or through carboxylated glycans, TLR4 and receptor for advanced glycated end products (RAGE).17,33
TLR4 is the main CLP receptor34 and is preferentially bound by the heterodimer.17 The signal transduction cascade is mediated by MyD88 and NF-κB, which translocate into the nucleus17,35,36 and promote the expression of pro-inflammatory cytokines, such as TNF-α, interleukin (IL)-1β, IL-6, IL-8, IL-23, chemokine (CXC)-8, and other CXCs17,37,38 (Figure 2). RAGE is also bound by CLP33 and activates inflammatory signaling pathways, including MAPK and NF-κB.35 Notably, the expression of S100A8 and S100A9 increases following NF-κB activation, thereby generating a positive feedback loop which amplifies inflammation via an autocrine and paracrine stimulation of the cells responsible for S100A8 and S100A9 production.17
A major function of S100A8 and S100A9 is the regulation of leukocyte chemotaxis and tissue infiltration.37 CLP induces the expression of integrin receptors on leukocytes, thus increasing their adhesion to fibrinogen, fibronectin, and endothelial cells.37,39
CLP causes a pro-inflammatory and thrombogenic response in the endothelium: it binds to endothelial cells through carboxylated glycans and TLR4 leading to cell activation. 40,41Following CLP activation, endothelial cells express CXCs, such as IL-8 and MCP-1, and further S100A8 and S100A9. Endothelial cells also express VCAMs, ICAMs, and selectins on their surface which results in a chemotactic gradient, which then attracts PMNs and favors their binding to the endothelium.37,39,42 CLP release leads to loss of cell–cell contacts and consequently alters the permeability of endothelium with leukocyte extravasation.42 It also triggers endothelial cell apoptosis and necrosis which are responsible for vascular and tissue damage.43
Putative role in adaptive immunity
Along with its classical role as an endogenous activator of innate immune response, CLP might represent a connection between inflammation and the adaptive immune response. CLP contributes to the induction of auto-reactive CD8+ T cells during the activation process by antigen-presenting cells.44 This molecule is a costimulatory enhancer together with CD40/CD40 ligand signaling and leads to the loss of tolerance of T cells.44 In a murine model of autoimmunity, the absence of S100A8 and S100A9 resulted in reduced IL-17 production by autoreactive CD8+ T cells and in lower autoantibody production.44In addition to its proinflammatory activity, CLP exerts also a regulatory function in the adaptive immune system. CLP overexpression in dendritic cells (DCs) is associated with an impaired T cell proliferation.45 A study by Chih-Ru et al.,46 showed that CLP is the endogenous ligand of CD69 expressed on regulatory T cells. The interaction between CLP and CD69 favors the differentiation of CD4+ T cells into regulatory T cells, thus hindering the exacerbation of T cell response. Furthermore, CLP regulates cytokine production, particularly supporting the expression of transforming growth factor – ß which is an anti-inflammatory cytokine.46
CLP in rheumatic diseases
Rheumatoid arthritis
S100A8 and S100A9 are the most up-regulated proteins in rheumatoid arthritis (RA) synovial tissue and synovial fluid, 47–49 where they are produced by macrophages, PMNs, synovial fibroblasts, and chondrocytes.24,47–51 S100A8 and S100A9 are involved in the amplification of the inflammatory process, neutrophil and monocyte recruitment, cartilage destruction, and bone resorption.
Macrophages producing S100A8 and S100A920,37,52 are present in the crucial sites of joint destruction, in the synovial membrane and the cartilage-pannus junction,20,40,53,54 where they cause an imbalance in favor of bone resorption.55
The interaction of TLR4 with its ligands, including CLP, induces synovial fibroblast proliferation and the production of metalloproteinase (MMP)-1, IL-6, and further S100A8 and S100A9.50,56 Activated chondrocytes also express CLP which, in turn, induces a catabolic effect on these cells via TLR4 signaling and the activation of NF-kB.22,52 This activation leads to proteoglycan depletion, prevention of new cartilage formation and, eventually, chondrocyte death.17,22,24,57,58 CLP also causes the degranulation of PMNs and the release of MMPs and collagenases, thus contributing to cartilage degradation.59 Osteoclast differentiation is also affected by CLP primarily through a TLR4-mediated signal and S100A8-induced osteoclastic bone resorption in a murine model.60
CLP levels are higher in the serum of RA patients compared with healthy subjects or patients with osteoarthritis (OA), spondyloarthritis (SpA), systemic lupus erythematosus (SLE), and pseudogout.47,61,62 Still, no cut-off levels have been identified to help in the diagnosis of RA. High levels of CLP are observed in RA synovial fluid, even >5 µg/ml, and might differentiate RA from OA and other inflammatory arthritides.47,63–66 Notably, proteomic analysis has revealed the presence of S100A9 in synovial fluid from RA patients, but not in other disease controls.67
CLP serum levels correlate with disease activity and are associated with markers of more severe RA in many studies.48,49,61,64,66,68–76 CLP has been found to be higher in the serum of rheumatoid factor48,49,69,70,71,73 and anti-citrullinated peptide positive patients23,49,64,72 compared with seronegative ones.23 CLP correlates with inflammatory indices, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and serum amyloid A protein (SAA) and inversely with TNF-α inhibitors through levels.74,76,77 CLP serum levels seem to perform even better compared to CRP and ESR. In fact, CLP has showed to correlate better with all clinical indices, such as 28 joint-disease activity score and simplified disease activity index.48,74–78
CLP serum levels can decrease in response to treatment with both conventional and biologic disease modifying anti-rheumatic drugs.72,74–80 Indeed, CLP serum levels have shown a prompt and more marked response to inflammatory changes compared with classic inflammatory indices, thus representing a sensitive biomarker for assessing treatment response.72,74 This finding is supported by evidence of S100A8 and S100A9 expression in PMNs decreasing after treatment with TNF-α inhibitors.79 Baseline CLP serum levels were higher in responders to treatment compared with non-responders and their decrease was predictive of treatment response in some studies,74,75,80 but not in all.73,80
A controversial effect of corticosteroids on CLP serum levels has been observed. Effective corticosteroid treatment decreases CLP serum levels in autoimmune diseases.81 However, Klingberg et al.82 reported an increase in CLP levels along with the increase in white blood cells (WBC) count after corticosteroid administration. There is evidence that corticosteroids can induce the expression of S100A8 in monocytes, DCs, and synovial macrophages in RA.83 Corticosteroids might therefore promote the expression of CLP also in circulating PMNs.
CLP has been independently associated with ultrasonography assessment scores, particularly in large joints.72 A recent study by Inciarte-Mundo et al.84 showed that CLP was associated with synovitis at the ultrasonography assessment in RA and psoriatic arthritis (PSA) patients in remission. CLP serum levels had the highest discriminatory capacity compared to CRP and ESR and a cut-off of 1.66 µg/ml for synovitis presence was also identified.84 Notably, CLP is the only biomarker which has been shown to correlate with radiographic scores48,49 and there is some evidence that baseline CLP might be predictive of radiographic damage.48,49,85 In a recent study by Vogl et al.,86 CLP was tested as a marker of inflammation in a murine model of arthritis by optical molecular imaging. It appeared sensitive in the identification of sub-clinical disease activity in the joints.
Notably, CLP might have a role as a therapeutic target in RA. In an arthritis murine model, the blockade of S100A9 through a monoclonal antibody was effective.87 This finding supports the putative role of CLP as a treatment target in RA and, possibly, in other immune-mediate arthritides.
Spondyloarthritis
Heterogeneous studies on CLP in SpA are available. Some include all subtypes of SpA and others are focused on ankylosing spondylitis (AS) alone. Although the results in SpA are similar to those obtained in PSA, studies regarding PSA will be separately discussed.
CLP is highly expressed in the synovial tissue of SpA patients, and it mirrors the presence of PMNs and monocytes which are responsible for its production.88 The distribution of S100A8 and S100A9 in the synovial tissue is characteristic of SpA, being mainly localized in perivascular areas of the synovial sub-lining layer.54,89 In a study by De Rycke et al.,89 CLP was increased in the synovial fluid of inflamed joints where it positively correlated with local markers of inflammation, such as SAA and WBC.
Some authors have reported increased CLP serum levels in SpA patients,54,89–91 while others have found similar levels in AS and controls.92,93 Other studies have described lower or similar CLP serum levels in patients with SpA compared with RA.88,94 One study by Cypers et al.94 found high CLP serum levels associated with peripheral involvement in SpA, which could explain the almost normal levels observed in patients with only axial involvement. However, this observation was not confirmed in another study by De Rycke et al.89 and needs to be supported by further evidence. It was shown that serum CLP positively correlates with CRP, ESR, WBC, and platelet count in SpA patients.54,89,94,95 However, CLP does not seem to be a reliable disease activity biomarker in SpA. Indeed, almost no correlation was found between CLP and Ankylosing Spondylitis Disease Activity Score,82Bath AS disease activity index, or Bath AS functional index.89,91,92,94–96
Notably, CLP is secreted not only in the joints, but also in other inflamed tissues such as the gastrointestinal mucosa. Up to 50% of patients with SpA have subclinical bowel inflammation, which is associated with increased serum levels of CLP.97 The mucosal contribution to the increase in circulating CLP levels may explain the modest correlation with SpA disease activity.94
CLP decreases rapidly upon effective treatment with TNF-α-inhibitors and secukinumab in both axial and peripheral SpA and it has been suggested as a useful biomarker for disease monitoring, where it performs even better than high-sensitivity CRP.91,95 Baseline CLP was also found to be an independent predictor of radiographic spinal progression in axial SpA in a cohort of German patients.95 CLP concentration >0.5 µg/mL was associated with worsening of the modified Stoke Ankylosing Spondylitis Spine Score and the development or progression of syndesmophytes at the two year follow-up.95
Psoriatic arthritis
Serum levels of S100A8, S100A9, and CLP are increased in PSA patients compared with healthy controls. Like other inflammatory arthritis, PSA is characterized by monocytes and PMNs infiltration in the synovial tissue,98which are responsible for CLP production. In PSA, CLP is produced also in the skin by keratinocytes21 and CLP serum levels correlate with the extent of skin involvement.99 However, high circulating CLP levels appear to be associated with articular rather than skin involvement, since they are higher in PSA patients compared with psoriatic patients. The activation of the monocyte/macrophage system might be responsible for the increase in CLP in patients with arthritis compared with psoriasis.54,100
As in other SpA, in PSA, CLP is typically localized in perivascular areas in the synovial tissue.54 Such distribution might be related to the distinctive synovial macro-vascular changes found in PSA, which are not observed in RA.101
CLP seems to be a useful biomarker since it correlates with clinical measures, including the number of involved joints,100 the Ritchie Articular Index, and systemic inflammatory indices.54 In a study by Kane et al.54 in PSA patients, CLP was a sensitive and specific indicator of treatment response, as previously reported in SpA.54 CLP was even more sensitive than clinical joint scores in assessing response to MTX treatment.54 Notably, Madland et al.70 found that high CLP serum levels were associated with peripheral radiographic abnormalities thereby suggesting a more erosive disease.
Adult-onset still’s disease
Adult-onset still’s disease (AOSD) is characterized by high levels of inflammation, and ferritin is the most commonly used laboratory biomarker, although it is not specific for AOSD diagnosis and follow-up. Two studies are available on CLP in AOSD patients.102,103 In the study by Guo et al.,102 serum levels of CLP were higher in AOSD patients than in controls or other autoimmune disorders, including RA, SLE, and Sjögren’s syndrome (SS), thereby supporting CLP use as a diagnostic biomarker.102 In both studies, CLP showed to have a positive correlation with laboratory biomarkers particularly ferritin102,103 and to a lesser extent leukocyte count, CRP, and liver enzymes.103A negative correlation was found with haemoglobin.102 CLP was also associated with disease activity scores and treatment response.102,103 No association was found between CLP and clinical manifestations, apart from sore throat, which is one of the early disease manifestations.102
Gout
A study by Holzinger et al.104 demonstrated that S100A8 and S100A9 proteins are actively secreted by monocytes at moderate monosodium urate monohydrate (MSU) crystal concentrations. At high MSU crystal concentrations, these proteins are also passively released following neutrophil and monocyte death.104,105
IL-1β plays a pivotal role in MSU crystals-induced inflammation. CLP provides a danger signal which induces the expression of IL-1β precursor in vitro. IL-1β is activated through cleavage by the inflammasome after a second signal from MSU crystals.104 This function was further confirmed in a murine model of MSU-induced inflammation, where S100A9-knockout mice had lower IL-1β secretion and moderately reduced recruitment of neutrophils and monocytes compared with wild-type mice.104 The moderate impairment of IL-1β secretion suggests that other endogenous triggers may be involved and that S100A8 and S100A9 proteins have a role in maintaining and amplifying rather than in inducing the inflammatory process.
In the study by Holzinger et al., CLP serum levels ≥2000 ng/ml were found in patients with active gout; similar levels were found in patients with RA, SpA, PSA, and calcium pyrophosphate dehydrate deposition disease (CPPD). A correlation between CLP in the serum and in the synovial fluid was observed. In the synovial fluid, CLP levels were significantly higher in gout compared with RA, SpA, PSA, and OA, but similar to those observed in CPPD.104 CLP correlates with disease activity in gout, being high in the acute phase, and decreasing after treatment with non-steroidal anti-inflammatory drugs and betamethasone. Serum levels were higher in gouty patients compared with controls during the inter-critical phase of the disease suggesting the persistence of subclinical inflammation. A potential source of CLP production could be the S100A8 and S100A9 positive cells found in the tophi of gouty patients.104,106 Thus, CLP seems to be a better biomarker of gout than IL-1β, which is unstable in serum and therefore not suitable for use in clinical practice.107
Osteoarthritis
There is a lot of evidence of CLP being involved in cartilage damage in OA. Synovial lining macrophages play a crucial role in the OA process and in cartilage damage.108 For example, they release IL-1β, which is pivotal in cartilage destruction.109 S100A8 and S100A9 are the most expressed proteins by macrophages14 and they have been shown to induce a specific inflammatory response in human chondroblasts, mediated by TLR4.52 Furthermore, they cause a catabolic phenotype in murine chondrocytes, characterized by the release of MMP-1, MMP-3, MMP-9, and the inhibition of matrix molecule production.22,57,110 S100A8 and S100A9 stimulate other cells, such as fibroblast-like type B cells to produce MMPs.111 In a murine OA model, S100A9-knockout mice had reduced damage compared with wild-type controls, which further confirms the role of these proteins in cartilage destruction.24 Furthermore, in synovial biopsies from OA patients, levels of S100A8 and S100A9 correlated with markers of tissue damage, such as synovial lining thickness, sub-intima cellularity, and cartilage destruction.57
Synovial CLP expression seems to be increased only if synovial inflammation is present. Vogl et al.57 demonstrated that in a murine model of collagenase-induced OA, which is characterized by synovial inflammation, the expression of S100A8 and S100A9 was higher compared with another model of OA, without synovial inflammation. Also, CLP serum levels were found higher in OA patients with synovial inflammation compared with those without.112
CLP levels have been found at high levels also in the synovial fluid (up to 5–7 µg/ml).42 A moderate CLP increase has been observed in the serum of OA patients compared with healthy controls, particularly in early OA.37,57,113 Notably, in the study by Vogl et al.57, high CLP serum levels were also predictive of cartilage damage: a cutoff value of 0.6 µg/ml was associated with an odds ratio of 7.5.
Systemic lupus erythematosus
In SLE, CLP is expressed by monocytes, PMNs and also plasmocytoid DCs upon immune complex stimulation.114 SLE plasmocytoid DCs and leukocytes, except T cells, express the CLP complex on their cell surface114 bound to heparan sulphate proteoglycans and carboxylated glycans.40 Along with active secretion, CLP is released in NETs30,31 and passively by necrotic PMNs19 CLP might stimulate the adaptive immune system in SLE through TLR4 signaling in auto-reactive CD8+ T cells leading to the increase in IL-17 expression.44
CLP expression is up-regulated in SLE kidneys and skin. It has been found in renal tissue from SLE patients with glomerulonephritis, especially with proliferative lesions, and in the epidermis where it is produced by keratinocytes lesions.4,44,115 Skin stressors, including UV exposure,116lead to an inflammatory response, which is usually part of the physiological mechanism of wound healing, but might also be responsible for autoimmune disregulation in skin lesions.21
Patients with SLE have higher serum CLP levels compared with healthy individuals and also SS patients.117,118 Serum CLP has been found correlated with disease activity,3,114,118 particularly with SLE activity index (SLEDAI) score.3,118 CLP serum levels have been also found higher in patients with inactive disease compared with controls and correlating with the systemic lupus international collaborating clinics/American College of Rheumatology (SLICC/ACR) damage index.119 This association supports the idea that the persistence of subclinical inflammation can be responsible for disease progression.120
CLP seems associated with some clinical manifestations in SLE. Haga et al.3 described high CLP serum levels associated with arthritis and positive anti-double strand DNA antibodies.3 In a study by Tyden et al.,119 CLP serum levels were high in SLE patients with acute myocardial infarction and cerebro-vascular events, probably due to the involvement of CLP in the initiation and progression of atherosclerosis through RAGE and TLR4.121 However, CLP circulating levels were not increased in patients with venous thromboembolism,119 despite CLP being implicated in the thrombogenic response of the endothelium. Vessel inflammation might be an additional source of CLP122and could perpetrate a positive feedback loop maintaining both inflammation and atherogenesis in SLE patients. Thus, elevated CLP serum levels in SLE could be helpful in identifying patients at risk of cardiovascular events who might benefit from preventive treatment.
Interestingly, there is evidence that CLP might be an effective treatment target in SLE. Quinoline-3-carboxamides, which bind S100A9 inhibiting CLP interaction with RAGE and TLR4, are about to enter a phase II study for the treatment of SLE.123
Sjögren syndrome
Increased serum levels of CLP in SS patients compared with healthy subjects were first reported by Kuruto et al.117 and subsequently confirmed by other studies,102,124except one study which did not specifically refer to primary SS.61 Nordal et al.125 showed that CLP serum levels correlated with some indices of disease activity, especially with the fatigue score, but not with ESR and CRP; no association between CLP and arthritis was observed. CLP was found in saliva from SS patients at a concentration higher than in healthy subjects, and CLP levels were higher in the saliva than in the serum of SS patients.61,124,126 Notably, faecal CLP is increased in SS patients compared with normal subjects.127 Indeed, CLP is also produced by exocrine glands of the gastrointestinal mucosa in SS patients.
CLP was also found in salivary gland biopsies from SS patients.124,125 CLP might be locally released by inflammatory cells, as well as actively secreted by epithelial cells.128
The evidence that RAGE, a receptor of CLP, is over-expressed by myoepithelial cells in labial salivary glands of SS patients confirms the implication of CLP in the development of local inflammation.129 No correlation has been found between CLP expression in the salivary glands and the lymphocytic focus score,61,125 CLP concentration in the saliva might be a promising marker of local inflammatory activity in SS.
Systemic sclerosis
CLP plays a central role in skin injury by maintaining persistent inflammation and triggering the pro-fibrotic response. S100A8 and S100A9 are produced by injured keratinocytes21 and by plasmocytoid DCs via TLR4.114 In addition, they are highly expressed in infiltrating mononuclear cells in the early stages of systemic sclerosis (SSc).130CLP is able to activate fibroblasts, which proliferate and produce pro-fibrotic cytokines as well as further CLP.21,130 CLP receptors, TLR4, and RAGE are highly expressed on the surface of fibroblasts and probably mediate CLP signal in these cells.130,131 RAGE is implicated in the development of lung fibrosis132 and its expression on the fibroblast cell surface has been correlated with SSc severity.133 TLR4 signaling has been found to induce fibroblast proliferation134 and the activation of plasmocytoid DCs.114 Notably, TLR4 inhibition hindered the fibrotic process in a model of LPS-induced lung fibrosis.135
High concentrations of S100A8 and S100A9 have been found in the skin, bronchoalveolar lavage (BAL), saliva130,131,136–138 and also in the peripheral blood cells of SSc patients.117,127,139 In a cohort of Asian patients, serum CLP levels were higher in those with diffuse cutaneous SSc compared with limited SSc,130 while in Caucasian patients CLP levels were increased only in limited SSc associated with lung fibrosis.139 Although no clear correlation with disease activity was found, CLP serum levels seem to be associated with specific and severe manifestations of SSc, including lung fibrosis, kidney involvement (only S100A8), myositis and myalgia (only S100A9), and arthritis and arthralgia.130 Xu et al.130 reported high S100A8 and S100A9 serum levels in diffuse SSc with anti-Scl70, anti-hystone, or anti-U1RNP antibodies. Van Bon et al.139 confirmed the association between high S100A8 serum levels and anti-Scl70, which is a marker of severe disease. The production of CLP in the gastrointestinal mucosa of SSc patients was also shown. Indeed, CLP was increased in the faeces of SSc patients compared with other autoimmune disorders and was correlated with the severity of digestive symptoms.127,140
Importantly, the association between CLP serum levels and lung fibrosis is supported by higher CLP and S100A9 concentrations in the BAL of SSc patients with lung involvement compared with patients without lung involvement and healthy controls.136,138 However, this result was not confirmed by other authors.141 Furthermore, high CLP concentrations in the BAL were associated with extensive fibrosis by high-resolution computed tomography and were correlated with BAL eosinophil count.138 Thus, since CLP is a marker of severe disease, and especially of lung fibrosis, it might be useful in identifying patients who need a tight follow up.
Idiopathic inflammatory myopathies
Although the major idiopathic inflammatory myopathies (IIM) subsets, polymyositis (PM), dermatomyositis (DM), inclusion body myositis (IBM), and juvenile dermatomyositis (JDM) are characterized by different pathogenic mechanisms, they share some common features, including a similar cytokine expression pattern and the important role of macrophages in muscle lesions.142
In fact, S100A8 and S100A9 proteins are expressed by macrophages in muscles where they induce the release of inflammatory mediators.143–145 A clear association between S100A8 and S100A9 expression and the degeneration of muscular fibers was shown in muscle biopsies from PM, DM, and IBM patients.145 CLP inhibits the differentiation and proliferation of myoblasts in vitro through the reduction of myotube formation and expression of myocyte differentiation markers.145,146 At high levels, CLP induces apoptosis of myoblasts by caspase-3 activation.145
Some indirect evidence of the role of CLP in IIM derives from the observation that TLR4 is involved in the pathogenesis of IIM147,148 and in the promotion of autophagy,149 which is implicated in myositis, particularly in IBM.13,19 TLR4, the main CLP receptor, is highly expressed in muscle tissue from patients with IIM and was more expressed in PM and DM than in JDM, IBM, and controls.150
In a study by Nistala et al.,144 S100A8 was shown to induce the secretion of MCP-1 and IL-6 from skeletal muscle cells in vitro. Indeed, MCP-1 mediates the activation of monocytes and memory T cells and the differentiation of local B cells into plasma cells in the muscles of DM patients.151 Once recruited and activated, myeloid cells amplify the inflammatory signal by producing CLP.145 Thus, CLP could represent a link between the innate immune stimulation and activation of cells of the adaptive immune system.
Behçet’s disease
Serum levels of CLP were found higher in a cohort of 47 patients with Behçet’s disease (BD) compared with healthy controls.152 CLP was also tested as a disease biomarker, but no correlations were found with disease activity scores or quality of life indices in BD patients.152
Uveitis and oral aphthous ulcerations might be potential sources of S100A8 and S100A9 production in BD. In fact, raised CLP serum levels have been found in patients with uveitis153–155 and it has been shown that mucosal squamous epithelial cells can express CLP under inflammatory conditions.156–158
CLP might have a role in the pathogenesis of vascular involvement in BD.42,43,159 CLP is implicated in neutrophil recruitment and extravasation leading to tissue injury.160 The thrombogenic phenotype, induced by CLP-activated endothelial cells, could contribute to the thrombotic manifestations of BD, reported in up to 40% of the patients.161
Antineutrophil cytoplasm antibody-associated vasculitis
In two studies including antibody-associated vasculitis (AAV) patients, CLP was found highly expressed on the surface of circulating leukocytes along with high serum levels of this protein in patients with AAV compared with controls.162,163 CLP serum levels correlated with disease activity but not with antineutrophil cytoplasm antibodies (ANCA), CRP or WBC.162,163 CLP decreased during treatment but did not get back into the normal range, suggesting the persistence of subclinical inflammation.162,163 Importantly, the increase of CLP serum levels seems to predict a disease relapse with a higher likelihood than ANCA serum levels.162,164
CLP is expressed in the kidneys of patients with AAV-associated glomerulophritis. CLP positive leukocytes and macrophages are present in glomeruli and their concentration correlates with the severity of histological inflammation. A prominent CLP expression has been observed in areas of focal necrosis and active crescents, but not in sclerotic glomeruli.4,162,165 A pathogenetic role of CLP is endorsed by an experimental vasculitis model with S100A9-knockout mice showing a significant decrease in the inflammatory vascular lesions.166 Furthermore, TLR4, the major CLP ligand, is expressed in glomerular endothelial cells and has been involved in the development of renal injury in animal models.41,162
Giant cell arteritis and polymyalgia rheumatica
A few studies with a low number of patients were focused on CLP in giant cell arteritis (GCA) and polymyalgia rheumatica (PMR).79,167,165 In all these studies, serum levels of CLP were higher in PMR and GCA than in controls. As expected, serum levels correlated with inflammatory biomarkers and decreased in response to treatment.81,167,168 Notably, CLP lowered after prednisone initiation and the decrease was inversely correlated with oral prednisone dose.81
In a study by Brun et al.,81 CLP was found abundantly expressed in the adventitia and media of affected arteries. Like in other vasculitis, CLP probably mediates the activation of endothelial cells in an early disease stage, contributes to the maintenance of vessel inflammation, and leads to leukocyte extravasation from the vasa vasorum into the adventitia, which is pivotal in GCA.167 CLP might also damage arterial smooth muscle cells in the adventitia and media. In fact, infiltrating macrophages produce CLP168 which can induce the apoptosis of myoblasts.145 In addition to the production in the arterial wall, in PMR other inflamed tissues, such as bursae and synovial tendon sheaths, might be responsible for the release of CLP and the increase in serum levels.
Conclusions
CLP is considered an acute-phase protein which is not synthesized in the liver but produced by activated PMNs in the circulation and inflamed tissues. In contrast to cytokines such as IL-6, TNF-α or IL-1β, CLP is relatively stable and easily measurable in the serum, which makes this protein a candidate biomarker in inflammatory diseases. Nevertheless, a comparison of CLP levels between the studies is not possible. In clinical studies, CLP levels have been detected with either commercial kits or home-made ELISAs. Every test has a different sensitivity and different limits to define a positive CLP, ranging from 3 ng/ml and 2.9 µg/ml.76,119 A standardized method to measure CLP concentration is required. A potential diagnostic role of CLP has not been clarified yet. CLP serum levels are particularly high in AOSD, but cut-off levels need to be identified and tested in larger populations. CLP levels in other fluids, such as saliva and synovial fluid, might be helpful in the diagnosis of SS and gout or RA, respectively. However, concomitant non-autoimmune inflammatory conditions must be considered, particularly infections, which can be associated with rheumatic diseases and can also raise CLP serum levels.118
Of interest is the possible role of S100A8 and/or S100A9 as a target of treatment. There is evidence that a number of immunomodulators, including TNF-α inhibitors or JAK/STAT inhibitors,169 may reduce CLP expression. In murine models of autoimmune disorders, the direct or indirect blockade of S100A8 or S100A9 exerted a beneficial effect.
CLP seems to be more accurate than CRP, as it is able to detect minimal residual inflammation and can predict disease relapse in some autoimmune diseases including SLE, BD, and AAV. High CLP serum levels are also associated with worse structural outcomes in RA and, to a lesser extent, in SpA. Thus, they might have a role in the therapeutic decision, especially in the treatment tapering.170 Furthermore, high CLP serum levels are associated with some severe manifestations of connective tissue diseases, such as glomerulonephritis in SLE and AAV and lung fibrosis in SSc, so can identify patients who need an accurate screening and tight follow-up. Although its pathogenic role remains to be elucidated, CLP has demonstrated to be a highly sensitive biomarker of inflammation. In future, once cut-off levels will be identified in the different rheumatic diseases, CLP might replace classical markers of systemic inflammation.
Acknowledgments
The authors wish to thank Anne Lewis for revising the text.
Authors’ contributions
FO, BR, and DA made substantial contributions to the literature review, and drafted and revised the manuscript. LF and DA made substantial contributions to the literature review. CB, LP, and AD helped to draft and revise the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
There was no funding to support this minireview.
References
- 1.Goebeler M, Roth J, van den Bos C, Ader G, Sorg C. Increase of calcium levels in epithelial cells induces translocation of calcium-binding proteins migration inhibitory factor-related protein 8 (MRP8) and MRP14 to keratin intermediate filaments. Biochem J 1995; 309: 419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem 1991; 266: 7706–13. [PubMed] [Google Scholar]
- 3.Haga HJ, Brun JG, Berntzen HB, Cervera R, Khamashta M, Hughes GR. Calprotectin in patients with systemic lupus erythematosus: relation to clinical and laboratory parameters of disease activity. Lupus 1993; 2: 247–50. [DOI] [PubMed] [Google Scholar]
- 4.Frosch M, Vogl T, Waldherr R, Sorg C, Sunderkoütter C, Roth J. Expression of MRP8 and MRP14 by macrophages is a marker for severe forms of glomerulonephritis. J Leukoc Biol 2004; 75: 198–206. [DOI] [PubMed] [Google Scholar]
- 5.Horvath I, Jia X, Johansson P, Wang C, Moskalenko R, Steinau A, Forsgren L, Wagberg T, Svensson J, Zetterberg H, Morozova-Roche L. Pro-inflammatory S100A9 protein as a robust biomarker differentiating early stages of cognitive impairment in Alzheimer’s disease. ACS Chem Neurosci 2016; 7: 34–9. [DOI] [PubMed] [Google Scholar]
- 6.Markowitz J, Carson WE., III Review of S100A9 biology and its role in cancer. Biochim Biophys Acta 2013; 1835: 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 2009; 86: 557–66. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen SJ, Sorensen IJ, Lambert RG, Hermann KG, Garnero P, Johansen JS, Madsen OR, Hansen A, Hansen MS, Thamsborg G, Andersen LS, Majgaard O, Loft AG, Erlendsson J, Asmussen KH, Jurik AG, Møller J, Hasselquist M, Mikkelsen D, Østergaard M. Radiographic progression is associated with resolution of systemic inflammation in patients with axial spondylarthritis treated with tumor necrosis factor alpha inhibitors: a study of radiographic progression, inflammation on magnetic resonance imaging, and circulating biomarkers of inflammation, angiogenesis, and cartilage and bone turnover. Arthritis Rheum 2011; 63: 3789–800. [DOI] [PubMed] [Google Scholar]
- 9.Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut 2009; 58: 859–68. [DOI] [PubMed] [Google Scholar]
- 10.Myklebust G, Gran JT. A prospective study of 287 patients with polymyalgia rheumatica and temporal arteritis: clinical and laboratory manifestations at onset of disease and at the time of diagnosis. Br J Rheumatol 1996; 35: 1161–8. [DOI] [PubMed] [Google Scholar]
- 11.Mariani A, Marsili M, Nozzi M, Faricelli R, Chiarelli F, Breda L. Serum calprotectin: review of its usefulness and validity in paediatric rheumatic diseases. Clin Exp Rheumatol 2015; 33: 109–14. [PubMed] [Google Scholar]
- 12.Marenholz I, Lovering RC, Heizmann CW. An update of the S100 nomenclature. Biochim Biophys Acta 2006; 1763: 1282–3. [DOI] [PubMed] [Google Scholar]
- 13.Roth J, Burwinkel F, van den Bos C, Goebeler M, Vollmer E, Sorg C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood 1993; 82: 1875–83. [PubMed] [Google Scholar]
- 14.Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, Gerke V, Manitz MP, Nacken W, Werner S, Sorg C, Roth J. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 2004; 104: 4260–8. [DOI] [PubMed] [Google Scholar]
- 15.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum 2004; 50: 3762–71. [DOI] [PubMed] [Google Scholar]
- 16.Korndorfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha- helices can determine specific association of two EF-hand proteins. J Mol Biol 2007; 370: 887–98. [DOI] [PubMed] [Google Scholar]
- 17.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 2007; 13: 1042–9. [DOI] [PubMed] [Google Scholar]
- 18.Frosch M, Strey A, Vogl T, Wulffraat NM, Kuis W, Sunderkötter C, Harms E, Sorg C, Roth J. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum 2000; 43: 628–37. [DOI] [PubMed] [Google Scholar]
- 19.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem 1997; 272: 9496–502. [DOI] [PubMed] [Google Scholar]
- 20.Yen T, Harrison CA, Devery JM, Leong S, Iismaa SE, Yoshimura T, Geczy CL. Induction of the S100 chemotactic protein, CP-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood 1997; 90: 4812–21. [PubMed] [Google Scholar]
- 21.Nukui T, Ehama R, Sakaguchi M, Sonegaw H, Katagiri C, Hibino T, Huh NH. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J Cell Biochem 2008; 104: 453–64. [DOI] [PubMed] [Google Scholar]
- 22.Van Lent PL, Grevers LC, Blom AB, Arntz OJ, van de Loo FA, van der Kraan P, Abdollahi-Roodsaz S, Srikrishna G, Freeze H, Sloetjes A, Nacken W, Vogl T, Roth J, van den Berg WB. Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum 2008; 58: 3776–87. [DOI] [PubMed] [Google Scholar]
- 23.Youssef P, Roth J, Frosch M, Costello P, Fitzgerald O, Sorg C, Bresnihan B. Expression of myeloid related proteins (MRP) 8 and 14 and the MRP8/14 heterodimer in rheumatoid arthritis synovial membrane. J Rheumatol 1999; 26: 2523–8. [PubMed] [Google Scholar]
- 24.Van Lent PL, Grevers L, Blom AB, Sloetjes A, Mort JS, Vogl T, Nacken W, van den Berg WB, Roth J. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann Rheum Dis 2008; 67: 1750–8. [DOI] [PubMed] [Google Scholar]
- 25.Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14). J Infect Dis 2000; 182: 1272–5. [DOI] [PubMed] [Google Scholar]
- 26.Clark MA, Plank LD, Connolly AB, Streat SJ, Hill AA, Gupt R, Monk DN, Shenkin A, Hill GL. Effect of a chimeric antibody to tumor necrosis factor-alpha on cytokine and physiologic responses in patients with severe sepsis – a randomized, clinical trial. Crit Care Med 1998; 26: 1650–9. [DOI] [PubMed] [Google Scholar]
- 27.Burwinkel F, Roth J, Goebeler M, Bitter U, Wrocklage V, Vollmer E, Roessner A, Sorg C, Bocker W. Ultrastructural localization of the S-100-like proteins MRP8 and MRP14 in monocytes is calcium-dependent. Histochemistry 1994; 101: 113–20. [DOI] [PubMed] [Google Scholar]
- 28.Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J 2005; 19: 467–9. [DOI] [PubMed] [Google Scholar]
- 29.Kerkhoff C, Klempt M, Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9). Biochim Biophys Acta 1998; 1448: 200–11. [DOI] [PubMed] [Google Scholar]
- 30.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extra-cellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 2009; 5: e1000639–e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, Heesemann J, Ebel F. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigates. MicrobesInfect 2010; 12: 928–936. [DOI] [PubMed] [Google Scholar]
- 32.Bjork P, Bjork A, Vogl T, Stenstrom M, Liberg D, Olsson A, Roth J, Ivars F, Leanderson T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol 2009; 7: e97–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta 2009; 1793: 993–1007. [DOI] [PubMed] [Google Scholar]
- 34.Fassl SK, Austermann J, Papantonopoulou O, Riemenschneider M, Xue J, Bertheloot D, Freise N, Spiekermann C, Witten A, Viemann D, Kirschnek S, Stoll M, Latz E, Schultze JL, Roth J, Vogl T. Transcriptome assessment reveals a dominant role for TLR4 in the activation of human monocytes by the alarmin MRP8. J Immunol 2015; 194: 575–83. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res 2011; 9: 133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steenvoorden MM, Toes RE, Ronday HK, Huizinga TW, Degroot J. RAGE activation induces invasiveness of RA fibroblast-like synoviocytes in vitro. Clin Exp Rheumatol 2007; 25: 740–2. [PubMed] [Google Scholar]
- 37.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S, Makino H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther 2006; 8: R69–R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryckman C, McColl SR, Vandal K, de Medicis R, Lussier A, Poubelle PE, Tessier PA. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum 2003; 48: 2310–20. [DOI] [PubMed] [Google Scholar]
- 39.Anceriz N, Vandal K, Tessier PA. S100A9 mediates neutrophil adhesion to fibronectin through activation of beta2 integrins. Biochem Biophy Res Commun 2007; 354: 84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson MJ, Tessier P, Poulsom R, Hogg N. The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J Biol Chem 2002; 277: 3658–65. [DOI] [PubMed] [Google Scholar]
- 41.Summers SA, van der Veen BS, O’Sullivan KM, Gan PY, Ooi JD, Heeringa P, Satchell SC, Mathieson PW, Saleem MA, Visvanathan K, Holdsworth SR, Kitching AR. Intrinsic renal cell and leukocyte-derived TLR4 aggravate experimental anti-MPO glomerulonephritis. Kidney Int 2010; 78: 1263–74. [DOI] [PubMed] [Google Scholar]
- 42.Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B, Gerke V, Sorg C, Roth J. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 2005; 105: 2955–62. [DOI] [PubMed] [Google Scholar]
- 43.Viemann D, Barczyk K, Vogl T, Fischer U, Sunderkötter C, Schulze-Osthoff K, Roth J. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood 2007; 109: 2453–60. [DOI] [PubMed] [Google Scholar]
- 44.Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, Kuhn A, Foell D, Sorokin L, Luger TA, Roth J, Beissert S. The toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med 2010; 16: 713–7. [DOI] [PubMed] [Google Scholar]
- 45.Averill MM, Barnhart S, Becker L, Li X, Heinecke JW, Leboeuf RC, Hamerman JA, Sorg C, Kerkhoff C, Bornfeldt KE. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation 2011; 123: 1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chih-Ru L, Tong-You WW, Hsien-Yu T, Ying-Ta W, Pei-Yu W, Shui-Tein C. Glycosylation-dependent interaction between CD69 and S100A8/S100A9 complex is required for regulatory T-cell differentiation. FASEB J 2015; 29: 5006–17. [DOI] [PubMed] [Google Scholar]
- 47.Baillet A, Trocme C, Berthier S, Arlotto M, Grange L, Chenau J, Quétant S, Sève M, Berger F, Juvin R, Morel F, Gaudin P. Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatology 2010; 49: 671–82. [DOI] [PubMed] [Google Scholar]
- 48.Hammer HB, Odegard S, Fagerhol MK, Landewé R, van der Heijde D, Uhlig T, Mowinckel P, Kvien TK. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis 2007; 66: 1093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammer HB, Ødegård S, Syversen SW, Landewé R, van der Heijde D, Uhlig T, Mowinckel P, Kvien TK. Calprotectin (a major S100 leucocyte protein) predicts 10-year radiographic progression in patients with rheumatoid arthritis. Ann Rheum Dis 2010; 69: 150–4. [DOI] [PubMed] [Google Scholar]
- 50.Carrión M, Juarranz Y, Martínez C, González-Alvaro I, Pablos JL, Gutiérrez-Canas I, Gomariz RP. IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatology 2003; 52: 2177–86. [DOI] [PubMed] [Google Scholar]
- 51.Zreiqat H, Howlett CR, Gronthos S, Hume D, Geczy CL. S100A8/S100A9 and their association with cartilage and bone. J Mol Histol 2007; 38: 381–91. [DOI] [PubMed] [Google Scholar]
- 52.Schelbergen RF, Blom AB, van den Bosch MH, Slöetjes A, Abdollahi-Roodsaz S, Schreurs BW, Mort JS, Vogl T, Roth J, van den Berg WB, van Lent PL. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum 2012; 64: 1477–87. [DOI] [PubMed] [Google Scholar]
- 53.Pap T, Muller-Ladner U, Gay RE, Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res 2000; 2: 361–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kane D, Roth J, Frosch M, Vogl T, Bresnihan B, FitzGerald O. Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum 2003; 48: 1676–85. [DOI] [PubMed] [Google Scholar]
- 55.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 2007; 7: 429–42. [DOI] [PubMed] [Google Scholar]
- 56.Neumann E, Lefèvre S, Zimmermann B, Gay S, Müller-Ladner U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol Med 2010; 16: 458–68. [DOI] [PubMed] [Google Scholar]
- 57.Van Lent PL, Blom AB, Schelbergen RF, Slöetjes A, Lafeber FP, Lems WF, Cats H, Vogl T, Roth J, van den Berg WB. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum 2012; 64: 1466–76. [DOI] [PubMed] [Google Scholar]
- 58.Nishimoto N, Ito A, Ono M, Tagoh H, Matsumoto T, Tomita T, Ochi T, Yoshizaki K. IL-6 inhibits the proliferation of fibroblastic synovial cells from rheumatoid arthritis patients in the presence of soluble IL-6 receptor. Int Immunol 2000; 12: 187–93. [DOI] [PubMed] [Google Scholar]
- 59.Simard JC, Simon MM, Tessier PA, Girard D. Damage- associated molecular pattern S100A9 increases bactericidal activity of human neutrophils by enhancing phagocytosis. J Immunol 2011; 186: 3622–31. [DOI] [PubMed] [Google Scholar]
- 60.Grevers LC, de Vries TJ, Vogl T, Abdollahi-Roodsaz S, Sloetjes AW, Leenen PJ, Roth J, Everts V, van den Berg WB, van Lent PL. S100A8 enhances osteoclastic bone resorption in vitro through activation of Toll-like receptor 4. Arthritis Rheum 2011; 63: 1365–75. [DOI] [PubMed] [Google Scholar]
- 61.Brun JG, Jonsson R, Haga HJ. Measurement of plasma calprotectin as an indicator of arthritis and disease activity in patients with inflammatory rheumatic diseases. J Rheumatol 1994; 21: 733–8. [PubMed] [Google Scholar]
- 62.Drynda S, Ringel B, Kekow M, Kühne C, Drynda A, Glocker MO, Thiesen HJ, Kekow J. Proteome analysis reveals disease-associated marker proteins to differentiate RA patients from other inflammatory joint diseases with the potential to monitor anti-TNFalpha therapy. Pathol Res Pract 2004; 200: 165–71. [DOI] [PubMed] [Google Scholar]
- 63.Uchida T, Fukawa A, Uchida M, Fujita K, Saito K. Application of a novel protein biochip technology for detection and identification of rheumatoid arthritis biomarkers in synovial fluid. J Proteome Res 2002; 1: 495–9. [DOI] [PubMed] [Google Scholar]
- 64.de Seny D, Fillet M, Ribbens C, Marée R, Meuwis MA, Lutteri L, Chapelle JP, Wehenkel L, Louis E, Merville MP, Malaise M. Monomeric calgranulins measured by SELDI-TOF mass spectrometry and calprotectin measured by ELISA as biomarkers in arthritis. Clin Chem 2008; 54: 1066–75. [DOI] [PubMed] [Google Scholar]
- 65.Lee DG, Woo JW, Kwok SK, Cho ML, Park SH. MRP8 promotes Th17 differentiation via upregulation of IL-6 production by fibroblast-like synoviocytes in rheumatoid arthritis. Exp Mol Med 2013; 45: e20–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berntzen HB, Munthe E, Fagerhol MK. A longitudinal study of the leukocyte protein L1 as an indicator of disease activity in patients with rheumatoid arthritis. J Rheumatol 1998; 16: 1416–20. [PubMed] [Google Scholar]
- 67.Sinz A, Bantscheff M, Mikkat S, Ringel B, Drynda S, Kekow J, Thiesen HJ, Glocker MO. Mass spectrometric proteome analyses of synovial fluids and plasmas from patients suffering from rheumatoid arthritis and comparison to reactive arthritis or osteoarthritis. Electrophoresis 2002; 23: 3445–56. [DOI] [PubMed] [Google Scholar]
- 68.Berntzen HB, Munthe E, Fagerhol MK. A longitudinal study of the leukocyte protein L1 as an indicator of disease activity in patients with rheumatoid arthritis. J Rheumatol 1989; 16: 1416–20. [PubMed] [Google Scholar]
- 69.Brun JG, Haga HJ, Boe E, Kallay I, Lekven C, Berntzen HB, Fagerhol MK. Calprotectin in patients with rheumatoid arthritis: relation to clinical and laboratory variables of disease activity. J Rheumatol 1992; 19: 85962–85962. [PubMed] [Google Scholar]
- 70.Madland TM, Hordvik M, Haga HJ, Jonsson R, Brun JG. Leukocyte protein calprotectin and outcome in rheumatoid arthritis. Scand J Rheumatol 2002; 31: 351–4. [DOI] [PubMed] [Google Scholar]
- 71.Hammer HB, Haavardsholm EA, Kvien TK. Calprotectin (a major leucocyte protein) is associated with the levels of anti-CCP and rheumatoid factor in a longitudinal study of patients with very early rheumatoid arthritis. Scand J Rheumatol 2008; 37: 179–82. [DOI] [PubMed] [Google Scholar]
- 72.Hammer HB, Fagerhol MK, Wien TN, Kvien TK. The soluble bio-marker calprotectin (a S100 protein) is associated to ultrasonographic synovitis scores and is sensitive to change in patients with rheumatoid arthritis treated with adalimumab. Arthritis Res Ther 2011; 13: R178–R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Arias M, Pascual-Salcedo D, Ramiro S, Ueberschlag ME, Jermann TM, Cara C, Martín-Mola E, Balsa A. Calprotectin in rheumatoid arthritis: association with disease activity in a cross-sectional and a longitudinal cohort. Mol Diagn Ther 2013; 17: 49–56. [DOI] [PubMed] [Google Scholar]
- 74.Andrés Cerezo L, Mann H, Pecha O, Pleštilová L, Pavelka K, Vencovský J, Senolt L. Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent-onset rheumatoid arthritis. Arthritis Res Therapy 2011; 13: R122–R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi IY, Gerlag DM, Herenius MJ, Thurlings RM, Wijbrandts CA, Foell D, Vogl T, Roth J, Tak PP, Holzinger D. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis 2015; 74: 499–505. [DOI] [PubMed] [Google Scholar]
- 76.Inciarte-Mundo J, Ruiz-Esquide V, Hernandez MV, Canete JD, Cabrera-Villalba SR, Ramirez J, Yagu J, Sanmarti R. Calprotectin more accurately discriminates the disease status of rheumatoid arthritis patients receiving tocilizumab than acute phase reactants. Rheumatology 2015; 54: 2239–4. [DOI] [PubMed] [Google Scholar]
- 77.Inciarte-Mundo J, Hernandez MV, Ruiz-Esquide V, Cabrera-Villalba SR, Ramirez J, Cuervo A, Pascal M, Yague J, Canete JD, Sanmarti R. Serum calprotectin versus acute-phase reactants in the discrimination of inflammatory disease activity in rheumatoid arthritis patients receiving tumor necrosis factor inhibitors. Arthritis Care Res 2015; 7: 899–906. [DOI] [PubMed] [Google Scholar]
- 78.Ometto F, Botsios C, Raffeiner B, Sfriso P, Bernardi L, Todesco S, Doria A, Punzi L. Methods used to assess remission and low disease activity in rheumatoid arthritis. Autoimmun Rev 2010; 9: 161–4. [DOI] [PubMed] [Google Scholar]
- 79.Meugnier E, Coury F, Tebib J, Ferraro-Peyret C, Rome S, Bienvenu J, Vidal H, Sibilia J, Fabien N. Gene expression profiling in peripheral blood cells of patients with rheumatoid arthritis in response to anti-TNF-alpha treatments. Physiol Genomics 2011; 43: 365–71. [DOI] [PubMed] [Google Scholar]
- 80.Obry A, Lequerre T, Hardouin J, Boyer O, Fardellone P, Philippe P, Le Loët X, Cosette P, Vittecoq O. Identification of S100A9 as biomarker of responsiveness to the methotrexate/etanercept combination in rheumatoid arthritis using a proteomic approach. PLoS One 2014; 9: e115800–e115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brun JG, Madland TM, Gran JT, Myklebust G. A longitudinal study of calprotectin in patients with polymyalgia rheumatica or temporal arteritis: relation to disease activity. Scand J Rheumat 2005; 34: 125–8. [DOI] [PubMed] [Google Scholar]
- 82.Klingberg E, Carlsten H, Hilme E, Hedberg M, Forsblad-d’Elia H. Calprotectin in ankylosing spondylitis – frequently elevated in faeces, but normal in serum. Scand J Gastroenterol 2012; 47: 435–44. [DOI] [PubMed] [Google Scholar]
- 83.Hsu K, Passey RJ, Endoh Y, Rahimi F, Youssef P, Yen T, Geczy CL. Regulation of S100A8 by glucocorticoids. J Immunol 2005; 174: 2318–26. [DOI] [PubMed] [Google Scholar]
- 84.Inciarte-Mundo J, Ramirez J, Hernandez MV, Ruiz-Esquide V, Cuervo A, Cabrera-Villalba SR, Pascal M, Yague J, Canete JD, Sanmarti R. Calprotectin and TNF through serum levels identify power Doppler ultrasound synovitis in rheumatoid arthritis and psoriatic arthritis patients in remission or with low disease activity. Arthritis Res Ther 2016; 18: 160–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liao H, Wu J, Kuhn E, Chin W, Chang B, Jones MD, O’Neil S, Clauser KR, Karl J, Hasler F, Roubenoff R, Zolg W, Guild BC. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Rheum 2004; 50: 3792–803. [DOI] [PubMed] [Google Scholar]
- 86.Vogl T, Eisenblätter M, Völler T, Zenker S, Hermann S, van Lent P, Faust A, Geyer C, Petersen B, Roebrock K, Schäfers M, Bremer C, Roth J. Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity. Nat Commun 2014; 5: 4593–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cesaro A, Anceriz N, Plante A, Page N, Tardif MR, Tessier PA. An inflammation loop orchestrated by S100A9 and calprotectin is critical for development of arthritis. PLoS One 2012; 7: e45478–e45478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kruithof E, De Rycke L, Vandooren B, De Keyser F, Fitzgerald O, McInnes I, Tak PP, Bresnihan B, Veys EM, Baeten D. Identification of synovial biomarkers of response to experimental treatment in early-phase clinical trials in spondylarthritis. Arthritis Rheum 2006; 54: 1795–804. [DOI] [PubMed] [Google Scholar]
- 89.De Rycke L, Baeten D, Foell D, Kruithof E, Veys EM, Roth J, De Keyser F. Differential expression and response to anti-TNF treatment of infiltrating versus resident tissue macrophage subsets in auto-immune arthritis. J Pathol 2005; 206: 17–27. [DOI] [PubMed] [Google Scholar]
- 90.Oktayoglu P, Bozkurt M, Mete N, Caglayan M, Em S, Nas K. Elevated serum levels of calprotectin (myeloid-related protein 8/14) in patients with ankylosing spondylitis and its association with disease activity and quality of life. J Invest Med 2014; 62: 880–4. [DOI] [PubMed] [Google Scholar]
- 91.Turina MC, Yeremenko N, Paramarta JE, De Rycke L, Baeten D. Calprotectin (S100A8/9) as serum biomarker for clinical response in proof-of-concept trials in axial and peripheral spondyloarthritis. Arthritis Res Ther 2014; 16: 413–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duran A, Kobak S, Sen N, Aktakka S, Atabay T, Orman M. Fecal calprotectin is associated with disease activity in patients with ankylosing spondylitis. Bosn J Basic Med Sci 2015; 16: 71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klingberg E, Carlsten H, Hilme E, Hedberg M, Forsblad-d’Elia H. Calprotectin in ankylosing spondylitis – frequently elevated in faeces, but normal in serum. Scand J Gastroenterol 2012; 47: 435–44. [DOI] [PubMed] [Google Scholar]
- 94.Cypers H, Varkas G, Beeckman S, Debusschere K, Vogl T, Roth J, Drennan MB, Lavric M, Foell D, Cuvelier CA, De Vos M, Delanghe J, Van den Bosch F, Elewaut D. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann Rheum Dis 2016;75:1357–62. [DOI] [PMC free article] [PubMed]
- 95.Turina MC, Sieper J, Yeremenko N, Conrad K, Haibel H, Rudwaleit M, Baeten D, Poddubnyy D. Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann Rheum Dis 2014; 73: 1746–8. [DOI] [PubMed] [Google Scholar]
- 96.Klingberg E, Carlsten H, Hilme E, Hedberg M, Forsblad-d’Elia H. Calprotectin in ankylosing spondylitis – frequently elevated in faeces, but normal in serum. Scand J Gastroenterol 2012; 47: 435–44. [DOI] [PubMed] [Google Scholar]
- 97.Van Praet L, Jacques P, Van den Bosch F, Elewaut D. The transition of acute to chronic bowel inflammation in spondyloarthritis. Nat Rev Rheumatol 2012; 8: 288–95. [DOI] [PubMed] [Google Scholar]
- 98.Veale D, Yanni G, Rogers S, Barnes L, Bresnihan B, FitzGerald O. Reduced synovial membrane macrophage numbers, ELAM-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum 1993; 36: 893–900. [DOI] [PubMed] [Google Scholar]
- 99.Benoit S, Toksoy A, Ahlmann M, Schmidt M, Sunderkotter C, Foell D, Pasparakis M, Roth J, Goebeler M. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol 2006; 155: 62–6. [DOI] [PubMed] [Google Scholar]
- 100.Aochi S, Tsuji K, Sakaguchi M, Huh N, Tsuda T, Yamanishi K, Komine M, Iwatsuki K. Markedly elevated serum levels of calcium-binding S100A8/A9 proteins in psoriatic arthritis are due to activated monocytes/macrophages. J Am Acad Dermatol 2011; 64: 879–87. [DOI] [PubMed] [Google Scholar]
- 101.Reece RJ, Canete JD, Parsons WJ, Emery P, Veale DJ. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum 1999; 42: 1481–4. [DOI] [PubMed] [Google Scholar]
- 102.Guo Q, Zha X, Li C, Jia Y, Zhu L, Guo J, Su Y. Serum calprotectin a promising diagnostic marker for adult-onset Still’s disease. Clin Rheumatol 2016; 35: 73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jung SY, Park YB, Ha YJ, Lee KH, Lee SK. Serum calprotectin as a marker for disease activity and severity in adult-onset Still’s disease. J Rheumatol 2010; 37: 1029–34. [DOI] [PubMed] [Google Scholar]
- 104.Holzinger D, Frosch M, Kastrup A, Prince FH, Otten MH, Van Suijlekom-Smit LW, ten Cate R, Hoppenreijs EP, Hansmann S, Moncrieffe H, Ursu S, Wedderburn LR, Roth J, Foell D, Wittkowski H. The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann Rheum Dis 2012; 71: 974–80. [DOI] [PubMed] [Google Scholar]
- 105.Akahoshi T, Nagaoka T, Namai R, Sekiyama N, Kondo H. Prevention of neutrophil apoptosis by monosodium urate crystals. Rheumatol Int 1997; 16: 231–5. [DOI] [PubMed] [Google Scholar]
- 106.Dalbeth N, Pool B, Gamble GD, Smith T, Callon KE, McQueen FM, Cornish J. Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum 2010; 62: 1549–56. [DOI] [PubMed] [Google Scholar]
- 107.Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, Wittkowski H, Bek S, Hartmann N, Bosset S, Hawkins PN, Jung T. In vivo regulation of interleukin 1 in patients with cryopyrin-associated periodic syndromes. J Exp Med 2009; 206: 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blom AB, van Lent PL, Libregts S, Holthuysen AE, van der Kraan PM, van Rooijen N, van den Berg WB. Crucial role of macrophages in matrix metallo-proteinase–mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum 2007; 56: 147–57. [DOI] [PubMed] [Google Scholar]
- 109.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol 2007; 213: 626–34. [DOI] [PubMed] [Google Scholar]
- 110.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum 2010; 62: 647–57. [DOI] [PubMed] [Google Scholar]
- 111.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum 2001; 44: 585–94. [DOI] [PubMed] [Google Scholar]
- 112.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010; 6: 625–35. [DOI] [PubMed] [Google Scholar]
- 113.Mahler EAM, Zweers MC, van Lent PL, Blom AB, van den Hoogen FH, van den Berg WB, Roth J, Vogl T, Bijlsma JWJ, van den Ende CHM, den Broeder AA. Association between serum levels of the proinflammatory protein S100A8/A9 and clinical and structural characteristics of patients with established knee, hip, and hand osteoarthritis. Scand J Rheumatol 2015; 44: 56–60. [DOI] [PubMed] [Google Scholar]
- 114.Lood C, Stenström M, Tydén H, Gullstrand B, Källberg E, Leanderson T, Truedsson L, Sturfelt G, Ivars F, Bengtsson AA. Protein synthesis of the pro-inflammatory S100A8/A9 complex in plasmacytoid dendritic cells and cell surface S100A8/A9 on leukocyte subpopulations in systemic lupus erythematosus. Arthritis Res Ther 2011; 13: R60–R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gabrielsen TO, Dale I, Brandtzaeg P, Hoel PS, Fagerhol MK, Larsen TE, Thune PO. Epidermal and dermal distribution of a myelomonocytic antigen (L1) shared by epithelial cells in various inflammatory skin diseases. J Am Acad Dermatol 1986; 15: 173–9. [DOI] [PubMed] [Google Scholar]
- 116.Bando M, Hiroshima Y, Kataoka M, Shinohara Y, Herzberg MC, Ross KF, Nagata T, Kido J. Interleukin-1 regulates antimicrobial peptide expression in human keratinocytes. Immunol Cell Biol 2007; 85: 532–37. [DOI] [PubMed] [Google Scholar]
- 117.Kuruto R, Nozawa R, Takeishi K, Aral K, Yokota T, Takasaki Y. Myeloid calcium binding proteins: expression in the differentiated HL-60 cells and detection in sera of patients with connective tissue diseases. J Biochem 1990; 108: 650–3. [DOI] [PubMed] [Google Scholar]
- 118.Soyfoo MS, Roth J, Vogl T, Pochet R, Decaux G. Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus. J Rheumatol 2009; 36: 2190–4. [DOI] [PubMed] [Google Scholar]
- 119.Tyden H, Lood C, Gullstrand B, Jönsen A, Nived O, Sturfelt G, Truedsson L, Ivars F, Leanderson T, Bengtsson AA. Increased serum levels of S100A8/A9 and S100A12 are associated with cardiovascular disease in patients with inactive systemic lupus erythematosus. Rheumatology 2013;52:2048–55. [DOI] [PubMed]
- 120.Zen M, Iaccarino L, Gatto M, Bettio S, Nalotto L, Ghirardello A, Punzi L, Doria A. Prolonged remission in Caucasian patients with SLE: prevalence and outcomes. Ann Rheum Dis 2015; 74: 2117–22. [DOI] [PubMed] [Google Scholar]
- 121.Ehlermann P, Eggers K, Bierhaus A, Most P, Weichenhan D, Greten J, Nawroth PP, Katus HA, Remppis A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol 2006; 5: 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hollan I, Scott H, Saatvedt K, Prayson R, Mikkelsen K, Nossent HC, Kvelstad IL, Liang MH, Førre OT. Inflammatory rheumatic disease and smoking are predictors of aortic inflammation: a controlled study of biopsy specimens obtained at coronary artery surgery. Arthritis Rheum 2007; 56: 2072–9. [DOI] [PubMed] [Google Scholar]
- 123.Bjork P, Bjork A, Vogl T, Stenstrom M, Liberg D, Olsson A, Roth J, Ivars F, Leanderson T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3- carboxamides. PLoS Biol 2009; 7: e97–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cuida M, Halse AK, Johannessen AC, Tynning T, Jonsson R. Indicators of salivary gland inflammation in primary Sjögren’s syndrome. Eur J Oral Sci 1997; 105: 228–233. [DOI] [PubMed] [Google Scholar]
- 125.Nordal HH, Brun JG, Halse AK, Madland TM, Fagerhol MK, Jonsson R. Calprotectin (S100A8/A9), S100A12, and EDTA-resistant S100A12 complexes (ERAC) in primary Sjoügren’s syndrome. Scand J Rheumatol 2014; 43: 76–8. [DOI] [PubMed] [Google Scholar]
- 126.Sweet SP, Denbury AN, Challacombe SJ. Salivary calprotectin levels are raised in patients with oral candidiasis or Sjogren’s syndrome but decreased by HIV infection. Oral Microbiol Immunol 2001; 16: 119–123. [DOI] [PubMed] [Google Scholar]
- 127.Andreasson K, Scheja A, Saxne T, Ohlsson B, Hesselstrand R. Faecal calprotectin: a biomarker of gastrointestinal disease in systemic sclerosis. J Intern Med 2011; 270: 50–7. [DOI] [PubMed] [Google Scholar]
- 128.Eversole LR, Miyasaki KT, Christensen RE. Keratinocyte expression of calprotectin in oral inflammatory mucosal diseases. J Oral Pathol Med 1993; 22: 303–7. [DOI] [PubMed] [Google Scholar]
- 129.Katz J, Stavropoulos F, Bhattacharyya I, Stewart C, Perez FM, Caudle RM. Receptor of advanced glycation end product (RAGE) expression in the minor salivary glands of patients with Sjoügren’s syndrome: a preliminary study. Scand J Rheumatol 2004; 33: 174–8. [DOI] [PubMed] [Google Scholar]
- 130.Xu X, Wu WY, Tu WZ, Chu HY, Zhu XX, Liang MR, Xue Y, Wang JC, Zou HJ. Increased expression of S100A8 and S100A9 in patients with diffuse cutaneous systemic sclerosis. A correlation with organ involvement and immunological abnormalities. Clin Rheumatol 2013; 32: 1501–10. [DOI] [PubMed] [Google Scholar]
- 131.Nikitorowicz-Buniak J, Shiwen X, Denton CP, Abraham D, Stratton R. Abnormally differentiating keratinocytes in the epidermis of systemic sclerosis patients show enhanced secretion of CCN2 and S100A9. J Invest Dermatol 2014; 134: 2693–702. [DOI] [PubMed] [Google Scholar]
- 132.He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, Yamaya M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2007; 293: L1427–36. [DOI] [PubMed] [Google Scholar]
- 133.Yoshizaki A, Komura K, Iwata Y, Ogawa F, Hara T, Muroi E, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, Sato S. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: association with disease severity. J Clin Immunol 2009; 29: 180–9. [DOI] [PubMed] [Google Scholar]
- 134.He Z, Zhu Y, Jiang H. Inhibiting toll-like receptor 4 signaling ameliorates pulmonary fibrosis during acute lung injury induced by lipopolysaccharide: an experimental study. Respir Res 2009; 10: 126–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He Z, Gao Y, Deng Y, Li W, Chen Y, Xing S, Zhao X, Ding J, Wang X. Lipopolysaccharide induces lung fibroblast proliferation through Toll-like receptor 4 signaling and the phosphoinositide3-kinase-Akt pathway. PLoS One 2012; 7: e35926–e35926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fietta A, Bardoni A, Salvini R, Passadore I, Morosini M, Cavagna L, Codullo V, Pozzi E, Meloni F, Montecucco C. Analysis of bronchoalveolar lavage fluid proteome from systemic sclerosis patients with or without functional, clinical and radiological signs of lung fibrosis. Arthritis Res Ther 2006; 8: R160–R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Giusti L, Bazzichi L, Baldini C, Ciregia F, Mascia G, Giannaccini G, Del Rosso M, Bombardieri S, Lucacchini A. Specific proteins identified in whole saliva from patients with diffuse systemic sclerosis. J Rheumatol 2007; 34: 2063–9. [PubMed] [Google Scholar]
- 138.Hesselstrand R, Wildt M, Bozovic G, Andersson-Sjöland A, Andréasson K, Scheia A, Westergren-Thorsson G, Bjermer L, Wuttge DM. Biomarkers from bronchoalveolar lavage fluid in systemic sclerosis patients with interstitial lung disease relate to severity of lung fibrosis. Respir Med 2013; 107: 1079–86. [DOI] [PubMed] [Google Scholar]
- 139.Van Bon L, Cossu M, Loof A, Gohar F, Wittkowski H, Vonk M, Roth J, van den Berg W, van Heerde W, Broen JCA, Radstake TRDJ. Proteomic analysis of plasma identifies the Toll-like receptor agonists S100A8/A9 as a novel possible marker for systemic sclerosis phenotype. Ann Rheum Dis 2014; 73: 1585–89. [DOI] [PubMed] [Google Scholar]
- 140.Marie I, Leroi AM, Menard JF, Levesque H, Quillard M, Ducrotte P. Fecal calprotectin in systemic sclerosis and review of the literature. Autoimmunity Rev 2015; 14: 547–54. [DOI] [PubMed] [Google Scholar]
- 141.Bargagli E, Olivieri C, Prasse A, Bianchi N, Magi B, Cianti R, Bini L, Rottoli P. Calgranulin B (S100A9) levels in bronchoalveolar lavage fluid of patients with interstitial lung diseases. Inflammation 2008; 3: 351–4. [DOI] [PubMed] [Google Scholar]
- 142.Ghirardello A, Borella E, Beggio M, Franceschini F, Fredi M, Doria A. Myositis autoantibodies and clinical phenotypes. Auto Immun Highlights 2014; 23: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rostasy KM, Piepkorn M, Goebel HH, Menck S, Hanefeld F, Schulz-Schaeffer WJ. Monocyte/macrophage differentiation in dermatomyositis and polymyositis. Muscle Nerve 2004; 30: 225–30. [DOI] [PubMed] [Google Scholar]
- 144.Nistala K, Varsani H, Wittkowski H, Vogl T, Krol P, Shah V, Mamchaoui K, Brogan PA, Roth J, Wedderburn LR. Myeloid related protein induces muscle derived inflammatory mediators in juvenile dermatomyositis. Arthrit Res Ther 2013; 15: R131–R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Seeliger S, Vogl T, Engels IH, Schroder JM, Sorg C, Sunderkotter C, Roth J. Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle diseases. Am J Pathol 2003; 163: 947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bardouille C, Lehmann J, Heimann P, Jockusch H. Growth and differentiation of permanent and secondary mouse myogenic cell lines on microcarriers. Appl Microbiol Biotechnol 2001; 55: 556–62. [DOI] [PubMed] [Google Scholar]
- 147.Kim GT, Cho ML, Park YE, Yoo WH, Kim JH, Oh HJ, Kim DS, Baek SH, Lee SH, Lee JH, Kim HY, Kim SI. Expression of TLR2, TLR4, and TLR9 in dermatomyositis and polymyositis. Clin Rheumatol 2010; 29: 273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brunn A, Zornbach K, Hans VH, Haupt WF, Deckert M. Toll-like receptors promote inflammation in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol 2012; 71: 855–67. [DOI] [PubMed] [Google Scholar]
- 149.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007; 450: 1253–7. [DOI] [PubMed] [Google Scholar]
- 150.Cappelletti C, Galbardi B, Kapetis D, Vattemi G, Guglielmi V, Tonin P, Salerno F, Morandi L, Tomelleri G, Mantegazza R, Bernasconi P. Autophagy, inflammation and innate immunity in inflammatory myopathies. PLoS One 2014; 9: e111490–e111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.De Paepe B, De Bleecker JL. Beta-chemokine receptor expression in idiopathic inflammatory myopathies. Muscle Nerve 2005; 31: 621–7. [DOI] [PubMed] [Google Scholar]
- 152.Oktayoglu P, Mete N, Caglayan M, Bozkurt M, Bozan T, Em S, Nas K. Elevated serum levels of calprotectin (MRP8/ MRP14) in patients with Behçet’s disease and its association with disease activity and quality of life. Scand J Clin Labor Invest 2015; 75: 106–12. [DOI] [PubMed] [Google Scholar]
- 153.Chan TK, Dick AD, Forrester JV, Herriot R. Antineutrophil cytoplasmic antibodies in chronic idiopathic intraocular inflammatory disease. Ocul Immunol Inflamm 1996; 4: 83–90. [DOI] [PubMed] [Google Scholar]
- 154.Akyol N, De˘er O, Erdöl H, Gedik Y, Orem A, Imamoglu HI. The importance of plasma polymorphonu- clear (PMN) elastase determination in patients with uveitis. Acta Ophthalmol Scand 1997; 75: 287–9. [DOI] [PubMed] [Google Scholar]
- 155.Olson JA, Forrester M, Cloheddy PA, Golden BE, Hettiot R, Forrester JV. Calprotectin is raised in endogenous posterior uveitis. Ocul ImmunolInflamm 1996; 4: 91–8. [DOI] [PubMed] [Google Scholar]
- 156.Brandtzaeg P, Gabrielsen TO, Dale I, Muller F, Steinbakk M, Fagerhol MK. The leucocyte protein L1 (calprotectin): a putative nonspecific defence factor at epithelial surfaces. Adv Exp Med Biol 1995; 371A: 201–6. [DOI] [PubMed] [Google Scholar]
- 157.Schlegel Gómez R, Langer P, Pelka M, von den Driesch P, Johannessen AC, Simon M., Jr Variational expression of functionally different macrophage markers (27E10, 25F9, RM3/1) in normal gingiva and inflammatory periodontal disease. J Clin Periodontol 1995; 22: 341–6. [DOI] [PubMed] [Google Scholar]
- 158.Zheng Y, Hou J, Peng L, Zhang X, Jia L, Wang X, Wei S, Meng H. The Pro-Apoptotic and Pro-Inflammatory Effects of Calprotectin on Human Periodontal Ligament Cells. PloS One 2014; 9: e110421–e110421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Borman P, Tuncay F, Kocaoǧlu S, Okumuš M, Güngör E, Ekşioǧlu M. The subclinic autonomic dysfunction in patients with Behçet disease: an electrophysiological study. Clin Rheumatol 2012; 31: 41–7. [DOI] [PubMed] [Google Scholar]
- 160.Zouboulis CC, May T. Pathogenesis of Adamantiades-Behçet’s disease. Med Microbiol Immunol 2003; 192: 149–55. [DOI] [PubMed] [Google Scholar]
- 161.Kwon SR, Lim MJ, Park SG, Moon YS, Park W. Decreased protein S activity is related to the disease activity of Behcet’s disease. Rheumatol Int 2006; 27: 39–43. [DOI] [PubMed] [Google Scholar]
- 162.Pepper RJ, Hamour S, Chavele KM, Todd SK, Rasmussen N, Flint S, Lyons PA, Smith KG, Pusey CD, Cook HT, Salama AD. Leukocyte and serum S100A8/S100A9 expression reflects disease activity in ANCA-associated vasculitis and glomerulonephritis. Kidney Int 2013; 83: 1150–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Draibe JB, Pepper RJ, Merkel PA, Salama AD, Investigators R-I. Serum calprotectin and disease relapse in ANCA-associated vasculitis. Arthritis Rheumatol 2014; 66: S818–S818. [Google Scholar]
- 164.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis–a meta-analysis. Rheumatology 2012; 51: 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rastaldi MP, Ferrario F, Crippa A, Dell’Antonio G, Casartelli D, Grillo C, D’Amico G. Glomerular monocyte-macrophage features in ANCA-positive renal vasculitis and cryoglobulinemic nephritis. J Am Soc Nephrol 2000; 11: 2036–43. [DOI] [PubMed] [Google Scholar]
- 166.Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RRS, Hogg N, Lippy P, Simon DI. Myeloid-related protein 8/14 is critical for the biological response to vascular injury. Circulation 2009; 120: 427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Foell D, Hernandez-Rodriguez J, Sanchez M, Vogl T, Cid MC, Roth J. Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. J Pathol 2004; 204: 311–6. [DOI] [PubMed] [Google Scholar]
- 168.Weyand CM, Ma-Krupa W, Goronzy JJ. Immunopathways in giant cell arteritis and polymyalgia rheumatica. Autoimmun Rev 2004; 3: 46–53. [DOI] [PubMed] [Google Scholar]
- 169.Carrión M, Juarranz Y, Seoane IV, Martínez C, González-Álvaro I, Pablos JL, Gutiérrez-Cañas I, Gomariz RP. VIP modulates IL-22R1 expression and prevents the contribution of rheumatoid synovial fibroblasts to IL-22-mediated joint destruction. J Mol Neurosci 2014; 52: 10–7. [DOI] [PubMed] [Google Scholar]
- 170.Raffeiner B, Botsios C, Ometto F, Bernardi L, Stramare R, Todesco S, Sfriso P, Punzi L. Effects of half dose etanercept (25 mg once a week) on clinical remission and radiographic progression in patients with rheumatoid arthritis in clinical remission achieved with standard dose. Clin Exp Rheumatol 2015; 33: 63–8. [PubMed] [Google Scholar]