Abstract
The ability to quickly diagnose hemorrhagic shock is critical for favorable patient outcomes. Therefore, it is important to understand the time course and involvement of the various physiological mechanisms that are active during volume loss and that have the ability to stave off hemodynamic collapse. This review provides new insights about the physiology that underlies blood loss and shock in humans through the development of a simulated model of hemorrhage using lower body negative pressure. In this review, we present controlled experimental results through utilization of the lower body negative pressure human hemorrhage model that provide novel insights on the integration of physiological mechanisms critical to the compensation for volume loss. We provide data obtained from more than 250 human experiments to classify human subjects into two distinct groups: those who have a high tolerance and can compensate well for reduced central blood volume (e.g. hemorrhage) and those with low tolerance with poor capacity to compensate.We include the conceptual introduction of arterial pressure and cerebral blood flow oscillations, reflex-mediated autonomic and neuroendocrine responses, and respiration that function to protect adequate tissue oxygenation through adjustments in cardiac output and peripheral vascular resistance. Finally, unique time course data are presented that describe mechanistic events associated with the rapid onset of hemodynamic failure (i.e. decompensatory shock).
Impact Statement
Hemorrhage is the leading cause of death in both civilian and military trauma. The work submitted in this review is important because it advances the understanding of mechanisms that contribute to the total integrated physiological compensations for inadequate tissue oxygenation (i.e. shock) that arise from hemorrhage. Unlike an animal model, we introduce the utilization of lower body negative pressure as a noninvasive model that allows for the study of progressive reductions in central blood volume similar to those reported during actual hemorrhage in conscious humans to the onset of hemodynamic decompensation (i.e. early phase of decompensatory shock), and is repeatable in the same subject. Understanding the fundamental underlying physiology of human hemorrhage helps to test paradigms of critical care medicine, and identify and develop novel clinical practices and technologies for advanced diagnostics and therapeutics in patients with life-threatening blood loss.
Keywords: Tissue oxygenation, resuscitation, trauma, vital signs, lower body negative pressure
Introduction
Hemorrhagic shock represents a clinical condition of inadequate tissue perfusion resulting from an acute reduction in central blood volume (central hypovolemia).1,2 Shock is usually described by clinical markers such as severe hypotension (systolic blood pressure <90 mmHg), pronounced tachycardia (heart rate >120 bpm), metabolic acidosis (blood lactate > 2 – 3 mmol/L or base deficit < −4 mmol/L) countered by respiratory alkalosis (respiration rate >20 breaths/min with reduced EtCO2), decreased pulse pressure, cold and clammy skin, and altered mental state (e.g. disorientation, confusion).3,4 However, there is significant individual patient variability in the compensatory response to blood loss and time course for development of shock that can render these standard metrics inconsistent and misleading to the process of diagnosis.
As such, it is the objective of this review article to present evidence obtained from various experiments conducted in our laboratory over the past 30 years on healthy humans. In an attempt to provide the reader with new insights on the physiology of blood loss in humans, mechanisms of compensation for progressive tissue hypoperfusion associated with reductions in circulating volume, and eventual onset of hemorrhagic shock due to inadequate tissue oxygenation will be reviewed.
Development of a human model of hemorrhage
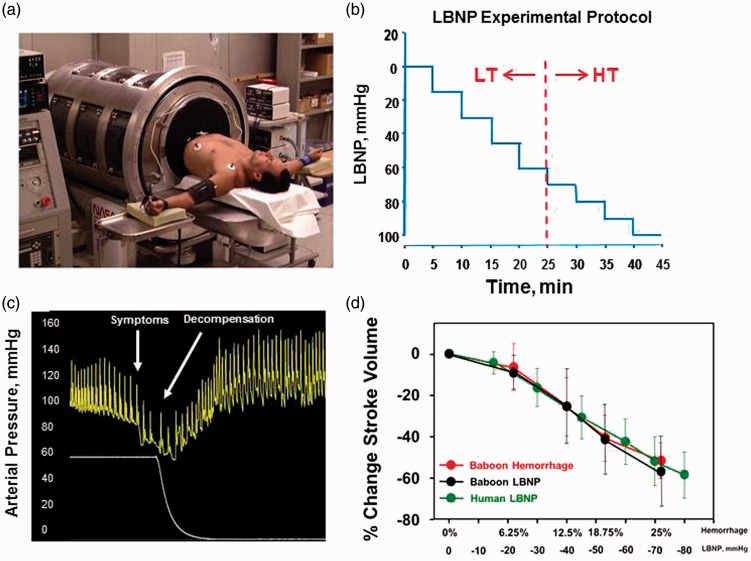
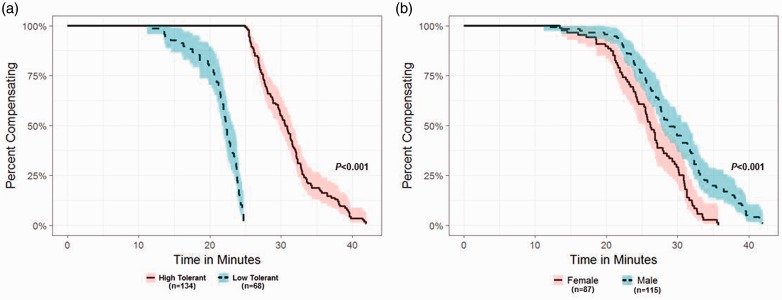
The model developed for the study of human hemorrhage physiology evolved from the use of a technology known as lower body negative pressure (LBNP). A healthy human subject is placed in a pressure chamber with the torso exposed, and waist wrapped in a neoprene “skirt” (Figure 1(a)). The experimental procedure for testing tolerance consists of stepwise reductions in pressure inside the chamber (Figure 1(b)), which produces a reduction in central blood volume. The progressive hypovolemia continues until symptoms of presyncope are attained (e.g. dizziness, loss of peripheral vision, disorientation, impaired responsiveness, systolic blood pressure < 80 mmHg). When the negative pressure is released, central blood volume returns quickly to normal baseline levels (Figure 1(c)). The completion of several hundred LBNP experiments and development of a database have revealed the classification of individuals into two general categories: two-thirds of the population display high tolerance (HT) in their ability to compensate for reduced central blood volume while the remaining one-third have a low tolerance (LT) to reduced central blood volume5,6. As such, we use the criteria of those subjects who compensate beyond a −60 mmHg LBNP level as HT and those who reach presyncope at LBNP ≤ −60 mmHg as LT (Figure 1(b)). This classification is supported by Kaplan–Meier survival curve analysis of our human LBNP database that reveals a clear separation of tolerances between individuals who failed or succeeded to complete 60 mmHg LBNP (Figure 2(a)). The functional distinction between HT and LT individuals is important to the understanding of hemorrhage physiology in that it eloquently illustrates normal variability in compensatory human responses.
Figure 1.
(a) Human subject undergoing lower body negative pressure (LBNP). (b) Depiction of standard protocol with definition of high tolerant and lower tolerant subjects. (c) Representative figure of an arterial pressure waveform during LBNP leading to decompensation. (d) Percent change in stroke volume from baseline during four steps of hemorrhage ( ) and LBNP (•) in baboons corresponding to 6.25% (n = 14), 12.5% (n = 14), 18.75% (n = 14) and 25% (n = 12) total blood volume loss. Human responses to six levels of LBNP are superimposed (
) and LBNP (•) in baboons corresponding to 6.25% (n = 14), 12.5% (n = 14), 18.75% (n = 14) and 25% (n = 12) total blood volume loss. Human responses to six levels of LBNP are superimposed ( ). Data are expressed as mean ± SD. Modified from Convertino et al.27 and Hinojosa-Laborde et al.9
). Data are expressed as mean ± SD. Modified from Convertino et al.27 and Hinojosa-Laborde et al.9
Figure 2.
Kaplan–Meier analysis of healthy human subjects undergoing LBNP until symptoms of presyncope are evident. Both panels utilize the same data. (a) Analysis of time to presyncope in minutes in subjects categorized as high tolerant or low tolerant. (b) Analysis of time to presyncope (minutes) in subjects categorized as male or female. (A color version of this figure is available in the online journal.)
One unique and valuable feature of defining a presyncope endpoint with this model of human hemorrhage is that it has allowed us to quantify each individual’s unique capacity to compensate before the onset of hemodynamic failure. This “compensatory reserve” can be defined and quantified as the difference between a baseline state and maximal physiological response. This definition of the reserve to compensate provides the ability to quantifiably distinguish individuals with HT (good compensators) from those with LT (poor compensators) (Figure 2(a)), as well as corroborate our previous observations that females demonstrate lower tolerance to central hypovolemia compared to males (Figure 2(b)).7
Comparison of LBNP and actual hemorrhage
Using this model, we have been able to demonstrate that hemodynamic responses to actual progressive hemorrhage can be accurately mimicked by LBNP in non-human primates and humans (Figure 1(d)). The direct comparison with actual hemorrhage has allowed for an equivalent volume of blood loss to be accurately estimated during human LBNP.8 As such, LBNP has repeatedly proven to simulate physiological responses to actual blood loss.9
Cerebral perfusion associated with high and low tolerance to hemorrhage
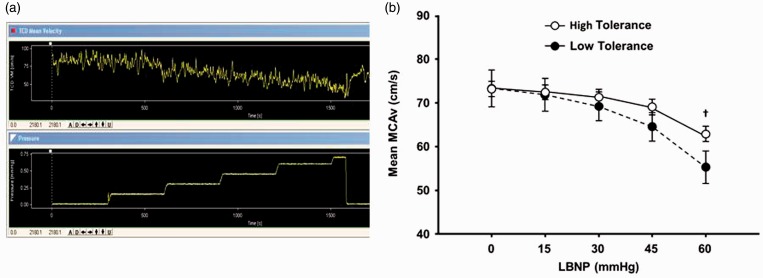
An individual’s tolerance to central hypovolemia during LBNP (simulated blood loss) largely reflects a reached “threshold” during a progressive reduction in cerebral blood flow and oxygenation.10 A tracing of a continuous transcranial Doppler (TCD) measurement obtained from a human subject undergoing progressive LBNP is presented in Figure 3(a). The TCD provides a measure of mean cerebral artery velocity (MCAv) which is progressively reduced as the subject’s central blood volume decreases. The experiment is abruptly terminated when the subject begins to express presyncopal symptoms, an indication of reaching a physiological threshold (MCAv < 10 cm/s) for inadequate cerebral oxygenation. Figure 3(b) illustrates that one of the mechanisms for HT physiology is the slower rate of MCAv reduction during progressive LBNP compared to LT subjects.
Figure 3.
(a) Real-time Transcranial Doppler (TCD) recording (top panel) of cerebral blood flow velocity (CBFV) in a human subject at rest (∼80 cm·s−1) and during progressive LBNP who decompensates at −70 mmHg LBNP (lower panel recording) at a CBFV of ∼5 cm·s−1. (b) Mean medial cerebral artery blood flow velocity (MCAv) during progressive reductions in central blood volume in high-tolerant and low-tolerant subjects. †P < 0.05 compared with corresponding LT value. Modified from Rickards et al.15 (A color version of this figure is available in the online journal.)
Hemodynamics underlying high and low tolerance to hemorrhage
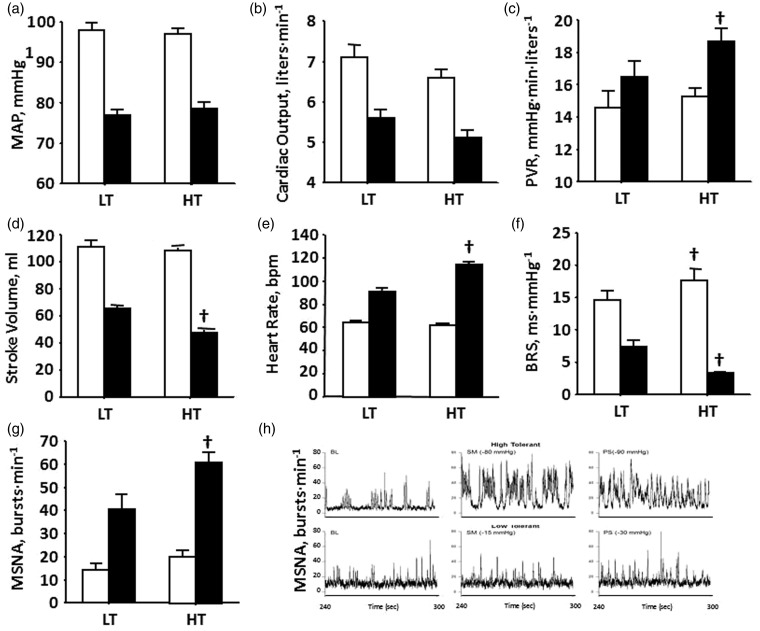
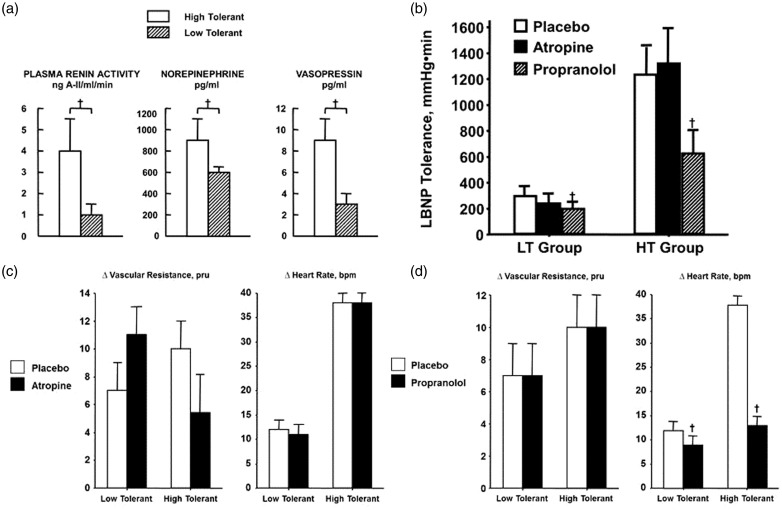
Physiological compensation is the ability to call upon the numerous mechanisms that maintain tissue and cerebral perfusion during central hypovolemia to prevent inadequate tissue oxygenation (i.e. shock). Tissue perfusion is dependent on maintaining arterial pressure (Figure 4(a)), which is protected by mechanisms that affect cardiac output and peripheral vascular resistance (PVR). Although the longer duration to hemodynamic failure results in greater reduction in stroke volume of HT individuals (Figure 4(d)), cardiac output is similar to that in LT subjects (Figure 3(b)) because of a greater reserve for elevated heart rate in HT (Figure 4(e)). Additionally, HT individuals display higher increases in PVR (Figure 4(c)). Greater tachycardia and vasoconstriction in HT individuals are associated with greater cardiac baroreflex-mediated vagal withdrawal (Figure 4(f)), sympathetic nerve activity (Figure 4(g) and (h)), and circulating neuroendocrine vasopressors (Figure 5(a)).
Figure 4.
(a) Mean arterial pressure (MAP), (b) cardiac output, (c) peripheral vascular resistance (PVR), (d) stroke volume, (e) heart rate, (f) baroreflex sensitivity (BRS), and (g) muscle sympathetic nerve activity (MSNA) at baseline (open bars) and at decompensation (closed bars) in high-tolerant (HT) and low-tolerant (LT) subject groups. Bars and lines represent mean ± 1 sem. †P < 0.001 compared with corresponding LT value. Modified from Convertino et al.28 (h) MSNA recordings at baseline (BL), sub-maximal LBNP (SM), and presyncope (PS) in HT (top panel) and LT (bottom panel) subjects
Figure 5.
(a) Plasma renin-angiotensin activity, norepinephrine, and vasopressin levels at decompensation in high-tolerant (open bars) and low-tolerant (lined bars) subject groups. Bars and lines represent mean ± 1 sem. † P < 0.05 compared with corresponding LT value. Modified from Convertino and Sather.29 (b) LBNP tolerances during placebo (open bar), atropine (closed bar), and propranolol (lined bar) treatments in low (LT, n = 5) and high (HT, n = 6) LBNP-tolerant individuals. Symbols are mean ± 1 sem. †P < 0.05 compared with corresponding LT value. Modified from Convertino and Sather.23 (c) Changes (Δ) in peripheral vascular resistance and heart rate during placebo (open bar) and atropine (closed bar) treatments in low (n = 5) and high (n = 6) LBNP-tolerant individuals. Symbols are mean ± 1 sem. Modified from Convertino.30 (d) Changes (Δ) in peripheral vascular resistance and heart rate during placebo (open bar) and propranolol (closed bar) treatments in low (n = 5) and high (n = 6) LBNP-tolerant individuals. Symbols are mean ± 1 sem. †P < 0.05 compared with corresponding LT value. Modified from Convertino.30
Underlying autonomic mechanisms of cardiovascular compensations – the importance of sympathetic adrenergic control
In addition to differences in neuroendocrine responses (Figure 5(a)), Figure 5 presents data obtained from HT and LT subjects during experiments in our laboratory in which vagal and sympathetic blockade of cardiac and vascular responses were measured during tests of LBNP tolerance in an effort to assess the autonomic contribution to compensation for reduced central blood volume (e.g. hemorrhage). These experiments corroborated that greater elevations in heart rate, caused in part by reflex antagonism of the sympathetic nervous system,11 are associated with higher tolerance to LBNP. However, while cardiac cholinergic (vagal) blockade with the use of atropine had no impact on vascular resistance (Figure 5(c)), heart rate (Figure 5(c)), and tolerance (Figure 5(b)), cardiac β-adrenoreceptor (sympathetic) blockade with the use of propranolol dramatically attenuated the normal elevation in heart rate (Figure 5(d)) and was subsequently associated with a pronounced decrease in tolerance to progressive reductions in central blood volume in both HT and LT individuals (Figure 5(b)). These results provided compelling evidence that sympathetically mediated tachycardia represents a critical mechanism for tolerating acute hemorrhage in addition to, and independent of, peripheral vascular constriction.
The role of sympathetically mediated oscillatory flow as a compensatory mechanism
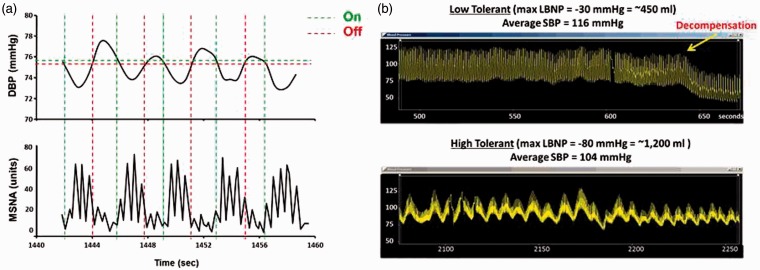
During homeostasis and compensation, a tightly coupled, rhythmic and dynamic relationship exists between arterial pressure and sympathetic outflow. A decrease in arterial pressure quickly initiates increased sympathetic nerve activity, in part, by decreasing inhibitory afferent activity to nucleus of the solitary tract.12 The subsequent arterial vasoconstriction results in increased vascular resistance and a compensatory elevation in arterial pressure, and the ascending arterial pressures initiate a feedback reduction in sympathetic outflow.13 Figure 6(a) depicts this relationship with representative diastolic blood pressure (DBP) and muscle sympathetic nerve activity (MSNA) tracings from a healthy human subject during central hypovolemia induced by 60 mmHg LBNP. The rhythmic relationship between ascending DBP and increased MSNA bursts is clearly visible (horizontal green broken lines). Similar arterial blood pressure oscillations are highly associated with an increased tolerance to central hypovolemia.14–16 Figure 6(b) represents two arterial pressure tracings taken from healthy human subjects during a standard LBNP protocol. The top panel represents a LT subject who experience decompensation at 30 mmHg LBNP which equated to a blood loss of only ∼450 mL. Note the consistent and static nature of the arterial pressure waveforms and absence of pronounced oscillations. This relationship is consistent with a lack of increased sympathetic activation in an attempt to compensate for volume loss. The second subject (bottom panel) has completed the LBNP protocol to an estimated blood loss of approximately 1200 mL, but was able to successfully compensate and did not experience presyncope symptoms or decompensation. This subject’s tracing demonstrates the consistent arterial pressure oscillations that are characteristic of compensatory patterns observed in HT individuals during volume loss. As such, pronounced oscillatory patterns of circulatory pressure and flow represent a sensitive “on–off” sympathetically mediated feedback mechanism designed to maintain tissue oxygenation despite the compromise to blood flow under severe hypovolemic conditions.15 In addition, there is evidence that maintaining coherence between arterial pressure and sympathetic nerve activity represents an important compensatory mechanism during hypovolemia.16
Figure 6.
(a) Continuous recordings demonstrating the tight coupling between arterial diastolic blood pressure oscillations (DBP; upper tracing) and muscle sympathetic nerve activity (MSNA; lower tracing) that act to provide alternating perfusion to the brain and peripheral tissue under conditions of low circulating blood volume. Modified from Convertino.31 (b) Arterial waveform recordings demonstrate that pronounced oscillatory patterns are associated with high tolerance to progressive reduction in central blood volume (bottom recording) compared to low tolerance (top recording). Modified from Convertino et al.27
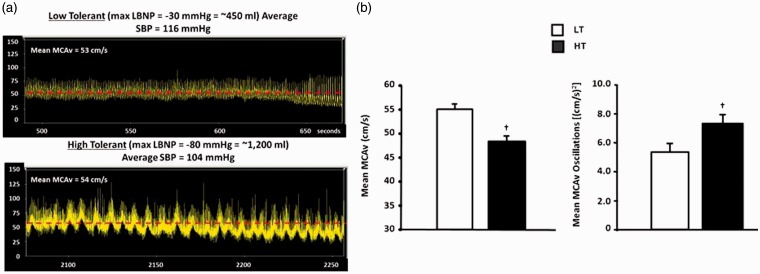
The same relationship between oscillations in arterial blood pressure and tolerance to central hypovolemia also translates to protection of cerebral blood flow. Figure 7(a) shows a representative tracing of MCAv from the same two subjects during the same experiment referenced in Figure 5(b). The LT individual (Figure 7(a), top panel) expressed presyncope symptoms after a LBNP-induced reduction in central blood volume equivalent to only ∼450 mL blood loss. Similar to the arterial pressure recordings (Figures 6(b) and 7(a)), few oscillatory patterns are visible. In contrast, the HT individual (Figure 7(a), lower panel) exhibits an obvious and continuous oscillatory pattern of MCAv, again like the arterial pressure waveforms (Figure 6(b)). Interestingly, there is convincing evidence that these patterns of oscillation are more important than absolute velocity. In Figure 6(b), MCAv is compared with quantified MCAv oscillations in HT and LT individuals. The HT subjects had a lower MCAv overall, but increased quantified oscillations. These data speak to the physiological importance of measuring dynamic relationships such as oscillations in pressure and flow to accurately assess the clinical status of a hemorrhaging patient rather than relying on average responses.
Figure 7.
(a) Arterial waveform recordings of the medial cerebral artery blood flow velocity (MCAv) demonstrate that pronounced oscillatory patterns are associated with high tolerance to progressive reduction in central blood volume (bottom recording) compared to low tolerance (top recording). Modified from Convertino et al.16 (b) Individuals with high tolerance to central hypovolemia (closed bars) display greater oscillations in mean medial cerebral artery blood flow velocity (MCAv) at the onset of decompensation (right panel) compared to individuals with low tolerance (open bars), but tolerate lower levels of mean MCAv (left panel). Symbols are mean ± 1 sem. †P < 0.05 compared with corresponding LT value. Modified from Rickards et al.15 (A color version of this figure is available in the online journal.)
Underlying respiratory mechanisms of cardiovascular compensations
The respiratory system also contributes valuable mechanisms to assist with cardiovascular compensation to prevent shock during hemorrhage. Blood flow is continuously regulated by negative and positive pressures. During normal breathing, an inhalation results from the diaphragm moving down and creating a negative pressure within the thorax that encourages air to be moved into the lungs. Consequently, the reversal of this process during exhalation expels air from the lungs as the result of positive pressure. Through an anatomical paraventricular plexis, the changes in intrathoracic pressure (ITP) are transferred to the brain. Consequently, intracranial pressure (ICP) also follows the pattern of ITP (decreasing during inhalation, and increasing during expiration), assisting blood flow in the brain.
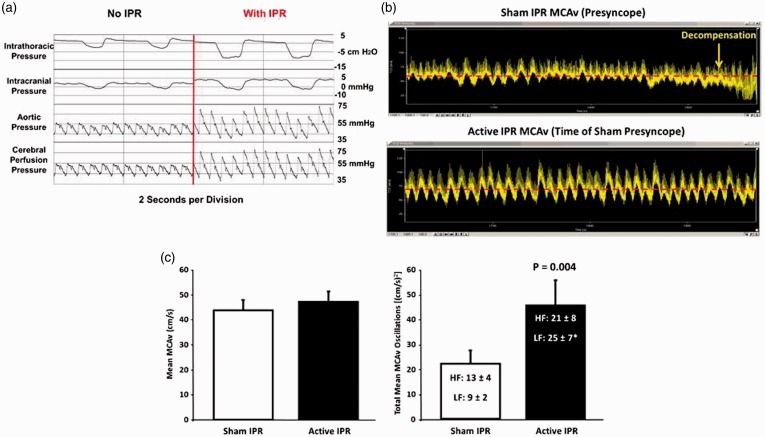
Using our human model of hemorrhage with LBNP, we have been able to investigate the relationship between respiration and hemodynamics during progressive reductions in central blood volume. We have demonstrated that we can “optimize” the respiratory pump by further reducing ITP with the application of increased resistance during inspiration, a process known as intrathoracic pressure regulation (IPR).17 IPR applied during reductions in central blood volume results in increased cardiac filling and output,18 higher arterial blood,18 and elevated aortic and cerebral perfusion pressures (Figure 8(a)). In addition, reductions in ITP produced by breathing against resistance are associated with greater cerebral blood flow oscillations and increased tolerance to central hypovolemia.18 This relationship between the respiratory pump and enhanced cerebral circulation is best illustrated in Figure 8(b) and (c). Delayed expression of syncope symptoms (i.e. maintained adequate cerebral oxygenation) and increased tolerance to progressive loss of central blood volume is associated with significantly greater MCAv oscillations when ITP is decreased with application of IPR (Figure 8(b), bottom panel) compared to no IPR (Figure 8(b), top panel). Similar to the comparison of HT to LT individuals (Figure 7(b)), increased tolerance in the same individual is associated with larger patterns of MCAv oscillation without differences in absolute average MCAv (Figure 8(c)). Taken together, the data make a compelling case for the important contribution of the respiratory pump in maintaining adequate tissue perfusion during hemorrhage.17
Figure 8.
(a) Results from experiments conducted using an animal (porcine) model of 40% controlled hemorrhage demonstrate that reduced intrathoracic pressure lowers right atrial pressure, which in turn pulls more venous blood back into the thorax and increases arterial pressure and reduces intracranial pressure, which together increase cerebral perfusion. Modified from Convertino et al.17 (b) Arterial waveform recordings demonstrate that a delay in the onset of symptoms and hemodynamic decompensation is associated with increases in CBFV oscillations when intrathoracic pressure is reduced by breathing against resistance (bottom recording) compared to breathing with normal intrathoracic pressure in the same subject (top recording). Modified from Convertino et al.16 (c) Individuals with high tolerance to central hypovolemia (closed bars) display greater oscillations in mean medial cerebral artery blood flow velocity (MCAv) at the onset of decompensation (right panel) compared to individuals with low tolerance (open bars) despite similar levels of mean blood flow (left panel). Symbols are mean ± 1 sem. Modified from Rickards et al.32 (A color version of this figure is available in the online journal.)
Onset of decompensatory shock – the “last ditch effort”
The historical approach of using traditional vital sign measurements obtained from trauma patients suffering from hemorrhage have limited the usefulness of clinical trials designed to identify the mechanisms underlying the onset of hypovolemic shock. Our approach of using an experimentally controlled LBNP protocol as a model of progressive hemorrhage leading to presyncope in all subjects provides the unique opportunity to investigate mechanisms that contribute to the initial stage of hemodynamic decompensation in humans. In combination with computer processing techniques that provide the capability to collect and analyze electronic data in refined time sequence (milliseconds), we sought new insights into mechanisms that contribute to the transition from compensation to hemorrhage into a state of decompensatory shock.
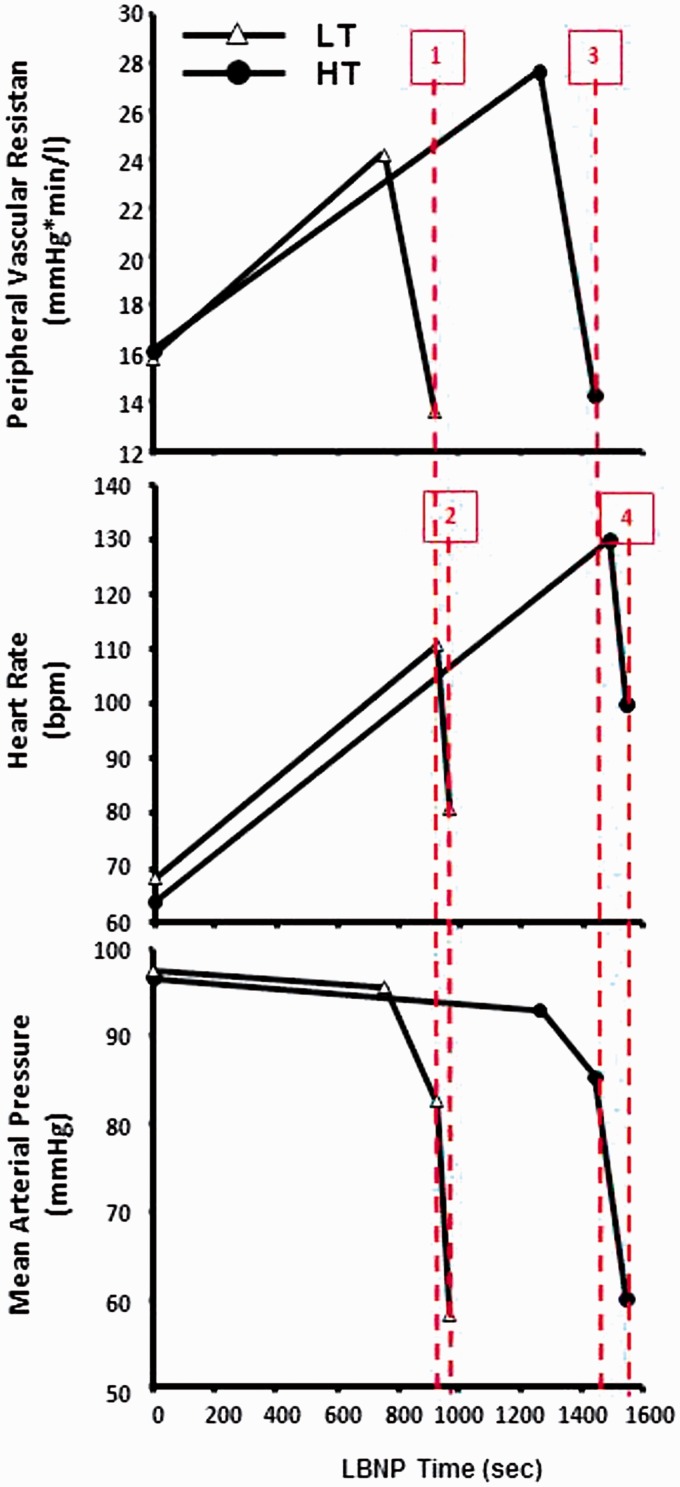
The eventual onset of relative hypotension secondary to progressive blood loss marks the initiation of circulatory shock when perfusion pressure fails to maintain adequate tissue perfusion.19,20 The hemodynamic data collected by Barcroft and colleagues in 1944 during a controlled hemorrhage of 1080 cc in a human volunteer who subsequently experienced severe hypotension (Systolic Blood Pressure [SBP] < 40 mmHg) and syncope revealed a pronounced bradycardia (HR < 60 bpm) and drop in PVR.21 However, data obtained from clinical studies have failed to consistently demonstrate responses of slowed heart rate and drop in systemic vascular resistance. Using our laboratory LBNP model of hemorrhage, we studied the time course of heart rate, vascular resistance, and sympathetic nerve activities to test the hypothesis that bradycardia and vasodilation may represent prerequisite mechanisms underlying the rapid and pronounced hypotension that results in decompensatory shock. As expected, we corroborated earlier findings that subjects with LT to central hypovolemia reached their maximal rise in HR and PVR at a similar rate but earlier than HT subjects (Figure 9), demonstrating their limited capacity to compensate. However, we were able to demonstrate that the reduction in systemic perfusion (arterial) pressure is marked by two mechanistic phases of hypotension. After a steady vasoconstriction mediated by elevation in vasoactive hormones and sympathetic nerve activity (Figure 4(g) and (h)), there is a sudden reduction in mean arterial pressure from ∼94 mmHg to ∼83 mmHg at 924 s in LT subjects and ∼86 mmHg at 1445 s in HT subjects coincidental with a reduced peripheral vascular resistance (Figure 8, Lines 1 and 3). This level of perfusion pressure can easily sustain adequate oxygenation to the tissue such as the brain20 as suggested by our observation that the onset time of reduced peripheral vascular resistance occurred 47 s and 101 s before expression of syncopal symptoms by the LT and HT subjects, respectively. Since SNA remained elevated during the initial hypotension (unpublished data), this vasodilation most likely reflected the strong vasodilator effect of local metabolites (e.g. H+, CO2) generated from increased dependence on anaerobic metabolism required to meet the energy needs of hypoxic tissue. Given our observations that (1) mean arterial perfusion pressure remained adequate during vasodilation, and (2) there existed a significant time delay in both subject groups between reduced PVR and the onset of syncopal symptoms, we conclude that peripheral vasodilation is protective of oxygen delivery to hypoxic tissue but does not contribute to hemodynamic decompensation following progressive reductions in central blood volume.
Figure 9.
Systemic peripheral vascular resistance (top panel), heart rate (middle panel), and mean arterial pressure (bottom panel) during progressive LBNP time (seconds) in individuals with low tolerance (LT, n = 59, open Δ) and high tolerance (HT, n = 113, closed •). The first phase of hypotension is associated with a sudden reduction in peripheral vascular resistance (vertical lines 1 and 3 for LT and HT) followed by the second phase of hypotension that is associated with a sudden reduction in heart rate (vertical lines 2 and 4 for LT and HT). Data extracted from Ryan et al.33 (A color version of this figure is available in the online journal.)
The second phase of hypotension is marked by a rapid reduction in mean arterial pressure to ≥60 mmHg that occurred in both HT and LT subjects following a severe bradycardia (Figure 9, Lines 2 and 4). This rapid slowing of heart rate was coincident with an equally rapid sympathetic withdrawal and minimal vagal activation as suggested by depressed cardiac baroreflex sensitivity at presycope (Figure 4(f)). This level of perfusion pressure resulted in unsustainable cerebral tissue oxygenation, as reflected by the onset of presyncopal symptoms <10 s following a ∼30% drop in mean arterial pressure in both HT and LT subjects. Our findings are consistent with those of previously reported events of bradycardia observed in individuals with reduced circulating blood volume under controlled experimental conditions in humans21,22 and a reduced LBNP tolerance when tachycardia is blocked by propranolol during progressively reduced central blood volume.23 Although the sudden bradycardia has been explained by the impact of low ventricular filling that initiates the Bezold–Jarish reflex,24 our experiment extends previous findings in that we demonstrate for the first time that in addition to vagal activation, sympathetically mediated bradycardia appears to be a prerequisite physiological mechanism that contributes to hemodynamic decompensation in all humans. There is also literature supporting the notion that an “empty heart” might contribute to cardiac receptor stimulation resulting in afferent activation involved in a bradycardia and subsequent “vasovagal” syncope.25 As such, bradycardia represents a “last ditch effort” to save the organism by optimizing cardiac filling time and reducing cardiac work in critically low circulating volume states when coronary blood flow is compromised.26
Summary
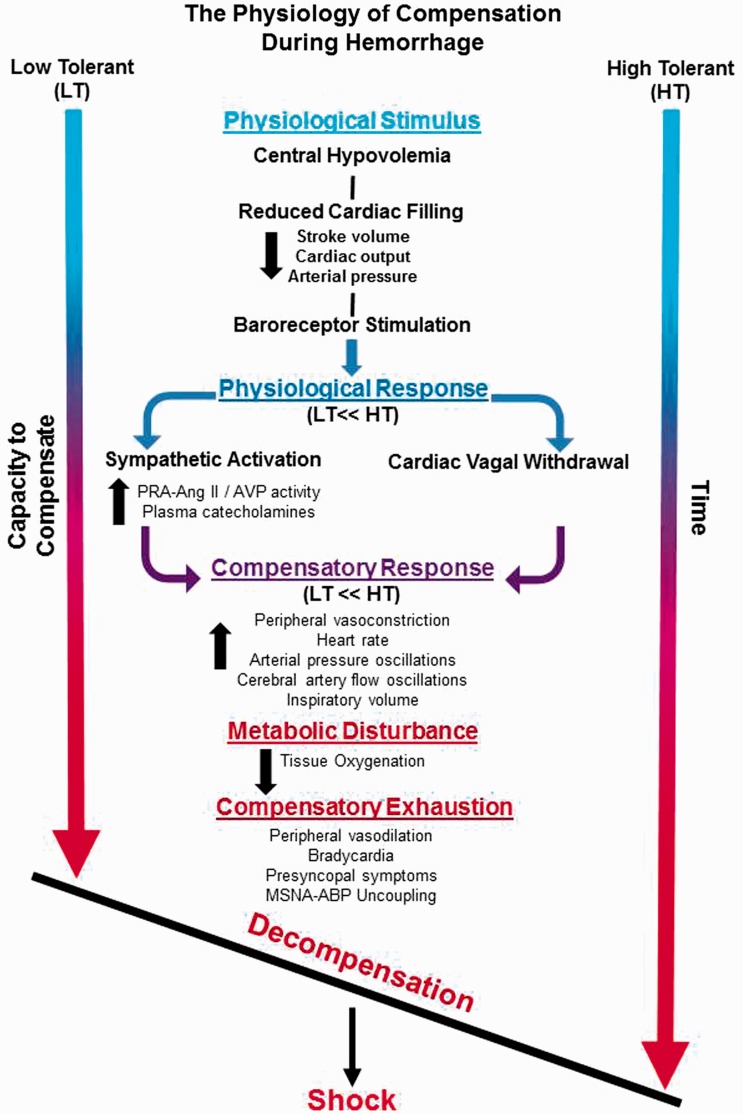
Development of a human model of hemorrhage has provided a unique opportunity to investigate the underlying physiology that defines the individual capacity to avoid the life-threatening clinical condition of inadequate tissue oxygenation known as “shock.” The experimental approach of progressively reducing central blood volume to the point of hemodynamic decompensation with the use of lower body negative pressure has revealed stark distinctions in the physiological compensatory responses between individuals with high compared with low tolerances to blood loss. A conceptual schematic highlighting these key interactions is presented in Figure 10. High tolerance to hemorrhage is defined by a capacity to maintain systemic perfusion pressure and reduce the rate of cerebral hypoperfusion by: (1) protecting cardiac output with greater elevations in heart rate associated with greater cardiac vagal withdrawal and sympathetically mediated adrenergic stimulation; (2) greater increases in systemic peripheral vascular resistance associated with higher sympathetic nerve activation and levels of circulating vasopressor endocrine responses; (3) alternating blood flow between the brain and peripheral tissue with greater sympathetically mediated oscillatory patterns of systemic pressure and flow; and (4) enhancing cardiac filling and cerebral perfusion pressure gradient by optimizing the respiratory pump. When the capacity for these compensatory responses is exhausted, an active vasodilation drops resistance to blood flow allowing for increased perfusion of peripheral tissue. When cardiac filling is no longer adequate to maintain systemic pressure and flow, a reflex-mediated pronounced bradycardia leads to the initiation of decompensatory shock.
Figure 10.
Conceptual figure depicting the key interactions from a physiological stimulus that elicits hypovolemia, such as hemorrhage, that results in hemodynamic decompensation and ultimately shock. Note that LT individuals compared to HT individuals have a reduced physiological and compensatory response, and thus reach a threshold of decompensation at a faster rate.
Acknowledgments
The authors thank the scientists and engineers who were instrumental in conducting the laboratory experiments and analysis of data. Support and funding for this work was provided in part by an appointment to the Internship/Research Participation Program at the United States Army Institute of Surgical Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA, and grants from the US Army Medical Research and Materiel Command Combat Casualty Care Research Program (grant nos D-0010-2001-IS; E52-0019-2005; E52-0015-2005; D-023-2011-USAISR; D-009-2014-USAISR). The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Authors’ contributions
AS and VC drafted the manuscript. AS, JH and VC prepared figures and edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Burgess FW, Sborov MJ, Calcagni DR. Hemorrhage, shock, and fluid resuscitation. In: Zajtchuk R, Bellamy RF (eds) Anesthesia and perioperative care of the combat casualty. Washington, DC: Office of The Surgeon General at TMM Publications, 1995, pp. 95–7.
- 2.Wilson RF (ed.). Handbook of trauma: pitfalls and pearls. Philadelphia, PA: Lippincott Williams & Wilkins, 1999.
- 3.Soller BR, Sliwa J, Yang Y, Zou F, Ryan KL, Rickards CA, Convertinob VA. Simultaneous spectroscopic determination of forearm muscle pH and oxygen saturation during simulated haemorrhage. Mortality 2012; 3: 4–4. [Google Scholar]
- 4.Ryan K, Rickards CA, Hinojosa-Laborde C, Cooke WH, Convertino V. Sympathetic responses to central hypovolemia: new insights from microneurographic recordings. Front Physiol 2012; 3: 110–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard JT, Janak JC, Hinojosa-Laborde C, Convertino VA. Specificity of compensatory reserve and tissue oxygenation as early predictors of tolerance to progressive reductions in central blood volume. Shock 2016; 46: 68–73. [DOI] [PubMed] [Google Scholar]
- 6.Janak JC, Howard JT, Goei KA, Weber R, Muniz GW, Hinojosa-Laborde C, Convertino VA. Predictors of the onset of hemodynamic decompensation during progressive central hypovolemia: comparison of the peripheral perfusion index, pulse pressure variability, and compensatory reserve index. Shock 2015; 44: 548–553. [DOI] [PubMed] [Google Scholar]
- 7.Hinojosa-Laborde C, Aden JK, Goei KA, Convertino VA. Evidence for a higher risk of hypovolemia-induced hemodynamic instability in females: implications for decision support during prehospital triage. Milit Med 2015;180:19–23. [DOI] [PubMed]
- 8.Hinojosa-Laborde C, Howard JT, Mulligan J, Grudic GZ, Convertino VA. Comparison of compensatory reserve during lower-body negative pressure and hemorrhage in nonhuman primates. Am J Physiol 2016;310:R1154–1159. [DOI] [PMC free article] [PubMed]
- 9.Hinojosa-Laborde C, Shade RE, Muniz GW, Bauer C, Goei KA, Pidcoke HF, Chung KK, Cap AP, Convertino VA. Validation of lower body negative pressure as an experimental model of hemorrhage. J Appl Physiol 2014; 116: 406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kay VL, Rickards CA. The role of cerebral oxygenation and regional cerebral blood flow on tolerance to central hypovolemia. Am J Physiol Regul Integr Comp Physiol 2016; 310: 375–83. [DOI] [PubMed] [Google Scholar]
- 11.White DW, Raven PB. Autonomic neural control of heart rate during dynamic exercise: revisited. J Physiol 2014; 592: 2491–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev 1973; 53: 159–227. [DOI] [PubMed] [Google Scholar]
- 13.Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA. Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. J Physiol 2009;587:4987–99. [DOI] [PMC free article] [PubMed]
- 14.Convertino VA, Moulton SL, Grudic GZ, Rickards CA, Hinojosa-Laborde C, Gerhardt RT, Blackbourne LH, Ryan KL. Use of advanced machine-learning techniques for noninvasive monitoring of hemorrhage. J Trauma 2011; 71: S25–32. [DOI] [PubMed] [Google Scholar]
- 15.Rickards CA, Ryan KL, Cooke WH, Convertino VA. Tolerance to central hypovolemia: the influence of oscillations in arterial pressure and cerebral blood velocity. J Appl Physiol 2011; 111: 1048–58. [DOI] [PubMed] [Google Scholar]
- 16.Convertino VA, Rickards CA, Ryan KL. Responses of sympathetic nerve activity to presyncope: new insights about mechanisms of fainting. J Gravit Physiol 2010; 17: P27–30. [Google Scholar]
- 17.Convertino VA, Ryan KL, Rickards CA, Glorsky SL, Idris AH, Yannopoulos D, Metzger A, Lurie KG. Optimizing the respiratory pump: harnessing inspiratory resistance to treat systemic hypotension. Respir Care 2011; 56: 846–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan KL, Cooke WH, Rickards CA, Lurie KG, Convertino VA. Breathing through an inspiratory threshold device improves stroke volume during central hypovolemia in humans. J Appl Physiol 2008; 104: 1402–9. [DOI] [PubMed] [Google Scholar]
- 19.Ward KR, Tiba MH, Ryan KL, Torres Filho IP, Rickards CA, Witten T, Soller BR, Ludwig DA, Convertino VA. Oxygen transport characterization of a human model of progressive hemorrhage. Resuscitation 2010; 81: 987–93. [DOI] [PubMed] [Google Scholar]
- 20.Finfer S, Vincent J-L. Critical care: an all-encompassing specialty. N Engl J Med 2013; 369: 669–70. [DOI] [PubMed] [Google Scholar]
- 21.Barcroft H, Edholm OG, Mcmichael J, Sharpey-Schafer EP. Posthaemorrhagic fainting: study by cardiac output and forearm flow. Lancet 1944; 243: 489–91. [Google Scholar]
- 22.Secher NH, Sander JK, Werner C, Warberg J, Bie P. Bradycardia during severe but reversible hypovolemic shock in man. Circ Shock 1983; 14: 267–74. [PubMed] [Google Scholar]
- 23.Convertino VA, Sather TM. Effects of cholinergic and β-adrenergic blockade on orthostatic tolerance in healthy subjects. Clin Auton Res 2000; 10: 327–36. [DOI] [PubMed] [Google Scholar]
- 24.Campagna JA, Carter C. Clinical relevance of the Bezold–Jarisch reflex. Anesthesiology 2003; 98: 1250–60. [DOI] [PubMed] [Google Scholar]
- 25.Sharpey-Schafer EP, Hayter CJ, Barlow ED. Mechanism of acute hypotension from fear or nausea. Br Med J 1958; 2: 878–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little RA, Kirkman E, Driscoll P, Hanson J, Mackway-Jones K. Preventable deaths after injury: why are the traditional’vital’signs poor indicators of blood loss? J Accid Emerg Med 1995; 12: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Convertino VA, Wirt MD, Glenn JF, Lein BC. The compensatory reserve for early and accurate prediction of hemodynamic compromise. Shock 2016; 45: 580–90. [DOI] [PubMed] [Google Scholar]
- 28.Convertino VA, Rickards CA, Ryan KL. Autonomic mechanisms associated with heart rate and vasoconstrictor reserves. Clin Auton Res 2012; 22: 123–30. [DOI] [PubMed] [Google Scholar]
- 29.Convertino VA, Sather TM. Vasoactive neuroendocrine responses associated with tolerance to lower body negative pressure in humans. Clin Physiol 2000; 20: 177–84. [DOI] [PubMed] [Google Scholar]
- 30.Convertino VA. Neurohumoral mechanisms associated with orthostasis: Reaffirmation of the significant contribution of the heart rate response. Frontier Physiol 2014; 5: 236–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Convertino VA. Blood pressure measurement for accurate assessment of patient status in emergency medical settings. Aviat Space Environ Med 2012; 83: 614–19. [DOI] [PubMed] [Google Scholar]
- 32.Rickards CA, Ryan KL, Cooke WH, Lurie KG, Convertino VA. Inspiratory resistance delays the reporting of symptoms with central hypovolemia: association with cerebral blood flow. AJP Regul Integr Comp Physiol 2007; 293: R243–50. [DOI] [PubMed] [Google Scholar]
- 33.Ryan KL, Rickards CA, Hinojosa-Laborde C, Convertino VA. Time course of compensatory physiological responses to central hypovolemia varies with tolerance. FASEB J 2012; 26: 1080.5 (abstract)–1080.5 (abstract). [Google Scholar]