Abstract
Lymphangiogenesis is a recognized hallmark of inflammatory processes in tissues and organs as diverse as the skin, heart, bowel, and airways. In clinical and animal models wherein the signaling processes of lymphangiogenesis are manipulated, most studies demonstrate that an expanded lymphatic vasculature is necessary for the resolution of inflammation. The fundamental roles that lymphatics play in fluid clearance and immune cell trafficking from the periphery make these results seemingly obvious as a mechanism of alleviating locally inflamed environments: the lymphatics are simply providing a drain. Depending on the tissue site, lymphangiogenic mechanism, or induction timeframe, however, evidence shows that inflammation-associated lymphangiogenesis (IAL) may worsen the pathology. Recent studies have identified lymphatic endothelial cells themselves to be local regulators of immune cell activity and its consequential phenotypes – a more active role in inflammation regulation than previously thought. Indeed, results focusing on the immunocentric roles of peripheral lymphatic function have revealed that the basic drainage task of lymphatic vessels is a complex balance of locally processed and transported antigens as well as interstitial cytokine and immune cell signaling: an interplay that likely defines the function of IAL. This review will summarize the latest findings on how IAL impacts a series of disease states in various tissues in both preclinical models and clinical studies. This discussion will serve to highlight some emerging areas of lymphatic research in an attempt to answer the question relevant to an array of scientists and clinicians of whether IAL helps to fuel or extinguish inflammation.
Impact statement
Inflammatory progression is present in acute and chronic tissue pathologies throughout the body. Lymphatic vessels play physiological roles relevant to all medical fields as important regulators of fluid balance, immune cell trafficking, and immune identity. Lymphangiogenesis is often concurrent with inflammation and can potentially aide or worsen disease progression. How new lymphatic vessels impact inflammation and by which mechanism is an important consideration in current and future clinical therapies targeting inflammation and/or vasculogenesis. This review identifies, across a range of tissue-specific pathologies, the current understanding of inflammation-associated lymphangiogenesis in the progression or resolution of inflammation.
Keywords: Vascular endothelial growth factor receptor-3, lymphatic, vascular endothelial growth factor-D, metabolic syndrome, endometriosis, hypertension
Introduction
The importance of the lymphatic vasculature and its fundamental roles have often been limited to the focus of narrow fields. For vascular biologists and tissue physiologists, lymphatics are part of the body’s circulation.1 For cancer researchers and clinicians, lymphatics are a route for metastatic cells to spread.2 For immunologists, lymphatics carry immune cells, deliver antigens, and provide a site for immune cell interactions.3 In each of these highlighted areas, lymphangiogenesis – the growth of new lymphatic vessels – is a potential mechanism for increasing that particular role or function. What has become increasingly appreciated, however, is that these roles are highly integrated and lymphatic “function” extends to all aspects of tissue homeostasis. The best examples of this integration are presented in instances of acute or chronic inflammation wherein lymphatics and lymphangiogenesis play a contextually dependent beneficial or detrimental role. To illustrate the importance of lymphatic biology and physiology, this review will identify how inflammation-associated lymphangiogenesis (IAL) extinguishes or propagates inflammatory progression in multiple tissues and diseases.
Lymphatic vessel and endothelial cell purpose
Lymphatic endothelial cell (LEC) biology and lymphangiogenesis have been extensively examined in a host of excellent reviews in the past decade (see the literature4–7). To understand how IAL specifically impacts inflammatory progression, only a brief and basic summary of lymphatic roles and lymphatic biology is necessary. The smallest lymphatic vessels, lymphatic capillaries, reside in nearly all vascularized tissues as the low pressure sink to which extravasated fluid from blood vessels is removed from the interstitium.1 This interstitial fluid, or what becomes primary lymph, contains all transportable macromolecules of the tissue. This cocktail is then intrinsically and extrinsically pumped along larger collecting lymphatic vessels to sentinel lymph nodes and is returned to blood circulation further downstream.8 Disruption of the lymphatics anywhere along this path by genetic manipulation or surgical resection results in peripheral lymphedema.9 Consequently, fluid balance, and the resulting biomechanical and biochemical environment regulated by flow, is viewed as the predominant lymphatic role. Lymphatics also serve as a conduit for immune cell trafficking from the periphery by providing a route to the regional lymph nodes. The macromolecules transported via this path include cytokines, tissue fragments, hormones, and foreign antigens essential for both regular immune maintenance – tissue tolerance – and acquired immune responses.3 The role of “fluid balance” is therefore more complex considering that disruption of flow impacts interstitial transport and that the composition of the fluid and the immune cells that traffic this route are both responsible for the classical lymphatic role in immune regulation. As a result, in instances of lymphatic malfunction such as lymphedema, many processes are disrupted: hydrostatic fluid balance is altered, cells do not experience interstitial flow, immune cells fail to properly traffic, and peripheral antigen transport is significantly delayed. The result: failure of both acute and chronic immune responses.9–11 Recently, more active roles for LECs in immune regulation have been receiving more attention.12–14 As was eloquently reviewed by Card et al.,3 LECs have the ability to themselves take up and process antigens, alter immune cell phenotype and function, serve as a site of immune cell interaction, and secrete cytokines that propagate immune responses both locally and in the downstream node. These three roles of fluid balance, antigen and immune cell clearance, and immune response regulation are therefore not fully distinct. In reality, these functions are intimately linked and conceptually important in understanding how lymphatic vessels and the extent of IAL induce beneficence or harm.
Lymphangiogenesis regulation
Lymphangiogenesis is the development of the initial lymphatic vasculature, the growth of new vessels, and the hyperplasia of existing structures. Adult lymphangiogenesis is limited to acute and chronic conditions of inflammation and tissue remodeling.15 Adult lymphatic vessels are otherwise quiescent explaining the lack of a lymphatic vascular phenotype upon genetic ablation of the predominant lymphatic growth factor, vascular endothelial growth factor (VEGF)-C in adult mice.16 The mechanism by which lymphangiogenesis occurs, most notably the cell source of new LECs, has recently been described to be much more complex than the traditional vascular sprouting described in blood angiogenesis and is very much tissue specific.16–20 Multiple cell types may participate or assist in lymphangiogenesis as a source of lymphatic growth factors that induce LEC proliferation6,15 and equally many antiproliferative cells and cytokines have been described.15,21 The predominant and consistently key signaling mechanism for lymphangiogenesis is VEGF receptor (VEGFR)-3 activation by its ligands VEGF-C and VEGF-D.4,7 LEC VEGFR-3 signaling is requisite in developmental and adult models of lymphangiogenesis.5,22 Serum levels of VEGF-C and VEGF-D are elevated in inflammatory disease further confirming a driving role in IAL.23–25 Focusing on VEGFR-3 signaling perhaps oversimplifies the mechanisms of IAL in which truly a host of pro- and antifactors are spatially and temporally at play; however, VEGFR-3 signaling is the gold standard for targeting lymphatic growth in preclinical studies and is now being manipulated in clinical approaches seeking to induce or resolve IAL.
Defining IAL
Lymphatic vessel roles make the system of increasing interest to the broader scientific community. Pathology slides are now routinely labeled for lymphatic protein markers LYVE-1 or podoplanin by clinical and research labs alike and have identified changes to lymphatic size and density across a range of diseases. As previously mentioned, adult lymphangiogenesis is limited to states of acute or chronic tissue inflammation (or wound healing which we do not specifically cover herein26,27). Most often, this inflammation and the consequences of the resulting IAL are dependent upon the tissue in which the remodeling is occurring. For this purpose, we accept IAL to be either expansion or generation of lymphatic capillary networks or the destabilization and hyperplasia of larger vessels. Thus, while lymphangiogenesis should increase fluid clearance, it is noted in multiple cases presented herein that IAL may merely result in expanded but less functional lymphatics. This review will highlight specific tissue pathologies in an attempt to give a broad overview of the contextually dependent beneficial and detrimental effects of IAL. Two well-studied IAL manifestations that are not necessarily limited to a particular region of the body, lymph node expansion and peritumoral lymphangiogenesis, are easily illustrated environments that provide an excellent basis to define potential roles of IAL and pathological lymphatic functions prior to discussing tissue-specific effects.
Inflammatory lymph node remodeling
Lymph node remodeling in response to afferent peripheral inflammation is a well-characterized process. Changes in the lymph node environment can include volume expansion from increased fluid burden, expansion of the LEC-structured lymphatic sinuses, expansion of the fibroblastic reticular cell network, and proliferation of lymphocyte populations.15 These changes are mechanically mediated and driven by vascular growth factors, cytokines, and CCL21-expressing T-cells entering with the afferent lymph as well as by lymph node reticular fibroblasts and B-cells (and later by neutrophils).15 CCL21 is a noted LEC-secreted chemokine that guides leukocytes to lymphatic vessels for tissue egress; its levels are indicative in this review of both LEC numbers and activation.5 Induction of proper lymph node expansion is a necessary part of resolving afferent inflammation and is documented in most IAL models in this review.28 Once the peripheral inflammation is resolved, lymph node volumes recede and lymphatic structures regress, a process driven at least in part by changes in the T-cell secretome.29,30 Nodal expansion and subsequent resolution are thus a beneficial model by which to judge IAL.
Lessons from cancer research
Increased tumor-associated lymphatic vessel density and sentinel lymph node expansion correlate with higher incidences of metastases and poorer survival statistics in multiple cancer forms.31–33 Lymphatic expansion by VEGF-C- or VEGF-D-expressing tumors is overall detrimental to patients, leading to dilation and hyperplasia of lymphatic vasculature,2,34 increased lymph flow,35,36 and increased lymphatic metastases.2,37 One beneficial consequence of this process is that future therapies could potentially target the tumor spread via the upstream lymphatic network: a novel strategy of prevention and treatment of metastatic lymph nodes that shows promise in preclinical models.38
Tumor-associated lymphatics are, however, more than a metastatic highway. When tumors form, one component promoting their rapid progression is the ability to hijack immune signaling pathways.39–41 This mimicry effect prohibits the host’s innate immune response by activating more regulatory T-cells and preventing them from functioning against cancer cells, leading to tumor tolerance of the host’s immune defenses.39–41 Metastatic tumors are also known to secrete the leukocyte attractant CCL21,42 a chemokine highly expressed by LECs, that serves to propagate leukocyte-driven lymphangiogenesis and essentially makes the tumor a rogue tertiary lymphoid organ (TLO).43 The increased local LEC-immune interaction is essential for immunotolerance and the tumor’s ability to evade normal immune responses.43–46 Considering that the roles of lymphatics in tumor progression involve both local fluid and immune regulation, their effects serve as a model for understanding the impact of IAL on other disease states.
IAL in tissue inflammation pathologies
Immunohistochemical labeling has identified lymphatic vessels in most tissues – recent appreciation of functional lymphatics in the eye and brain only highlight the system’s potential47,48 – and a multitude of case reports identify increased or expanded lymphatic vessels with inflammation. For many tissues, a more complete characterization of lymphangiogenesis and, in most cases, direct evidence of the benefits or harm of IAL through the application of VEGFR-3 ligands (genetically, ectopically, or virally) or blockade of this pathway, is covered in multiple pathologies. In others, we cite literature wherein lymphatics themselves or lymphatic roles are referenced in the disease and extrapolate how changes in lymphatic density might impact tissue disease state.
Lymphangiogenesis in airway inflammation
Airway lymphatic vessels are necessary to regulate immune responses to the external environment and maintain fluid balance in the respiratory mucosa. Inhaled pathogens, including air pollution particles,49 are transported via lymph to downstream lymph nodes for immune surveillance. Rodent models using Mycoplasma pulmonis infection have offered great insight into the mechanisms and function of IAL in the lung and airways.50 Infection results in robust VEGFR-3 signaling and B- and T-lymphocyte-dependent lymphatic expansion that persists even after resolution of the infection.51,52 While these lymphatics were morphologically different than native vessels (suggestive of altered function53), blocking IAL during infection results in increased mucosal edema.51 Conversely, VEGF-C-induction of severe pulmonary lymphangiectasia, a condition of monstrous lymphatic expansion, also increased lung edema,54 demonstrating that the quality of these newly developed vessels dictates their beneficence. Mycobacterium tuberculosis-induced granulomas also increase VEGF-C expression, resulting in local IAL.55 Tuberculosis highlights active immune roles of LECs, with nodal IAL being requisite for both antigen-specific T-cell responses55 and, interestingly, the ability of M. tuberculosis to directly infect LECs for the purpose of proliferation.56 In allergic rhinitis or asthma models, neither lymphangiogenesis nor vascular remodeling is consistently demonstrated.57,58 Changes to lymphatic morphology have been reported in chronic obstructive pulmonary disease and could play a role in sustaining or priming inflammation, though no systematic study has yet been made of lymphatic changes.59 What role bronchiolar lymphatic density plays in chronic asthma, obstructive pulmonary diseases, or how IAL impacts later responses to allergens or infection is not entirely clear.
Lymphangioleiomyomatosis (LAM) is systemic cystic disease but is often initially diagnosed through its pulmonary implications that include chylothorax (lymphatic, not hemal, fluid leakage). LAM pulmonary cysts are characterized by local IAL and massively elevated serum VEGF-D levels – 2- to 10-fold higher than healthy patients – may serve as a biomarker for the condition.23,60 Human samples from other respiratory diseases such as pulmonary fibrosis and pneumonia samples have shown increased lymphatic density, lymphoid follicles, and elevated LEC-associated CCL21–CCR7 signaling.61 Whether lymphatics serve as LAM sites and what role VEGF-D-mediated IAL plays in LAM and other lung pathologies remains to be elucidated.
Dermal inflammation and lymphangiogenesis
In the skin, IAL and changes to lymphatic patterning have been identified in both chronic62 and acute63 inflammatory skin disorders including psoriasis, atopic dermatitis, and systemic sclerosis.62 Altered dermal lymphatics are also involved in dermal sodium balance64 and tolerance sensitization.11
Acute dermal UVB-induced inflammation is characterized by enlarged lymphatic vessels and increased permeability leading to expanded inflammation and dermal edema.63,65 Blocking VEGFR-3, inhibiting lymphatic expansion, prolonged tissue swelling, and VEGF-C overexpression attenuated fluid accumulation through increased lymphatic vessel density.63,66 While these models are well characterized, conditions such as atopic dermatitis and urticaria have also been correlated with vasculogenic factors, such as VEGF and semaphorins, that suggest a role for lymphangiogenesis in each disease.62 In samples of human skin with psoriasis, the lymphatic vessels were enlarged,67–69 VEGF-C was highly overexpressed, and skin mast cell numbers were elevated.67–70 In acute mouse models, limiting IAL by inhibition of VEGFR-3 signaling increased inflammation and the infiltration, but presumably not the exit, of CD11b+ immune cells.71 Conversely, overexpression of VEGF-C led to increased lymphangiogenesis and an overall reduction in chronic skin inflammation.71,72 Consequently, in chronic skin disease models, lymphangiogenesis appears to inhibit or resolve inflammation.
Additionally, IAL has been implicated in contact hypersensitivity. K14-VEGFR-3-Ig transgenic mice lack dermal lymphatic vessels and have decreased solute transport and dendritic cell migration to the draining lymph nodes.11 These mice showed greater swelling upon initial contact hypersensitization – demonstrating the importance of the lymphatic vessels in dermal fluid balance – but failed to tolerize to hypersensitization upon further challenge.11 While these mice could elicit robust T-cell responses to dermal immunizations, the response was delayed and, with age, these mice developed autoantibodies to dermal proteins.11 These studies demonstrate that healthy lymphatic transport is necessary for maintaining long-term immune tolerance.
Not only does lymphatic density play a role in inflammatory skin diseases, but they have also been implicated in homeostatic and blood pressure regulatory control systems in dermal cutaneous tissue.64 Salt-sensitive individuals store excess electrolytes (Na+ and Cl−) in the skin interstitium that ultimately induce increased VEGF-C expression from infiltrating macrophages and, consequently, lymphangiogenesis. In turn, the excess electrolytes are cleared by these new lymphatics.64 Studies have also shown, however, that deletion of TonEBP (tonicity-responsive enhancer binding protein) from monocytes led to a lack of VEGF-C secretion, decreased clearance of electrolytes, and increased blood pressure.64 Wiig et al. confirmed this effect by also inhibiting VEGFR-3, thereby decreasing cutaneous lymphatic vessel density and inducing hypertension by preventing sufficient clearance of excess electrolytes. VEGF-C overexpression had the opposite effect, increasing the uptake of electrolytes and water in the skin to further confirm the homeostatic role of dermal lymphatic remodeling.64
Lymphangiogenesis in intestinal inflammation
Gut lymphatics are not only responsible for surveying the massive bacterial load of the intestinal lumen, and the immunologic and inflammatory maintenance of such, but also play a lymphatic role that has not yet been touched upon: that of dietary lipid absorption in the intestine. Intestinal enterocytes take up digested and emulsified long chain dietary fatty acids from the lumen and repackage the fats into chylomicrons and very low-density lipoprotein particles that enter the initial lymphatic lacteal found within each villus. This chyle-rich lymph is transported through the submucosal lymphatics, large conducting mesenteric lymphatic vessels, and eventually on to the blood circulation at the thoracic duct. VEGFR-3-mediated deficiencies in the structure and maintenance of the lacteals disrupt lipid absorption, while changes in diet and hydration primarily affect mesenteric lymph flow rates.73 Genetic and inflammatory alterations of lymphatic architecture result in lymph leakage.74 Lymph leaked into the peritoneum is rich in soluble antigens and lipophilic bacterial products (e.g. lipopolysaccharide), and hence initiates the inflammatory immune cascade of macrophage and dendritic cell activation: a cycle of inflammation that likely further alters lymphatic function.74,75 Lymph leaked in the mesentery is also fatty acid-rich and highly adipogenic, causing expansion of the adipose tissue surrounding the collecting mesenteric vessels.74,76
In chronic inflammatory bowel diseases from ulcerative colitis to Crohn’s disease, lacteals dilate, submucosal edema occurs, and initial lymphatic capillaries proliferate.73 It appears to be unlikely that IAL improves lymphatic drainage function under these conditions that manifest with rampant edema and failure of dendritic cells to migrate from the tissue,77 but this may be partially caused by downstream reductions in pumping or lymphatic obstructions with lymphangitis.73,78 In Crohn’s mesenteric tissue biopsies, granulomatous lymphangitis further propagates inflammation as CD20+ B-cell rich TLOs reside along the mesenteric lymphatic vessels inhibiting flow and likely modulating the local immune response.78 As in Crohn’s, animal models of ileitis and colitis also exhibit lymphangiogenesis in the lymph nodes and lymphatic-associated TLOs, in addition to defective lymphatic drainage and pumping, as well as sustained mucosal edema and inflammation.79,80 Expansion of the mesenteric adipose tissue, termed “fat-wrapping” or “creeping fat,” is a hallmark of Crohn’s. Poor lymphatic function, accompanied by leaking mesenteric lymph and coupled with increased cytokine accumulation from the surrounding inflamed tissue, creates an adipogenic environment similar to that in well-characterized peripheral lymphedema models.73,81,82 Conversely, mouse models with congenital intestinal lymphatic dysplasia or targeted deletion of LECs both exhibit increased intestinal inflammation.21 Blockade of IAL using an antibody against VEGFR-3 has also led to increased leukocyte accumulation and edema in a spontaneous mouse irritable bowel disease model.83 Destabilization of the existing lymphatic vasculature thus reduces the critical maintenance roles of gut lymphatics suggesting that IAL factors are overall detrimental to bowel diseases.
Lymphangiogenesis in cardiovascular disease
In addition to lymphatics being part of the body’s greater circulatory loop, local lymphatics are critical in maintaining the health of the cardiovascular system. The lymphatic vasculature is particularly extensive within the heart and near the region of the vena cava, where lymphatic fluid is eventually returned to blood circulation.1 Recently, myocardial infarctions (MI), an all-too-common cardiac pathology, have been identified as an inflammation-associated event with lymphatics playing a significant healing role. Post-MI inflammation is accompanied by increased VEGFR-3 signaling and lymphangiogenesis, particularly at the border of the scarred/infarct region.17 Furthermore, VEGF-C administration after a myocardial infarct event induces lymphangiogenesis, improves cardiac function, and increases the drainage of the non-infarct region thereby improving prognosis.17,84 Recently, delivery of adenoviral VEGF-D has also demonstrated improvement in cardiac function and is currently progressing to the clinic for further study. This work promisingly links cardiac lymphangiogenesis to improved patient health and future clinical practice.85,86
Atherosclerosis is characterized by a buildup of cholesterol plaques in arterial walls.87 Local lymphatic expansion (or lack thereof) and immune cell trafficking have been named key players in the inflammatory aspect of this disease.88 One characteristic of the pathology is the conversion of macrophages to foam cells by the ingestion of lipids in the plaque and their inability to exit the tissue in the absence of lymphatic vessels.89,90 Lymphatic vessels may also provide a route to clear cholesterol from the atherosclerotic lesion via reverse cholesterol transport (RCT). RCT is the process by which cholesterol is taken up by high-density lipoprotein (HDL) in the periphery and transported to the liver for processing and degradation.91,92 Recent studies have implicated the lymphatics as the primary transport system by which peripheral HDL enters circulation.91,93 Lymphatic density may thus control the rate of tissue cholesterol clearance; several animal studies have demonstrated a disruption in RCT with a disruption of lymphatic vessels.91,93 Lymphatics have also been implicated in venous health, with lymphatic ligation increasing venous lipid retention and TNF-alpha-mediated degeneration.94 Martel et al.93 also confirmed significantly impaired cholesterol removal from transplanted atherosclerotic aortas in which VEGFR-3 had been blocked. Mice with inherently reduced lymphatic density and impaired VEGFR-3 signaling also demonstrate increased atherosclerotic phenotypes.95 It should be noted that the role of the lymphatics in this process is potentially one of local immune regulation or even active HDL transport from the tissue space by LECs themselves.91 Collectively, the presence of lymphatic vessels and lymphangiogenesis appears to be beneficial in conditions of atherosclerosis. As such, increasing lymphangiogenesis at an atherosclerotic site could prove to be therapeutic in the clinic.
Considering their close association with arterial walls, it is reasonable to ponder whether lymphatics play a role in vascular inflammation, venous disease, and hypertension. We have already discussed how dermal lymphatics play a role in sodium storage and, hence, potentially salt-sensitive hypertension.96 In other organ systems, lymphatic function may locally regulate endocrine tissue homeostasis and their hormone secretion and transport, such as the renin–angiotensin–aldosterone system present in the kidney.97 Recent work in the kidney, for example, has implicated changes in renal T-cell populations and inflammation with hypertension in both human samples and animal models.98 With the lymphatics regulating immune phenotypes, tissue inflammation, cholesterol transport, and salt retention, lymphangiogenesis potentially plays complex and multitudinous roles in regulating cardiovascular health.
Lymphangiogenesis and renal disease
The field of renal health and kidney inflammation more specifically is associated with conditions such as hypertension, diabetes, and proteinuria.99 Little is published about the physiological functions of renal lymphatics, and less so in these disease states. In animal models, ligation of renal lymphatics results in renal edema, increased urine volume, and hypertension.100,101 Two case reports describing patients presenting with hypertension have diagnosed concurrent renal lymphangiectasia. In these cases, hypertension is attributed to the mechanical stress applied by hyperplastic collecting lymphatic vessels.102,103 In one case study, the hypertension was resolved upon removal of the collected fluid, while in another the patient’s hypertension was resolved with angiotensin-converting enzyme inhibitors, pain medication, and salt restriction.102,103 Lymphatic function in immune cell trafficking may present a clearer role: increased T-cell infiltration is necessary for development of Dahl salt-sensitive hypertension via aberrant activation of the renin–angiotensin system in rats.104–106 While inflammatory immune cells are a source of VEGFR-3 ligands, none of these studies directly implicate IAL, or a lack thereof, to hypertension specifically. We recently presented a model of VEGF-D-driven expansion of the renal lymphatic vasculature (Figure 1) that could potentially be used to assess how lymphatic function impacts blood pressure in a variety of hypertensive models.107 Based on the results in other tissues, inducing renal lymphangiogenesis may alleviate the kidney’s inflammatory burden and increase immune cell egress in these models.
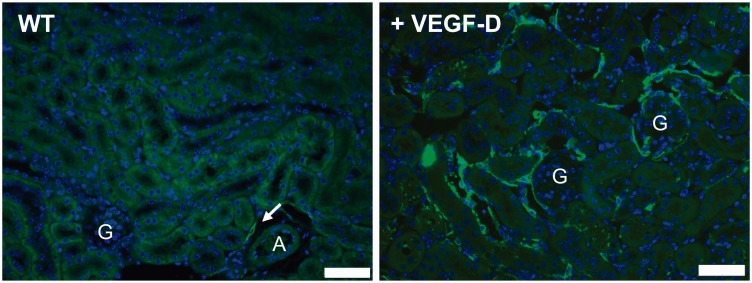
Figure 1.
Expansion of the renal lymphatic network. Lymphatic vessels are few in the murine renal cortex limited to tracking along interlobular arterioles (arrow, left). Overexpression of VEGF-D (right) by tubular epithelial cells induces a massive expansion of cortical lymphatic vessels (green, LYVE-1) providing a model to study the impact of lymphatic density in renal function and pathology. “A” indicates arteriole. “G” indicate glomeruli. Blue = DAPI. Scale bars = 50 µm. VEGF-D: vascular endothelial growth factor-D
Renal diseases often manifest with concurrent inflammation, fibrosis, and proteinuria. Proteinuria is not only a symptom but also directly damages tubular epithelia resulting in pro-inflammatory CC- and CXC-chemokine secretions.108 These chemokines also promote the recruitment of circulating leukocytes, such as regulatory T-cells, and therefore, indirectly promote IAL.109 Lymphangiogenesis has also been previously correlated with renal fibrosis in several studies.110–113 Renal lymphangiogenesis has been identified, however, prior to the development of marked fibrosis.114 Blocking CCL21, a LEC-secreted chemokine, reduces kidney fibrosis by preventing the infiltration of fibrocytes and macrophages.115 Together, these evidences suggest IAL occurs early with proteinuria and is potentially necessary, or merely an early indicator of, the development of subsequent fibrotic phenotypes. In contrast to other IAL events, lymphangiogenesis could be targeted to prevent proteinuria-mediated tubulointerstitial fibrosis; however, chronic lymphatic ligation worsens renal inflammation, fibrosis, and proteinuria100 so disruption of homeostatic lymphatic function may have severe consequences.
In healthy kidneys lacking any sort of pathology, relatively few lymphatic capillaries are present within the cortex of the kidney.99 Despite their relative scarcity, their chronic ligation is severely detrimental to renal function and inflammation.100 Chronic inflammatory conditions such as lupus nephritis, antineutrophil cytoplasmic antibody-related glomerulonephritis, tubulointerstitial nephritis, and IgA nephropathy show markedly increased populations of lymphatic vessels in the renal cortex compared to controls.111 Additionally, Type 2 diabetic nephropathy biopsies have shown both increased VEGF-C expression and elevated lymphatic density.111 A study focusing specifically on tubulointerstitial nephritis found that the number of lymphatic vessels was significantly correlated with the degree of fibrosis and that infiltrating monocytes were expressing VEGF-C.111 Biopsies taken from chronic interstitial nephritis or chronic IgA nephropathy patients showed significantly elevated lymphatic density compared to biopsies of acute tubulointerstitial nephropathy patients.116 These results suggest that sustained inflammation must be present for a substantial period of time prior to (or to initiate) lymphangiogenesis. Whether lymphatic expansion is actively involved or merely a marker of these pathologies, however, remains to be determined, though it is intriguing to speculate that by having fewer lymphatics when healthy, the kidney is ripe for IAL causing dysfunction.
Adipose tissue, the metabolic syndrome, and lymphatic function
Systemic manifestations of the metabolic syndrome – hyperlipidemia, hyperglycemia, and insulin resistance – all have their root in dysfunctional obese adipose tissue that is characterized by inadequate vascularization and increased fibrosis and inflammation.117 In mouse models targeting inflammation, either by reducing immune cell populations in adipose tissue or by genetic manipulation of inflammatory cytokines, systemic metabolism is improved.118 What role lymphatic vessels play in regulating the adipose tissue interstitium is still unknown in normal physiology, but feedback between the lymphatics and adipocytes is clear. Adipose tissue hormones, collectively adipokines, have potent effects on endothelial cell biology.76 Adiponectin, for example, increases nitric oxide synthase in LECs and adiponectin treatment aides in ameliorating lymphedema.119 How other adipokines, such as leptin, impact LEC biology and function is also of timely interest in many research groups.120 Multiple mouse models targeting lymphatic vessel development and maturation demonstrate increased adiposity accompanied by adult onset obesity.76 In lymphedema, when lymph flow is compromised, potentially massive adipose expansion occurs in the affected periphery.121 In inflammation, leaky lymphatics driven by IAL factors are commiserate with expansion of the adipose directly around the destabilized lymphatic vessel. Inflammation models have also demonstrated local and lymph node adiposity with IAL.73 Adipose–lymphatic interactions are not all malicious, however, as perinodal adipose tissue and adipose-resident monocytes along collecting lymphatic vessels play an important role in maintaining lymph node and immune surveillance functions, respectively.73,75
Measurements of lymph flow in generalized obesity demonstrate reduced lymphatic function, and its improvement with weight loss and exercise in the same animals.122,123 In lipedema, a condition of pathologic adipogenesis, lymphatic function is reduced and potentially plays a role in disease progression.15 A multitude of adipokines and growth factors are expressed in healthy and obese adipose and their relative distributions may impact local lymphatic integrity and function as well as IAL.76 VEGF-C and VEGF-D are heightened in obesity; a recent study of systemic VEGFR-3 blockade improved glucose regulation in part through reduced adipose tissue macrophage infiltration.24 Similarly, mice with constitutive dermal overexpression of VEGF-C exhibit hyperplastic lymphatic vessels, heightened adipose inflammation, and increased adiposity and insulin resistance.124 Recently, we developed a mouse model with inducible adipocyte-specific overexpression of VEGF-D and, while the mice demonstrated a massive expansion of lymphatic networks in adipose tissues, macrophage recruitment and inflammation were also increased.107 With high fat diet feeding that normally drives adipose tissue inflammation, fewer resident macrophages were found with increased lymphatic density (Figure 2). Uncoupling the negative chemokine aspects of these lymphatic growth factors from the potentially positive effects of increased lymphatic density is currently underway.
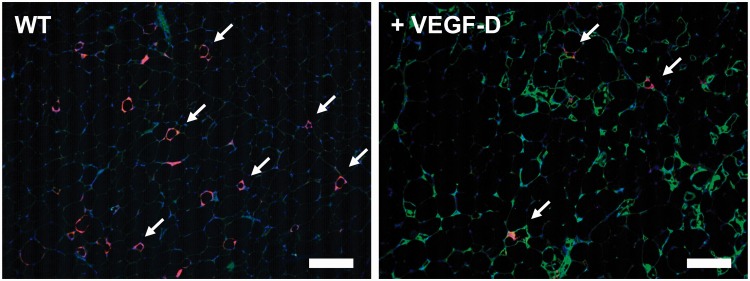
Figure 2.
Adipose tissue lymphangiogenesis. Obese murine inguinal adipose tissue lacks lymphatic capillaries (green, LYVE1) and contains macrophages formed crown-like structures (red, Mac2; arrows highlight some) in the inflamed tissue (left). VEGF-D overexpression induces lymphangiogenesis in adipose tissue (right), with IAL potentially providing a route for immune efflux with fewer Mac2+ areas (arrows, all noted) in equally obese adipose tissue. Blue = DAPI. Scale bars = 100 µm. VEGF-D: vascular endothelial growth factor-D
Though adipose inflammation may be a target for lymphangiogenesis in the metabolic syndrome, diabetic lymphatic function, or, perhaps more importantly, direct lymphangiogenic effects on beta cells’ insulin secretion and the pancreatic islets is less clear. Islets have intra-islet blood capillaries, but lymphatic capillaries are only found in the islet periphery. In two models in which VEGF-C or VEGF-D was directly overexpressed in beta cells, no lymphatic ingrowth to the islets was observed, despite marked extra-islet lymphatic expansion.34,125 In the commonly used model of streptozotocin (STZ)-induced islet toxicity, IAL was identified around the islets.126 Inhibiting IAL in the STZ model by blocking VEGFR-3 reduced islet cytokine levels as well as macrophage infiltration and preserved islet mass (and, by extension, insulin secretion).126 Whether islet IAL is good or bad may therefore depend on the underlying mechanism of beta cell loss, i.e. Type 1 versus Type 2 diabetes. No large-scale systematic study in humans has yet identified peripheral lymphatics to be functionally altered in diabetes, but in some patient samples and murine models, insulin resistance and obesity, not high fat diet alone, reduces lymphatic fluid transport.127,128 Since weight loss and exercise alone each rapidly improve obesity-associated lymphatic dysfunction,123,127 are lymphatic deficiencies merely reflective of greater tissue imbalance? The importance of lymphangiogenesis and LECs in modulating peripheral inflammation, however, still makes lymphatic expansion an attractive potential target in dysfunctional adipose tissue in obesity, lipedema, and lymphedema and in the peripheral vascular manifestations of diabetes.
IAL in female reproductive tissues
Lymphangiogenesis occurs in the ovaries and uterus with reproductive cycles and pregnancy26,129; however, inflammatory diseases of the female reproductive tissues are largely understudied and few murine models exist to recapitulate them. Endometriosis is a disease uniquely affecting women in which tissue resembling the uterine lining – the endometrium – is found in tissue outside the uterus, as far away as the lungs.130 One explanation is similar to the theory of LAM lesion spreading: that shed endometrial tissue and cells are transported via the lymphatic system to other regions of the body causing lesions at ectopic sites.131 Reichelt et al.132 demonstrated an increase in the lymphatic density of peritoneal lesions compared to normal peritoneum. In one study, VEGF-C and VEGF-D were both found to be significantly upregulated in endometriotic lesions and accompanied by significant lymphangiogenesis (and minimal blood angiogenesis) in ectopic lesions.132 Endometriosis is at least partially a chronic inflammatory disease, in which the inflammation is perpetuated, rather than resolved, by the lymphangiogenesis.132 Further work beyond male mouse models may identify what roles lymphatics play in endometriosis and other diseases of women’s health.
Transplantation neovascularization
One area where the role of lymphatics is often emphasized is in the context of tissue transplants. Transplant outcomes demonstrate the range of responses associated with IAL as often a mixture of harm and benefit depending on the context. In cardiac allografts, most often, lymphangiogenesis increases the chances of rejection, with blockage of VEGFR-3 signaling improving the outcome of the allograft.133 This result is likely due to the decrease in CCL21 expression and CD8+ T-cell (lymphocytes that regulate tolerance) infiltration upon blocking VEGFR-3.133 Another common transplant highlighting a potentially negative role of the lymphatics is that of corneal transplants. In instances of both human and animal keteroplasties, corneal lymphangiogenesis decreases the graft’s survival and is an indication of impending rejection.134–137 Several studies show that antivascular treatment both pre- and postprocedure improve the chances of corneal graft survival.136,138,139
The effect of IAL in renal transplants, however, is more ambiguous. In the three weeks following kidney transplant, the donor organ generates a new lymphatic system.140 This process is crucial, and low lymphatic density in the first postprocedural biopsy has been correlated with acute transplant rejection.141 This is likely due to the role of the lymphatics in fluid clearance, inflammation modulation, and immune cell clearance after the operation.142 Once the initial IAL takes place, however, persistent lymphangiogenesis and increased lymphatic density in regions of inflammation have been associated with transplant rejection.141 Whether the continuing lymphangiogenesis is merely a marker or a cause of rejection is currently unknown. One possible explanation is that, after achieving homeostatic fluid balance, the increased presence of lymphatics allows antigen-presenting cells to induce an allogenic response as in the cornea.142 As more is understood about the evolution and functions of these vessels following transplant, however, more specific therapies can be designed to improve the chances of allograft survival.
Conclusion
No region of the body is impenetrable to the flames of inflammation. The body’s natural response to tissue injury is inflammation, often accompanied with lymphangiogenesis, that is a required part of healing. This IAL and its associated responses, much like fire itself, can be classified into a few broad categories: absent, functional, and dysfunctional (Figure 3). In instances when IAL results in the development of functional vasculature, it serves its purpose of fluid and macromolecule reclamation and immune cell modulation and clearance resulting in restoration to a preinflammatory state. In this way, IAL performs its physiological duties similar to a beneficial fire that is extinguished when needed. Much like a wildfire, however, left to progress unchecked, IAL results in poorly functional vessels, a worsened immunologic state, and unresolved inflammation. As a result, the simple question of whether IAL is beneficial or harmful receives the unsatisfying answer of: it depends. Lymphatics are at the center in the interplay of fluid clearance, inflammatory cytokine removal, and innate and acquired immune regulation. Understanding IAL and its degree of beneficence on a disease- and tissue-basis is requisite in determining whether lymphangiogenesis will prove to be a valid therapeutic target for inflammation resolution and the treatment of the myriad of diseases plaguing today’s patient populations.
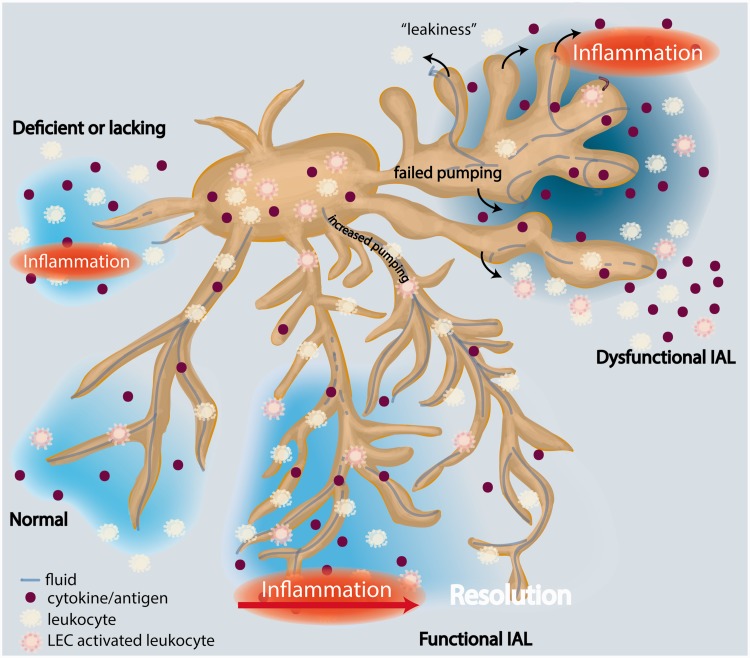
Figure 3.
Inflammation-associated lymphangiogenesis (IAL) and the modulation of tissue homeostasis. Normal, functional lymphatics clear fluid, macromolecules, and immune cells – both passively and actively – from the peripheral interstitium. Trafficking to the afferent lymph node allows for the propagation of specific immune responses. When lymphatics are deficient (left), as in lymphedema, failed clearance leads to chronic inflammation. With IAL in inflammation, an increased functional lymphatic network permits resolution as fluid and cytokines are cleared and leukocytes – activated locally by inflammation or through contact with lymphatic endothelial cells (LEC) – traffic to the lymph node. Disorganized lymphatic expansion in inflammation, however, results in lymph efflux, or “leakiness” of hyperplastic vessel (right). This reduced functional capacity fails to aid, and indeed can propagate, inflammation
Acknowledgements
JMR is supported in part by the American Heart Association (12SDG12050287) and a research grant from the University of Pennsylvania Orphan Disease Center (MDBR-16-118-GLA/GSD).
Authors’ contributions
All authors made substantial contributions to the literature research, writing, and editing of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 2012; 92: 1005–60. [DOI] [PubMed] [Google Scholar]
- 2.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001; 7: 192–8. [DOI] [PubMed] [Google Scholar]
- 3.Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest 2014; 124: 943–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 2014; 124: 878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K. Lymphatic system in cardiovascular medicine. Circ Res 2016; 118: 515–30. [DOI] [PubMed] [Google Scholar]
- 6.Cimpean AM, Raica M. Lymphangiogenesis and inflammation-looking for the “missing pieces” of the puzzle. Arch Immunol Ther Exp (Warsz) 2015; 63: 415–26. [DOI] [PubMed] [Google Scholar]
- 7.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014; 14: 159–72. [DOI] [PubMed] [Google Scholar]
- 8.Bouta EM, Li J, Ju Y, Brown EB, Ritchlin CT, Xing L, Schwarz EM. The role of the lymphatic system in inflammatory-erosive arthritis. Semin Cell Dev Biol 2015; 38: 90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res 2006; 72: 161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One 2012; 7: e49940–e49940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, Swartz MA. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol 2012; 189: 2181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthesy-Henrioud P, Capotosti F, Halin Winter C, Hugues S, Swartz MA. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J Immunol 2014; 192: 5002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kedl RM, Tamburini BA. Antigen archiving by lymph node stroma: a novel function for the lymphatic endothelium. Eur J Immunol 2015; 45: 2721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiansen AJ, Dieterich LC, Ohs I, Bachmann SB, Bianchi R, Proulx ST, Hollmen M, Aebischer D, Detmar M. Lymphatic endothelial cells attenuate inflammation via suppression of dendritic cell maturation. Oncotarget 2016; 7: 39421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan KW, Chong SZ, Angeli V. Inflammatory lymphangiogenesis: cellular mediators and functional implications. Angiogenesis 2014; 17: 373–81. [DOI] [PubMed] [Google Scholar]
- 16.Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR, Alitalo K. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med 2015; 7: 1418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dube KN, Bollini S, Matsuzaki F, Carr CA, Riley PR. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 2015; 522: 62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicenboim J, Malkinson G, Lupo T, Asaf L, Sela Y, Mayseless O, Gibbs-Bar L, Senderovich N, Hashimshony T, Shin M, Jerafi-Vider A, Avraham-Davidi I, Krupalnik V, Hofi R, Almog G, Astin JW, Golani O, Ben-Dor S, Crosier PS, Herzog W, Lawson ND, Hanna JH, Yanai I, Yaniv K. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 2015; 522: 56–61. [DOI] [PubMed] [Google Scholar]
- 19.Stanczuk L, Martinez-Corral I, Ulvmar MH, Zhang Y, Lavina B, Fruttiger M, Adams RH, Saur D, Betsholtz C, Ortega S, Alitalo K, Graupera M, Makinen T. cKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep 2015;10:1708–1721. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Corral I, Ulvmar MH, Stanczuk L, Tatin F, Kizhatil K, John SW, Alitalo K, Ortega S, Makinen T. Nonvenous origin of dermal lymphatic vasculature. Circ Res 2015; 116: 1649–54. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Kataru RP, Koh GY. Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest 2014; 124: 936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regan E, Sibley RC, Cenik BK, Silva A, Girard L, Minna JD, Dellinger MT. Identification of gene expression differences between lymphangiogenic and non-lymphangiogenic non-small cell lung cancer cell lines. PLoS One 2016; 11: e0150963–e0150963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radzikowska E, Jagus P, Skoczylas A, Sobiecka M, Chorostowska-Wynimko J, Wiatr E, Kus J, Roszkowski-Sliz K. Role of serum vascular endothelial growth factor D in discrimination of patients with polycystic lung diseases. Pol Arch Med Wewn 2013; 123: 533–8. [DOI] [PubMed] [Google Scholar]
- 24.Karaman S, Hollmen M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, Wolfrum C, Detmar M. Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Mol Metab 2015; 4: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Lu Y, Ma L, Cao X, Xiao J, Chen J, Jiao S, Gao Y, Liu C, Duan Z, Li D, He Y, Wei B, Wang H. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-kappaB signaling and protects against endotoxin shock. Immunity 2014; 40: 501–14. [DOI] [PubMed] [Google Scholar]
- 26.Rutkowski JM, Ihm JE, Lee ST, Kilarski WW, Greenwood VI, Pasquier MC, Quazzola A, Trono D, Hubbell JA, Swartz MA. VEGFR-3 neutralization inhibits ovarian lymphangiogenesis, follicle maturation, and murine pregnancy. Am J Pathol 2013; 183: 1596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutkowski JM, Boardman KC, Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol 2006; 291: H1402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 2006; 24: 203–15. [DOI] [PubMed] [Google Scholar]
- 29.Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH, Koh GY. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 2011; 34: 96–107. [DOI] [PubMed] [Google Scholar]
- 30.Mumprecht V, Roudnicky F, Detmar M. Inflammation-induced lymph node lymphangiogenesis is reversible. Am J Pathol 2012; 180: 874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das S, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci 2008; 1131: 235–41. [DOI] [PubMed] [Google Scholar]
- 32.Pepper MS, Tille JC, Nisato R, Skobe M. Lymphangiogenesis and tumor metastasis. Cell Tissue Res 2003; 314: 167–77. [DOI] [PubMed] [Google Scholar]
- 33.Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, Harris AL, Dirix LY, Vermeulen PB. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer 2006; 95: 1611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001; 20: 672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol 2007; 170: 774–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP, Jain RK. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res 2006; 66: 8065–75. [DOI] [PubMed] [Google Scholar]
- 37.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001; 7: 186–91. [DOI] [PubMed] [Google Scholar]
- 38.Kodama T, Matsuki D, Tada A, Takeda K, Mori S. New concept for the prevention and treatment of metastatic lymph nodes using chemotherapy administered via the lymphatic network. Sci Rep 2016; 6: 32506–32506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev 2006; 25: 357–71. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454: 436–44. [DOI] [PubMed] [Google Scholar]
- 41.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5: 263–74. [DOI] [PubMed] [Google Scholar]
- 42.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007; 11: 526–38. [DOI] [PubMed] [Google Scholar]
- 43.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010; 328: 749–52. [DOI] [PubMed] [Google Scholar]
- 44.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol 2006; 7: 344–53. [DOI] [PubMed] [Google Scholar]
- 45.Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol 2008; 26: 627–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol 2003; 170: 4638–48. [DOI] [PubMed] [Google Scholar]
- 47.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523: 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, Sung HK, Onay T, Chen H, Yamaguchi S, Economides AN, Flenniken A, Gale NW, Hong YK, Fawzi A, Liu X, Kume T, Quaggin SE. A lymphatic defect causes ocular hypertension and glaucoma in mice. J Clin Invest 2014; 124: 4320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreyling WG, Dirscherl P, Ferron GA, Heilmann P, Josten M, Miaskowski U, Neuner M, Reitmeir P, Ruprecht L, Schumann G, Takenaka S, Ziesenis A, Heyder J. Health effects of sulfur-related environmental air pollution. III. Nonspecific respiratory defense capacities. Inhal Toxicol 1999; 11: 391–422. [DOI] [PubMed] [Google Scholar]
- 50.McDonald DM, Yao LC, Baluk P. Dynamics of airway blood vessels and lymphatics: lessons from development and inflammation. Proc Am Thorac Soc 2011; 8: 504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest 2005; 115: 247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aurora AB, Baluk P, Zhang D, Sidhu SS, Dolganov GM, Basbaum C, McDonald DM, Killeen N. Immune complex-dependent remodeling of the airway vasculature in response to a chronic bacterial infection. J Immunol 2005; 175: 6319–26. [DOI] [PubMed] [Google Scholar]
- 53.Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol 2012; 180: 2561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao LC, Testini C, Tvorogov D, Anisimov A, Vargas SO, Baluk P, Pytowski B, Claesson-Welsh L, Alitalo K, McDonald DM. Pulmonary lymphangiectasia resulting from vascular endothelial growth factor-C overexpression during a critical period. Circ Res 2014; 114: 806–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harding J, Ritter A, Rayasam A, Fabry Z, Sandor M. Lymphangiogenesis is induced by mycobacterial granulomas via vascular endothelial growth factor receptor-3 and supports systemic T-cell responses against mycobacterial antigen. Am J Pathol 2015; 185: 432–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lerner TR, de Souza Carvalho-Wodarz C, Repnik U, Russell MR, Borel S, Diedrich CR, Rohde M, Wainwright H, Collinson LM, Wilkinson RJ, Griffiths G, Gutierrez MG. Lymphatic endothelial cells are a replicative niche for Mycobacterium tuberculosis. J Clin Invest 2016; 126: 1093–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eifan AO, Orban NT, Jacobson MR, Durham SR. Severe persistent allergic rhinitis. Inflammation but no histologic features of structural upper airway remodeling. Am J Respir Crit Care Med 2015; 192: 1431–9. [DOI] [PubMed] [Google Scholar]
- 58.Moldobaeva A, Jenkins J, Zhong Q, Wagner EM. Lymphangiogenesis in rat asthma model. Angiogenesis 2016;20:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mori M, Andersson CK, Graham GJ, Lofdahl CG, Erjefalt JS. Increased number and altered phenotype of lymphatic vessels in peripheral lung compartments of patients with COPD. Respir Res 2013; 14: 65–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu KF, Zhang P, Tian X, Ma A, Li X, Zhou J, Zeng N, Gui YS, Guo Z, Feng R, Zhang W, Sun W, Cai B. The role of vascular endothelial growth factor-D in diagnosis of lymphangioleiomyomatosis (LAM). Respir Med 2013; 107: 263–8. [DOI] [PubMed] [Google Scholar]
- 61.Yamashita M. Lymphangiogenesis and lesion heterogeneity in interstitial lung diseases. Clin Med Insights Circ Respir Pulm Med 2015; 9: 111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varricchi G, Granata F, Loffredo S, Genovese A, Marone G. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J Am Acad Dermatol 2015; 73: 144–53. [DOI] [PubMed] [Google Scholar]
- 63.Kajiya K, Detmar M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J Invest Dermatol 2006; 126: 919–21. [DOI] [PubMed] [Google Scholar]
- 64.Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Muller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 2013; 123: 2803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol 2006; 169: 1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kajiya K, Sawane M, Huggenberger R, Detmar M. Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced edema formation and skin inflammation by promoting lymphangiogenesis. J Invest Dermatol 2009; 129: 1292–8. [DOI] [PubMed] [Google Scholar]
- 67.Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med 1994; 180: 1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood 2004; 104: 1048–57. [DOI] [PubMed] [Google Scholar]
- 69.Ozdamar SO, Seckin D, Kandemir B, Turanli AY. Mast cells in psoriasis. Dermatology 1996; 192: 190–190. [DOI] [PubMed] [Google Scholar]
- 70.Liew SC, Das-Gupta E, Chakravarthi S, Wong SF, Lee N, Safdar N, Jamil A. Differential expression of the angiogenesis growth factors in psoriasis vulgaris. BMC Res Notes 2012; 5: 201–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, Werner S, Alitalo K, Detmar M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 2011; 117: 4667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huggenberger R, Ullmann S, Proulx ST, Pytowski B, Alitalo K, Detmar M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med 2010; 207: 2255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.von der Weid PY, Rainey KJ. Review article: lymphatic system and associated adipose tissue in the development of inflammatory bowel disease. Aliment Pharmacol Ther 2010; 32: 697–711. [DOI] [PubMed] [Google Scholar]
- 74.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol 2015; 194: 5200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zawieja SD, Wang W, Chakraborty S, Zawieja DC, Muthuchamy M. Macrophage alterations within the mesenteric lymphatic tissue are associated with impairment of lymphatic pump in metabolic syndrome. Microcirculation 2016;23:558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J 2009; 276: 5738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Middel P, Raddatz D, Gunawan B, Haller F, Radzun HJ. Increased number of mature dendritic cells in Crohn’s disease: evidence for a chemokine mediated retention mechanism. Gut 2006; 55: 220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Randolph GJ, Bala S, Rahier JF, Johnson MW, Wang PL, Nalbantoglu I, Dubuquoy L, Chau A, Pariente B, Kartheuser A, Zinselmeyer BH, Colombel JF. Lymphoid aggregates remodel lymphatic collecting vessels that serve mesenteric lymph nodes in Crohn disease. Am J Pathol 2016;186:3066–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Acedo SC, Gotardo EM, Lacerda JM, de Oliveira CC, de Oliveira Carvalho P, Gambero A. Perinodal adipose tissue and mesenteric lymph node activation during reactivated TNBS-colitis in rats. Dig Dis Sci 2011; 56: 2545–52. [DOI] [PubMed] [Google Scholar]
- 80.Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol 2006; 291: G566–74. [DOI] [PubMed] [Google Scholar]
- 81.Cuzzone DA, Weitman ES, Albano NJ, Ghanta S, Savetsky IL, Gardenier JC, Joseph WJ, Torrisi JS, Bromberg JF, Olszewski WL, Rockson SG, Mehrara BJ. IL-6 regulates adipose deposition and homeostasis in lymphedema. Am J Physiol Heart Circ Physiol 2014; 306: H1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aschen S, Zampell JC, Elhadad S, Weitman E, De Brot M, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg 2012; 129: 838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jurisic G, Sundberg JP, Detmar M. Blockade of VEGF receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm Bowel Dis 2013; 19: 1983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henri O, Pouehe C, Houssari M, Galas L, Nicol L, Edwards-Levy F, Henry JP, Dumesnil A, Boukhalfa I, Banquet S, Schapman D, Thuillez C, Richard V, Mulder P, Brakenhielm E. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation 2016; 133: 1484–97; discussion 97. [DOI] [PubMed] [Google Scholar]
- 85.Hassinen I, Kivela A, Hedman A, Saraste A, Knuuti J, Hartikainen JE, Yla-Herttuala S. Intramyocardial gene therapy directed to hibernating heart muscle using a combination of electromechanical mapping and positron emission tomography. Hum Gene Ther 2016;27:830–834. [DOI] [PubMed] [Google Scholar]
- 86.Nurro J, Halonen PJ, Kuivanen A, Tarkia M, Saraste A, Honkonen K, Lahteenvuo J, Rissanen TT, Knuuti J, Yla-Herttuala S. AdVEGF-B186 and AdVEGF-DDeltaNDeltaC induce angiogenesis and increase perfusion in porcine myocardium. Heart 2016; 102: 1716–20. [DOI] [PubMed] [Google Scholar]
- 87.Vinereanu D. Risk factors for atherosclerotic disease: present and future. Herz 2006; 31: 5–24. [PubMed] [Google Scholar]
- 88.Libby P, Hansson GK. Cell-mediated immunity in atherosclerosis. Curr Opin Lipidol 1997; 8: 301–11. [DOI] [PubMed] [Google Scholar]
- 89.Libby P. Inflammatory and immune mechanisms in atherogenesis. In: Moncada S, and Higgs A. (eds). The vascular endothelium II, Berlin, Heidelberg: Springer-Verlag, 2006, pp. 285–307. [Google Scholar]
- 90.Peters W, Charo IF. Involvement of chemokine receptor 2 and its ligand, monocyte chemoattractant protein-1, in the development of atherosclerosis: lessons from knockout mice. Curr Opin Lipidol 2001; 12: 175–80. [DOI] [PubMed] [Google Scholar]
- 91.Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab 2013; 17: 671–84. [DOI] [PubMed] [Google Scholar]
- 92.Huang LH, Elvington A, Randolph GJ. The role of the lymphatic system in cholesterol transport. Front Pharmacol 2015; 6: 182–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci-Thomas MG, Randolph GJ. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest 2013; 123: 1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanaka H, Yamamoto N, Suzuki M, Mano Y, Sano M, Zaima N, Sasaki T, Setou M, Unno N. Insufficient lymph drainage causes abnormal lipid accumulation and vein wall degeneration. Ann Vasc Dis 2016; 9: 277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vuorio T, Nurmi H, Moulton K, Kurkipuro J, Robciuc MR, Ohman M, Heinonen SE, Samaranayake H, Heikura T, Alitalo K, Yla-Herttuala S. Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler Thromb Vasc Biol 2014; 34: 1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Speed JS, Heimlich JB, Hyndman KA, Fox BM, Patel V, Yanagisawa M, Pollock JS, Titze JM, Pollock DM. Endothelin-1 as a master regulator of whole-body Na+ homeostasis. FASEB J 2015; 29: 4937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Navar LG. The kidney in blood pressure regulation and development of hypertension. Med Clin N Am 1997; 81: 1165–98. [DOI] [PubMed] [Google Scholar]
- 98.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 2014; 307: F499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yazdani S, Navis G, Hillebrands JL, van Goor H, van den Born J. Lymphangiogenesis in renal diseases: passive bystander or active participant? Expert Rev Mol Med 2014; 16: e15–e15. [DOI] [PubMed] [Google Scholar]
- 100.Zhang T, Guan G, Liu G, Sun J, Chen B, Li X, Hou X, Wang H. Disturbance of lymph circulation develops renal fibrosis in rats with or without contralateral nephrectomy. Nephrology (Carlton) 2008; 13: 128–38. [DOI] [PubMed] [Google Scholar]
- 101.Barer GR, Ward-Mcquaid JN. Demonstration of renal lymphatics in vivo by intravenous injection of dye: the effect of lymphatic ligature on the blood-pressure. Br J Urol 1957; 29: 171–4. [DOI] [PubMed] [Google Scholar]
- 102.Kumar K, Ahmad A, Singh M, Kumar A, Singh RP, Hussain M. Bilateral renal lymphangiectasia in a thirty-two-year-old woman. Nephrourol Mon 2015; 7: e21736–e21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blanc M, Schmutz G, Belzile F, Sabbagh R. Renal lymphangiectasia presenting with hypertension and polycythemia. Can Urol Assoc J 2014; 8: E163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL, PhysGen Knockout P. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 2014; 63: 559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luft FC, Dechend R, Muller DN. Immune mechanisms in angiotensin II-induced target-organ damage. Ann Med 2012; 44: S49–54. [DOI] [PubMed] [Google Scholar]
- 106.Rudemiller NP, Patel MB, Zhang JD, Jeffs AD, Karlovich NS, Griffiths R, Kan MJ, Buckley AF, Gunn MD, Crowley SD. C-C motif chemokine 5 attenuates angiotensin II-dependent kidney injury by limiting renal macrophage infiltration. Am J Pathol 2016;186:2846–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lammoglia GM, Van Zandt CE, Galvan DX, Orozco JL, Dellinger MT, Rutkowski JM. Hyperplasia, de novo lymphangiogenesis, and lymphatic regression in mice with tissue-specific, inducible overexpression of murine VEGF-D. Am J Physiol Heart Circ Physiol 2016; 311: H384–94. [DOI] [PubMed] [Google Scholar]
- 108.Moreno JA, Moreno S, Rubio-Navarro A, Gomez-Guerrero C, Ortiz A, Egido J. Role of chemokines in proteinuric kidney disorders. Expert Rev Mol Med 2014; 16: e3–e3. [DOI] [PubMed] [Google Scholar]
- 109.Chung AC, Lan HY. Chemokines in renal injury. J Am Soc Nephrol 2011; 22: 802–9. [DOI] [PubMed] [Google Scholar]
- 110.Matsui K, Nagy-Bojarsky K, Laakkonen P, Krieger S, Mechtler K, Uchida S, Geleff S, Kang DH, Johnson RJ, Kerjaschki D. Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: aminopeptidase p and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels. J Am Soc Nephrol 2003; 14: 1981–9. [DOI] [PubMed] [Google Scholar]
- 111.Sakamoto I, Ito Y, Mizuno M, Suzuki Y, Sawai A, Tanaka A, Maruyama S, Takei Y, Yuzawa Y, Matsuo S. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int 2009; 75: 828–38. [DOI] [PubMed] [Google Scholar]
- 112.Lee AS, Lee JE, Jung YJ, Kim DH, Kang KP, Lee S, Park SK, Lee SY, Kang MJ, Moon WS, Kim HJ, Jeong YB, Sung MJ, Kim W. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int 2013; 83: 50–62. [DOI] [PubMed] [Google Scholar]
- 113.Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, Mizuno T, Shimizu H, Fujita Y, Matsui K, Maruyama S, Imai E, Matsuo S, Takei Y. Transforming growth factor-beta induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int 2012; 81: 865–79. [DOI] [PubMed] [Google Scholar]
- 114.Yazdani S, Poosti F, Kramer AB, Mirkovic K, Kwakernaak AJ, Hovingh M, Slagman MC, Sjollema KA, de Borst MH, Navis G, van Goor H, van den Born J. Proteinuria triggers renal lymphangiogenesis prior to the development of interstitial fibrosis. PLoS One 2012; 7: e50209–e50209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA 2006; 103: 14098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heller F, Lindenmeyer MT, Cohen CD, Brandt U, Draganovici D, Fischereder M, Kretzler M, Anders HJ, Sitter T, Mosberger I, Kerjaschki D, Regele H, Schlondorff D, Segerer S. The contribution of B cells to renal interstitial inflammation. Am J Pathol 2007; 170: 457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab 2016; 23: 770–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol 2015; 208: 501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimizu Y, Shibata R, Ishii M, Ohashi K, Kambara T, Uemura Y, Yuasa D, Kataoka Y, Kihara S, Murohara T, Ouchi N. Adiponectin-mediated modulation of lymphatic vessel formation and lymphedema. J Am Heart Assoc 2013; 2: e000438–e000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sato A, Kamekura R, Kawata K, Kawada M, Jitsukawa S, Yamashita K, Sato N, Himi T, Ichimiya S. Novel mechanisms of compromised lymphatic endothelial cell homeostasis in obesity: the role of leptin in lymphatic endothelial cell tube formation and proliferation. PLoS One 2016; 11: e0158408–e0158408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Reconstr Surg 2014; 134: 154e–60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hespe GE, Kataru RP, Savetsky IL, Garcia Nores GD, Torrisi JS, Nitti MD, Gardenier JC, Zhou J, Yu JZ, Jones LW, Mehrara BJ. Exercise training improves obesity-related lymphatic dysfunction. J Physiol 2016; 594: 4267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nitti MD, Hespe GE, Kataru RP, Garcia Nores GD, Savetsky IL, Torrisi JS, Gardenier JC, Dannenberg AJ, Mehrara BJ. Obesity-induced lymphatic dysfunction is reversible with weight loss. J Physiol 2016;594:7073–7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karaman S, Hollmen M, Yoon SY, Alkan HF, Alitalo K, Wolfrum C, Detmar M. Transgenic overexpression of VEGF-C induces weight gain and insulin resistance in mice. Sci Rep 2016; 6: 31566–31566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kopfstein L, Veikkola T, Djonov VG, Baeriswyl V, Schomber T, Strittmatter K, Stacker SA, Achen MG, Alitalo K, Christofori G. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am J Pathol 2007; 170: 1348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yin N, Zhang N, Lal G, Xu J, Yan M, Ding Y, Bromberg JS. Lymphangiogenesis is required for pancreatic islet inflammation and diabetes. PLoS One 2011; 6: e28023–e28023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garcia Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, Gardenier JC, Savetsky IL, Aschen SZ, Nitti MD, Mehrara BJ. Obesity but not high-fat diet impairs lymphatic function. Int J Obes (Lond) 2016; 40: 1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haemmerle M, Keller T, Egger G, Schachner H, Steiner CW, Stokic D, Neumayer C, Brown MK, Kerjaschki D, Hantusch B. Enhanced lymph vessel density, remodeling, and inflammation are reflected by gene expression signatures in dermal lymphatic endothelial cells in type 2 diabetes. Diabetes 2013; 62: 2509–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Red-Horse K. Lymphatic vessel dynamics in the uterine wall. Placenta 2008; 29: S55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giudice LC, Kao LC. Endometriosis. Lancet 2004; 364: 1789–99. [DOI] [PubMed] [Google Scholar]
- 131.Javert CT. Pathogenesis of endometriosis based on endometrial homeoplasia, direct extension, exfoliation and implantation, lymphatic and hematogenous metastasis, including five case reports of endometrial tissue in pelvic lymph nodes. Cancer 1949; 2: 399–410. [DOI] [PubMed] [Google Scholar]
- 132.Reichelt U, Keichel S, Barcena de Arellano ML, Chiantera V, Schneider A, Mechsner S. High lymph vessel density and expression of lymphatic growth factors in peritoneal endometriosis. Reprod Sci 2012; 19: 876–82. [DOI] [PubMed] [Google Scholar]
- 133.Nykanen AI, Sandelin H, Krebs R, Keranen MA, Tuuminen R, Karpanen T, Wu Y, Pytowski B, Koskinen PK, Yla-Herttuala S, Alitalo K, Lemstrom KB. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation 2010; 121: 1413–22. [DOI] [PubMed] [Google Scholar]
- 134.Ling SQ, Qi CX, Li W, Xu JG, Kuang WH. Crucial role of corneal lymphangiogenesis for allograft rejection in alkali-burned cornea bed. Clin Exp Ophthalmol 2009; 37: 874–83. [DOI] [PubMed] [Google Scholar]
- 135.Tang XL, Sun JF, Wang XY, Du LL, Liu P. Blocking neuropilin-2 enhances corneal allograft survival by selectively inhibiting lymphangiogenesis on vascularized beds. Mol Vis 2010; 16: 2354–61. [PMC free article] [PubMed] [Google Scholar]
- 136.Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, Wiegand S, Chen L, Cursiefen C. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol 2010; 184: 535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng Y, Lin H, Ling S. Clinicopathological correlation analysis of (lymph) angiogenesis and corneal graft rejection. Mol Vis 2011; 17: 1694–700. [PMC free article] [PubMed] [Google Scholar]
- 138.Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, Kruse FE, Wiegand SJ, Dana MR, Streilein JW. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci 2004; 45: 2666–73. [DOI] [PubMed] [Google Scholar]
- 139.Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, Cursiefen C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res 2013; 34: 89–124. [DOI] [PubMed] [Google Scholar]
- 140.Mobley JE, O’Dell RM. The role of lymphatics in renal transplantation. Renal lymphatic regeneration. J Surg Res 1967; 7: 231–3. [DOI] [PubMed] [Google Scholar]
- 141.Stuht S, Gwinner W, Franz I, Schwarz A, Jonigk D, Kreipe H, Kerjaschki D, Haller H, Mengel M. Lymphatic neoangiogenesis in human renal allografts: results from sequential protocol biopsies. Am J Transplant 2007; 7: 377–84. [DOI] [PubMed] [Google Scholar]
- 142.Seeger H, Bonani M, Segerer S. The role of lymphatics in renal inflammation. Nephrol Dial Transplant 2012; 27: 2634–41. [DOI] [PubMed] [Google Scholar]