Abstract
Calcium is vital for many physiological functions including bone mineralization. Postnatal deposition of calcium into bone is greatest in infancy and continues through childhood and adolescence until peek mineral density is reached in early adulthood. Thereafter, bone mineral density remains static until it eventually declines in later life. A positive calcium balance, i.e. more calcium absorbed than excreted, is crucial to bone deposition during growth and thus to peek bone mineral density. Dietary calcium is absorbed from the intestine into the blood. It is then filtered by the renal glomerulus and either reabsorbed by the tubule or excreted in the urine. Calcium can be (re)absorbed across intestinal and renal epithelia via both transcellular and paracellular pathways. Current evidence suggests that significant intestinal and renal calcium transport changes occur throughout development. However, the molecular details of these alterations are incompletely delineated. Here we first briefly review the current model of calcium transport in the intestine and renal tubule in the adult. Then, we describe what is known with regard to calcium handling through postnatal development, and how alterations may aid in mediating a positive calcium balance. The role of transcellular and paracellular calcium transport pathways and the contribution of specific intestinal and tubular segments vary with age. However, the current literature highlights knowledge gaps in how specifically intestinal and renal calcium (re)absorption occurs early in postnatal development. Future research should clarify the specific changes in calcium transport throughout early postnatal development including mediators of these alterations enabling appropriate bone mineralization.
Impact statement
This mini review outlines the current state of knowledge pertaining to the molecules and mechanisms maintaining a positive calcium balance throughout postnatal development. This process is essential to achieving optimal bone mineral density in early adulthood, thereby lowering the lifetime risk of osteoporosis.
Keywords: Calcium transport, calcium homeostasis, intestine, kidney, bone and development
Introduction
Calcium is necessary for a multitude of essential physiological functions including bone mineralization, blood coagulation, neuronal transmission, muscle contraction, and intracellular signalling.1 Approximately 99% of total body calcium is found within bone where it is an essential component of the structural matrix. The remaining 1% of total body calcium is in corporal fluids and soft tissues.2 Given the essential structural role of calcium in bone, this micronutrient is especially important during periods of growth. Bone calcium deposition rate is highest in the fetus3 and postnatal deposition is greatest in the neonate with a roughly 3-fold decrease by one year of age. Another 50% decrease occurs by age 10 and bone deposition further declines to a rate ten-fold less than in the initial neonatal period by early adulthood.4 Infancy and childhood are thus critical years to achieving and maintaining a positive calcium balance enabling long-term bone health.5
Failure to achieve appropriate calcium deposition into bone confers an increased risk of osteopenia later in life.5 Although osteopenia is traditionally thought of as a problem of aging populations, an estimated 60% of this risk is attributed to the magnitude of peak bone mass reached in early adulthood.6 Importantly, after peak bone mineral density (BMD) is achieved in early adulthood, it remains largely stable as a neutral calcium balance is maintained. Women will experience bone mineral loss during pregnancy and lactation with subsequent gain afterwards and then further permanent loss around menopause. Men lose BMD approximately 10 years post the perimenopausal decline in women.4,7 Thus, bone disease in children is particularly concerning as a failure to achieve a healthy peak bone mineral content predisposes an individual to bone disease later in life.
Serum calcium is tightly regulated by a set of complex interactions between the intestine, kidneys, and bones.1 Ingested calcium is absorbed from the intestine into blood making it available for its various functional and structural roles. After calcium in plasma is filtered by the renal glomerulus, it is either reabsorbed back into the blood or excreted in the urine. If intestinal and renal calcium losses are greater than intestinal absorption, a negative balance ensues and calcium is sacrificed from bone to maintain blood levels. Conversely, a positive calcium balance is fundamental to ensuring adequate plasma calcium to mineralize bone throughout development.
Previous reviews have discussed calcium homeostasis during fetal development,3 during pregnancy and lactation,7 and in aging; in particular, how alterations contribute to the development of osteoporosis.8,9 However, much less has been written on how a positive calcium balance is achieved during postnatal development from infancy to adulthood to facilitate optimal peak bone mineral content. Here, we first briefly review the mechanisms of calcium transport in the intestine and renal tubule in adults. Then, we describe our current knowledge of calcium handling during postnatal development from infancy to adulthood and possible mechanisms mediating alterations in calcium transport during this time.
Intestinal absorption
Calcium transport across epithelia occurs along the length of the small intestine. However, each intestinal segment does not contribute uniformly to overall absorption. Intestinal perfusion studies in healthy adults demonstrate that, when normalized to segment length, the duodenum is the site of the highest rate of absorption.10 The jejunum and ileum also transport calcium in humans, the magnitude and direction of which depends on calcium intake.11 Colonic perfusion studies in healthy adults did not find significant absorption under conditions of normal calcium intake; however, this segment appears to contribute net absorption when dietary calcium is low.12,13 To date, studies in healthy human infants have not assessed the contribution of individual intestinal segments to calcium absorption.14,15
Studies in adult rodents have examined the contribution of intestinal segments to net absorption. In rats, Ussing chamber studies on duodenum revealed net absorption of calcium while net secretion has been observed in the jejunum and ileum.16–18 However, net absorption may occur in the ileum under conditions of low calcium intake.18,19 The cecum was found to be the major site of net absorption.20 No net flux has been found in proximal or distal colon.18,20 In mice, Ussing chamber studies found net secretion in the jejunum while the cecum contributed significantly to net absorption. Importantly, these studies often employed conditions lacking a transepithelial electrochemical gradient, where calcium flux across duodenum, ileum and colon was not significantly different to zero.21 However, it should be recognized that the overall contribution of each segment to calcium absorption will vary based upon intraluminal calcium solubility, calcium concentration, transit time, the transepithelial permeability to calcium and potential difference across the epithelia.22 Importantly, in vivo after a meal, there will be a large lumen to blood calcium gradient and the transepithelial potential difference across the intestine is lumen negative. Further, due to its length, the sojourn time is greatest in the ileum. Thus, this segment is said to be the site of greatest calcium absorption under physiological conditions of adequate calcium intake.22
Absorption from the intestinal lumen can follow one of two pathways. Transcellular absorption occurs via calcium influx into the enterocyte, intracellular shuttling, and finally basolateral extrusion. This pathway can therefore move calcium against a concentration gradient, for example when calcium intake is low. Absorption can also occur via a passive, paracellular route, whereby tight junction proteins facilitate or block the movement of calcium between epithelial cells.23 Ussing chamber studies in rats demonstrate approximately one-third of net absorption from the duodenum and half the absorption from the cecum occurs via the transcellular route.16,20 Calcium absorption and secretion into the jejunum and ileum occurs via the paracellular pathway.17
Transcellular calcium absorption
Studies delineating the proteins involved in intestinal calcium absorption have been largely carried out in animal models, predominantly rodents. The current model of transcellular calcium absorption involves apical entry into the enterocyte, intracellular binding to a buffering protein, shuttling across the cell, and finally basolateral extrusion. Calcium entry into the enterocyte is mediated, at least in part, by the transient receptor potential vanilloid 6 (Trpv6) calcium channel that is maximally open under hyperpolarizing conditions.1 In humans, TPRV6 mRNA has been observed exclusively in the duodenum of adults. However, Trpv6 mRNA has been identified in the duodenum, cecum, and colon of two- and three-month-old mice.21,24 Apical staining of TRPV6 occurs in the villi of duodenum but not ileum of rabbits.25 Trpv6 knockout mouse models have been generated. One model, reported by Bianco et al., displayed decreased intestinal calcium absorption compared to wildtype mice after oral gavage, when the mice were maintained on either a normal or low calcium diet. These animals had lower serum calcium when fed a low calcium diet. In addition, the Trpv6 knockout mice at 95 days of age had lower femoral BMD on a regular calcium-containing diet.26 Using the everted sac method, transcellular calcium transport in the duodenum of these Trpv6 knockout mice was significantly reduced when they were fed a low calcium diet.27 Although reduced, the presence of residual net luminal to serosal calcium flux in these Trpv6 knockout mice suggests the presence of another apical calcium influx mechanism. Another model genetically engineered to lack functional TRPV6 by expressing a non-functional channel pore displayed significantly reduced in vivo calcium absorption compared to wildtype mice. However, in this model femurs from six-month-old male mice did not show changes in cortical or trabecular mineralization.28 Calcium absorption was not completely abolished in this model either, providing further evidence of a compensatory role for the paracellular pathway or another apical influx mechanism.29
The apical L-type calcium channel, Cav1.3 may also contribute to intestinal calcium absorption under depolarizing conditions, thereby playing a complementary role to TRPV6.30 Consistent with this, immunocytochemistry of male rats demonstrates Cav1.3 in the jejunum and proximal ileum, but not in duodenum, cecum, or colon.31 Further, perfusion of adult rat jejunal loops confirmed that calcium flux was reduced by 72% upon addition of an L-type calcium channel blocker.31 Similarly, in Caco-2 cells, a model of human colonic epithelia, unidirectional calcium flux was increased by prolactin administration and this increase was prevented by pharmacological inhibition or knockdown of Cav1.3, an effect not observed by the knockdown of Trpv6.32
Intracellular buffering and shuttling of calcium in intestinal epithelial cells is mediated by Calbindin-D9K (CaBP9K).33 This facilitated diffusion of calcium across the cell prevents apoptosis due to increased cytosolic-free calcium.1,33 CaBP9K expression has been detected in adult human, rat, and mouse duodenum but not jejunum or ileum of either humans or rodents.21,24,34 Expression is also detected in the cecum and proximal colon of mature mice.21 Further, CaBP9K colocalizes with Trpv6 in rabbit duodenum.25 In vivo calcium absorption studies in 12-week-old CaBP9K knockout mice showed no difference to wildtype mice, suggesting that CaBP9K is not essential for intestinal calcium absorption.35
The basolateral extrusion of calcium from the enterocyte is mediated by plasma membrane Ca2+-ATPase (PMCA1) and the Na+/Ca2+ exchanger (NCX1) 1.1,36 In humans, PMCA1 is expressed in the duodenum, ileum, and colon, whereas in the mouse Pmca1 is expressed along all segments of the small and large intestine.24 In the duodenum of rabbits, PMCA colocalizes with TRPV6 and CaBP9K.25 PMCA1 is necessary for calcium absorption. Duodenal calcium transport, determined by the everted sac method, of intestinal specific Pmca1 KO mice was reduced by almost 50%. Consequently, this led to decreased total and femoral BMD in the knockout mice compared to wildtype mice.37
Paracellular calcium absorption
Paracellular calcium absorption is a passive process occurring down an electrochemical gradient. It is considered the major route of absorption when calcium intake is high.38 A luminal free calcium concentration of at least 1.74 mM is required to overcome the approximately −5 mV transepithelial potential difference present across the small intestine.23 Sodium-driven water absorption plays a significant role in this process as it increases the luminal calcium concentration from proximal to distal segments of the intestine and paracellular water flux itself facilitates paracellular calcium absorption via solvent drag.39 Consistent with this, mice null for the epithelial Na+/H+-exchanger (Nhe3), which mediates intestinal sodium and water absorption, have decreased calcium flux across duodenum and cecum.39
Paracellular flux requires not only a driving force but also a paracellular pore. Tight junction proteins called claudins (Cldn) form selective pores that mediate paracellular calcium movement.23 Claudins-2, -12, and -15 have been implicated in intestinal calcium absorption. All three of these claudins are expressed along the length of the small intestine and colon of adult mice although Cldn15 has higher expression in the proximal intestine.21,40–43 In humans, CLDN2 and CLDN15 mRNA are more highly expressed in the proximal segments of intestine, whereas CLDN12 is expressed throughout the small and large intestine.44 When overexpressed in an epithelial cell model, Cldn2 and Cldn12 increase the tight junction selectivity for cations, decrease transepithelial resistance and increase calcium permeability.40,45 Overexpression of Cldn2 but not Cldn12 also increases relative sodium permeability, suggesting that it forms a more specific cation permeable pore.40 Further, compensatory increases in Cldn2 and Cldn15 were observed in the duodenum of adult CaBP9k KO mice, suggesting the possibility of a cooperative interaction between the transcellular and paracellular calcium absorption pathways.46
Changes in intestinal calcium absorption during postnatal development
While most investigations of intestinal calcium absorption are carried out in adult subjects or mature animal models, existing studies highlight clear changes with age in both the absorption of calcium from intestinal segments and the expression of molecules participating in intestinal calcium absorption. Table 1 summarizes changes in expression of molecules implicated in intestinal calcium absorption throughout development. Dual isotope studies in very low birth weight infants found that the achievement of a net positive calcium balance was primarily determined by the magnitude of intestinal calcium absorption. The authors observed high intervariability of calcium balance between subjects, although a large range of birth weights (750–1750 g) lends the possibility that some variability might be due to differences in developmental age.14 Still, this study highlights that the intestine plays a major role in determining overall calcium balance in infants. Further, small and premature infants require higher dietary calcium in order to maintain a positive balance, consistent with an inability to upregulate the active, transcellular pathway and that infants rely on passive, paracellular absorption to meet their requirements.15 It is important to note, however, that intestinal absorption in preterm infants may be more representative of fetal physiology.3 None the less, a decline in the predominance of the paracellular pathway is observed from childhood to adulthood as the coefficient of intestinal calcium absorption (a measure of intestinal calcium permeability) measured by dual isotopes decreased by more than 50% between the ages of 12 and 80 years of age.47 Balance studies in rats found that the fraction of calcium absorption from the intestine was approximately 50% of intake at three weeks of age, peaked at 70% at five weeks, and then declined to about 40% by seven weeks. It is noteworthy that calcium retention mirrored intake.48 The results of these studies demonstrate that intestinal calcium absorption changes with age to meet the requirements for bone mineralization during periods of growth.
Table 1.
Expression of molecules implicated in intestinal calcium absorption throughout development
| Duodenum |
Jejunum |
Ileum |
Cecum |
Proximal Colon |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (weeks) | <3 | 3–8 | >8 | <3 | 3–8 | >8 | <3 | 3–8 | >8 | <3 | 3–8 | >8 | <3 | 3–8 | >8 | Reference |
| Transcellular | ||||||||||||||||
| Trpv6 | Yes | Yes | Yes* | ND | ND | No | ND | ND | No | ND | ND | Yes | ND | ND | Yes | 19,22,37,55,71 |
| Cav1.3 | ND | ND | No | ND | ND | Yes | ND | ND | Yes | ND | ND | No | ND | ND | No | 29 |
| CaBP9K | Yes | Yes | Yes* | ND | Yes | No | ND | ND | No | ND | Yes | Yes | ND | ND | Yes | 19,22,33,37,55,71 |
| Pmca1 | Yes | Yes | Yes* | ND | ND | Yes | ND | ND | Yes* | ND | ND | Yes | ND | ND | Yes* | 19,22,34,35,37,55 |
| Pmca4 | ND | ND | Yes | ND | ND | Yes | ND | ND | Yes | ND | ND | Yes | ND | ND | Yes | 34 |
| Ncx1 | ND | ND | Yes | ND | ND | Yes | ND | ND | Yes | ND | ND | Yes | ND | ND | Yes | 19 |
| Paracellular | ||||||||||||||||
| Cldn2 | ND | Yes | Yes* | Yes | Yes | Yes | ND | ND | Yes* | ND | ND | Yes* | ND | ND | Yes* | 19,37,38,42 |
| Cldn12 | ND | ND | Yes* | Yes | Yes | Yes | ND | ND | Yes* | ND | ND | Yes* | ND | ND | Yes* | 19,37,38,40,42 |
| Cldn15 | ND | ND | Yes* | Yes | Yes | Yes | ND | ND | Yes* | ND | ND | Yes* | ND | ND | Yes* | 19,37,38,40,42 |
| Cldn19 | ND | ND | No | Yes | ND | No | ND | ND | No | ND | ND | No | ND | ND | No | 40 |
| Nhe3 | ND | ND | Yes | ND | ND | Yes | ND | ND | Yes | ND | ND | Yes | ND | ND | Yes | 19 |
Note: Ages refer to age of rodent animal models. ND, no data.
Expression in adult human and rodent samples.
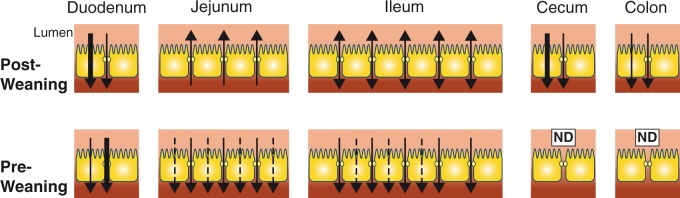
Complementing this work, functional measures of calcium transport across intestinal segments have been performed on rodents to measure changes with age. Using the everted sac technique in rat duodenum, greater net calcium absorption was observed in three- and five-week-old pups compared to 8–week- and 15-month-old mature animals.49 Using in situ ligated loops of rat duodenum, no evidence of saturable absorption was found in three- or five-day-old rats. In this study, the saturable component (which represents transcellular absorption) was first observed in rats at 19 days of age and became proportionally greater with increasing age until maximum flux was observed between 24 and 40 days of age.50 Expression of the intracellular calcium binding protein, CaBP followed the same pattern consistent with an increasing contribution of transcellular absorption from the duodenum with age.50 Similarly, using in situ loops of rat duodenum, there is no evidence of transcellular calcium uptake in pups at 14 days while linear regression of absorption at different luminal calcium concentrations suggests a transcellular pathway present in pups only at 18 days. By weaning at 21 days, intestinal calcium absorption had switched from predominantly non-saturable diffusion to predominantly transcellular.51 At one month of age, both paracellular and transcellular pathways appear to be present in the duodenum along with CaBP expression, while in the ileum, only non-saturable absorption seems to occur and CaBP is not present.51 Everted sac studies of rat duodenum similarly found active transport, measured as the serosal to mucosal ratio of 45Ca2+ flux, decreased 4.5-fold from 3 weeks to 3.5 months of age. This was accompanied by a similar decrease in CaBP protein.35,52 Active absorption from the duodenum of rats appears to continue to decrease through aging.53 A study of everted duodenum and jejunum from suckling (two-week), weanling (three-week), adolescent (six-week), and adult rats revealed that in the suckling rat, unidirectional lumen to serosal calcium flux is predominantly non-saturable suggesting paracellular absorption. At weaning, a transition to predominantly saturable absorption occurred, suggesting a larger contribution of a transcellular pathway.54 Concurrently, the calcium permeability of duodenum and jejunum decreases with age.54 Thus, the results of this study are indicative of a transition from initial passive diffusion to a transcellular process in the proximal small intestine through development, then back to diffusion in adulthood. Consistent with this, using the in situ ligated loop technique in 16-day-old rat pups, net calcium absorption was found from the duodenum, jejunum, and ileum with net secretion in the colon. The jejunum appeared to be the segment of greatest net absorption which increased when luminal sodium decreased, independent of mannitol flux.55 Thus, limited evidence supports transcellular calcium absorption from the distal small intestine prior to weaning. This is in contrast to net secretion from the jejunum and net absorption in the cecum in adult rodents.17,20,21 Together, the current evidence suggests that intestinal calcium absorption is significantly altered throughout early development, likely to maintain a positive calcium balance. In particular, weaning in rodents appears to be a time of rapid change. Figure 1 depicts the current state of evidence of net absorption and secretion by intestinal segment before and after weaning.
Figure 1.
Net calcium absorption and secretion by intestinal section during suckling and after weaning. A pictorial representation of current evidence. Absorption is represented by a downward arrow indicating net movement of calcium from lumen to serosa. When both transcellular and paracellular pathways are present in a segment, a larger arrow indicates the predominant pathway. Note that the transcellular pathway in the duodenum pre-weaning occurs after 1–2 weeks of age in rodents. The dashed line indicates that evidence supporting the pathway is limited. Evidence for calcium absorption in the cecum is from animal models only. Weaning is at 21 days in rodents. ND: no data. (A color version of this figure is available in the online journal.)
While functional changes in intestinal calcium absorption have been described throughout development, very few studies have examined the molecular details conferring these changes. When expression was examined at one, three, six, and eight weeks of age in mice, Trpv6 mRNA was detected only after one week with a peak at three weeks. A similar pattern was observed for both the gene and protein expression of CaBP9K. Unfortunately, the authors simply state “intestine,” so it is not clear which segment was examined or if a mixture of intestinal segments employed and thus specific conclusions about changes in specific segments from this work are difficult to make.56 In a separate study of mice, mRNA expression of Trpv6, CaBP9K, and Pmca1 in duodenum is first noted at 14 days and peaks at 21 days.57 At six weeks of age, CaBP9K was highly expressed in duodenum with a small amount in jejunum and cecum. At 44 weeks of age, CaBP9K expression is no longer detected in jejunum and cecum but remains in duodenum.35
With respect to paracellular transport, a single study has looked at the expression profile of claudins in the jejunum of mice at one day, two weeks, and one and three months of age. The authors noted a decrease in Cldn2 but increases in Cldn12 and Cldn15 with age. Of note, Cldn19 (a claudin that mediates calcium transport across the thick ascending limb of the renal tubule) was observed at 1 day and 14 days of age, whereas expression of this gene is not found in intestinal segments of adult mice.42 Together, expression studies highlight that the molecules mediating intestinal calcium absorption are not constant throughout development. Similar to results of functional studies, important changes appear to occur around the age of weaning, which are largely consistent with a change from paracellular to transcellular calcium flux in the duodenum and jejunum as well as a transition from net absorption to net secretion in the distal small bowel.
Potential mediators of changes in intestinal calcium absorption during postnatal development
Studies in rodents and humans demonstrate alterations in intestinal calcium absorption from neonatal through adult ages. However, what mediates the changes observed in expression of calcium transport genes that contribute the functional changes in absorption at these ages has not been delineated. Extensive research has been conducted into the role of vitamin D and its active hormone, calcitriol, in mediating intestinal calcium transport.58 It is well established that decreased serum calcium causes an increased release of parathyroid hormone (PTH), which acts on the kidney to increase transcription of Cyp27b1, which then converts vitamin D to its active form calcitriol, which increases intestinal calcium absorption (as reviewed in Kopic and Geibel2). In mice with a nonfunctional vitamin D receptor (VDR), the phenotype of poor weight gain and decreased BMD is only evident after weaning, highlighting that calcium absorption while suckling is not calcitriol dependent.3,59 In adult mice, calcitriol acts to increase intestinal calcium absorption by increasing the transcription of Trpv6 and CaBP9K in the duodenum and cecum.17,20,24,60 Expression of these genes is not detected in mice prior to weaning when calcium absorption occurs via passive, paracellular diffusion.3,61 Further, consistent with calcitriol not participating in intestinal calcium absorption pre-weaning, using the everted sac technique in duodenum, Halloran et al. observed that calcitriol injections increased active and total calcium uptake of six-week-old mice but not 14-day-old pups.62
It is recognized that lactose present in milk or the diet facilitates increased calcitriol-independent calcium absorption. This is of particular importance during the neonatal period (as reviewed in Kovacs3 and Allen63). Lactose also facilitates increased calcium absorption from the ileum of adult rats, a section of the intestine where the paracellular pathway predominates.64 In addition, ingested lactose augments calcium absorption thereby rescuing the calcium phenotype of VDR mutants.60,65 However, whether the absence of lactose after weaning mediates molecular alterations in calcium transport pathways is not known.
Calcitriol has also been proposed to affect the paracellular pathway thereby enhancing calcium absorption.40 This finding is based on the observation that VDR knockout mice have lower expression of Cldn2 and Cldn12 in the small intestine and colon, and that the treatment of a colonic epithelial cell culture model with calcitriol increased Cldn2 and Cldn12 expression.40 However, the injection of exogenous calcitriol into adult mice did not effect a change in Cldn2 or Cldn12 mRNA expression in duodenum.24 This apparent discrepancy may be reconciled by the idea that, in vivo, there is a maximal effect of calcitriol to upregulate the transcription of these genes. Regardless, the potential role of calcitriol as a regulator of paracellular calcium absorption throughout development has not been characterized, but given the lack of an apparent role in transcellular absorption it is unlikely important for the changes in paracellular absorption observed.
Prolactin is a peptide hormone that is present in high amounts in early breast milk, but has a declining concentration through lactation.66,67 It consists of several isoforms produced via alternative splicing, proteolytic cleavage, and post-translational modifications.68 Maternal prolactin is absorbed and bioactive in neonatal rats where the prolactin receptor is present on epithelial cells throughout the small and large intestines as well as the duodenum and colon of humans.67,69 Prolactin directly alters the expression and activity of mediators of both transcellular and paracellular calcium absorption in the intestine of both sexes via intracellular signalling molecules including PI3K.32,70,71 Provision of exogenous prolactin increases net intestinal calcium absorption at three weeks of age in rats but decreases fractional absorption at five and seven weeks. In the same study, inhibition of endogenous prolactin production with bromocriptine decreased fractional absorption at three and five weeks but increased absorption at seven weeks of age.48 Similarly, in suckling rat pups, bromocriptine treatment decreased net calcium absorption from jejunum while the administration of bromocriptine with exogenous prolactin returned net calcium absorption to baseline. Under conditions where only exogenous prolactin was present, net calcium absorption from the duodenum was absent.55 This study illustrates the potential for endogenous and exogenous prolactin to confer different effects on intestinal calcium absorption. In adult mice and in human cell culture models, endogenous prolactin appears to increase calcium flux through an L-type calcium channel, presumably Cav1.3.32,71 Moreover, the induction of hyperprolactinemia in mice led to increased femoral total calcium content whereas bromocriptine treatment lowered total lumbar calcium, suggesting a role for prolactin in mediating mineral accrual into bone.48 Taken together, these results infer that exogenous prolactin in breast milk may stimulate calcium absorption from the intestine by non-traditional mechanisms and its withdrawal may lead to the more typical calcium absorption mechanisms seen in adults.
Renal reabsorption
Calcium filtered by the kidney is reabsorbed along the nephron to maintain the serum concentration. The mature nephron efficiently reabsorbs most calcium so that only 1–2% of filtered calcium is excreted in urine.72 Similar to the intestine, renal reabsorption can occur via both paracellular and transcellular pathways. Most filtered calcium, about 70% in the proximal tubule and 20% in the thick ascending limb, is reabsorbed via the paracellular pathway.23 Microperfusion studies and characterization of the Nhe3 knockout mice highlight the coupling of sodium and water movement to paracellular calcium reabsorption in the proximal tubule and thick ascending limb.23,39 The remainder of calcium is reabsorbed in the distal nephron, in particular the distal convoluted tubule and connecting tubule via an active transcellular process.1,73
Transcellular calcium reabsorption
Active transcellular calcium reabsorption involves apical entry into the cell, intracellular binding and shuttling, and then basolateral extrusion. The transient receptor potential vanilloid 5 (Trpv5) calcium channel is expressed in the distal convoluted tubule and connecting tubule of rat kidney.74 In rabbit and rodent kidney, TRPV5 colocalizes with intracellular calcium binding protein, Calbindin-D28K (CaBP28K) as well as PMCA and NCX1.25,74,75 In Trpv5 knockout mice, CaBP28K is dispersed in the cell whereas in wildtype mice, it is more abundant apically, suggesting an interaction between the proteins.75 Trpv5 knockout and Trpv5/CaBP28K double knockout mice have increased urinary calcium excretion from the distal nephron and maintain plasma calcium levels via compensatory intestinal hyperabsorption of calcium.76,77 The Trpv5 knockout and double knockout mice compensate with increased expression of Trpv6 and CaBP9K in duodenum, suggesting a complementary regulation which is likely mediated by increased serum vitamin D76,77 CaBP28K knockout mice do not display a phenotype of altered calcium homeostasis. However, CaBP28K/VDR double knockout mice have slowed growth after one month of age. At two months of age, these double knockout mice had decreased tibia and femur length and total, trabecular, and cortical BMD compared to VDR knockout mice. These observations suggest that CaBP28K is not necessary to maintain calcium homeostasis.78 In human and mouse kidney, PMCA4 is expressed in the distal nephron, and in mice it is most highly expressed in cells with TRPV5.36 This suggests a role in calcium reabsorption, although its expression is not altered by calcium intake and Pmca4 knockout mice do not display an altered calcium phenotype, thereby inferring a housekeeping role.36,79 An Ncx1 knockout model is embryonically lethal due to its importance in cardiomyocyte development. However, work in cell culture models support the idea that NCX1 significantly contributes to basolateral calcium extrusion in the distal nephron.80,81
Similar to its role in the intestines, calcitriol, downstream of PTH, increases active calcium reabsorption in the distal nephron via transcriptional regulation (as reviewed in Hoenderop et al.1). In addition, PTH has calcitriol-independent actions on the distal nephron. It increases transcription of Trpv5, Calbindin-D28k, and Ncx as well as directly increases the open probability of the TRPV5 channel thereby increasing calcium reabsorption (as reviewed in Kopic and Geibel2).
Paracellular calcium reabsorption
As in the intestine, paracellular calcium reabsorption across the nephron occurs down an electrochemical gradient or via solvent drag.23 Cldn2 confers calcium permeability across the proximal tubule. Cldn2 knockout mice have decreased monovalent cation permeability in the proximal tubule and a 3-fold increase in fractional excretion of calcium (FECa).82 Schnermann et al. also showed that Cldn2 knockout mice have a 22.7% decrease in water permeability of the proximal tubule.83 Moreover, mice null for the transporter mediating the majority of sodium reabsorption from the proximal tubule, Nhe3, have a 2-fold increase in FECa. Together these results highlight how tightly coupled sodium, water, and calcium reabsorption is in the proximal tubule.39 What other claudins may contribute to paracellular calcium permeability across the proximal tubule is not known.
In the thick ascending limb, Claudins-16 and -19 localize to the tight junction and form a cation-permeable pore.84 Cldn14 is expressed in this nephron segment under conditions of increased plasma calcium. It blocks paracellular reabsorption of cations, including calcium.85,86 Microperfusion of cortical thick ascending limb from two-month-old mice showed that a high calcium diet caused increased transepithelial resistance (TER), decreased absolute calcium permeability, and a 3-fold increase in Cldn14 mRNA with no change to Cldn16 or Cldn19 expression.87 Subsequently, a high calcium diet led to a more than 3-fold increase in urinary calcium to creatinine ratio (Ca/Cr) and a 4-fold increase in FECa.87 In addition, pharmacological downregulation of Cldn14 expression in wildtype mice decreased total urinary calcium and FECa while microperfusion studies revealed decreased transepithelial resistance and increased cation permeability across the thick ascending limb.88 Together these studies highlight how paracellular calcium reabsorption can be regulated in the thick ascending limb via altering the permeability of the tight junction.
The calcium sensing receptor (CaSR) is expressed in all segments of the renal tubule; however, expression is greatest in the thick ascending limb (as reviewed in Riccardi and Valenti89). Activating CaSR mutations increase Cldn14 transcription via microRNA thereby causing downstream decreased calcium reabsorption.88 Disease-causing mutations in the CaSR highlight its important role in the regulation of renal calcium handling. Gain of function mutations result in autosomal dominant hypocalcemia.90 Conversely, loss of function mutations in the CaSR cause hypercalcemia ranging from benign in familial hypocalciuric hypercalcemia (FHH) to potentially fatal neonatal severe hyperparathyroidism (NSHPT).90 The potential role of the CaSR in mediating alterations in renal calcium handling during postnatal development has, however, not been determined.
Changes to renal calcium reabsorption during postnatal development
Studies in humans and mice highlight the existence of changes in renal calcium handling with age. Table 2 summarizes the changes in expression of molecules implicated in renal calcium reabsorption throughout postnatal development. Premature and low birth weight infants have a high incidence of renal calcifications on ultrasound. This is likely due to their urine calcium excretion that can be 3-fold higher than the upper limit of normal for term infants.91 In healthy children, urine Ca/Cr declines by 50% between one month and two years of age with a further reduction to 30% of neonatal values by 10 years of age.92 In a Swedish cohort, urine Ca/Cr was significantly higher in children aged 2–6 compared to children aged 7–18 and it significantly declined from 2 to 18 years of age. Importantly, urine Ca/Cr values did not correlate to milk intake suggesting inherit underlying differences in tubule transport physiology.93 Microperfusion of mouse proximal tubules shows a 36% reduction in relative sodium permeability (PNa/PCl) and a 200% increase in relative bicarbonate permeability (PHCO3/PCl) from 10 days of age to adult age in mice.94 Together, these studies strongly suggest developmental changes in renal tubular calcium handling. Figure 2 illustrates current evidence of altered renal calcium reabsorption during suckling and post-weaning.
Table 2.
Expression of molecules implicated in renal calcium reabsorption throughout development
| Age | <3 weeks | 3–8 weeks | >8 weeks | Reference |
|---|---|---|---|---|
| Transcellular | ||||
| Trpv5 | Yes | Yes | Yes | 37,54,69,73,74 |
| Trpv6 | Yes | ND | Yes | 54,72,73,87 |
| CaBP9K | Yes | Yes | Yes | 33,37,54,69,71–74,87 |
| CaBP28K | Yes | Yes | Yes | 37,54,71–74,87 |
| Pmca1 | ND | Yes | Yes | 37,74,87 |
| Pmca4 | ND | Yes | Yes | 34,74 |
| Ncx1 | ND | ND | Yes | 72,74,87 |
| Paracellular | ||||
| Cldn2 | Yes | Yes | Yes | 77,86 |
| Cldn6 | Yes | ND | No | 86 |
| Cldn9 | Yes | ND | No | 86 |
| Cldn14 | ND | Yes | Yes | 80–82 |
| Cldn16 | Yes | Yes | Yes | 37,79,82,86 |
| Cldn19 | ND | Yes | Yes | 37,79,82 |
Note: Ages refer to age of rodent animal models. ND: no data
Figure 2.
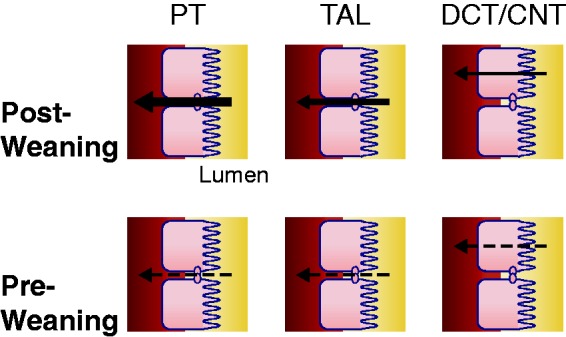
Renal calcium reabsorption during suckling and after weaning. An illustrative representation of the current knowledge. The leftward arrow indicates net movement of calcium from lumen to serosa represents reabsorption. The thickness of the arrow represents relative reabsorption between tubule segments and ages. The dashed line indicates that reabsorption is inferred from expression studies or limited functional studies. Weaning is at 21 days in rodents. PT: proximal tubule; TAL: thick ascending limb; DCT: distal convoluted tubule; CNT: connecting tubule. (A color version of this figure is available in the online journal.)
Few studies have investigated the molecular details of how renal calcium reabsorption changes with age. In mice, mRNA of Trpv6 in the kidney is highest at one week and declines 3-fold by three weeks of age. Conversely, Trpv5, CaBP9K, and CaBP28K mRNA increases by at least 2-fold from one week of age to peak at three weeks with a subsequent decline of 30% by six weeks of age. CaBP9K and CaBP28K protein expression follows a similar pattern.56 TRPV5 and CaBP28K expression declines further after 10 weeks while Trpv5 mRNA expression is not different from 3 to 12 months.95,96 Trpv5 knockout mice at 10 weeks have increased intestinal calcium absorption by in vivo assay.95 It may be that, if the renal tubule is unable to reabsorb calcium in the young, the intestine compensates via hyperabsorption to ensure a positive calcium balance is maintained. Together, these studies suggest that transcellular calcium reabsorption is highest during suckling and then declines through maturation. A higher FECa at early ages suggests that there are also changes to the paracellular pathway. Indeed, at one day of age, expression of Cldn6, Cldn9, and Cldn13 is detected in the kidney, whereas no expression is detected in adult kidney.94 When overexpressed in a kidney cell model, CLDN6 and CLDN9 led to increased TER and decreased permeability of chloride, PNa/PCl, and PHCO3/PCl.97 If cation permeability is indeed reduced across the proximal tubule of neonates due to altered claudin expression, this may explain the increased calcium excretion observed at young ages. However, further research is needed to clearly delineate the postnatal developmental changes that occur in renal tubule calcium transport.
Summary and Future Directions
Overall, the current literature highlights knowledge gaps in how intestinal calcium absorption occurs early in postnatal life in order to maintain a positive calcium balance. The roles of the transcellular and paracellular pathways vary significantly with age, although this has not been precisely delineated. Moreover, the contribution of individual segments of the small and large intestine during growth has not been determined. Expression patterns and further functional studies throughout postnatal development are needed, as is further attention to the signals conferring these changes. Similarly, developmental changes in renal calcium reabsorption have only been partially explored and more work investigating why infants and young children have much greater urinary calcium excretion warrants further attention.
Acknowledgements
The authors would like to thank Dr Allen Plain for reviewing the manuscript. MRB is funded by the Stollery Children’s Hospital Foundation through the Women and Children’s Health Research Institute (WCHRI). Work in the Alexander laboratory is funded by a grant from WCHRI, which is supported by the Stollery Children's Hospital Foundation, a grant from the Canadian Institutes of Health Research (CIHR, MOP 136891) and the National Science and Engineering Research Council of Canada (RGPIN-2015-05842). RTA is an Alberta Innovates Health Solutions (AIHS) Clinical Scholar and the Canada Research Chair in Renal Tubular Epithelial Transport Physiology.
Authors’ contributions
MRB wrote the first draft of the manuscript, RTA read and edited the manuscript for important scientific details.
Declaration of Conflict Of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hoenderop JGJ, Nilius B, Bindels RJM. Calcium absorption across epithelia. Physiol Rev 2005; 85: 373–422. [DOI] [PubMed] [Google Scholar]
- 2.Kopic S, Geibel JP. Gastric acid, calcium absorption, and their impact on bone health. Physiol Rev 2013; 93: 189–268. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs CS. Bone development and mineral homeostasis in the fetus and neonate: roles of the calciotropic and phosphotropic hormones. Physiol Rev 2014; 94: 1143–218. [DOI] [PubMed] [Google Scholar]
- 4.Bronner F, Pansu D. Nutritional aspects of calcium absorption. J Nutr 1999; 129: 9–12. [DOI] [PubMed] [Google Scholar]
- 5.Golden NH, Abrams SA. Optimizing bone health in children and adolescents. Pediatrics 2014; 134: e1229–e43. [DOI] [PubMed] [Google Scholar]
- 6.Bachrach LK, Levine MA, Cowell CT, Shaw NJ. Clinical indications for the use of DXA in pediatrics. Bone densitometry in growing patients. Totowa, NJ: Springer, 2007, pp.59–72.
- 7.Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev 2016; 96: 449–547. [DOI] [PubMed] [Google Scholar]
- 8.Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res 2016; 4: 16041–16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol 2010; 5: S23–30. [DOI] [PubMed] [Google Scholar]
- 10.Birge SJ, Peck WA, Berman M, Whedon GD. Study of calcium absorption in man: a kinetic analysis and physiologic model. J Clin Invest 1969; 48: 1705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman DA, Fordtran JS, Brinkley LJ, Zerwekh JE, Nicar MJ, Strowig SM, Pak CYC. Jejunal and ileal adaptation to alterations in dietary calcium: changes in calcium and magnesium absorption and pathogenetic role of parathyroid hormone and 1,25-dihydroxyvitamin D. J Clin Invest 1981; 67: 1599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandstrom B, Cederbiad A, Kivistoo B, Stenquist B, Andersson H. Retention of zinc and calcium from the human coIon. Am J Clin Nutr 1986; 44: 501–4. [DOI] [PubMed] [Google Scholar]
- 13.Grinstead WC, Pak CYC, Krejs GJ. Effect of 1,25-dihydroxyvitamin D3 on calcium absorption in the colon of healthy humans. Am J Physiol Gastrointest Liver Physiol 1984; 247: G189–G92. [DOI] [PubMed] [Google Scholar]
- 14.Abrams SA, Esteban NV, Vieira NE, Yergey AL. Calcium absorption and endogenous fecal excretion in low birth weight infants. Pediatr Res 1991; 29: 615–8. [DOI] [PubMed] [Google Scholar]
- 15.Hillman LS, Johnson LS, Lee DZ, Vieira NE, Yergey AL. Measurement of true absorption, endogenous fecal excretion, urinary excretion, and retention of calcium in term infants by using a dual-tracer, stable-isotope method. J Pediatr 1993; 123: 444–56. [DOI] [PubMed] [Google Scholar]
- 16.Karbach U, Schmitt A, Saner FH. Different mechanism of magnesium and calcium transport across rat duodenum. Dig Dis Sci 1991; 36: 1611–8. [DOI] [PubMed] [Google Scholar]
- 17.Karbach U. Paracellular calcium transport across the small intestine. J Nutr 1992; 122: 672–7. [DOI] [PubMed] [Google Scholar]
- 18.Favus MJ. Factors that influence absorption and secretion of calcium in the small intestine and colon. Am J Physiol 1985; 248: G147–G57. [DOI] [PubMed] [Google Scholar]
- 19.Nellans HN, Kimberg DV. Cellular and paracellular calcium transport in rat ileum: effects of dietary calcium. Am J Physiol Endocrinol Metab 1978; 235: E726–E726. [DOI] [PubMed] [Google Scholar]
- 20.Karbach U, Feldmeier H. The cecum is the site with the highest calcium absorption in rat intestine. Dig Dis Sci 1993; 38: 1815–24. [DOI] [PubMed] [Google Scholar]
- 21.Rievaj J, Pan W, Cordat E, Alexander RT. The Na+/H+ exchanger isoform 3 is required for active paracellular and transcellular Ca2+ transport across murine cecum. Am J Physiol Gastrointest Liver Physiol 2013; 305: G303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duflos C, Bellaton C, Pansu D, Bronner F. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J Nutr 1995; 125: 2348–55. [DOI] [PubMed] [Google Scholar]
- 23.Alexander RT, Rievaj J, Dimke H. Paracellular calcium transport across renal and intestinal epithelia. Biochem Cell Biol 2014; 92: 467–80. [DOI] [PubMed] [Google Scholar]
- 24.Lameris AL, Nevalainen PI, Reijnen D, Simons E, Eygensteyn J, Monnens L, Bindels RJ, Hoenderop JG. Segmental transport of Ca2+ and Mg2+ along the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 2015; 308: G206–16. [DOI] [PubMed] [Google Scholar]
- 25.Hoenderop JG, Hartog A, Stuiver M, Doucet A, Willems PH, Bindels RJ. Localization of the epithelial Ca2+ channel in rabbit kidney and intestine. J Am Soc Nephrol 2000; 11: 1171–8. [DOI] [PubMed] [Google Scholar]
- 26.Bianco SDC, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CHA, Wu J. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res 2007; 22: 274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng J-B, Jiang Y, Oh GT, Jeung E-B, Lieben L. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 2008; 149: 3196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Eerden BC, Weissgerber P, Fratzl-Zelman N, Olausson J, Hoenderop JG, Schreuders-Koedam M, Eijken M, Roschger P, de Vries TJ, Chiba H, Klaushofer K, Flockerzi V, Bindels RJ, Freichel M, van Leeuwen JP. The transient receptor potential channel TRPV6 is dynamically expressed in bone cells but is not crucial for bone mineralization in mice. J Cell Physiol 2012; 227: 1951–9. [DOI] [PubMed] [Google Scholar]
- 29.Woudenberg-Vrenken TE, Lameris AL, Weissgerber P, Olausson J, Flockerzi V, Bindels RJ, Freichel M, Hoenderop JG. Functional TRPV6 channels are crucial for transepithelial Ca2+ absorption. Am J Physiol Gastrointest Liver Physiol 2012; 303: G879–85. [DOI] [PubMed] [Google Scholar]
- 30.Kellett GL. Alternative perspective on intestinal calcium absorption: proposed complementary actions of Cav1.3 and TRPV6. Nutr Rev 2011; 69: 347–70. [DOI] [PubMed] [Google Scholar]
- 31.Morgan EL, Mace OJ, Helliwell PA, Affleck J, Kellett GL. A role for Cav1.3 in rat intestinal calcium absorption. Biochem Biophys Res Commun 2003; 312: 487–93. [DOI] [PubMed] [Google Scholar]
- 32.Thongon N, Nakkrasae L-i, Thongbunchoo J, Krishnamra N, Charoenphandhu. Enhancement of calcium transport in Caco-2 monolayer through PKCzeta-dependent Cav1.3-mediated transcellular and rectifying paracellular pathways by prolactin. Am J Physiol Cell Physiol 2009;296:C1373-82. [DOI] [PubMed]
- 33.Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol Gastrointest Liver Physiol 1986; 250: G561–G9. [DOI] [PubMed] [Google Scholar]
- 34.Barley NF, Radhika Prathalingam S, Zhi P, Legon S, Howard A, Walters JRF. Factors involved in the duodenal expression of the human calbindin-D9k gene. Biochem J 1999; 341: 491–500. [PMC free article] [PubMed] [Google Scholar]
- 35.Akhter S, Kutuzova GD, Christakos S, Deluca HF. Calbindin D 9k is not required for 1,25-dihydroxyvitamin D3 -mediated Ca2+ absorption in small intestine. Arch Biochem Biophys 2007; 460: 227–32. [DOI] [PubMed] [Google Scholar]
- 36.Alexander RT, Beggs MR, Zamani R, Marcussen N, Frische S, Dimke H. Ultrastructural and immunohistochemical localization of plasma membrane Ca2+-ATPase 4 in Ca2+-transporting epithelia. Am J Physiol Renal Physiol 2015; 309: F604–F16. [DOI] [PubMed] [Google Scholar]
- 37.Ryan ZC, Craig TA, Filoteo AG, Westendorf JJ, Cartwright EJ, Neyses L, Strehler EE, Kumar R. Deletion of the intestinal plasma membrane calcium pump, isoform 1, Atp2b1, in mice is associated with decreased bone mineral density and impaired responsiveness to 1, 25-dihydroxyvitamin D 3. Biochem Biophys Res Commun 2015; 467: 152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bronner F. Recent developments in intestinal calcium absorption. Nutr Rev 2009; 67: 109–13. [DOI] [PubMed] [Google Scholar]
- 39.Pan W, Borovac J, Spicer Z, Hoenderop JG, Bindels RJ, Shull GE, Doschak MR, Cordat E, Alexander RT. The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re) absorption. Am J Physiol Renal Physiol 2012; 302: F943–F56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H. Tight junction proteins claudin-2 and-12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 2008; 19: 1912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei Z, Maeda T, Tamura A, Nakamura T, Yamazaki Y, Shiratori H, Yashiro K, Tsukita S, Hamada H. EpCAM contributes to formation of functional tight junction in the intestinal epithelium by recruiting claudin proteins. Dev Biol 2012; 371: 136–45. [DOI] [PubMed] [Google Scholar]
- 42.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Express Pattern 2006; 6: 581–8. [DOI] [PubMed] [Google Scholar]
- 43.Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N. Differential expression and subcellular localization of claudin-7,− 8,− 12,− 13, and− 15 along the mouse intestine. J Histochem Cytochem 2006; 54: 933–44. [DOI] [PubMed] [Google Scholar]
- 44.Lameris AL, Huybers S, Kaukinen K, Mäkelä TH, Bindels RJ, Hoenderop JG, Nevalainen PI. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol 2013; 48: 58–69. [DOI] [PubMed] [Google Scholar]
- 45.Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 2002; 115: 4969–76. [DOI] [PubMed] [Google Scholar]
- 46.Hwang I, Yang H, Kang HS, Ahn C, Hong EJ, An BS, Jeung EB. Alteration of tight junction gene expression by calcium- and vitamin D-deficient diet in the duodenum of calbindin-null mice. Int J Mol Sci 2013; 14: 22997–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alevizaki CC, Ikkos DG, Singhelakis P. Progressive decrease of true intestinal calcium absorption with age in normal man. J Nucl Med 1973; 14: 760–2. [PubMed] [Google Scholar]
- 48.Piyabhan P, Krishnamra N, Limlomwongse L. Changes in the regulation of calcium metabolism and bone calcium content during growth in the absence of endogenous prolactin and during hyperprolactinemia: a longitudinal study in male and female Wistar rats. Can J Physiol Pharmacol 2000; 78: 757–65. [PubMed] [Google Scholar]
- 49.Krishnamra N, Lotinun S, Limlomwongse L. Acute effect and mechanism of action of prolactin on the in situ passive calcium absorption in rat. Bone Miner 1993; 23: 253–66. [DOI] [PubMed] [Google Scholar]
- 50.Pansu D, Bellaton C, Bronner F. Developmental changes in the mechanisms of duodenal calcium transport in the rat. Am J Physiol 1983; 244: G20–G6. [DOI] [PubMed] [Google Scholar]
- 51.Toverud SU, Dostal LA. Calcium absorption during development: experimental studies of the rat small intestine. J Pediatr Gastroenterol Nutr 1986; 5: 688–95. [PubMed] [Google Scholar]
- 52.Armbrecht HJ, Zenser TV, Bruns ME, Davis BB. Effect of age on intestinal calcium absorption and adaptation to dietary calcium. Am J Physiol Endocrinol Metab 1979; 236: E769–E74. [DOI] [PubMed] [Google Scholar]
- 53.Horst RL, Deluca HF, Jorgensen NA. The effect of age on calcium absorption and accumulation of 1,25-hydroxyvitamin D3 in intestinal mucosa of rats. Metab Bone Dis Relat Res 1978; 1: 29–33. [Google Scholar]
- 54.Ghishan FK, Parker P, Nichols S, Hoyumpa A. Kinetics of intestinal calcium transport during maturation in rats. Pediatr Res 1984; 18: 235–9. [DOI] [PubMed] [Google Scholar]
- 55.Amnattanakul S, Charoenphandhu N, Limlomwongse L, Krishnamra N, Amnattanakul S, Charoenphandhu N, Limlomwongse L, Krishnamra N. Endogenous prolactin modulated the calcium absorption in the jejunum of suckling rats. J Physiol Pharmacol 2005; 83: 595–604. [DOI] [PubMed] [Google Scholar]
- 56.Song Y, Peng X, Porta A, Takanaga H, Peng J-B, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1, 25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology 2003; 144: 3885–94. [DOI] [PubMed] [Google Scholar]
- 57.Lee G-S, Lee K-Y, Choi K-C, Ryu Y-H, Paik SG, Oh GT, Jeung E-B. Phenotype of a calbindin-D9k gene knockout is compensated for by the induction of other calcium transporter genes in a mouse model. J Bone Miner Res 2007; 22: 1968–78. [DOI] [PubMed] [Google Scholar]
- 58.Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol 2011; 347: 25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 1997; 16: 391–6. [DOI] [PubMed] [Google Scholar]
- 60.Van Cromphaut SJ, Dewerchin M, Hoenderop JGJ, Stockmans I, Van Herck E, Kato S, Bindels RJM, Collen D, Carmeliet P, Bouillon R. Duodenal calcium absorption in vitamin D receptor–knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A 2001; 98: 13324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dostal LA, Toverud SU. Effect of vitamin D3 on duodenal calcium absorption in vivo during early development. Am J Physiol Gastrointest Liver Physiol 1984; 246: G528–G34. [DOI] [PubMed] [Google Scholar]
- 62.Halloran BP, DeLuca HF. Calcium transport in small intestine during pregnancy and lactation. Am J Physiol Endocrinol Metab 1980; 239: E64–E8. [DOI] [PubMed] [Google Scholar]
- 63.Allen LH. Calcium bioavailability and absorption: a review. Am J Clin Nutr 1982; 35: 783–808. [DOI] [PubMed] [Google Scholar]
- 64.Sato R, Noguchi T, Naito H. Effect of lactose on calcium absorption from the rat small intestine with a non-flushed ligated loop. J Nutr Sci Vitaminol (Tokyo) 1983; 29: 365–73. [DOI] [PubMed] [Google Scholar]
- 65.Song Y, Kato S, Fleet JC. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr 2003; 133: 374–80. [DOI] [PubMed] [Google Scholar]
- 66.Cox DB, Owens RA, Hartmann PE. Blood and milk prolactin and the rate of milk synthesis in women. Exp Physiol 1996; 81: 1007–20. [DOI] [PubMed] [Google Scholar]
- 67.Ellis LA, Mastro AM, Picciano MF. Milk-borne prolactin and neonatal development. J Mammary Gland Biol Neoplasia 1996; 1: 259–69. [DOI] [PubMed] [Google Scholar]
- 68.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000; 80: 1523–631. [DOI] [PubMed] [Google Scholar]
- 69.Nagano M, Chastre E, Choquet A, Bara J, Gespach C, Kelly PA. Expression of prolactin and growth hormone receptor genes and their isoforms in the gastrointestinal tract. Am J Physiol 1995; 268: G431–42. [DOI] [PubMed] [Google Scholar]
- 70.Wongdee K, Teerapornpuntakit J, Sripong C, Longkunan A, Chankamngoen W, Keadsai C, Kraidith K, Krishnamra N, Charoenphandhu N. Intestinal mucosal changes and upregulated calcium transporter and FGF-23 expression during lactation: contribution of lactogenic hormone prolactin. Arch Biochem Biophys 2016; 590: 109–17. [DOI] [PubMed] [Google Scholar]
- 71.Dorkkam N, Wongdee K, Suntornsaratoon P, Krishnamra N, Charoenphandhu N. Prolactin stimulates the L-type calcium channel-mediated transepithelial calcium transport in the duodenum of male rats. Biochem Biophys Res Commun 2013; 430: 711–6. [DOI] [PubMed] [Google Scholar]
- 72.Dimke H, Hoenderop JGJ, Bindels RJM. Molecular basis of epithelial Ca2+ and Mg2+ transport: insights from the TRP channel family. J Physiol 2011; 589: 1535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dimke H, Hoenderop JG, Bindels RJ. Hereditary tubular transport disorders: implications for renal handling of Ca2+ and Mg2+. Clin Sci (Lond) 2010; 118: 1–18. [DOI] [PubMed] [Google Scholar]
- 74.Hoenderop JG, Müller D, Van Der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, Van Os CH, Bindels RJ. Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol 2001; 12: 1342–9. [DOI] [PubMed] [Google Scholar]
- 75.Lambers TT, Mahieu F, Oancea E, Hoofd L, de Lange F, Mensenkamp AR, Voets T, Nilius B, Clapham DE, Hoenderop JG, Bindels RJ. Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. EMBO J 2006; 25: 2978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoenderop JGJ, van Leeuwen JPTM, van der Eerden BCJ, Kersten FFJ, WCM van derKemp A, Mérillat A-M, Waarsing JH, Rossier BC, Vallon V, Hummler E. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 2003;112:1906-14. [DOI] [PMC free article] [PubMed]
- 77.Gkika D, Hsu Y-J, Van Der Kemp AW, Christakos S, Bindels RJ, Hoenderop JG. Critical role of the epithelial Ca2+ channel TRPV5 in active Ca2+ reabsorption as revealed by TRPV5/Calbindin-D 28K knockout mice. J Am Soc Nephrol 2006; 17: 3020–7. [DOI] [PubMed] [Google Scholar]
- 78.Zheng W, Xie Y, Li G, Kong J, Feng JQ, Li YC. Critical role of calbindin-D28k in calcium homeostasis revealed by mice lacking both vitamin D receptor and calbindin-D28k. J Biol Chem 2004; 279: 52406–13. [DOI] [PubMed] [Google Scholar]
- 79.van Loon EPM, Little R, Prehar S, Bindels RJM, Cartwright EJ, Hoenderop JGJ. Calcium extrusion pump PMCA4: a new player in renal calcium handling? PLoS One 2016; 11: e0153483–e0153483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 2001; 15: 1209–11. [DOI] [PubMed] [Google Scholar]
- 81.Bindels RJ, Ramakers PL, Dempster JA, Hartog A, van Os CH. Role of Na+/Ca2+ exchange in transcellular Ca2+ transport across primary cultures of rabbit kidney collecting system. Pflugers Archiv 1992; 420: 566–72. [DOI] [PubMed] [Google Scholar]
- 82.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M, Giebisch G, Muto3 ‘ S, Hatab M, Taniguchic J, Tsuruokad S, Moriwaki6 K, Saitouf M, Furuseb K, Sasaki6 H, Fujimurad A, Lmaicf M, Kusanoaf E, Tsukitaf S, Furuse M, Giebisch. Claudin-2 – deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A 2010;107:8011-6. [DOI] [PMC free article] [PubMed]
- 83.Schnermann J, Huang Y, Mizel D. Fluid reabsorption in proximal convoluted tubules of mice with gene deletions of claudin-2 and/or aquaporin1. Am J Physiol Renal Physiol 2013; 305: F1352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A 2009; 106: 15350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT. Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca2+ excretion. Am J Physiol Renal Physiol 2013; 304: F761–F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 2012; 31: 1999–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plain A, Wulfmeyer VC, Milatz S, Klietz A, Hou J, Bleich M, Himmerkus N. Corticomedullary difference in the effects of dietary Ca2+ on tight junction properties in thick ascending limbs of Henle’s loop. Pflügers Archiv 2016; 468: 293–303. [DOI] [PubMed] [Google Scholar]
- 88.Gong Y, Himmerkus N, Plain A, Bleich M, Hou J. Epigenetic regulation of microRNAs controlling CLDN14 expression as a mechanism for renal calcium handling. J Am Soc Nephrol 2015; 26: 663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riccardi D, Valenti G. Localization and function of the renal calcium-sensing receptor. Nat Rev Nephrol 2016; 12: 414–25. [DOI] [PubMed] [Google Scholar]
- 90.Mayr B, Schnabel D, Dorr HG, Schofl C. Gain and loss of function mutations of the calcium-sensing receptor and associated proteins: current treatment concepts. Eur J Endocrinol 2016; 174: R189–208. [DOI] [PubMed] [Google Scholar]
- 91.Jacinto JS, Modanlou HD, Crade M, Strauss AA, Bosu SK. Renal calcification incidence in very low birth weight infants. Pediatrics 1998; 81: 31–5. [PubMed] [Google Scholar]
- 92.Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard J-P. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr 1997; 131: 252–7. [DOI] [PubMed] [Google Scholar]
- 93.Esbjorner E, Jones I. Urinary calcium excretion in Swedish children. Acta Paediatr 1995; 84: 156–9. [DOI] [PubMed] [Google Scholar]
- 94.Abuazza G, Becker A, Williams SS, Chakravarty S, Truong H-T, Lin F, Baum M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol 2006; 291: F1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Abel M, Huybers S, Hoenderop JGJ, van der Kemp AWCM, van Leeuwen JPTM, Bindels RJM. Age-dependent alterations in Ca2+ homeostasis: role of TRPV5 and TRPV6. Am J Physiol Renal Physiol 2006; 291: F1177–F83. [DOI] [PubMed] [Google Scholar]
- 96.Cheng Z, Liang N, Chen T-H, Li A, Santa Maria C, You M, Ho H, Song F, Bikle D, Tu C, Shoback D, Chang W. Sex and age modify biochemical and skeletal manifestations of chronic hyperparathyroidism by altering target organ responses to Ca2+ and parathyroid hormone in mice. J Bone Miner Res 2013; 28: 1087–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sas D, Hu M, Moe OW, Baum M. Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells. Am J Physiol Regul Integr Comp Physiol 2008; 295: R1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]