Abstract
The Escherichia coli LacZ gene is a widely used reporter for gene regulation studies in transgenic mice. It encodes bacterial β-galactosidase (Bact β-Gal), which causes insoluble precipitates when exposed to chromogenic homologues of galactose. We and others have recently reported that Bact β-Gal detection with Salmon-Gal (S-Gal) in combination with nitro blue tetrazolium chloride (NBT) is very sensitive and not prone to interference by acidic endogenous β-galactosidases. Unfortunately, as we show here, the method appears to be inadequate for evaluation of Bact β-Gal expression in keratinized epithelial appendages but not in other keratinized epithelia. NBT in the reaction mixture, just as other tetrazolium salts, inevitably causes unwanted staining artifacts in lingual filiform papillae, penile spines, and hair fibers by interacting with keratin sulfhydryl-rich regions. The methodological limitation can be overcome in part by pretreating the tissues before the S-Gal/NBT staining with an iodine–potassium iodide solution. Alternatively, the use of iodonitrotetrazolium chloride instead of NBT in the S-Gal reaction mixture provides enough color resolution to distinguish the specific Bact β-Gal staining in orange from the artifact staining in dark red. In summary, we provide evidence that S-Gal/NBT histochemistry has limitations, when staining keratinized epithelial appendages.
Keywords: β-galactosidase, epithelial appendages, filiform papillae, hair fibers, keratinized stratified squamous epithelia, LacZ expression, penile spines, Salmon-Gal, tetrazolium salts
Introduction
Reporter genes are widely used to reveal target gene expression in whole mounts and tissue sections. The bacterial LacZ gene is among the most popular reporter genes used in this context. It encodes bacterial β-galactosidase (Bact β-Gal), an exoglycosidase that cleaves β-linked terminal galactosyl residues from a variety of natural and artificial substrates.1 In the presence of chromogenic homologues of galactose (e.g., 2-nitrophenyl β-d-galactopyranoside, 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside [X-Gal], and Salmon-Gal [S-Gal; 3,4-cyclohexenoesculetin β-d-galactopyranoside]), insoluble precipitates are formed. We and others have shown that S-Gal in combination with tetrazolium salts results in more sensitive and faster staining than X-Gal in combination with ferric and ferrous ions.2–4 Potential additional advantages of the S-Gal/tetrazolium staining are (1) reduced interference from lysosomal endogenous β-galactosidase species (Endo β-Gal) particularly in high Endo β-Gal–containing organs (e.g., epididymis, kidney, and intestine)5 and (2) better preservation of the histochemically treated tissues due to much shorter incubation times.3 In this study, we report a methodological drawback which we have noted while using this enhanced alternative Bact β-Gal detection system in our analyses of targeted gene expression patterns in LacZ+ transgenic mice. We report evidence indicating that the tetrazolium salts used in combination with S-Gal in the reaction mixture cause a Bact β-Gal–independent staining artifact in stratified epithelial modifications (e.g., filiform papillae, penile spines, and growing specialized hair fibers). The false-positive staining is likely caused by sulfhydryl-rich keratins and keratin-associated proteins.6,7 Our observations concerning keratinized epithelial appendages will be discussed in the context of known histochemical properties of tetrazolium salts. Approaches for overcoming the methodological limitations of S-Gal tetrazolium staining in stratified epithelium are provided.
Materials and Methods
Organ Sampling
We made use of C57BL/6 organ samples of wild-type (wt) littermate mice and LacZ+-tagged mice deficient for adhesion G protein–coupled receptor 111 (Gpr111LacZ/LacZ) originally collected in a follow-up study to the initial description of GPR111 mutant mice.8 No animals were sacrificed solely for the purpose of this report. Animal maintenance and carbon dioxide euthanasia in the follow-up study were in accordance with the ethical guidelines for animal experimentation set by the EU Laboratory Animal Directive, German Animal Welfare Act and approved by the local ethics committee for animal experimentation appointed by the government of the Free State of Saxony, Germany (Authorization No. T07/13).
Preparation of Cryostat Sections
Only tissues of freshly sacrificed adult mice were used. The tissues were snap frozen in liquid nitrogen and sectioned with a cryostat at −20C. The sections (10 µm thick) were mounted on glass slides, postfixed with ice cold ethanol–methanol (1:1) for 1 min, air-dried for 15 min at room temperature, and stored at −20C until further use. Before the cryostat sections were subjected to β-Gal detection (see below), the sections were thawed, air-dried for 15 min at room temperature, and immersed in 0.1-M PBS, pH 7.5.
β-Gal Detection
β-Gal activity was assessed in different buffer systems depending on the designated chromogenic homologue of galactose and the designated reaction pH, as previously described.3 These studies revealed that 0.5-M HEPES at pH 7.5 was an ideal buffer for use in the X-Gal staining protocol. The staining solution contained 50-mg (2.5 mM) X-Gal dissolved in 2.5-ml N,N-dimethylformamide, 15-mM NaCl, 1.3-mM MgCl2, 3-mM K3(Fe(CN)6), and 3-mM K4(Fe(CN)6) in a final volume of 50-ml HEPES, pH 7.5. The staining solution containing S-Gal consisted of 0.1% sodium deoxycholate, 0.2% IGEPAL CA-630 (octylphenoxy poly(ethyleneoxy)ethanol, branched; Sigma, Taufkirchen, Germany), 2-mM MgCl2, 50-mg (3 mM) S-Gal (Sigma), and either 0.4-mM nitro blue tetrazolium chloride (NBT; Sigma), tetranitroblue tetrazolium chloride (TNBT; Sigma), or iodonitrotetrazolium chloride (INT; Sigma) in a final volume of 50-ml PBS, pH 7.5. X-Gal staining of cryostat sections was routinely conducted in a humidified chamber overnight at 30C. Staining with S-Gal was performed in the chamber for 2 hr at 30C. In some reactions, either chromogenic homologues of galactose, corresponding salts (potassium ferro-/ferricyanide and tetrazolium salts), or both were omitted in the reaction mixtures. After incubation with the various reaction mixtures, sections were thoroughly washed and counterstained with nuclear fast red-aluminum sulfate solution in the case of β-Gal detection with X-Gal/FeCN or with Mayer’s hematoxlin solution when S-Gal tetrazolium salt was used.
Oxidation of Tissue Sections Before Detection of β-Gal
In selected cases, free sulfhydryl groups expected to be present in the tissue sections were oxidized before β-Gal detection following essentially the procedure of Barnett and Seligmann.9 The sections were immersed for a maximum of 4 hr in PBS, pH 7.5, containing 1.5-mM iodine and 2-mM potassium iodide, and rinsed.
Results
Expression of the LacZ reporter gene is frequently detected using the standard histochemical method involving X-Gal as the artificial substrate for Bact β-Gal. S-Gal/NBT staining is becoming popular as a superior alternative to the overnight X-Gal/FeCN staining procedure.2–4 Increasing interest in the faster and more sensitive S-Gal/NBT staining method warrants investigation into potential pitfalls associated with this method. We used herein selected epithelia from adhesion G protein–coupled receptor 111 knockout/LacZ reporter knockin mice (Gpr111LacZ/LacZ mice) and from their corresponding wt littermates to investigate potential pitfalls of using the S-Gal/NBT staining method. Gpr111LacZ/LacZ mice have previously been reported to display strong LacZ reporter gene expression in keratinized stratified squamous epithelia, such as the epidermis of the skin and the epithelia of the tongue, esophagus, and forestomach.8 In contrast, no reporter gene expression is observed in non-keratinized stratified squamous epithelia (e.g., corneal epithelium). In the present study, we used keratinized stratified squamous epithelia of the tongue, penis, esophagus, and skin from different sites including the back, abdomen, ear, snout, tail, and genital area.
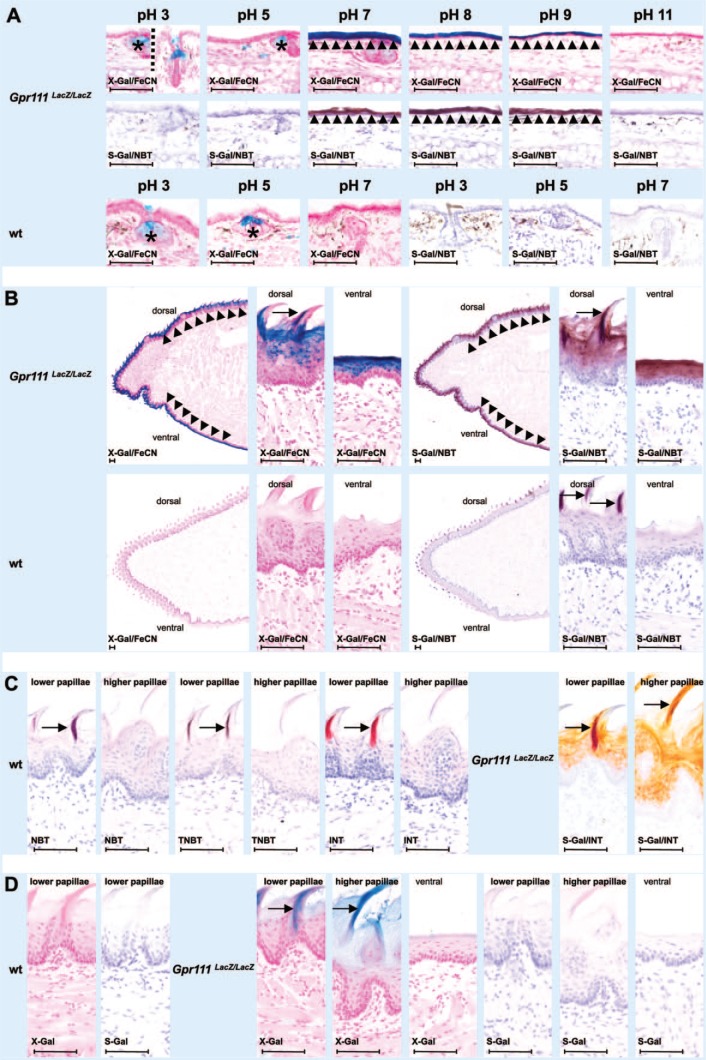
We used the skin of the ear to determine the optimal pH in which Bact β-Gal activity in Gpr111LacZ/LacZ mice produced high and reliable staining using X-Gal/FeCN and S-Gal/NBT (Fig. 1A). Bact β-Gal activity was found to be strong and specific in the epithelium of the ears of Gpr111LacZ/LacZ mice in the pH range of 7 to 9 independently of the staining method used (Fig. 1A, X-Gal/FeCN and S-Gal/NBT; first and second rows, respectively). An additional non–Bact β-Gal-specific staining reaction (marked by asterisks in Fig. 1A) was recognized in sebaceous glands in the vicinity of the opening shaft of hairs. This staining was only observed in X-Gal/FeCN-stained sections of Gpr111-deficient mice and wt littermates and was restricted to an acidic pH and not seen at pH 8, 9, or 11 (Fig. 1A, first and third rows from the top). The staining was most likely due to endogenous acidic forms of β-Gal reported to be present at high levels in sebaceous glands.10,11 Apart from the nonspecific X-Gal staining in sebaceous glands, the ear skin specimens of wt littermates displayed no further β-Gal expression independently of the method used and the pH in the reaction mixture.
Figure 1.
Comparative analysis of S-Gal tetrazolium salt and X-Gal potassium ferri-/ferrocyanide histochemistry in appendages of keratinized stratified squamous epithelia from GPR111-deficient LacZ-expressing mice (Gpr111LacZ/LacZ) and wt littermates. (A) S-Gal/NBT and X-Gal/FeCN staining at different pH. In a pH range 7 to 9, both histochemical methods result in detection of Bact β-Gal activity in the epithelial lining of the ear; at acidic pH, the presence of mammalian β-Gal in sebaceous gland affects only X-Gal/FeCN staining. (B) Comparative S-Gal/NBT and X-Gal/FeCN histochemistry of tongues from Gpr111LacZ/LacZ mice and wt littermates. Bact β-Gal–independent staining is observed in parts of filiform papillae in wt tissue. (C) Bact β-Gal–independent staining is limited to lower filiform papillae of the anterior part of the dorsum linguae. It can also be caused by tetrazolium salts other than NBT in these epithelial appendages. See specific S-Gal/INT staining in GPR111-deficient tissue for comparison of Bact β-Gal specific and nonspecific staining. (D) When the chromogenic substrates X-Gal and S-Gal are applied alone, faint specific staining is observed. Asterisks mark sebaceous glands. Arrowheads denote epithelial lining. Arrows point to filiform papillae. Abbreviations: Bact β-Gal, bacterial β-galactosidase; Gpr111LacZ/LacZ, adhesion G protein–coupled receptor 111 knockout/LacZ reporter knockin mice; INT, iodonitrotetrazolium chloride; NBT, nitro blue tetrazolium chloride; S-Gal, Salmon-Gal; TNBT, tetranitroblue tetrazolium chloride; wt, wild type; X-Gal, 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside. Scale bars: A–D = 80 µm.
We selected pH 7.5 as the pH for all of our subsequent experiments as Endo β-Gal activity was rarely a problem in X-Gal/FeCN-incubated sections at pH 7 and higher, and the intensity of staining was relatively comparable in the pH range 7 to 9.
Bact β-Gal–Independent Staining
In tongue sections from Gpr111LacZ/LacZ mice, both X-Gal/FeCN and S-Gal/NBT staining revealed LacZ expression in the epithelium covering the tongue dorsal and ventral surfaces (Fig. 1B, first row from the top). In contrast, and as expected, tongue sections from wt littermates did not reveal specific Bact β-Gal activity (Fig. 1B, second row from the top). However, and surprisingly, S-Gal/NBT staining (photomicrographs on the right), but not X-Gal/FeCN staining (photomicrographs on the left), resulted in a Bact β-Gal–independent hitherto not defined nonspecific partial staining at the tip and the dorsal rough surface of the tongue. It was apparent that the staining originated from the lower filiform papillae in the anterior part of the dorsal tongue surface when examined at higher magnification (arrows). The interpapillay epithelium, the higher and more bulky filiform papillae in the posterior part of the dorsal tongue surface, and the entire epithelium at the ventral surface of the tongue showed no staining. Interestingly, the lower filiform papillae were only partly stained. The staining varied from more anteriorly to more posteriorly between the papillae. To elucidate which component in the staining mixture was responsible for the observed β-Gal-independent staining, we performed S-Gal/NBT staining by omitting one or both of the two components S-Gal and NBT in the reaction mixture. The presence of only NBT or TNBT and INT as alternative tetrazolium salts in the reaction mixture resulted in the same type of β-Gal-independent staining (Fig. 1C, photomicrographs of wt sections on the left). Again the nonspecific staining was restricted to the lower filiform papillae in the anterior part of the dorsum linguae. The higher and more bulky filiform papillae of the posterior part of the dorsum linguae showed no reaction. Of note, in S-Gal/INT-stained tongue sections from Gpr111LacZ/LacZ mice (Fig. 1C, photomicrographs on the right), the β-Gal-independent staining in filiform papillae was dark red against the dull orange staining caused by Bact β-Gal in the epithelium. In contrast, the use of either X-Gal or S-Gal in the reaction mixture alone (i.e., without any FeCN or tetrazolium salt) showed no (Fig. 1D, wt tissue, photomicrographs on the left) or only faint (X-Gal, photomicrographs in the middle) or extremely faint (S-Gal, photomicrographs on the right) Bact β-Gal–specific staining (GPR111-deficient tissue). The faint Bact β-Gal–specific X-Gal staining was evident in both forms of filiform papillae (i.e., filiform papillae of both the anterior and posterior parts of the dorsum linguae). No staining was observed when the two reaction components, S-Gal and NBT, were omitted in the reaction mixture (not shown).
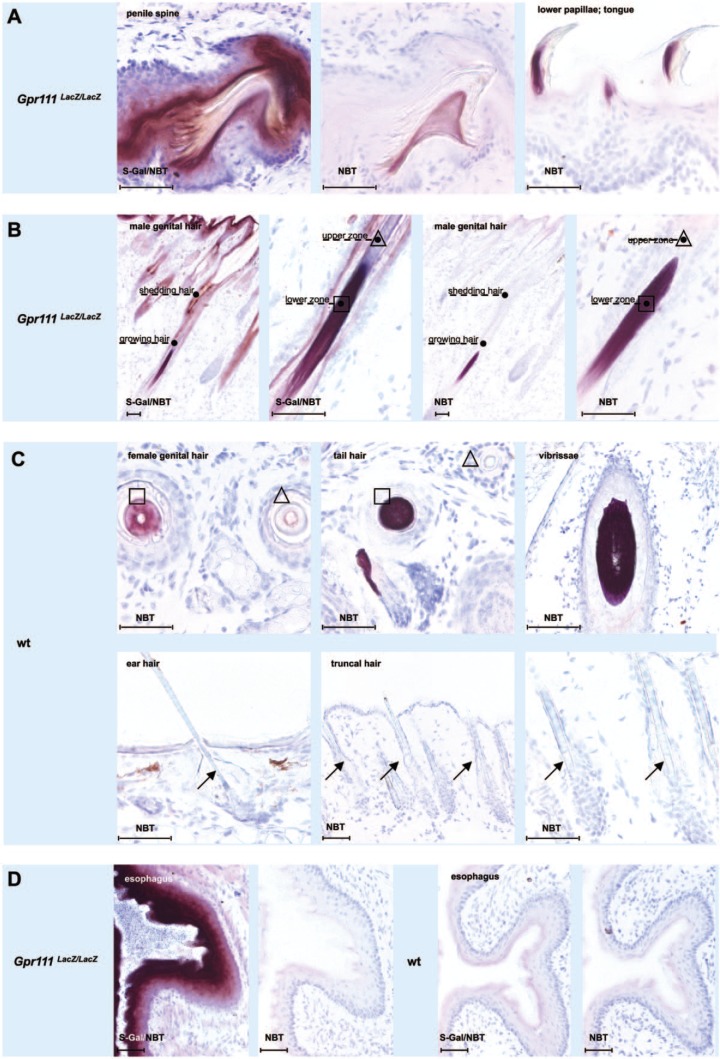
Filiform projections similar to those observed in the dorsum linguae cover (as so-called penile spines) the surface of the glans penis of rodents and all other mammals, with the exception of human males.6,12 As for the lower filiform papillae of the dorsum linguae, the presence of tetrazolium salts and absence of S-Gal in the reaction mixture was sufficient to exclusively stain parts of the penile filiform projections (Fig. 2A, compare photomicrographs of the S-Gal/NBT-treated Gpr111LacZ/LacZ section on the left with the only NBT-treated Gpr111LacZ/LacZ section in the middle). Of note, the only NBT staining of the penile projections (Fig. 2A, middle photomicrograph) was of a more symmetrical shape than the corresponding staining of the lingual filiform papillae (Fig. 2A, right photomicrograph).
Figure 2.
Nitro blue tetrazolium causes nonspecific staining in penile spines and hair fibers as well as other types of epithelial appendages made of hard keratins. (A) In penile spines, NBT staining occupies the spine center in the form of a conical dome. In contrast, a more anterior–posterior position is seen in lingual filiform papillae. (B) In genital hair shafts, NBT preferentially reacts in the lower zones of growing hair fibers. (C) NBT staining in hair fiber zones (squares) and total absence of NBT staining in hairs at other sites (arrows). (D) NBT does not stain keratinized stratified squamous epithelia without keratinized epithelial appendages, as exemplified here by esophageal mucosa. Squares and triangles mark growing and advanced zones of hair fibers, respectively. Arrows point to unstained hair shafts. Abbreviations: Gpr111LacZ/LacZ, adhesion G protein–coupled receptor 111 knockout/LacZ reporter knockin mice; NBT, nitro blue tetrazolium chloride; S-Gal, Salmon-Gal; wt, wild type. Scale bars: A–D = 100 µm.
Both of the keratinized epithelial appendages discussed above (i.e., filiform projections of the dorsum linguae and penis) contain a core of hard keratin proteins, a finding that suggests they have a close evolutionary relationship to hair fibers.7,13–15 Indeed, by exposing hair fibers from different parts of the body to the different compositions of the reaction mixture, a similar but more complex reaction pattern emerged in that surprising differences were observed in the reaction pattern between different types of hairs and between hairs in different hair cycle stages. Collectively, mainly growing specialized hairs of the body (e.g., hairs of genital area, tail, and snout) were intensively stained in the lower region of their fibers (Fig. 2B and first row from the top in Fig. 2C). The staining was not dependent on the presence of S-Gal in the reaction mixture, because it still occurred when Gpr111LacZ/LacZ (Fig. 2B, photomicrographs on the right) and wt littermate sections (Fig. 2C, first row of photomicrographs from the top) were only exposed to NBT. In contrast, different types of coat hairs, as well as hairs in the outer region of the ear, did not stain when only NBT was added to the reaction mixture (Fig. 2C, second row of photomicrographs from the top). In addition, no staining occurred in specialized hairs when they were being shed (Fig. 2B), or in keratinized stratified squamous epithelia without epithelial appendages such as the mucosa of the ventral tongue (Fig. 1B, second row of photomicrographs from the top), esophagus (Fig. 2D), forestomach, and vagina at estrus (not shown).
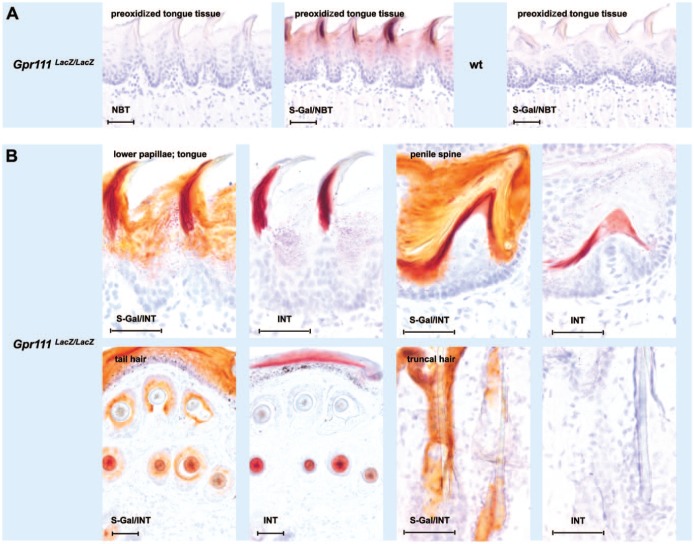
We next explored methods to prevent the Bact β-Gal–independent nonspecific staining with its potential to lead to false-positive results. Clearly, one possibility would be to detect LacZ reporter gene expression in epithelial tissue with keratinized appendages by using the less sensitive classical X-Gal/FeCN protocol (Fig. 1B, left photomicrographs in the first row from the top), or by using an immunofluorescence-based LacZ expression assay.16 Figure 3 proposes two alternative approaches, which would allow the continued use of the fast and very sensitive S-Gal/tetrazolium salt method. Filiform papillae, penile spines, and hair fibers are all reported to contain high amounts of sulfhydryl group–rich hard keratins and keratin-associated proteins in their more immature central or lower regions.13,17 These sulfhydryl group–rich proteins are cross-linked by disulfide bonds at maturation to harden and stabilize the keratin proteins.18,19 Sulfhydryl groups have been reported to strongly reduce tetrazolium salts. When the corresponding tissue sections from Gpr111LacZ/LacZ and wt littermate mice were oxidized for more than 5 min with iodine–potassium iodide solution before the incubation with S-Gal-free reaction mixture, the nonspecific NBT-caused staining in filiform papillae was absent. However, when the sections were incubated with S-Gal/NBT-containing reaction mixture after the preoxidation treatment for 5 min Bact β-Gal activity could still be observed. In addition, the use of INT instead of NBT in the reaction mixture presents another alternative approach. A direct comparison of the staining achieved when S-Gal/INT was present in the reaction mixture as opposed to only INT revealed that only tissue sections from Gpr111LacZ/LacZ mice stained with S-Gal/INT contained orange beside dark red colored areas. The dark red coloring was probably due to the reaction of the tetrazolium salts with sulfhydryl-rich proteins (Fig. 3B, first three pairs of photomicrographs; Fig. 1C, photomicrographs on the right). Of note, in coat hairs which did not stain when only NBT was added to the reaction mixture (see above and Fig. 2C, second row from the top), no dark red stained areas were seen (Fig. 3B, second row from the top, last pair of photomicrographs on the right).
Figure 3.
Attempts to differentiate between Bact β-Gal specific and nonspecific staining in keratinized epithelial appendages using the S-Gal tetrazolium salt method. (A) The Bact β-Gal–independent S-Gal/NBT staining in filiform papillae of the anterior part of the dorsum linguae can largely be prevented by preoxidation of the tissue with iodine–potassium iodide solution. (B) Bact β-Gal specific and nonspecific staining can be estimated in epithelial appendages from Gpr111LacZ/LacZ mice by color differences, if INT is used in the S-Gal/tetrazolium salt reaction. Note, no dark red color component is present in epithelial appendages free of Bact β-Gal nonspecific signals, as exemplified by truncal hairs (bottom row, right two photomicrographs). Abbreviations: Bact β-Gal, bacterial β-galactosidase; Gpr111LacZ/LacZ, adhesion G protein–coupled receptor 111 knockout/LacZ reporter knockin mice; INT, iodonitrotetrazolium chloride; NBT, nitro blue tetrazolium chloride; S-Gal, Salmon-Gal; wt, wilde type. Scale bars: A, B = 100 µm.
Discussion
S-Gal was initially designed to replace X-Gal in blue-white selection of recombinant bacterial colonies with the LacZ+ phenotype. We and others provided evidence that this chromogenic substrate for β-Gal is also suitable for analysis of LacZ reporter gene expression in LacZ+ transgenic murine tissue and that in combination with tetrazolium salts, its use is even superior to the most widely used combination of X-Gal and ferric and ferrous ions.3,4
However, there is a major disadvantage of this method, as we report herein. In keratinized epithelial appendages, histochemistry involving S-Gal/tetrazolium salts may lead to false-positive data. In this study, we focused on keratinized stratified squamous epithelia of Gpr111LacZ/LacZ mice and wt littermates.
We first optimized the pH conditions and incubation times of our β-Gal detection systems by taking advantage of Gpr111LacZ/LacZ expression in ear skin tissue. Incubation of tissue sections in a reaction mixture containing S-Gal/NBT for 2 hr and X-Gal/FeCN staining overnight resulted in specific epithelial Bact β-Gal staining, if the pH in the reaction mixture ranged between 7 and 9 (Fig. 1A, first and second rows of photomicrographs from the top). X-Gal/FeCN incubation, but not S-Gal/NBT incubation, produced an additional staining in sebaceous glands at acidic pH (Fig. 1A, asterisks). This acidic β-Gal activity was also present in sebaceous glands of wt littermates (Fig. 1A, third row of photomicrographs from the top, asterisks), indicating that this activity was probably of endogenous origin, which is in line with findings in other species.10,11 We selected pH 7.5 as the pH for all of the subsequent experiments as Endo β-Gal activity was rarely detected following incubation at pH 7 and higher, and epithelial staining intensity was relatively comparable between pH 7 and 9.
In sections of GPR111-deficient tongue, X-Gal/FeCN and S-Gal/NBT staining resulted in a strong and homogeneous reaction of the entire dorsal and ventral epithelium (Fig. 1B). X-Gal/FeCN staining of corresponding wt sections resulted in no staining. However, S-Gal/NBT staining of wt sections produced a focal intermittent Bact β-Gal–independent staining. The same type of nonspecific staining was found in GPR111-deficient tissue as well as in wt tissue when S-Gal was omitted from the reaction mixture. Thus, conversion of S-Gal by β-Gal into a chromogenic precipitate was not involved in this nonspecific staining.
The Bact β-Gal–independent reaction was confined to numerous projections called filiform papilla on the anterior dorsal surface of the tongue. The interpapillary epithelium, the epithelium on the ventral surface of the tongue, and, most notably, filiform papillae in the posterior part of the dorsum linguae remained unstained. The staining in the anterior filiform papillae was compartmentalized with either the anterior or posterior part of the filiform papillae being stained. The different staining patterns obtained for filiform papillae on the anterior and posterior parts of the murine tongue substantiated that filiform papillae of the anterior and posterior parts of rodent tongue are indeed structurally and probably functionally different.20
In epithelial GPR111-deficient and wt appendages of the penis and skin, similar types of staining were observed as in filiform papillae of the anterior part of the dorsum linguae. The epithelia of GPR111-deficient penis and skin were strongly and homogeneously stained when S-Gal/NBT were included in the reaction mixture, whereas when S-Gal/NBT were used in the reaction mixture, the staining of wt tissue was distinct and independent of the presence of Bact β-Gal. The same type of signal was produced in wt tissue, as well as in GPR111-deficient tissue when only NBT was present in the reaction mixture (Fig. 2A–C). The S-Gal-independent staining was restricted to penile spines and hair fibers. The keratinized stratified squamous epithelium adjacent to these structures was unstained, just as the entire keratinized stratified squamous epithelium sections of the esophagus (Fig. 2D). Of note, the NBT staining in the penile spine showed no anterior–posterior compartmentalization in contrast to that which was observed in filiform papillae of the dorsum linguae (see above, Fig. 2A).
As the Bact β-Gal–independent staining occurred regardless of the presence or absence of S-Gal in the reaction mixture, we focused on the possibility that NBT was responsible for the nonspecific staining. Several authors have suggested that the presence of sulfhydryl-rich hair-like keratins in penile spines and lingual filiform papillae may be responsible for nonspecific staining.13,14 Positive staining of the mouse lingual filiform papillae with antibody AE14 substantiates the presence of sulfhydryl-rich proteins in the epithelial projections.15,21
Filiform papillae and penile spines thus appear to be composed of the same trichophytic hard keratins as hair shafts in addition to epidermal soft keratins.6,14,15,22,23 Trichophytic hard keratins can be identified by their high sulfhydryl and disulfide content.9,19 The Bact β-Gal–independent staining of NBT, as well as the other tetrazolium salts tested in this study, could therefore be based on the high content of reducing groups in these epithelial appendages resulting in water-insoluble formazan dyes. Indeed, tetrazolium salts are responsible for nonspecific control reactions in the quantitative histochemical analysis of NAD(P)+-dependent dehydrogenase activities because of reducing agents in the tissue sections or cells and/or dehydrogenation of endogenous substrates.24–27 When we preoxidized GPR111-deficient tissue sections with iodine–potassium iodide solution for more than 5 min, no staining resulted if sections were then tested with only NBT in the reaction mixture. In contrast, Bact β-Gal–specific staining still resulted when the sections were incubated with S-Gal/NBT after 5 min of preoxidation treatment.
The different and diverse NBT staining patterns that we observed in the absence of S-Gal in filiform papillae and penile spines (e.g., stained filiform papillae of the anterior as opposed to nonstained filiform papillae of the posterior part of the dorsum linguae) may be related to different keratinization patterns.20 Indeed, filiform papillae of the anterior part of the dorsum linguae are composed of a compartment with mainly skin-type keratins and of a compartment with mainly hair-type keratins.13–15,23 Penile spines, however, appear to be composed of only hair-type keratins6 and the epithelium in the interpapillary fields of pure esophageal keratins.
Similarly, the observation of different and diverse NBT staining patterns in hair follicles could be due to differences in the hair cycle stage analyzed, or structural and functional differences between the different hair types found in the murine body.28,29 It is of note that the strong NBT staining was observed in growing hairs and in the hair zone which is known to contain high amounts of sulfhydryl groups.18 No NBT staining was observed in the cortex of hairs leaving the site. These hairs contain only slight amounts of sulfhydryl groups.
Collectively, we demonstrate that NBT and the alternative tetrazolium salts TNBT and INT result in unwanted staining of keratinized epithelial appendages when used in Bact β-Gal detection assays but not in other keratinized epithelia. It is of note that the three protruding epithelial appendages involved (filiform papillae of tongue, penile spines, and hair fibers) are anatomical sites known to be developmentally, structurally, and functionally related. They all express hard keratins that are rich in sulfhydryl plus disulfide groups.30
In summary, staining for β-Gal activity is widely used for assessing LacZ gene expression in LacZ+ transgenic mice. Our article points to a disadvantage when using S-Gal as the chromogenic β-Gal substrate. S-Gal-independent staining associated with hair shafts and dermal-papilla-like structures results when this substrate is used in combination with tetrazolium salts in LacZ expression screens. Our findings indicate that when S-Gal is used in combination with tetrazolium salts to assess β-Gal activity in LacZ+ transgenic keratinized epithelial appendages, special attention should be paid to identify false-positive staining from keratinized epithelial appendages.
Acknowledgments
We are grateful for the support of Professor Jaime Rivera, Department of Cell and Developmental Biology, University of Massachusetts. He helped us to get Salmon-Gal/tetrazolium salt histochemistry working nicely in our laboratory. We are thankful to Jana Brendler and Anja Schmid who support us in our laboratory’s daily work. We are also grateful to the staff of the transgenic and experimental animal facility for taking care of our mice.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AMR developed the concept of the study, designed the histochemical experiments, analyzed the data, and prepared the manuscript. OB critically interpreted the data, revised the drafted manuscript, and wrote its final version together with AMR. CM performed all the histochemical experiments. JW and SP took care about the Gpr111LacZ/LacZ mice and their wild-type littermates, and further characterized these animals. Together with AS, they commented on the data, their implications, and the manuscript at all its stages.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This particular scientific work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Its execution was jointly financially supported by the Institute of Anatomy and the Institute of Biochemistry, Faculty of Medicine, University of Leipzig, Germany.
Literature Cited
- 1. Juers DH, Matthews BW, Huber RE. LacZ β-galactosidase: structure and function of an enzyme of historical and molecular biological importance. Protein Sci. 2012;21:1792–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sundararajan S, Wakamiya M, Behringer RR, Rivera-Pérez JA. A fast and sensitive alternative for β-galactosidase detection in mouse embryos. Development. 2012;139:4484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merkwitz C, Blaschuk O, Schulz A, Ricken AM. Comments on methods to suppress endogenous β-galactosidase activity in mouse tissues expressing the LacZ reporter gene. J Histochem Cytochem. 2016;64(10):579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trifonov S, Yamashita Y, Kase M, Maruyama M, Sugimoto T. Overview and assessment of the histochemical methods and reagents for the detection of β-galactosidase activity in transgenic animals. Anat Sci Int. 2016;91:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conchie J, Findlay J, Levvy GA. Mammalian glycosidases. Distribution in the body. Biochem J. 1959;71:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patrizi G, Munger BL. The maturation of cortical keratin in filiform hairs of the rat penis. J Ultrastruct Res. 1966;14:329–244. [DOI] [PubMed] [Google Scholar]
- 7. Steflik DE, Singh BB, Mckinney RV Jr, Boshell JL. Correlated TEM, SEM, and histological observations of filiform papillae of the cow tongue. Acta Anat (Basel). 1983;117:21–30. [DOI] [PubMed] [Google Scholar]
- 8. Prömel S, Waller-Evans H, Dixon J, Zahn D, Colledge WH, Doran J, Carlton MB, Grosse J, Schöneberg T, Russ AP, Langenhan T. Characterization and functional study of a cluster of four highly conserved orphan adhesion-GPCR in mouse. Dev Dyn. 2012;241:1591–602. [DOI] [PubMed] [Google Scholar]
- 9. Barnett RJ, Seligman AM. Histochemical demonstration of sulfhydryl and disulfide groups of protein. J Natl Cancer Inst. 1954;14:769–803. [PubMed] [Google Scholar]
- 10. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pati S, Jain S, Behera M, Acharya AP, Panda SK, Senapati S. X-gal staining of canine skin tissues: a technique with multiple possible applications. J Nat Sci Biol Med. 2014;5:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McLean CY, Reno PL, Pollen AA, Bassan AI, Capellini TD, Guenther C, Indjeian VB, Lim X, Menke DB, Schaar BT, Wenger AM, Bejerano G, Kingsley DM. Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature. 2011;471:216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farbman AI. The dual pattern of keratinization in filiform papillae on rat tongue. J Anat. 1970;106:233–42. [PMC free article] [PubMed] [Google Scholar]
- 14. Dhouailly D, Sun TT. The mammalian tongue filiform papillae: a theoretical model for primitive hairs. In: van Neste D, LaChapelle JM, Antoine JL. editors. Trends in human hair growth and alopecia research. Boston: Kluwer Academic; 1989. p. 29–34. [Google Scholar]
- 15. Dhouailly D, Xu C, Manabe M, Schermer A, Sun TT. Expression of hair-related keratins in a soft epithelium: subpopulations of human and mouse dorsal tongue keratinocytes express keratin markers for hair-, skin- and esophageal-types of differentiation. Exp Cell Res. 1989;181:141–58. [DOI] [PubMed] [Google Scholar]
- 16. Mahony D, Karunaratne S, Rothnagel JA. Improved detection of lacZ reporter gene expression in transgenic epithelia by immunofluorescence microscopy. Exp Dermatol. 2002;11:153–8. [DOI] [PubMed] [Google Scholar]
- 17. Kawabe TT, Buhl AE. A method to detect areas high in sulfhydryl groups in mouse epithelium. Microsc Res Tech. 1993;26:513–6. [DOI] [PubMed] [Google Scholar]
- 18. Hardy MH. The histochemistry of hair follicles in the mouse. Am J Anat. 1952;90:285–337. [DOI] [PubMed] [Google Scholar]
- 19. Taneda A, Ogawa H, Hashimoto K. The histochemical demonstration of protein-bound sulfhydryl groups and disulfide bonds in human hair by a new staining method (DACM staining). J Invest Dermatol. 1980;75:365–9. [DOI] [PubMed] [Google Scholar]
- 20. Kutuzov H, Sicher H. The filiform and the conical papillae of the tongue in the white rat. Anat Rec. 1951;110:275–88. [DOI] [PubMed] [Google Scholar]
- 21. Lynch MH, O’Guin WM, Hardy C, Mak L, Sun TT. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. J Cell Biol. 1986;103:2593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baratz RS, Farbman AI. Morphogenesis of rat lingual filiform papillae. Am J Anat. 1975;143:283–301. [DOI] [PubMed] [Google Scholar]
- 23. Manabe M, Lim HW, Winzer M, Loomis CA. Architectural organization of filiform papillae in normal and black hairy tongue epithelium: dissection of differentiation pathways in a complex human epithelium according to their patterns of keratin expression. Arch Dermatol. 1999;135:177–81. [DOI] [PubMed] [Google Scholar]
- 24. Butcher RG, Van Noorden CJ. Reaction rate studies of glucose-6-phosphate dehydrogenase activity in sections of rat liver using four tetrazolium salts. Histochem J. 1985;17:993–1008. [DOI] [PubMed] [Google Scholar]
- 25. Van Noorden CJ, Butcher RG. Histochemical localization of NADP-dependent dehydrogenase activity with four different tetrazolium salts. J Histochem Cytochem. 1984;32:998–1004. [DOI] [PubMed] [Google Scholar]
- 26. Van Noorden CJ, Butcher RG. Linearity in dehydrogenase reaction rate studies in tissue sections is affected by loss of endogenous substrates during the reaction. J Histochem Cytochem. 1987;35:1401–4. [DOI] [PubMed] [Google Scholar]
- 27. Van Noorden CJ, Butcher RG. The involvement of superoxide anions in the nitro blue tetrazolium chloride reduction mediated by NADH and phenazine methosulfate. Anal Biochem. 1989;176:170–4. [DOI] [PubMed] [Google Scholar]
- 28. Müller-Röver S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. [DOI] [PubMed] [Google Scholar]
- 29. Sundberg JP, Hogan ME. Hair types and subtypes in the laboratory mouse. In: Sundberg JP. editor. Handbook of mouse mutations with skin and hair abnormalities. Boca Raton: CRC Press; 1994. p. 57–68. [Google Scholar]
- 30. Langbein L, Rogers MA, Winter H, Praetzel S, Schweizer J. The catalog of human hair keratins. II. Expression of the six type II members in the hair follicle and the combined catalog of human type I and II keratins. J Biol Chem. 2001;276:35123–332. [DOI] [PubMed] [Google Scholar]