Abstract
Physical activity (PA) may modify the genetic effects that give rise to increased risk of obesity. To identify adiposity loci whose effects are modified by PA, we performed genome-wide interaction meta-analyses of BMI and BMI-adjusted waist circumference and waist-hip ratio from up to 200,452 adults of European (n = 180,423) or other ancestry (n = 20,029). We standardized PA by categorizing it into a dichotomous variable where, on average, 23% of participants were categorized as inactive and 77% as physically active. While we replicate the interaction with PA for the strongest known obesity-risk locus in the FTO gene, of which the effect is attenuated by ~30% in physically active individuals compared to inactive individuals, we do not identify additional loci that are sensitive to PA. In additional genome-wide meta-analyses adjusting for PA and interaction with PA, we identify 11 novel adiposity loci, suggesting that accounting for PA or other environmental factors that contribute to variation in adiposity may facilitate gene discovery.
Author summary
Decline in daily physical activity is thought to be a key contributor to the global obesity epidemic. However, the impact of sedentariness on adiposity may be in part determined by a person’s genetic constitution. The specific genetic variants that are sensitive to physical activity and regulate adiposity remain largely unknown. Here, we aimed to identify genetic variants whose effects on adiposity are modified by physical activity by examining ~2.5 million genetic variants in up to 200,452 individuals. We also tested whether adjusting for physical activity as a covariate could lead to the identification of novel adiposity variants. We find robust evidence of interaction with physical activity for the strongest known obesity risk-locus in the FTO gene, of which the body mass index-increasing effect is attenuated by ~30% in physically active individuals compared to inactive individuals. Our analyses indicate that other similar gene-physical activity interactions may exist, but better measurement of physical activity, larger sample sizes, and/or improved analytical methods will be required to identify them. Adjusting for physical activity, we identify 11 novel adiposity variants, suggesting that accounting for physical activity or other environmental factors that contribute to variation in adiposity may facilitate gene discovery.
Introduction
In recent decades, we have witnessed a global obesity epidemic that may be driven by changes in lifestyle such as easier access to energy-dense foods and decreased physical activity (PA) [1]. However, not everyone becomes obese in obesogenic environments. Twin studies suggest that changes in body weight in response to lifestyle interventions are in part determined by a person’s genetic constitution [2–4]. Nevertheless, the genes that are sensitive to environmental influences remain largely unknown.
Previous studies suggest that genetic susceptibility to obesity, assessed by a genetic risk score for BMI, may be attenuated by PA [5, 6]. A large-scale meta-analysis of the FTO obesity locus in 218,166 adults showed that being physically active attenuates the BMI-increasing effect of this locus by ~30% [7]. While these findings suggest that FTO, and potentially other previously established BMI loci, may interact with PA, it has been hypothesized that loci showing the strongest main effect associations in genome-wide association studies (GWAS) may be the least sensitive to environmental and lifestyle influences, and may therefore not make the best candidates for interactions [8]. Yet no genome-wide search for novel loci exhibiting SNP×PA interaction has been performed. A genome-wide meta-analysis of genotype-dependent phenotypic variance of BMI, a marker of sensitivity to environmental exposures, in ~170,000 participants identified FTO, but did not show robust evidence of environmental sensitivity for other loci [9]. Recent genome-wide meta-analyses of adiposity traits in >320,000 individuals uncovered loci interacting with age and sex, but also suggested that very large sample sizes are required for interaction studies to be successful [10].
Here, we report results from a large-scale genome-wide meta-analysis of SNP×PA interactions in adiposity in up to 200,452 adults. As part of these interaction analyses, we also examine whether adjusting for PA or jointly testing for SNP’s main effect and interaction with PA may identify novel adiposity loci.
Results
Identification of loci interacting with PA
We performed meta-analyses of results from 60 studies, including up to 180,423 adults of European descent and 20,029 adults of other ancestries to assess interactions between ~2.5 million genotyped or HapMap-imputed SNPs and PA on BMI and BMI-adjusted waist circumference (WCadjBMI) and waist-hip ratio (WHRadjBMI) (S1–S5 Tables). Similar to a previous meta-analysis of the interaction between FTO and PA [7], we standardized PA by categorizing it into a dichotomous variable where on average ~23% of participants were categorized as inactive and ~77% as physically active (see Methods and S6 Table). On average, inactive individuals had 0.99 kg/m2 higher BMI, 3.46 cm higher WC, and 0.018 higher WHR than active individuals (S4 and S5 Tables).
Each study first performed genome-wide association analyses for each SNP’s effect on BMI in the inactive and active groups separately. Corresponding summary statistics from each cohort were subsequently meta-analyzed, and the SNP×PA interaction effect was estimated by calculating the difference in the SNP’s effect between the inactive and active groups. To identify sex-specific SNP×PA interactions, we performed the meta-analyses separately in men and women, as well as in the combined sample. In addition, we carried out meta-analyses in European-ancestry studies only and in European and other-ancestry studies combined.
We used two approaches to identify loci whose effects are modified by PA. In the first approach, we searched for genome-wide significant SNP×PA interaction effects (PINT<5x10-8). As shown in Fig 1, this approach yielded the highest power to identify cross-over interaction effects where the SNP’s effect is directionally opposite between the inactive and active groups. However, this approach has low power to identify interaction effects where the SNP’s effect is directionally concordant between the inactive and active groups (Fig 1). We identified a genome-wide significant interaction between rs986732 in cadherin 12 (CDH12) and PA on BMI in European-ancestry studies (betaINT = -0.076 SD/allele, PINT = 3.1x10-8, n = 134,767) (S7 Table). The interaction effect was directionally consistent but did not replicate in an independent sample of 31,097 individuals (betaINT = -0.019 SD/allele, PINT = 0.52), and the pooled association P value for the discovery and replication stages combined did not reach genome-wide significance (NTOTAL = 165,864; PINT-TOTAL = 3x10-7) (S1 Fig). No loci showed genome-wide significant interactions with PA on WCadjBMI or WHRadjBMI. CDH12 encodes an integral membrane protein mediating calcium-dependent cell-cell adhesion in the brain, where it may play a role in neurogenesis [11]. While CDH12 rs4701252 and rs268972 SNPs have shown suggestive associations with waist circumference (P = 2x10-6) and BMI (P = 5x10-5) in previous GWAS [12, 13], the SNPs are not in LD with rs986732 (r2<0.1).
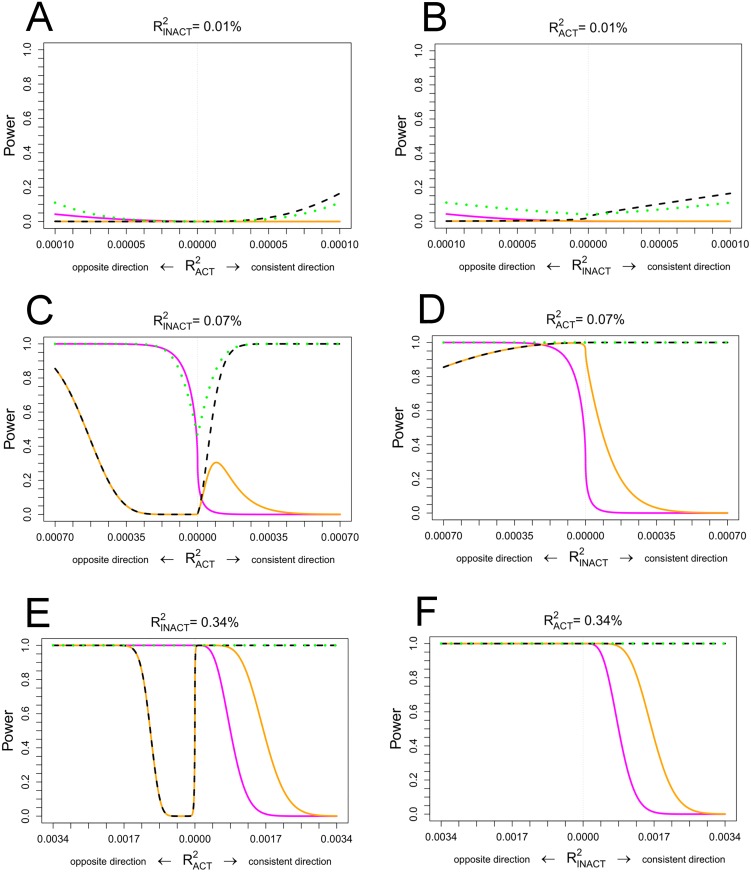
Fig 1. Power to identify PA-adjusted main, joint or GxPA interaction effects in 200,000 individuals (45,000 inactive, 155,000 active).
The plots compare power to identify genome-wide significant main effects (PadjPA<5x10-8, dashed black), joint effects (PJOINT<5x10-8, dotted green) or GxPA interaction effects (PINT<5x10-8, solid magenta) as well as the power to identify Bonferroni-corrected interaction effects (PINT<0.05/number of loci, solid orange) for the SNPs that reached a genome-wide significant PA-adjusted main effect association (PadjPA<5x10-8). The power computations were based on analytical power formulae provided elsewhere [50] and were conducted a-priori based on various types of known realistic BMI effect sizes [51]. Panels A, C, E: Assuming an effect in inactive individuals similar to a small (, comparable to the known BMI effect of the NUDT3 locus), medium (, comparable to the known BMI effect of the BDNF locus) and large (, comparable to the known BMI effect of the FTO locus) realistic effect on BMI and for various effects in physically active individuals (varied on the x axis); Panels B,D,F: Assuming an effect in physically active individuals similar to the small, medium and large realistic effects of the NUDT3, BDNF and FTO loci on BMI and for various effects in inactive individuals (varied on x axis).
In our second approach, we tested interaction for loci showing a genome-wide significant main effect on BMI, WCadjBMI or WHRadjBMI (S7–S12 Tables). We adjusted the significance threshold for SNP×PA interaction by Bonferroni correction (P = 0.05/number of SNPs tested). As shown in Fig 1, this approach enhanced our power to identify interaction effects where there is a difference in the magnitude of the SNP’s effect between inactive and active groups when the SNP’s effect is directionally concordant between the groups. We identified a significant SNP×PA interaction of the FTO rs9941349 SNP on BMI in the meta-analysis of European-ancestry individuals; the BMI-increasing effect was 33% smaller in active individuals (betaACTIVE = 0.072 SD/allele) than in inactive individuals (betaINACTIVE = 0.106 SD/allele, PINT = 4x10-5). The rs9941349 SNP is in strong LD (r2 = 0.87) with FTO rs9939609 for which interaction with PA has been previously established in a meta-analysis of 218,166 adults [7]. We identified no loci interacting with PA for WCadjBMI or WHRadjBMI.
In a previously published meta-analysis [7], the FTO locus showed a geographic difference for the interaction effect where the interaction was more pronounced in studies from North America than in those from Europe. To test for geographic differences in the present study, we performed additional meta-analyses for the FTO rs9941349 SNP, stratified by geographic origin (North America vs. Europe). While the interaction effect was more pronounced in studies from North America (betaINT = 0.052 SD/allele, P = 5x10-4, N = 63,896) than in those from Europe (betaINT = 0.028 SD/allele, P = 0.006, N = 109,806), we did not find a statistically significant difference between the regions (P = 0.14).
Explained phenotypic variance in inactive and active individuals
We tested whether the variance explained by ~1.1 million common variants (MAF≥1%) differed between the inactive and active groups for BMI, WCadjBMI, and WHRadjBMI [14]. In the physically active individuals, the variants explained ~20% less of variance in BMI than in inactive individuals (12.4% vs. 15.7%, respectively; Pdifference = 0.046), suggesting that PA may reduce the impact of genetic predisposition to adiposity overall. There was no significant difference in the variance explained between active and inactive groups for WCadjBMI (8.6% for active, 9.3% for inactive; Pdifference = 0.70) or WHRadjBMI (6.9% for active, 8.0% for inactive; Pdifference = 0.59).
To further investigate differences in explained variance between the inactive and active groups, we calculated variance explained by subsets of SNPs selected based on significance thresholds (ranging from P = 5x10-8 to P = 0.05) of PA-adjusted SNP association with BMI, WCadjBMI or WHRadjBMI [15] (S13 Table). We found 17–26% smaller explained variance for BMI in the active group than in the inactive group at all P value thresholds (S13 Table).
Identification of novel loci when adjusting for PA or when jointly testing for SNP main effect and interaction with PA
Physical activity contributes to variation in BMI, WCadjBMI, and WHRadjBMI, hence, adjusting for PA as a covariate may enhance power to identify novel adiposity loci. To that extent, each study performed genome-wide analyses for association with BMI, WCadjBMI, and WHRadjBMI while adjusting for PA. Subsequently, we performed meta-analyses of the study-specific results. We discovered 10 genome-wide significant loci (2 for BMI, 1 for WCadjBMI, 7 for WHRadjBMI) that have not been reported in previous GWAS of adiposity traits (Table 1, S2–S4 Figs).
Table 1. Novel loci achieving genome-wide significance (P<5x10-8) in meta-analyses for PA-adjusted SNP main effect (PadjPA) or the joint test of SNP main effect and SNP-PA interaction (Pjoint).
| Trait | Marker | Nearest Gene | Chr | Pos (hg19) | Trait increasing/decreasing allele | Trait increasing allele’s frequency | Analysis | NadjPA | BetaadjPA | SEadjPA | PadjPA | Pint | Pjoint |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novel loci achieving genome-wide significance in European-ancestry meta-analyses | |||||||||||||
| BMI | rs1720825 | MRAS | 3 | 138,108,083 | A/G | 0.20 | Overall | 178833 | 0.026 | 0.0047 | 2.98E-08 | 1.62E-01 | 3.67E-08 |
| Women | 102854 | 0.0281 | 0.006 | 2.84E-06 | 7.27E-02 | 3.35E-06 | |||||||
| Men | 47544 | 0.024 | 0.0069 | 4.91E-04 | 9.95E-01 | 1.30E-02 | |||||||
| BMI | rs1934100 | ELAVL2 | 9 | 23,234,308 | A/T | 0.68 | Overall | 140811 | 0.0179 | 0.0049 | 2.43E-04 | 3.99E-02 | 2.15E-04 |
| Women | 85142 | 0.0048 | 0.006 | 4.18E-01 | 9.89E-01 | 7.37E-01 | |||||||
| Men | 41958 | 0.0377 | 0.0074 | 3.18E-07 | 8.84E-04 | 3.70E-08 | |||||||
| WCadjBMI | rs7176527 | ZSCAN2 | 15 | 85,140,794 | C/T | 0.81 | Overall | 130413 | 0.0317 | 0.0054 | 5.98E-09 | 1.79E-01 | 2.80E-08 |
| Women | 77349 | 0.0303 | 0.007 | 1.37E-05 | 9.36E-01 | 1.28E-04 | |||||||
| Men | 52918 | 0.0342 | 0.0084 | 4.55E-05 | 3.23E-02 | 7.50E-06 | |||||||
| WHRadjBMI | rs4650943 | PAPPA2 | 1 | 176,414,781 | A/G | 0.53 | Overall | 113963 | 0.0267 | 0.0048 | 2.34E-08 | 1.77E-01 | 5.76E-08 |
| Women | 69016 | 0.0301 | 0.006 | 4.66E-07 | 7.79E-03 | 1.57E-07 | |||||||
| Men | 44430 | 0.0212 | 0.0073 | 3.55E-03 | 2.73E-01 | 5.64E-03 | |||||||
| WHRadjBMI | rs2300481 | MEIS1 | 2 | 66,782,467 | T/C | 0.39 | Overall | 110881 | 0.0267 | 0.0048 | 2.41E-08 | 5.80E-01 | 3.93E-08 |
| Women | 66519 | 0.0288 | 0.0059 | 1.19E-06 | 4.71E-01 | 1.47E-06 | |||||||
| Men | 43845 | 0.0258 | 0.0073 | 4.14E-04 | 1.00E+00 | 2.82E-03 | |||||||
| WHRadjBMI | rs167025 | ARHGEF28 | 5 | 73,433,308 | A/G | 0.33 | Overall | 117603 | 0.0179 | 0.0048 | 2.13E-04 | 8.01E-01 | 4.64E-04 |
| Women | 70494 | 0.0023 | 0.006 | 7.01E-01 | 4.50E-01 | 7.32E-01 | |||||||
| Men | 46591 | 0.0427 | 0.0074 | 6.24E-09 | 1.34E-01 | 3.73E-09 | |||||||
| WHRadjBMI | rs3094013 | HCP5 | 6 | 31,434,366 | G/A | 0.87 | Overall | 149338 | 0.0269 | 0.0061 | 1.06E-05 | 4.98E-01 | 6.93E-05 |
| Women | 84538 | 0.0104 | 0.0078 | 1.82E-01 | 4.50E-01 | 3.78E-01 | |||||||
| Men | 64138 | 0.0494 | 0.009 | 4.51E-08 | 8.91E-01 | 7.87E-07 | |||||||
| WHRadjBMI | rs6976930 | BAZ1B | 7 | 72,885,810 | G/A | 0.81 | Overall | 145913 | 0.0294 | 0.0051 | 1.03E-08 | 5.28E-01 | 1.87E-08 |
| Women | 83184 | 0.0326 | 0.0066 | 7.70E-07 | 7.00E-01 | 2.02E-06 | |||||||
| Men | 62149 | 0.0254 | 0.0075 | 7.69E-04 | 5.93E-01 | 3.10E-03 | |||||||
| WHRadjBMI | rs10786152 | PLCE1 | 10 | 95,893,514 | A/G | 0.52 | Overall | 147123 | 0.0224 | 0.004 | 1.79E-08 | 8.76E-02 | 1.44E-08 |
| Women | 83884 | 0.0192 | 0.0051 | 1.56E-04 | 5.81E-02 | 1.41E-04 | |||||||
| Men | 62722 | 0.0255 | 0.0058 | 1.32E-05 | 6.38E-01 | 5.89E-05 | |||||||
| WHRadjBMI | rs889512 | CTRB2 | 16 | 75,242,012 | C/G | 0.88 | Overall | 117417 | 0.031 | 0.0074 | 2.70E-05 | 4.26E-01 | 1.13E-04 |
| Women | 70315 | 0.0506 | 0.0091 | 2.87E-08 | 9.96E-02 | 1.09E-07 | |||||||
| Men | 46440 | -0.0022 | 0.0114 | 8.50E-01 | 5.06E-01 | 7.80E-01 | |||||||
| Novel loci achieving genome-wide significance in all-ancestry meta-analyses | |||||||||||||
| BMI | rs754635 | CCK | 3 | 42,305,131 | G/C | 0.87 | Overall | 151282 | 0.0356 | 0.0062 | 1.07E-08 | 1.21E-01 | 3.28E-07 |
| Women | 91241 | 0.026 | 0.0079 | 9.66E-04 | 1.25E-01 | 8.69E-04 | |||||||
| Men | 62741 | 0.0486 | 0.0093 | 1.61E-07 | 2.98E-01 | 3.68E-06 | |||||||
Chr: chromosome; Pos(hg19): position based on human assembly 19; NadjPA, BetaadjPA, SEadjPA, or PadjPA: sample size, effect size, standard error, or P value, respectively, in the physical activity adjusted SNP main effect model; PA: physical activity; WCadjBMI: BMI-adjusted waist circumference; WHRadjBMI: BMI-adjusted waist-hip ratio; Pint: P value for SNP-PA interaction; Pjoint: P value for the joint test of SNP main effect and SNP-PA interaction.
To establish whether additionally accounting for SNP×PA interactions would identify novel loci, we calculated the joint significance of PA-adjusted SNP main effect and SNP×PA interaction using the method of Aschard et al [16]. As illustrated in Fig 1, the joint test enhanced our power to identify loci where the SNP shows simultaneously a main effect and an interaction effect. We identified a novel BMI locus near ELAVL2 in men (PJOINT = 4x10-8), which also showed suggestive evidence of interaction with PA (PINT = 9x10-4); the effect of the BMI-increasing allele was attenuated by 71% in active as compared to inactive individuals (betaINACTIVE = 0.087 SD/allele, betaACTIVE = 0.025 SD/allele) (Table 1, S2–S4 Figs).
To evaluate the effect of PA adjustment on the results for the 11 novel loci, we performed a look-up in published GIANT consortium meta-analyses for BMI, WCadjBMI, and WHRadjBMI that did not adjust for PA [17, 18] (S22 Table). All 11 loci showed a consistent direction of effect between the present PA-adjusted and the previously published PA-unadjusted results, but the PA-unadjusted associations were less pronounced despite up to 40% greater sample size, suggesting that adjustment for PA may have increased our power to identify these loci.
The biological relevance of putative candidate genes in the novel loci, based on our thorough searches of the literature, GWAS catalog look-ups, and analyses of eQTL enrichment and overlap with functional regulatory elements, are described in Tables 2 and 3. As the novel loci were identified in a PA-adjusted model, where adjusting for PA may have contributed to their identification, we examined whether the lead SNPs in these loci are associated with the level of PA. More specifically, we performed look-ups in GWAS analyses for the levels of moderate-to-vigorous intensity leisure-time PA (n = 80,035), TV-viewing time (n = 28,752), and sedentary behavior at work (n = 59,381) or during transportation (n = 15,152) [personal communication with Marcel den Hoed, Marilyn Cornelis, and Ruth Loos]. However, we did not find significant associations when correcting for the number of loci that were examined (P>0.005) (S16 Table).
Table 2. Genes of biological interest within 500 kb of lead SNPs associated with BMI.
| CCK (rs754635): The lead SNP is located in intron 1 of the CCK gene that encodes cholecystokinin, a gastrointestinal peptide that stimulates the digestion of fat and protein in the small intestine by inhibiting gastric emptying, inducing the release of pancreatic enzymes, increasing production of hepatic bile, and causing contraction of the gallbladder. Cholecystokinin induces satiety and reduces the amount of food consumed when administered prior to a meal [52, 53]. In a candidate gene study, four common variants in CCK were associated with increased meal size [54], but the variants are not in LD with rs754635 (r2<0.1). A GWAS of BMI in 62,246 individuals of East Asian ancestry showed a suggestive association (P = 2x10-7) for the rs4377469 SNP in high LD with our lead SNP (r2 = 0.7) [55]. |
| ELAVL2 (rs1934100): The lead SNP showed an association with BMI only in men (Table 1). The only nearby gene ELAVL2 (455 kb away) is a conserved neuron-specific RNA-binding protein involved in stabilization or enhanced translation of specific mRNAs with AU-rich elements in the 3’-untranslated region [56]. While ELAVL2 is implicated in neuronal differentiation [56], potential mechanisms linking this function to obesity remain unclear. |
| MRAS (rs1720825): The lead SNP is an intronic variant in MRAS. The MRAS rs1199333 SNP, in high LD with rs1720825 (r2 = 0.85), has shown suggestive association with typical sporadic amyotrophic lateral sclerosis in a Chinese Han population (P = 4x10-6, S14 Table). Other MRAS SNPs have been associated with risk of coronary artery disease [57] but they are not in LD with rs1720825 (r2<0.06). MRAS encodes a member of the membrane-associated Ras small GTPase protein family that function as signal transducers in multiple processes of cell growth and differentiation and are involved in energy expenditure, adipogenesis, muscle differentiation, insulin signaling and glucose metabolism [58–60]. Mice with Mras knockout develop a severe obesity phenotype [61]. The SNP rs1199334, in high LD with our lead SNP rs1720825 (r2 = 0.90), has been identified as the SNP most strongly associated with the cis-expression of centrosomal protein 70kDa (CEP70) in subcutaneous adipose tissue (P = 2x10-7) (S15 Table). CEP70 encodes a centrosomal protein that is critical for the regulation of mitotic spindle assembly, playing an essential role in cell cycle progression [62]. |
Table 3. Genes of biological interest within 500 kb of lead SNPs associated with WCadjBMI or WHRadjBMI.
| ZSCAN2 (rs7176527): Twenty two genes lie within 500kb of the WCadjBMI-associated lead SNP (S3 Fig). The nearest gene, ZSCAN2, contains several copies of a zinc finger motif commonly found in transcriptional regulatory proteins. The rs7176527 SNP is in LD (r2>0.80) with five SNPs (rs3762168, rs2762169, rs12594450, rs72630460, and rs16974951) that are enhancers in multiple tissues in the data from Roadmap Epigenomics Consortium [22]. The rs7176527 SNP is a cis-eQTL for the putative transcriptional regulator SCAND2 [63] in the intestine, prefrontal cortex, and lymphocytes (S15 Table). |
| PAPPA2 (rs4650943): Seven genes lie within 500kb of the lead SNP (S3 Fig). The nearest gene, PAPPA2, is 18 kb upstream of rs4650943 and codes for a protease that locally regulates insulin-like growth factor availability through cleavage of IGF binding protein 5, most commonly found in bone tissue. In murine models, the PAPP-A2 protein has been shown to influence overall body size and bone growth, but not glucose metabolism or adiposity [64–66]. |
| MEIS1 (rs2300481): The only gene within 500 kb of the lead SNP is MEIS1 encoding a homeobox protein that plays an important role in normal organismal growth and development. Two variants in high LD with the lead SNP (r2 = 0.95) have been identified for association with PR interval of the heart (S14 Table). Another variant, in low LD with rs2300481 (r2 = 0.25), has been associated with restless leg syndrome [67]–a sleeping disorder that may cause weight gain [68]. |
| ARHGEF28 (rs167025): The lead SNP showed an association with WHRadjBMI in men only (Table 1). There are two protein-coding genes within 500kb of rs167025. The nearest gene is ARHGEF28, 195 kb downstream, encoding Rho guanine nucleotide exchange factor 28. This exchange factor has been shown to destabilize low molecular weight neurofilament mRNAs in patients with amyotrophic lateral sclerosis, leading to degeneration and death of motor neurons controlling voluntary muscle movement [69, 70]. The ENC1 gene, 490 kb away, encodes Ectoderm-neural cortex protein 1, an actin-binding protein required for adipocyte differentiation [71] |
| HCP5 (rs3094013): The lead SNP showed an association with WHRadjBMI in men only (Table 1). The rs3094013 SNP is located in the MHC complex on chromosome 6, and the region within 500kb contains 124 genes (S3 Fig). The known WHRadjBMI-increasing allele rs3099844, in strong LD with our lead SNP (r2≥0.8), has previously been associated with increased HDL-cholesterol levels [72]. Candidate gene studies suggest that rs1800629 in tumor necrosis factor (TNF), which is 109 kb upstream and in moderate LD (r2 = 0.64) with the lead SNP, may interact with physical activity to decrease serum CRP levels [73, 74]. We did not, however, find an interaction between rs1800629 and physical activity on WHRadjBMI (P = 0.3). |
| BAZ1B (rs6976930): There are 31 genes within 500kb the lead SNP rs6976930 (S3 Fig) which is in high LD (r2>0.8) with GWAS hits associated with protein C levels, triglycerides, serum urate levels, lipid metabolism, metabolic syndrome, and gamma-glutamyl transferase levels (S14 Table). The rs6976930 SNP shows an eQTL association with MLXIPL expression in omental (P = 7x10-22) and subcutaneous adipose tissue (P = 4x10-14). MLXIPL is 122 kb downstream of rs6976930 and codes for a transcription factor that binds carbohydrate response motifs, increasing transcription of genes involved in glycolysis, lipogenesis, and triglyceride synthesis [75, 76]. |
| PLCE1 (rs10786152): There are 8 genes within 500 kb of the lead SNP (S3 Fig). The lead SNP lies within the intron of PLCE1 encoding a phospholipase involved in cellular growth and differentiation and gene expression among many other biological processes involving phospholipids [77]. Variants in this gene have been shown to cause nephrotic syndrome, type 3 [78]. Nearby variants rs9663362 and rs932764 (r2 = 1.0 and 0.85, respectively) have been previously associated with systolic and diastolic blood pressure (S14 Table). |
| CTRB2 (rs889512): The lead SNP showed an association with WHRadjBMI in women only (Table 1). There are 17 genes within 500 kb (S3 Fig). The nearby rs4888378 SNP has been associated with carotid intima-media thickness in women but not in men, and BCAR1 (breast cancer anti-estrogen resistance protein 1) has been implicated as the causal gene [79]. The rs488378 SNP is not, however, in LD with our lead SNP (r2<0.1). The SNP rs7202877, in moderate LD with rs889512 (r2 = 0.6), is a risk variant for type 1 diabetes (S14 Table). The data from Roadmap Epigenomics Consortium [22] suggest that five variants in strong LD (r2>0.8) with our lead SNP rest in known regulatory regions, including rs9936550 within an active enhancer region and rs72802352 in a DNAse hypersensitive region for human skeletal muscle cells and myoblasts; and rs147630228 and rs111869668 within active enhancer regions for the pancreas. Additionally, rs111869668 rests within binding motifs for CEBPB and CEBPD (CCAAT enhancer-binding protein-Beta and Delta) which are enhancer proteins involved in adipogenesis [80, 81]. |
Identification of secondary signals
In addition to uncovering 11 novel adiposity loci, our PA-adjusted GWAS and the joint test of SNP main effect and SNP×PA interaction confirmed 148 genome-wide significant loci (50 for BMI, 58 for WCadjBMI, 40 for WHRadjBMI) that have been established in previous main effect GWAS for adiposity traits (S7–S12 Tables, S4 Fig). The lead SNPs in eight of the previously established loci (5 for BMI, 3 for WCadjBMI), however, showed no LD or only weak LD (r2<0.3) with the published lead SNP, suggesting they could represent novel secondary signals in known loci (S17 Table). To test whether these eight signals are independent of the previously published signals, we performed conditional analyses [19]. Three of the eight SNPs we examined, in/near NDUFS4, MEF2C-AS1 and CPA1, were associated with WCadjBMI with P<5x10-8 in our PA-adjusted GWAS even after conditioning on the published lead SNP, hence representing novel secondary signals in these loci (S17 Table).
Enrichment of the identified loci with functional regulatory elements
Epigenetic variation may underlie gene-environment interactions observed in epidemiological studies [20] and PA has been shown to induce marked epigenetic changes in the genome [21]. We examined whether the BMI or WHRadjBMI loci reaching P<1x10-5 for interaction with PA (13 loci for BMI, 5 for WHRadjBMI) show overall enrichment with chromatin states in adipose, brain and muscle tissues available from the Roadmap Epigenomics Consortium [22]. However, we did not find significant enrichment (S18 and S19 Tables), which may be due to the limited number of identified loci. The lack of significant findings may also be due to the assessment of chromatin states in the basal state, which may not reflect the dynamic changes that occur when cells are perturbed by PA [23].
We also tested whether the loci reaching P<5x10-8 in our PA-adjusted GWAS of BMI or WHRadjBMI show enrichment with chromatin states and found significant enrichment of the BMI loci with enhancer, weak transcription, and polycomb-repressive elements in several brain cell lines, and with enhancer elements in three muscle cell lines (S20 and S21 Tables). We also found significant enrichment of the WHRadjBMI loci with enhancer elements in three adipose and six muscle cell lines, with active transcription start sites in two adipose cell lines, and with polycomb-repressive elements in seven brain cell lines. The enrichment of our PA-adjusted main effect results with chromatin annotations in skeletal muscle in particular, the tissue most affected by PA, could highlight regulatory mechanisms that may be influenced by PA.
Discussion
In this genome-wide meta-analysis of more than 200,000 adults, we do not find evidence of interaction with PA for loci other than the established FTO locus. However, when adjusting for PA or jointly testing for SNP main effect and interaction with PA, we identify 11 novel adiposity loci, suggesting that accounting for PA or other environmental factors that contribute to variation in adiposity may increase power for gene discovery.
Our results suggest that if SNP×PA interaction effects for common variants exist, they are unlikely to be of greater magnitude than observed for FTO, the BMI-increasing effect of which is attenuated by ~30% in physically active individuals. The fact that common SNPs explain less of the BMI variance among physically active compared to inactive individuals indicates that further interactions may exist, but larger meta-analyses, more accurate and precise measurement of PA, and/or improved analytical methods will be required to identify them. We found no difference between inactive and active individuals in variance explained by common SNPs in aggregate for WCadjBMI or WHRadjBMI, and no loci interacted with PA on WCadjBMI or WHRadjBMI. Therefore, PA may not modify genetic influences as strongly for body fat distribution as for overall adiposity. Furthermore, while differences in variance explained by common variants may be due to genetic effects being modified by PA, it is important to note that heritability can change in the absence of changes in genetic effects, if environmental variation differs between the inactive and active groups. Therefore, the lower BMI variance explained in the active group could be partly due to a potentially greater environmental variation in this group.
While we replicated the previously observed interaction between FTO and PA [7], it remains unclear what biological mechanisms underlie the attenuation in FTO’s effect in physically active individuals, and whether the interaction is due to PA or due to confounding by other environmental exposures. While some studies suggest that FTO may interact with diet [24–26], a recent meta-analysis of 177,330 individuals did not find interaction between FTO and dietary intakes of total energy, protein, carbohydrate or fat [27]. The obesity-associated FTO variants are located in a super-enhancer region [28] and have been associated with DNA methylation levels [29–31], suggesting that this region may be sensitive to epigenetic effects that could mediate the interaction between FTO and PA.
In genome-wide analyses for SNP main effects adjusting for PA, or when testing for the joint significance of SNP main effect and SNPxPA interaction, we identify 11 novel adiposity loci, even though our sample size was up to 40% smaller than in the largest published main effect meta-analyses [17, 18]. Our findings suggest that accounting for PA may facilitate the discovery of novel adiposity loci. Similarly, accounting for other environmental factors that contribute to variation in adiposity could lead to the discovery of additional loci.
In the present meta-analyses, statistical power to identify SNPxPA interactions may have been limited due to challenges relating to the measurement and statistical modeling of PA [5]. Of the 60 participating studies, 56 assessed PA by self-report while 4 used wearable PA monitors. Measurement error and bias inherent in self-report estimates of PA [32] can attenuate effect sizes for SNP×PA interaction effects towards the null [33]. Measurement using PA monitors provides more consistent results, but the monitors are not able to cover all types of activities and the measurement covers a limited time span compared to questionnaires [34]. As sample size requirements increase nonlinearly when effect sizes decrease, any factor that leads to a deflation in the observed interaction effect estimates may make their detection very difficult, even when very large population samples are available for analysis. Finally, because of the wide differences in PA assessment tools used among the participating studies, we treated PA as a dichotomous variable, harmonizing PA into inactive and active individuals. Considerable loss of power is anticipated when a continuous PA variable is dichotomized [35]. Our power could be enhanced by using a continuous PA variable if a few larger studies with equivalent, quantitative PA measurements were available.
In summary, while our results suggest that adjusting for PA or other environmental factors that contribute to variation in adiposity may increase power for gene discovery, we do not find evidence of SNP×PA interaction effects stronger than that observed for FTO. While other SNP×PA interaction effects on adiposity are likely to exist, combining many small studies with varying characteristics and PA assessment tools may be inefficient for identifying such effects [5]. Access to large cohorts with quantitative, equivalent PA variables, measured with relatively high accuracy and precision, may be necessary to uncover novel SNP×PA interactions.
Methods
Main analyses
Ethics statement
All studies were conducted according to the Declaration of Helsinki. The studies were approved by the local ethical review boards and all study participants provided written informed consent for the collection of samples and subsequent analyses.
Outcome traits—BMI, WCadjBMI and WHRadjBMI
We examined three anthropometric traits related to overall adiposity (BMI) or body fat distribution (WCadjBMI and WHRadjBMI) [36] that were available from a large number of studies. Before the association analyses, we calculated sex-specific residuals by adjusting for age, age2, BMI (for WCadjBMI and WHRadjBMI traits only), and other necessary study-specific covariates, such as genotype-derived principal components. Subsequently, we normalized the distributions of sex-specific trait residuals using inverse normal transformation.
Physical activity
Physical activity was assessed and quantified in various ways in the participating studies of the meta-analysis (S1 and S6 Tables). Aiming to amass as large a sample size as possible, we harmonized PA by categorizing it into a simple dichotomous variable—physically inactive vs. active—that could be derived in a relatively consistent way in all participating studies, and that would be consistent with previous findings on gene-physical activity interactions and the relationship between activity levels and health outcomes. In studies with categorical PA data, individuals were defined inactive if they reported having a sedentary occupation and being sedentary during transport and leisure-time (<1 h of moderate intensity leisure-time or commuting PA per week). All other individuals were defined physically active. Previous studies in large-scale individual cohorts have demonstrated that the interaction between FTO, or a BMI-increasing genetic risk score, with physical activity, is most pronounced approximately at this activity level [6, 37, 38]. In studies with continuous PA data, PA variables were standardized by defining individuals belonging to the lowest sex- and age-adjusted quintile of PA levels as inactive, and all other individuals as active. The study-specific coding of the dichotomous PA variable in each study is described in S6 Table.
Study-specific association analyses
We included 42 studies with genome-wide data, 10 studies with Metabochip data, and eight studies with both genome-wide and Metabochip data. If both genome-wide and Metabochip data were available for the same individual, we only included the genome-wide data (S1 Table). Studies with genome-wide genotyped data used either Affymetrix or Illumina arrays (S2 Table). Following study-specific quality control measures, the genotype data were imputed using the HapMap phase II reference panel (S2 Table). Studies with Metabochip data used the custom Illumina HumanCardio-Metabo BeadChip containing ~195K SNPs designed to support large-scale follow-up of known associations with metabolic and cardiovascular traits [39]. Each study ran autosomal SNP association analyses with BMI, WCadjBMI and WHRadjBMI across their array of genetic data using the following linear regression models in men and women separately: 1) active individuals only; 2) inactive individuals only; and 3) active and inactive individuals combined, adjusting for the PA stratum. In studies that included families or closely related individuals, regression coefficients were estimated using a variance component model that modeled relatedness in men and women combined, with sex as a covariate, in addition to the sex-specific analyses. The additive genetic effect for each SNP and phenotype association was estimated using linear regression. For studies with a case-control design (S1 Table), cases and controls were analyzed separately.
Quality control of study-specific association results
All study-specific files for the three regression models listed above were processed through a standardized quality control protocol using the EasyQC software [40]. The study-specific quality control measures included checks on file completeness, range of test statistics, allele frequencies, trait transformation, population stratification, and filtering out of low quality data. Checks on file completeness included screening for missing alleles, effect estimates, allele frequencies, and other missing data. Checks on range of test statistics included screening for invalid statistics such as P-values >1 or <0, negative standard errors, or SNPs with low minor allele count (MAC, calculated as MAF*N, where MAF is the minor allele frequency and N is the sample size) and where SNPs with MAC<5 in the inactive or the active group were removed. The correctness of trait transformation to inverse normal was examined by plotting 2/median of the standard error with the square root of the sample size. Population stratification was examined by calculating the study specific genomic control inflation factor (λGC) [41]. If a study had λGC>1.1, the study analyst was contacted and asked to revise the analyses by adjusting for principal components. The allele frequencies in each study were examined for strand issues and miscoded alleles by plotting effect allele frequencies against the corresponding allele frequencies from the HapMap2 reference panel. Finally, low quality data were filtered out by removing monomorphic SNPs, imputed SNPs with poor imputation quality (r2_hat <0.3 in MACH [42], observed/expected dosage variance <0.3 in BIMBAM [43], proper_info <0.4 in IMPUTE [44]), and genotyped SNPs with a low call-rate (<95%) or that were out of Hardy-Weinberg equilibrium (P<10−6).
Meta-analyses
Beta-coefficients and standard errors were combined by an inverse-variance weighted fixed effect method, implemented using the METAL software [45]. We performed meta-analyses for each of the three models (active, inactive, active + inactive adjusted for PA) in men only, in women only, and in men and women combined. Study-specific GWAS results were corrected for genomic control using all SNPs. Study-specific Metabochip results as well as the meta-analysis results for GWAS and Metabochip combined were corrected for genomic control using 4,425 SNPs included on the Metabochip for replication of associations with QT-interval, a phenotype not correlated with BMI, WCadjBMI or WHRadjBMI, after pruning of SNPs within 500 kb of an anthropometry replication SNP. We excluded SNPs that 1) were not available in at least half of the maximum sample size in each stratum; 2) had a heterogeneity I2 >75%, or 3) were missing chromosomal and base position annotation in dbSNP.
Calculation of the significance of SNP×PA interaction and of the joint significance of SNP main effect and SNP×PA interaction
To identify SNP×PA interactions, we used the EasyStrata R package [46] to test for the difference in meta-analyzed beta-coefficients between the active and inactive groups for the association of each SNP with BMI, WCadjBMI and WHRadjBMI. Easystrata tests for differences in effect estimates between the active and inactive strata by subtracting one beta from the other (βactive−βinactive,) and dividing by the overall standard error of the difference as follows:
where r is the Spearman rank correlation coefficient between βactive and βinactive for all genome-wide SNPs. The joint significance of the SNP main and SNP×PA interaction effects was estimated using the method by Aschard et al. [16] which is a joint test for genetic main effects and gene-environment interaction effects where gene-environment interaction is calculated as the difference in effect estimates between two exposure strata, accounting for 2 degrees of freedom.
Testing for secondary signals
Approximate conditional analyses were conducted using GCTA version 1.24 [19]. In the analyses for SNPs identified in our meta-analyses of European-ancestry individuals only, LD correlations between SNPs were estimated using a reference sample comprised of European-ancestry participants of the Atherosclerosis Risk in Communities (ARIC) study. In the analyses for SNPs identified in our meta-analyses of all ancestries combined, the reference sample comprised 93% of European-ancestry individuals and 6% of African ancestry participants from ARIC, as well as 1% of CHB and JPT samples from the HapMap2 panel, to approximate the ancestry mixture in our all ancestry meta-analyses. To test if our identified SNPs were independent secondary signals that fell within 1 Mbp of a previously established signal, we used the GCTA—cojo-cond command to condition our lead SNPs on each previously established SNP in the same locus.
Replication analysis for the CDH12 locus
The replication analysis for the CDH12 locus included participants from the EPIC-Norfolk (NINACTIVE = 4,755, NACTIVE = 11,526) and Fenland studies (NINACTIVE = 1,213, NACTIVE = 4,817), and from the random subcohort of the EPIC-InterAct Consortium (NINACTIVE = 2,154, NACTIVE = 6,632). PA stratum-specific estimates of the association of CDH12 with BMI were assessed and meta-analyzed by fixed effects meta-analyses, and the differences between the PA-strata were determined as described above.
Examining the influence of BMI, WCadjBMI and WHRadjBMI-associated loci on other complex traits and their potential functional roles
NHGRI-EBI GWAS catalog lookups
To identify associations of the novel BMI, WCadjBMI or WHRadjBMI loci with other complex traits in published GWAS, we extracted previously reported GWAS associations within 500 kb and r2>0.6 with any of the lead SNPs, from the GWAS Catalog of the National Human Genome Research Institute and European Bioinformatics Institute [47] (S14 Table).
eQTLs
We examined the cis-associations of the novel BMI, WCadjBMI or WHRadjBMI loci with the expression of nearby genes from various tissues by performing a look-up in a library of >100 published expression datasets, as described previously by Zhang et al [48]. In addition, we examined cis-associations using gene expression data derived from fasting peripheral whole blood in the Framingham Heart Study [49] (n = 5,206), adjusting for PA, age, age2, sex and cohort. For each novel locus, we evaluated the association of all transcripts ±1 Mb from the lead SNP. To minimize the potential for false positives, we only considered associations where our lead SNP or its proxy (r2>0.8) was either the peak SNP associated with the expression of a gene transcript in the region, or in strong LD (r2>0.8) with the peak SNP.
Overlap with functional regulatory elements
We used the Uncovering Enrichment Through Simulation method to combine the genetic association data with the Roadmap Epigenomics Project segmentation data [22]. First, 10,000 sets of random SNPs were selected among HapMap2 SNPs with a MAF >0.05 that matched the original input SNPs based on proximity to a transcription start site and the number of LD partners (r2>0.8 in individuals of European ancestry in the 1000 Genomes Project). The LD partners were combined with their original lead SNPs to create 10,000 sets of matched random SNPs and their respective LD partners. These sets were intersected with the 15-state ChromHMM data from the Roadmap Epigenomics Project and resultant co-localizations were collapsed from total SNPs down to loci, which were then used to calculate an empirical P value when comparing the original SNPs to the random sets. We examined the enrichment for all loci reaching P<10−5 for SNP×PA interaction combined, and for all loci reaching P<5x10-8 in the PA-adjusted SNP main effect model combined. In addition, we examined the variant-specific overlap with regulatory elements for each of the index SNPs of the novel BMI, WCadjBMI and WHRadjBMI loci and variants in strong LD (r2>0.8).
Estimation of variance explained in inactive and active groups
We compared variance explained for BMI, WCadjBMI and WHRadjBMI between the active and inactive groups using two approaches. First, we used a method previously reported by Kutalik et al [15], and selected subsets of SNPs based on varying P value thresholds (ranging from 5x10-8 to 0.05) from the SNP main effect model adjusted for PA. Each subset of SNPs was clumped into independent regions using a physical distance criterion of <500kb, and the most significant lead SNP within the respective region was selected. For each lead SNP, the explained variance was calculated as:
in the active and inactive groups separately, where N is the sample size and P is the P value for SNP main effect in active or inactive strata. Finally, the variance explained by each subset of SNPs in the active and inactive strata was estimated by summing up the variance explained by the SNPs.
Second, we applied the LD Score regression tool developed by Bulik-Sullivan et al [14] to quantify the proportion of inflation due to polygenicity (heritability) rather than confounding (cryptic relatedness or population stratification) using meta-analysis summary results. LD Score regression leverages LD between causal and index variants to distinguish true signals by regressing meta-analysis summary results on an ‘LD Score’, i.e. the cumulative genetic variation that an index SNP tags. To obtain heritability estimates by PA strata, we regressed our summary results from the genome-wide meta-analyses of BMI, WCadjBMI and WHRadjBMI, stratified by PA status (active and inactive), on pre-calculated LD Scores available in HapMap3 reference samples of up to 1,061,094 variants with MAF≥1% and N>10th percentile of the total sample size.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability
All genome-wide association meta-analysis results files are available at the GIANT Consortium website: www.broadinstitute.org/collaboration/giant.
Funding Statement
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services. Funding for this study was provided by the Aase and Ejner Danielsens Foundation; Academy of Finland (102318; 104781, 120315, 123885, 129619, 286284, 134309, 126925, 121584, 124282, 129378, 117787, 250207, 258753, 41071, 77299, 124243, 1114194, 24300796); Accare Center for Child and Adolescent Psychiatry; Action on Hearing Loss (G51); Agence Nationale de la Recherche; Agency for Health Care Policy Research (HS06516); Age UK Research into Ageing Fund; Åke Wiberg Foundation; ALF/LUA Research Grant in Gothenburg; ALFEDIAM; ALK-Abello´ A/S (Hørsholm, Denmark); American Heart Association (13POST16500011, 10SDG269004); Ardix Medical; Arthritis Research UK; Association Diabète Risque Vasculaire; AstraZeneca; Australian Associated Brewers; Australian National Health and Medical Research Council (241944, 339462, 389927, 389875, 389891, 389892, 389938, 442915, 442981, 496739, 552485, 552498); Avera Research Institute; Bayer Diagnostics; Becton Dickinson; Biobanking and Biomolecular Resources Research Infrastructure (BBMRI –NL, 184.021.007); Biocentrum Helsinki; Boston Obesity Nutrition Research Center (DK46200); British Heart Foundation (RG/10/12/28456, SP/04/002); Canada Foundation for Innovation; Canadian Institutes of Health Research (FRN-CCT-83028); Cancer Research UK; Cardionics; Center for Medical Systems Biology; Center of Excellence in Complex Disease Genetics and SALVECenter of Excellence in Genomics (EXCEGEN); Chief Scientist Office of the Scottish Government; City of Kuopio; Cohortes Santé TGIR; Contrat de Projets État-Région; Croatian Science Foundation (8875); Danish Agency for Science, Technology and Innovation; Danish Council for Independent Research (DFF–1333-00124, DFF–1331-007308); Danish Diabetes Academy; Danish Medical Research Council; Department of Psychology and Education of the VU University Amsterdam; Diabetes Hilfs- und Forschungsfonds Deutschland; Dutch Brain Foundation; Dutch Ministry of Justice; Emil Aaltonen Foundation; Erasmus Medical Center; Erasmus University; Estonian Government (IUT20-60, IUT24-6); Estonian Ministry of Education and Research (3.2.0304.11-0312); European Commission (230374, 284167, 323195, 692145, FP7 EurHEALTHAgeing-277849, FP7 BBMRI-LPC 313010, nr 602633, HEALTH-F2-2008-201865-GEFOS, HEALTH-F4-2007-201413, FP6 LSHM-CT-2004-005272, FP5 QLG2-CT-2002-01254, FP6 LSHG-CT-2006-01947, FP7 HEALTH-F4-2007-201413, FP7 279143, FP7 201668, FP7 305739, FP6 LSHG-CT-2006-018947, HEALTH-F4-2007-201413, QLG1-CT-2001-01252); European Regional Development Fund; European Science Foundation (EuroSTRESS project FP-006, ESF, EU/QLRT-2001-01254); Faculty of Biology and Medicine of Lausanne; Federal Ministry of Education and Research (01ZZ9603, 01ZZ0103, 01ZZ0403, 03ZIK012, 03IS2061A); Federal State of Mecklenburg - West Pomerania; Fédération Française de Cardiologie; Finnish Cultural Foundation; Finnish Diabetes Association; Finnish Foundation of Cardiovascular Research; Finnish Heart Association; Food Standards Agency; Fondation de France; Fonds Santé; Genetic Association Information Network of the Foundation for the National Institutes of Health; German Diabetes Association; German Federal Ministry of Education and Research (BMBF, 01ER1206, 01ER1507); German Research Council (SFB-1052, SPP 1629 TO 718/2-1); GlaxoSmithKline; Göran Gustafssons Foundation; Göteborg Medical Society; Health and Safety Executive; Heart Foundation of Northern Sweden; Icelandic Heart Association; Icelandic Parliament; Imperial College Healthcare NHS Trust; INSERM, Réseaux en Santé Publique, Interactions entre les déterminants de la santé; Interreg IV Oberrhein Program (A28); Italian Ministry of Economy and Finance; Italian Ministry of Health (ICS110.1/RF97.71); John D and Catherine T MacArthur Foundation; Juho Vainio Foundation; King's College London; Kjell och Märta Beijers Foundation; Kuopio University Hospital; Kuopio, Tampere and Turku University Hospital Medical Funds (X51001); Leiden University Medical Center; Lilly; LMUinnovativ; Lundbeck Foundation; Lundberg Foundation; Medical Research Council of Canada; MEKOS Laboratories (Denmark); Merck Santé; Mid-Atlantic Nutrition Obesity Research Center (P30 DK72488); Ministère de l’Économie, de l’Innovation et des Exportations; Ministry for Health, Welfare and Sports of the Netherlands; Ministry of Cultural Affairs of the Federal State of Mecklenburg-West Pomerania; Ministry of Education and Culture of Finland (627;2004-2011); Ministry of Education, Culture and Science of the Netherlands; MRC Human Genetics Unit; MRC-GlaxoSmithKline Pilot Programme Grant (G0701863); Municipality of Rotterdam; Netherlands Bioinformatics Centre (2008.024); Netherlands Consortium for Healthy Aging (050-060-810); Netherlands Genomics Initiative; Netherlands Organisation for Health Research and Development (904-61-090, 985-10-002, 904-61-193, 480-04-004, 400-05-717, Addiction-31160008, Middelgroot-911-09-032, Spinozapremie 56-464-14192); Netherlands Organisation for Health Research and Development (2010/31471/ZONMW); Netherlands Organisation for Scientific Research (10-000-1002, GB-MW 940-38-011, 100-001-004, 60-60600-97-118, 261-98-710, GB-MaGW 480-01-006, GB-MaGW 480-07-001, GB-MaGW 452-04-314, GB-MaGW 452-06-004, 175.010.2003.005, 175.010.2005.011, 481-08-013, 480-05-003, 911-03-012); Neuroscience Campus Amsterdam; NHS Foundation Trust; Novartis Pharmaceuticals; Novo Nordisk; Office National Interprofessionel des Vins; Paavo Nurmi Foundation; Påhlssons Foundation; Päivikki and Sakari Sohlberg Foundation; Pierre Fabre; Republic of Croatia Ministry of Science, Education and Sport (108-1080315-0302); Research Centre for Prevention and Health, the Capital Region for Denmark; Research Institute for Diseases in the Elderly (014-93-015, RIDE2); Roche; Russian Foundation for Basic Research (NWO-RFBR 047.017.043); Rutgers University Cell and DNA Repository (NIMH U24 MH068457-06); Sanofi-Aventis; Scottish Executive Health Department (CZD/16/6); Siemens Healthcare; Social Insurance Institution of Finland (4/26/2010); Social Ministry of the Federal State of Mecklenburg-West Pomerania; Société Francophone du Diabète; State of Bavaria; Stroke Association; Swedish Diabetes Association; Swedish Foundation for Strategic Research; Swedish Heart-Lung Foundation (20140543); Swedish Research Council (2015-03657); Swedish Medical Research Council (K2007-66X-20270-01-3, 2011-2354); Swedish Society for Medical Research; Swiss National Science Foundation (33CSCO-122661, 33CS30-139468, 33CS30-148401); Tampere Tuberculosis Foundation; The Marcus Borgström Foundation; The Royal Society; The Wellcome Trust (084723/Z/08/Z, 088869/B/09/Z); Timber Merchant Vilhelm Bangs Foundation; Topcon; Torsten and Ragnar Söderberg's Foundation; UK Department of Health; UK Diabetes Association; UK Medical Research Council (MC_U106179471, G0500539, G0600705, G0601966, G0700931, G1002319, K013351, MC_UU_12019/1); UK National Institute for Health Research BioResource Clinical Research Facility and Biomedical Research Centre; UK National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre; UK National Institute for Health Research (RP-PG-0407-10371); Umeå University Career Development Award; United States – Israel Binational Science Foundation Grant (2011036); University Hospital Oulu (75617); University Medical Center Groningen; University of Tartu (SP1GVARENG); National Institutes of Health (AG13196, CA047988, HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, HHSC271201100004C, HHSN268200900041C, HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, HHSN268201500001I, HL36310, HG002651, HL034594, HL054457, HL054481, HL071981, HL084729, HL119443, HL126024, N01-AG12100, N01-AG12109, N01-HC25195, N01-HC55015, N01-HC55016, N01-HC55018, N01-HC55019, N01-HC55020, N01-HC55021, N01-HC55022, N01-HD95159, N01-HD95160, N01-HD95161, N01-HD95162, N01-HD95163, N01-HD95164, N01-HD95165, N01-HD95166, N01-HD95167, N01-HD95168, N01-HD95169, N01-HG65403, N02-HL64278, R01-HD057194, R01-HL087641, R01-HL59367, R01HL-086694, R01-HL088451, R24-HD050924, U01-HG-004402, HHSN268200625226C, UL1-RR025005, UL1-RR025005, UL1-TR-001079, UL1-TR-00040, AA07535, AA10248, AA11998, AA13320, AA13321, AA13326, AA14041, AA17688, DA12854, MH081802, MH66206, R01-D004215701A, R01-DK075787, R01-DK089256, R01-DK8925601, R01-HL088451, R01-HL117078, R01-DK062370, R01-DK072193, DK091718, DK100383, DK078616, 1Z01-HG000024, HL087660, HL100245, R01DK089256, 2T32HL007055-36, U01-HL072515-06, U01-HL84756, NIA-U01AG009740, RC2-AG036495, RC4-AG039029, R03 AG046389, 263-MA-410953, 263-MD-9164, 263-MD-821336, U01-HG004802, R37CA54281, R01CA63, P01CA33619, U01-CA136792, U01-CA98758, RC2-MH089951, MH085520, R01-D0042157-01A, MH081802, 1RC2-MH089951, 1RC2-MH089995, 1RL1MH08326801, U01-HG007376, 5R01-HL08767902, 5R01MH63706:02, HG004790, N01-WH22110, U01-HG007033, UM1CA182913, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, 44221); USDA National Institute of Food and Agriculture (2007-35205-17883); Västra Götaland Foundation; Velux Foundation; Veterans Affairs (1 IK2 BX001823); Vleugels Foundation; VU University’s Institute for Health and Care Research (EMGO+, HEALTH-F4-2007-201413) and Neuroscience Campus Amsterdam; Wellcome Trust (090532, 091551, 098051, 098381); Wissenschaftsoffensive TMO; and Yrjö Jahnsson Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series. 2000;894:i–xii, 1–253. Epub 2001/03/10. [PubMed] [Google Scholar]
- 2.Bouchard C, Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. The Journal of nutrition. 1997;127(5 Suppl):943S–7S. Epub 1997/05/01. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, et al. The response to long-term overfeeding in identical twins. The New England journal of medicine. 1990;322(21):1477–82. Epub 1990/05/24. 10.1056/NEJM199005243222101 [DOI] [PubMed] [Google Scholar]
- 4.Hainer V, Stunkard AJ, Kunesova M, Parizkova J, Stich V, Allison DB. Intrapair resemblance in very low calorie diet-induced weight loss in female obese identical twins. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2000;24(8):1051–7. Epub 2000/08/22. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad S, Rukh G, Varga TV, Ali A, Kurbasic A, Shungin D, et al. Gene x physical activity interactions in obesity: combined analysis of 111,421 individuals of European ancestry. PLoS genetics. 2013;9(7):e1003607 Epub 2013/08/13. 10.1371/journal.pgen.1003607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Zhao JH, Luan J, Ekelund U, Luben RN, Khaw KT, et al. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS medicine. 2010;7(8). Epub 2010/09/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilpelainen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS medicine. 2011;8(11):e1001116 Epub 2011/11/10. 10.1371/journal.pmed.1001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott RA, Chu AY, Grarup N, Manning AK, Hivert MF, Shungin D, et al. No interactions between previously associated 2-hour glucose gene variants and physical activity or BMI on 2-hour glucose levels. Diabetes. 2012;61(5):1291–6. Epub 2012/03/15. 10.2337/db11-0973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Loos RJ, Powell JE, Medland SE, Speliotes EK, Chasman DI, et al. FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490(7419):267–72. Epub 2012/09/18. 10.1038/nature11401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkler TW, Justice AE, Graff M, Barata L, Feitosa MF, Chu S, et al. The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study. PLoS genetics. 2015;11(10):e1005378 Epub 2015/10/02. 10.1371/journal.pgen.1005378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selig S, Lidov HG, Bruno SA, Segal MM, Kunkel LM. Molecular characterization of Br-cadherin, a developmentally regulated, brain-specific cadherin. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2398–403. Epub 1997/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heard-Costa NL, Zillikens MC, Monda KL, Johansson A, Harris TB, Fu M, et al. NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE Consortium. PLoS genetics. 2009;5(6):e1000539 Epub 2009/06/27. 10.1371/journal.pgen.1000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng MC, Hester JM, Wing MR, Li J, Xu J, Hicks PJ, et al. Genome-wide association of BMI in African Americans. Obesity (Silver Spring, Md). 2012;20(3):622–7. Epub 2011/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature genetics. 2015;47(3):291–5. Epub 2015/02/03. 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutalik Z, Whittaker J, Waterworth D, Beckmann JS, Bergmann S. Novel method to estimate the phenotypic variation explained by genome-wide association studies reveals large fraction of the missing heritability. Genetic epidemiology. 2011;35(5):341–9. Epub 2011/04/06. 10.1002/gepi.20582 [DOI] [PubMed] [Google Scholar]
- 16.Aschard H, Hancock DB, London SJ, Kraft P. Genome-wide meta-analysis of joint tests for genetic and gene-environment interaction effects. Human heredity. 2010;70(4):292–300. Epub 2011/02/05. 10.1159/000323318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. Epub 2015/02/13. 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96. Epub 2015/02/13. 10.1038/nature14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Ferreira T, Morris AP, Medland SE, Madden PA, Heath AC, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nature genetics. 2012;44(4):369–75, s1–3. Epub 2012/03/20. 10.1038/ng.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003;33 Suppl:245–54. Epub 2003/03/01. [DOI] [PubMed] [Google Scholar]
- 21.Ling C, Ronn T. Epigenetic adaptation to regular exercise in humans. Drug discovery today. 2014;19(7):1015–8. Epub 2014/03/19. 10.1016/j.drudis.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 22.Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317–30. Epub 2015/02/20. 10.1038/nature14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell metabolism. 2012;15(3):405–11. Epub 2012/03/13. 10.1016/j.cmet.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 24.Ahmad T, Lee IM, Pare G, Chasman DI, Rose L, Ridker PM, et al. Lifestyle interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes care. 2011;34(3):675–80. Epub 2011/01/27. 10.2337/dc10-0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corella D, Arnett DK, Tucker KL, Kabagambe EK, Tsai M, Parnell LD, et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. The Journal of nutrition. 2011;141(12):2219–25. Epub 2011/11/04. 10.3945/jn.111.143826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfalt E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. The American journal of clinical nutrition. 2009;90(5):1418–25. Epub 2009/09/04. 10.3945/ajcn.2009.27958 [DOI] [PubMed] [Google Scholar]
- 27.Qi Q, Kilpelainen TO, Downer MK, Tanaka T, Smith CE, Sluijs I, et al. FTO genetic variants, dietary intake and body mass index: insights from 177,330 individuals. Human molecular genetics. 2014;23(25):6961–72. Epub 2014/08/12. 10.1093/hmg/ddu411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. The New England journal of medicine. 2015;373(10):895–907. Epub 2015/08/20. 10.1056/NEJMoa1502214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almen MS, Jacobsson JA, Moschonis G, Benedict C, Chrousos GP, Fredriksson R, et al. Genome wide analysis reveals association of a FTO gene variant with epigenetic changes. Genomics. 2012;99(3):132–7. Epub 2012/01/12. 10.1016/j.ygeno.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 30.Bell CG, Finer S, Lindgren CM, Wilson GA, Rakyan VK, Teschendorff AE, et al. Integrated genetic and epigenetic analysis identifies haplotype-specific methylation in the FTO type 2 diabetes and obesity susceptibility locus. PloS one. 2010;5(11):e14040 Epub 2010/12/03. 10.1371/journal.pone.0014040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toperoff G, Aran D, Kark JD, Rosenberg M, Dubnikov T, Nissan B, et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Human molecular genetics. 2012;21(2):371–83. Epub 2011/10/14. 10.1093/hmg/ddr472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmerhorst HJ, Brage S, Warren J, Besson H, Ekelund U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. The international journal of behavioral nutrition and physical activity. 2012;9:103 Epub 2012/09/04. 10.1186/1479-5868-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundberg M, Hallqvist J, Diderichsen F. Exposure-dependent misclassification of exposure in interaction analyses. Epidemiology (Cambridge, Mass). 1999;10(5):545–9. Epub 1999/09/01. [PubMed] [Google Scholar]
- 34.Skender S, Ose J, Chang-Claude J, Paskow M, Bruhmann B, Siegel EM, et al. Accelerometry and physical activity questionnaires—a systematic review. BMC public health. 2016;16:515 Epub 2016/06/17. 10.1186/s12889-016-3172-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ragland DR. Dichotomizing continuous outcome variables: dependence of the magnitude of association and statistical power on the cutpoint. Epidemiology (Cambridge, Mass). 1992;3(5):434–40. Epub 1992/09/01. [DOI] [PubMed] [Google Scholar]
- 36.Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Steinthorsdottir V, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nature genetics. 2010;42(11):949–60. Epub 2010/10/12. 10.1038/ng.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andreasen CH, Stender-Petersen KL, Mogensen MS, Torekov SS, Wegner L, Andersen G, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57(1):95–101. Epub 2007/10/19. 10.2337/db07-0910 [DOI] [PubMed] [Google Scholar]
- 38.Vimaleswaran KS, Li S, Zhao JH, Luan J, Bingham SA, Khaw KT, et al. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. The American journal of clinical nutrition. 2009;90(2):425–8. Epub 2009/06/26. 10.3945/ajcn.2009.27652 [DOI] [PubMed] [Google Scholar]
- 39.Voight BF, Kang HM, Ding J, Palmer CD, Sidore C, Chines PS, et al. The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS genetics. 2012;8(8):e1002793 Epub 2012/08/10. 10.1371/journal.pgen.1002793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler TW, Day FR, Croteau-Chonka DC, Wood AR, Locke AE, Magi R, et al. Quality control and conduct of genome-wide association meta-analyses. Nature protocols. 2014;9(5):1192–212. Epub 2014/04/26. 10.1038/nprot.2014.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55(4):997–1004. Epub 2001/04/21. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genetic epidemiology. 2010;34(8):816–34. Epub 2010/11/09. 10.1002/gepi.20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan Y, Stephens M. Practical issues in imputation-based association mapping. PLoS genetics. 2008;4(12):e1000279 Epub 2008/12/06. 10.1371/journal.pgen.1000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nature genetics. 2007;39(7):906–13. Epub 2007/06/19. 10.1038/ng2088 [DOI] [PubMed] [Google Scholar]
- 45.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics (Oxford, England). 2010;26(17):2190–1. Epub 2010/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkler TW, Kutalik Z, Gorski M, Lottaz C, Kronenberg F, Heid IM. EasyStrata: evaluation and visualization of stratified genome-wide association meta-analysis data. Bioinformatics (Oxford, England). 2015;31(2):259–61. Epub 2014/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic acids research. 2014;42(Database issue):D1001–6. Epub 2013/12/10. 10.1093/nar/gkt1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Gierman HJ, Levy D, Plump A, Dobrin R, Goring HH, et al. Synthesis of 53 tissue and cell line expression QTL datasets reveals master eQTLs. BMC genomics. 2014;15:532 Epub 2014/06/30. 10.1186/1471-2164-15-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joehanes R, Ying S, Huan T, Johnson AD, Raghavachari N, Wang R, et al. Gene expression signatures of coronary heart disease. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(6):1418–26. Epub 2013/03/30. 10.1161/ATVBAHA.112.301169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Behrens G, Winkler TW, Gorski M, Leitzmann MF, Heid IM. To stratify or not to stratify: power considerations for population-based genome-wide association studies of quantitative traits. Genetic epidemiology. 2011;35(8):867–79. Epub 2011/11/30. 10.1002/gepi.20637 [DOI] [PubMed] [Google Scholar]
- 51.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nature genetics. 2010;42(11):937–48. Epub 2010/10/12. 10.1038/ng.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. Journal of comparative and physiological psychology. 1973;84(3):488–95. Epub 1973/09/01. [DOI] [PubMed] [Google Scholar]
- 53.Muurahainen N, Kissileff HR, Derogatis AJ, Pi-Sunyer FX. Effects of cholecystokinin-octapeptide (CCK-8) on food intake and gastric emptying in man. Physiology & behavior. 1988;44(4–5):645–9. Epub 1988/01/01. [DOI] [PubMed] [Google Scholar]
- 54.de Krom M, van der Schouw YT, Hendriks J, Ophoff RA, van Gils CH, Stolk RP, et al. Common genetic variations in CCK, leptin, and leptin receptor genes are associated with specific human eating patterns. Diabetes. 2007;56(1):276–80. Epub 2006/12/29. 10.2337/db06-0473 [DOI] [PubMed] [Google Scholar]
- 55.Okada Y, Kubo M, Ohmiya H, Takahashi A, Kumasaka N, Hosono N, et al. Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nature genetics. 2012;44(3):302–6. Epub 2012/02/22. 10.1038/ng.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao FB, Keene JD. Hel-N1/Hel-N2 proteins are bound to poly(A)+ mRNA in granular RNP structures and are implicated in neuronal differentiation. Journal of cell science. 1996;109 (Pt 3):579–89. Epub 1996/03/01. [DOI] [PubMed] [Google Scholar]
- 57.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nature genetics. 2015;47(10):1121–30. Epub 2015/09/08. 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cha JY, Kim HJ, Yu JH, Xu J, Kim D, Paul BD, et al. Dexras1 mediates glucocorticoid-associated adipogenesis and diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(51):20575–80. Epub 2013/12/04. 10.1073/pnas.1320454110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murgia M, Serrano AL, Calabria E, Pallafacchina G, Lomo T, Schiaffino S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nature cell biology. 2000;2(3):142–7. Epub 2000/03/09. 10.1038/35004013 [DOI] [PubMed] [Google Scholar]
- 60.Zhang W, Thompson BJ, Hietakangas V, Cohen SM. MAPK/ERK signaling regulates insulin sensitivity to control glucose metabolism in Drosophila. PLoS genetics. 2011;7(12):e1002429 Epub 2012/01/14. 10.1371/journal.pgen.1002429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freyer J, Behrensen M, Zouhair A, Schunkert H, Erdmann J. Abstract 15307: Mras-knockout leads to obesity and a lack of B-cell function. Circulation. 2012;126:A15307. [Google Scholar]
- 62.Shi X, Sun X, Liu M, Li D, Aneja R, Zhou J. CEP70 protein interacts with gamma-tubulin to localize at the centrosome and is critical for mitotic spindle assembly. The Journal of biological chemistry. 2011;286(38):33401–8. Epub 2011/07/29. 10.1074/jbc.M111.252262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dupuy D, Duperat VG, Arveiler B. SCAN domain-containing 2 gene (SCAND2) is a novel nuclear protein derived from the zinc finger family by exon shuffling. Gene. 2002;289(1–2):1–6. Epub 2002/05/31. [DOI] [PubMed] [Google Scholar]
- 64.Christians JK, Bath AK, Amiri N. Pappa2 deletion alters IGFBPs but has little effect on glucose disposal or adiposity. Growth hormone & IGF research: official journal of the Growth Hormone Research Society and the International IGF Research Society. 2015;25(5):232–9. Epub 2015/07/15. [DOI] [PubMed] [Google Scholar]
- 65.Christians JK, de Zwaan DR, Fung SH. Pregnancy associated plasma protein A2 (PAPP-A2) affects bone size and shape and contributes to natural variation in postnatal growth in mice. PloS one. 2013;8(2):e56260 Epub 2013/03/05. 10.1371/journal.pone.0056260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conover CA, Boldt HB, Bale LK, Clifton KB, Grell JA, Mader JR, et al. Pregnancy-associated plasma protein-A2 (PAPP-A2): tissue expression and biological consequences of gene knockout in mice. Endocrinology. 2011;152(7):2837–44. Epub 2011/05/19. 10.1210/en.2011-0036 [DOI] [PubMed] [Google Scholar]
- 67.Winkelmann J, Czamara D, Schormair B, Knauf F, Schulte EC, Trenkwalder C, et al. Genome-wide association study identifies novel restless legs syndrome susceptibility loci on 2p14 and 16q12.1. PLoS genetics. 2011;7(7):e1002171 Epub 2011/07/23. 10.1371/journal.pgen.1002171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hargens TA, Kaleth AS, Edwards ES, Butner KL. Association between sleep disorders, obesity, and exercise: a review. Nature and science of sleep. 2013;5:27–35. Epub 2013/04/27. 10.2147/NSS.S34838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Droppelmann CA, Wang J, Campos-Melo D, Keller B, Volkening K, Hegele RA, et al. Detection of a novel frameshift mutation and regions with homozygosis within ARHGEF28 gene in familial amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2013;14(5–6):444–51. Epub 2013/01/05. [DOI] [PubMed] [Google Scholar]
- 70.Keller BA, Volkening K, Droppelmann CA, Ang LC, Rademakers R, Strong MJ. Co-aggregation of RNA binding proteins in ALS spinal motor neurons: evidence of a common pathogenic mechanism. Acta neuropathologica. 2012;124(5):733–47. Epub 2012/09/04. 10.1007/s00401-012-1035-z [DOI] [PubMed] [Google Scholar]
- 71.Zhao L, Gregoire F, Sul HS. Transient induction of ENC-1, a Kelch-related actin-binding protein, is required for adipocyte differentiation. The Journal of biological chemistry. 2000;275(22):16845–50. Epub 2000/05/29. [DOI] [PubMed] [Google Scholar]
- 72.Kristiansson K, Perola M, Tikkanen E, Kettunen J, Surakka I, Havulinna AS, et al. Genome-wide screen for metabolic syndrome susceptibility Loci reveals strong lipid gene contribution but no evidence for common genetic basis for clustering of metabolic syndrome traits. Circulation Cardiovascular genetics. 2012;5(2):242–9. Epub 2012/03/09. 10.1161/CIRCGENETICS.111.961482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kilpelainen TO, Laaksonen DE, Lakka TA, Herder C, Koenig W, Lindstrom J, et al. The rs1800629 polymorphism in the TNF gene interacts with physical activity on the changes in C-reactive protein levels in the Finnish Diabetes Prevention Study. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2010;118(10):757–9. Epub 2010/04/03. [DOI] [PubMed] [Google Scholar]
- 74.Lakka HM, Lakka TA, Rankinen T, Rice T, Rao DC, Leon AS, et al. The TNF-alpha G-308A polymorphism is associated with C-reactive protein levels: the HERITAGE Family Study. Vascular pharmacology. 2006;44(5):377–83. Epub 2006/04/04. 10.1016/j.vph.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 75.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. The Journal of biological chemistry. 2006;281(39):28721–30. Epub 2006/08/04. 10.1074/jbc.M601576200 [DOI] [PubMed] [Google Scholar]
- 76.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell metabolism. 2006;4(2):107–10. Epub 2006/08/08. 10.1016/j.cmet.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 77.Lopez I, Mak EC, Ding J, Hamm HE, Lomasney JW. A novel bifunctional phospholipase c that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. The Journal of biological chemistry. 2001;276(4):2758–65. Epub 2000/10/07. 10.1074/jbc.M008119200 [DOI] [PubMed] [Google Scholar]
- 78.Hinkes B, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, Nurnberg G, et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nature genetics. 2006;38(12):1397–405. Epub 2006/11/07. 10.1038/ng1918 [DOI] [PubMed] [Google Scholar]
- 79.Boardman-Pretty F, Smith AJ, Cooper J, Palmen J, Folkersen L, Hamsten A, et al. Functional Analysis of a Carotid Intima-Media Thickness Locus Implicates BCAR1 and Suggests a Causal Variant. Circulation Cardiovascular genetics. 2015;8(5):696–706. Epub 2015/08/16. 10.1161/CIRCGENETICS.115.001062 [DOI] [PubMed] [Google Scholar]
- 80.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nature reviews Molecular cell biology. 2011;12(11):722–34. Epub 2011/09/29. 10.1038/nrm3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roy SK, Hu J, Meng Q, Xia Y, Shapiro PS, Reddy SP, et al. MEKK1 plays a critical role in activating the transcription factor C/EBP-beta-dependent gene expression in response to IFN-gamma. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(12):7945–50. Epub 2002/06/06. 10.1073/pnas.122075799 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All genome-wide association meta-analysis results files are available at the GIANT Consortium website: www.broadinstitute.org/collaboration/giant.