Abstract
Molecular mechanisms of human ductal plate (DP) development and differentiation (DD) are unclear. The author immunohistochemically investigated expressions of cholangiocellular antigens (CEA, CA19-9, EMA, MUC1, MUC2, MUC5AC, MUC6, mucins, CK7, and CK19), hepatocellular antigens (HepPar1, AFP, CK8, and CK18), hepatic stellate/progenitor cell (HSC) antigens or stem cell (SC) antigens (C-erbB2, CD56, chromogranin, synaptophysin, bcl2, NSE, NCAM, KIT, and PDGFRA), and proliferating antigen (Ki67) in 32 human fetal livers (HFL). The DD of human intrahepatic bile duct (IBD) could be categorized into four stages: DP, remodeling DP, remodeled DP, and immature IBD. All the molecules examined were expressed in the DP and DP derivatives. These results suggest that human DP or DP derivatives have capacities to differentiate into cholangiocellular, hepatocellular, HSC, SC, and neuroendocrine lineages. The data also suggest that NCAM, KIT/SC factor-signaling, NSE, HGF/MET signaling, PDGFa/PDGFRA signaling, chromogranin, synaptophysin, and CD56 play important roles in DD of DP and biliary cells of HFL. DP, DP derivatives, and IBD in HFL have proliferative capacity.
Keywords: Human, embryo, fetus, ductal plate, development, differentiation
Introduction
The author has investigated the fetal development and differentiation (DD) of intrahepatic bile ducts (IBDs) in humans.1–17 Similar studies of fetal DD of IBD in humans have been reported by Desmet’s group18–21 and Gerber’s group.22–24 The author’s studies1–17 and other studies18–24 have revealed that the IBDs in human fetal livers (HFL) are derived from fetal ductal plate (DP) which is a double-layered cylindrical structure located in the interface between hepatoblasts and portal mesenchyme.1–24 The DP undergoes remodeling, and some portion of DP gives rise to future IBDs. The remnants of DP disappear by apoptosis.7 The remodeled DP further gives rise to mature IBD resembling those of adult IBD.1–24 The author demonstrated that intrahepatic peribiliary glands (IPG), which were discovered by the author,25–37 are also derived from DP in HFL.1,5 The author also proved that pancreatic acinar cells clusters may be derived from remodeling DP and remodeled DP in HFL.1,5,6 The author demonstrated that the process of normal DD of human fetal IBD involves many molecular mechanisms including apoptosis, apoptosis-related proteins, DP cell proliferation, pancreatic digestive enzymes, such as α-amylase, trypsinogen, and lipase, some proteinases including matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases, peribiliary vascular plexus, carbohydrate structures of many glycoproteins, mucin core antigen (MUC) apomucin expression, expression of cytokeratin (CK), E-cadherin-catenin systems, double-stranded RNA-activated protein kinase, midkine, truncated midkine, type IV collagen, laminin, tenascin, trypsin, chymotrypsin, transforming growth factor-α and its receptor, and pancreatic amylase mRNA.1–17
The developmental failures of these human fetal IBD or DP give rise to the persistence of biliary structures in postnatal human livers. Such structures are called DP malformations (DPM) or hepatobiliary fibropolycystic disease, which involves congenital hepatic fibrosis, polycystic diseases (adult and infantile) of the liver and kidneys, congenital biliary atresia, von-Meyenburg complex, and Caroli’s disease.18–21,38–43
DP is also seen in ductular reactions44–46 and DPM in some liver hamartoma45 and cholangiocarcinoma.47 The DP in ductular reaction of focal nodular hyperplasia interestingly may express KIT,46 a receptor of stem cell factor (SCF).48
Recent evidence has suggested the presence of hepatic stellate/progenitor cell (HSC) in human as well as in animal livers.49–53 The HSC is present in ductules and Herring ductules adjacent to liver parenchyma in humans.49–53 Recent evidence has also suggested that a significant percentage of liver cancers arises from HSC.49–53 The HSC expresses several specific antigens including KIT (CD117), CD34, NCAM (CD56), OV6, Thy1 (CD90), CK14, CD133, ALDH, and M2PK,48–52 and these antigens are good markers of HSC.49–53
Recently, Carpentier et al.54 have suggested that mouse embryonic DP cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. However, this hypothesis has not been investigated in humans. The author herein examined the possibility that human DP may contain HSC, hepatocellular antigens, cholangiocellular antigens, stem cell (SC) antigens, or neuroendocrine antigens, and the possibility that human DP and its derivatives give rise to HSC, cholangiocytes, hepatocytes, SC, or neuroendocrine cells, by the immunohistochemical study using many commercially available antibodies that are relatively specific for individual cells. Recently, the author has investigated NCAM, KIT, and PDGFRA.55–76 The signaling pathways of KIT/SCF, hepatocyte growth factor (HGF) and its ligand MET (HGF/MET), and platelet-derived growth factor-α (PDGFa) and its ligand platelet-derived growth factor receptor-α (PDGFRA) (PDGFa/PDGFRA) have been thought to play key roles in DD in addition to tumorigenesis and regeneration of certain human tissues. In addition, the endocrine characters and CK expressions remain to be established in HFL. The author immunohistochemically examined herein the expression of these diverse molecules. The aim of the present study is to explore whether human embryonic DP can show DD into hepatoblasts, hepatocytes, cholangiocytes, peribiliary glands, SC, HSC, and neuroendocrine cells. The objective was carried out by expressions of proteins related to these precursor molecules, using immunohistochemistry. The present study is an observation study.
Materials and methods
The author recently collected 32 HFLs at various hospitals. They are abortions (spontaneous and artificial), intrauterine fetal death, and autopsies. The gestational ages (weeks) of the 32 fetal livers were as follows: 7, 8, 9 (n = 2), 10 (n = 3), 11 (n = 2), 12 (n = 3), 13 (n = 2), 14 (n = 2), 15 (n = 2), 16 (n = 2), 17, 18, 19, 21, 23, 24, 26, 29, 30, 36, 38, and 40. The sex was unclear. Informed consent was obtained from each mother. The HFL specimens obtained were immediately fixed in formalin and embedded in paraffin. Many 3-µm thin sections were cut, and they were subjected to hematoxylin and eosin (HE) stain, mucus histochemistry, and immunohistochemistry.
Mucins were investigated by mucin histochemical stains including mucicarmine, diastase-periodic acid-Schiff (d-PAS), PAS, alcian blue (AB) at pH 2.5 and pH 1.0, and combined d-PAS and AB at pH 2.5 and pH1.0.
An immunohistochemical study was performed with the use of Envision method as previously described.55–78 The antibodies used were as follows: anti-NCAM (CD56; clone MOC1, Dako Corp, dilution = 1:150), anti-KIT (CD117; polyclonal, Dako Corp, dilution = 1:100), anti-MET (clone SC10, Santa Cruz Lab, Santa Cruz, CA, USA, dilution = 1:100), anti-PDGFRA (polyclonal, Santa Cruz Lab, dilution = 1:100), anti-CK7 (clone OV-TL, Dako Corp, dilution = 1:200), anti-CK8 (Clone 35bH11, Dako Corp, dilution = 1:150), anti-CK18 (clone DC10, Dako Corp, dilution = 1:200), anti-CK19 (clone BA17, Dako Corp, dilution = 1:100), anti-chromogranin (clone DAK-A3, Dako Corp, dilution = 1:100), anti-synaptophysin (polyclonal, Dako Corp, dilution = 1:150), anti-NSE (polyclonal, Dako Corp, dilution = 1:100), anti-HepPar1 (clone OCH1E5, Dako Corp, dilution = 1:50), anti-AFP (rabbit polyclonal, Dako Corp), anti-CEA (clone II-7, Dako Corp, dilution =1:150), anti-CA19-9 (polyclonal, TFB Laboratory, Tokyo, Japan, dilution = 1:200), anti-EMA monoclonal antibody (clone E29, Dako Corp, dilution = 1:100), anti-bcl2 monoclonal antibody (clone 3.1, Novocastra Laboratories, New Castle Upon Tyne, UK, dilution = 1:40), anti-ErbB2 rabbit polyclonal antibody (Dako Corp, dilution = 1:100), anti-MUC1 (clone Ma695, Novocastra Laboratories, New Castle Upon Tyne, UK, dilution = 1:100), anti-MUC2 (clone Ccp58, Novocastra, dilution = 1:100), anti-MUC5AC (clone CLH2, Novocastra, dilution = 1:200), and anti-MUC6 (clone CLH5, Novocastra, dilution = 1:200). Microwave pretreatment was performed by each immunohistochemical run. Positive and negative appropriate control tissues were stained in each run.
The following are well-known corresponding antigens of each lineage:52–68
Cholangiocellular lineage antigens: sulfated mucins (detected by AB pH 1.0) carboxylated mucins (detected by AB pH2.5), neutral mucins (detected by d-PAS), MUC1, MUC2, MUC5AC, MUC6, CEA, CA19-9, EMA, CK7, CK8, CK18, CK19.
Hepatocellular lineage antigens: HepPar1, AFP, CK8, and CK18.
HSC and SC lineage antigens: KIT, PDGFRA, MET, CerbB2, bcl2, NCAM, NSE, chromogranin, and synaptophysin.
Proliferation antigen: Ki67 (clone MIB-1) (cells counted were 1000 and labeling index (LI) was calculated).
Neuroendocrine antigens: chromogranin, synaptophysin, NCAM, and NSE.
Signal transduction: HGF/MET, PDGFa/PDGFRA, and SCF/KIT.
Results
Histology and histochemistry
Histologically, the processes of human IBD development can be categorized into the following four stages: DP, remodeling DP, remodeled DP, and mature IBDs. DP was composed of single- or double-layered cylindrical structures located in the interface between hepatoblasts and mesenchyme of portal tracts (Figure 1(a) and (b)). Remodeling DP showed disappearance of some cells of DP and development of tubular structures of future IBD, which was moving into portal mesenchyme (Figure 1(b)). Remodeled DP was characterized by almost disappearance of DP and by moving tubular structures into portal mesenchyme. Mature IBDs were mature bile ducts similar to adult IBD. These processes progressed from hilar IBDs to peripheral IBDs. The periods of these processes in portal tracts near the hepatic hilus were as follows: DP stages, 7–10 gestational weeks (GW); remodeling DP stage, 11–15 GW; remodeled DP stage, 16–24 GW; and mature IBD stage, 26–40 GW. However, these stage periods differed from case to case as well as from portal tract to another. Interestingly, differentiation of IPG and pancreatic acinar cells from remodeling and remodeled DP was seen frequently and occasionally in the hilar areas, respectively. With regard to mucin histochemistry, cells of DP, remodeling DP, and remodeled DP were not stained by mucicarmine, PAS, AB at pH2.5 and pH1.0. However, DP showed magenta products in d-PAS and combined d-PAS/AB at pH2.5 (Figure 1(d)). In contrast, mature bile ducts were positive with all these techniques, indicating the presence of neutral, carboxylated, and sulfated mucins. Of interest, cells of DP occasionally positive for acidic mucins as revealed by AB at pH2.5 and pH1.0 and combined d-PAS/AB at pH2.5. These results showed that DP is positive for sulfated, carboxylated, and neutral mucins. The staining pattern was mostly cytoplasmic in positive cases.
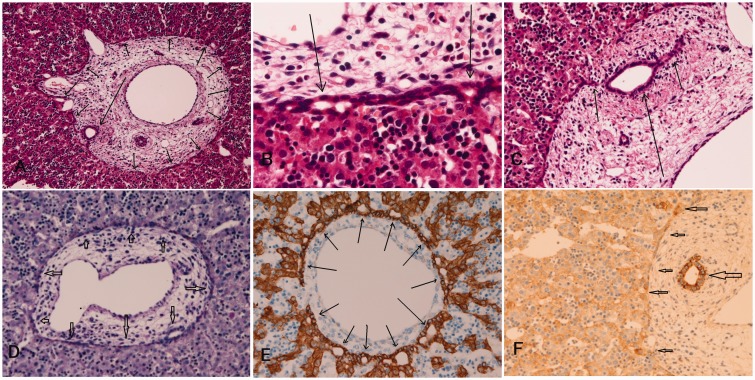
Figure 1.
The HE histologies of ductal plate and intrahepatic biliary cells in human fetal livers. (a) The ductal plate is composed of single or double-layered cylindrical structures located in the interface between hepatoblasts and mesenchyme of the portal tract (arrows: 8 gestational weeks (GW)). (b) The ductal plate is composed of single or double-layered cuboidal cells (arrows, 9 GW). (c) The remodeling ductal plate shows disappearance of some cells of the ductal plate and development of tubular structures of future bile ducts, which is moving into the portal mesenchyme. (c, arrows, 14 GW). (d) PAS-positive products are seen in the ductal plate (arrows, 9 GW). (e) Cytokeratin 18 is positive in hepatoblasts and ductal plate (arrows, 10 GW). (f) Cytokeratin 19 is seen mainly in the ducts but is also present faintly in periportal hepatocytes and ductal plate (arrows) HE stain. (a) HE, × 40. (b) HE, × 350. (c) HE, × 150. (d) PAS, × 200. (e) CK18, × 200. (f) CK7, × 200. (A color version of this figure is available in the online journal.)
Immunohistochemistry
Cytokeratins
Cells of DP, remodeling DP, remodeled DP, and mature IBD were strongly positive for CK8 and CK18 (Figure 1(e)), while they were weakly positive for CK7 and CK19. Of interest, CK7 and CK19, both of which are cholangiocytic lineage antigens, were faintly expressed in periportal primitive hepatocytes near DP (Figure 1(f)). The staining pattern was cytoplasmic with membranous accentuation.
NCAM, KIT, MET, and PDGFRA
Characteristically, NCAM was always expressed in some cells of DP (Figure 2(a)) and remodeling DP but not in remodeled DP and mature IBD. KIT was infrequently (12/32 cases) expressed weakly in some cells of DP and remodeling DP but not in remodeled DP and mature IBD. NCAM expression was also seen in some hepatoblasts and hematopoietic progenitor cells of the parenchyma, and neurons in portal mesenchyme. KIT was expressed also in some hepatoblasts and hematopoietic cells of the parenchyma (Figure 2(b)) and mast cells in portal mesenchyme. MET was strongly expressed in DP (Figure 3(c)), remodeling DP, remodeled DP, and mature IBD. MET was also strongly expressed in primitive hepatocytes and primitive hematopoietic cells (Figure 2(c)). MET was not expressed or very weakly expressed in portal mesenchyme, portal veins, sinusoids, and hepatic veins. The expression of PDGFRA was the same as that of MET. PDGFRA was strongly expressed in DP (Figure 2(d)), remodeling DP, remodeled DP, and mature IBD. PDGFRA was also strongly expressed in primitive hepatocytes and primitive hematopoietic cells. PDGFRA was not expressed or very weakly expressed in portal mesenchyme, portal veins, sinusoids, and hepatic veins (Figure 2(d)). The expression pattern of all these molecules was membranous, but it was cytoplasmic with membranous accentuation in some cases.
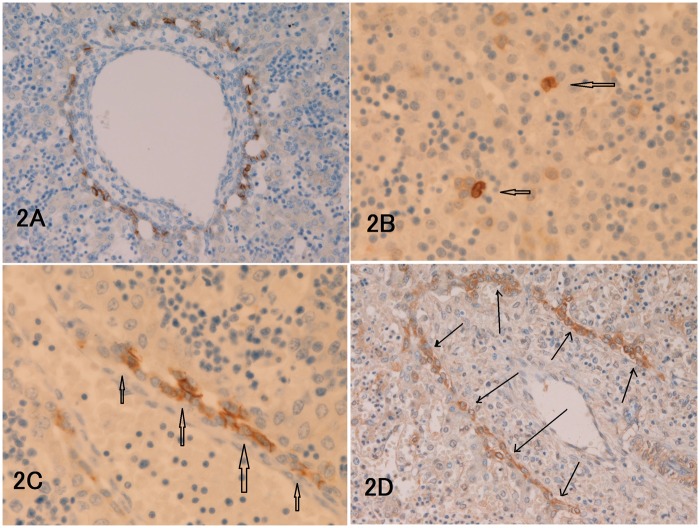
Figure 2.
(a) NCAM is always expressed in some cells of ductal plate (9 gestational weeks (GW)). (b) KIT expression is seen in some hepatoblasts and hematopoietic progenitor cells of the parenchyma (arrows, 12 GW). (c) The cells of ductal plate (arrows) are strongly positive for MET (9 GW). (d): The cells of remodeling ductal plate (arrows) indicated by arrows are strongly positive for PDGFRA (13 GW). The staining pattern is mostly membranous. (a, d) × 200. (b, c) × 400. (A color version of this figure is available in the online journal.)
Figure 3.
MUC1 (a, 13 gestational weeks (GW)), CEA (b, 11 GW), CA19-9 (c, 12 GW), EMA (d, 10 GW), cytokeratin 7(e, 11 GW), and HepPar1 (f, 8 GW) are expressed in ductal plate. (a–f) × 200. HepPar1 is expressed in hepatoblasts ductal plate; however, HepPar1 is expressed weakly in cells of portal mesenchyme. The staining pattern is mostly cytoplasmic. (A color version of this figure is available in the online journal.)
Cholangiocellular antigens: MUC1, MUC2, MUC5AC, MUC6, CEA, CA19-9, EMA, CK7, CK8, CK18, and CK19
MUC1 (polymorphic epithelial mucin) was present in DP (Figure 3(a)), remodeling DP, remodeled DP, and immature IBD. MUC5AC (gastric foveolar mucin) and MUC6 (pyloric gland-type mucin) were present only in DP. MUC5AC and MUC6 were absent in remodeling DP, remodeled DP, and immature IBD. No expression of MUC2 (goblet cell mucin) was seen throughout fetal IBD development. CEA (Figure 3(b)) and CA19-9 (Figure 3(c)) were expressed in DP, remodeling DP, remodeled DP, and immature IBD. The cytoplasmic expressions of CEA and CA19-9 were recognized in DP, while their expression patterns were luminal surface and cytoplasmic in remodeling DP, remodeled DP, and immature IBD. EMA (terminally differentiated biliary cell marker) was seen in DP (Figure 3(d)), remodeling DP, and remodeled DP but not seen in immature IBDs. The intensity and extent of EMA reactivity were relatively strong and broad in DP, while those were weak and focal in remodeling DP and remodeled DP. It is well known that EMA is strongly positive in adult bile ducts but negative in adult hepatocytes. Thus, EMA expression is relatively strong in precursor biliary elements, but its expression decreases as IBD development progresses. CK7 (Figure 3(e)), CK8, CK18 (Figure 1(f)), and CK19 were strongly expressed in any stages of DP development: DP, remodeling DP, remodeled DP, and immature IBD. The staining pattern of these antigens was mostly cytoplasmic.
Hepatocellular antigen expression (HepPar1, AFP, CK8, and CK19)
HepPar1 was expressed in DP (Figure 3(f)) but not in remodeling DP, remodeled DP, and immature IBD. However, portal mesenchyme was also weakly positive for HepPar1. AFP was expressed in primitive fetal cholangiocytes including cells of DP, remodeling DP, remodeled DP, and immature IBDs. CK8 and CK18 (Figure 1(f)) were clearly present in DP, remodeling DP, remodeled DP, and immature IBD.
HSC or SC lineage antigens (KIT, PDGFRA, MET, CerbB2, bcl2, NCAM, NSE, chromogranin, and synaptophysin)
KIT was expressed weakly in some cells of DP and remodeling DP but not in remodeled DP and immature IBD. PDGFRA was strongly expressed in DP, remodeling DP, remodeled DP, and immature IBD. ErbB2 was always expressed in DP (Figure 4(a)) but was not seen in remodeling DP, remodeled DP, and immature IBD. The expression was cytoplasmic. Bcl2 was always and apparently expressed in DP (Figure 4(b)) but was absent in remodeling DP, remodeled DP, and immature IBD. MET was expressed in DP, remodeling DP, remodeled DP, and immature IBD. The cells of DP showed obvious immunoreactive chromogranin, synaptophysin, NSE (Figure 4(c)), and CD56 (Figure 4(d)). These four neuroendocrine antigens were also found in cells of remodeling DP, remodeled DP, and immature IBD. The expression pattern was cytoplasmic with focal membranous accentuation.
Figure 4.
Positive expression of ErbB-1 (a, 7 GW), bcl2 (b, 14 GW), NSE (c, 16 GW), CD56 (d, 11 GW), chromogranin A (e, 13 GW), and Ki67 (9 GW) in human ductal plate. a, b, d: × 200. c,d, f: × 350. The expression pattern was cytoplasmic with membranous accentuation. (A color version of this figure is available in the online journal.)
Neuroendocrine antigens (chromogranin, synaptophysin, CD56, and NSE)
Cells of human DP showed obvious immunoreactive chromogranin (Figure 4(e)), synaptophysin, NSE, and CD56. Expressions of NSE and CD56 in remodeling DP were seen, although they were rare phenomena. In general, no expressions of these four neuroendocrine antigens were found in remodeling DP, remodeled DP, and immature IBD. Interestingly, the expressions of chromogranin, synaptophysin, NSE, and CD56 were occasionally found in the primitive hepatocytes (hepatoblasts). The hepatopoietic cells were always negative for these four neuroendocrine antigens. Also, interestingly, expressions of these four neuroendocrine molecules were focally seen in some unknown mesenchymal cells of portal mesenchyme in remodeling DP, remodeled DP, and immature IBD. These unknown cells expressing neuroendocrine antigens in portal mesenchyme were not seen in DP stage. Nerve fibers were consistently positive for chromogranin, synaptophysin, NSE, and CD56 in portal mesenchyme in the stages of remodeling DP, remodeled DP, and immature IBD. However, in the portal tracts of DP stage, all the immunostainings of chromogranin, synaptophysin, NSE, and CD56 tended not to identify the nerve fibers. Nerve fibers were almost always negative in parenchyma. The expression was cytoplasmic and membranous.
Proliferation antigen Ki67
The mean ± SD of the MIB-1 LI was as follows: 3.3% ± 1.8% in DP (Figure 4(f)), 4.2% ± 2.8% in remodeling DP, 3.6% ±2.1% in remodeled DP, and 5.3% ± 3.1% in cells of immature IBD. The hepatoblasts and hemopoietic cells showed circa 60% for hepatoblasts and circa 20% for hemopoietic cells. The expression pattern was nucleus.
Signal transduction antigens
The receptors (MET, KIT, PDGFRA, and ErbB-2) of signal transduction molecules were present in DP and DP derivatives.
Discussion
Only new findings are written in this section.
Limitations of the present study
Because no interventions were allowed in humans, the studies in humans are always descriptive. This is the limitation of the present study.
Histology
The histological investigations of present study are almost the same of the previous studies.1–24 Although Carpentier et al.54 stressed that they never identified apoptosis by TUNEL method in mouse DP, the presence of apoptosis has been observed in HFLs and fetal liver of various animals.7,79–81 The author observed that human IBD development started from the hilar portal areas to the peripheral portal tracts: the stages are early in IBD development in hilar portal tracts and late in IBD development in peripheral portal tracts. The author also demonstrated the gestational periods of the each process of IBD development in human. The author observed that IPG were developed from the DP and remodeling DP. The author observed that this phenomenon frequently occurs in hilar portal tracts,1 but this phenomenon has not been described in other researchers. This is the third report of such a phenomenon.1,5 Also, the author identified that pancreatic acinar cells developed from the remodeling DP and remodeled DP, although its occurrence was relatively infrequent. Two reports by the author demonstrated that human adult livers occasionally contain areas of clusters of pancreatic acinar cells.31 The present findings highly suggest that pancreatic acinar cells develop from DP and matured in adult livers.
Mucin histochemistry and immunohistochemistry of CKs
Mucin studies have not been reported in DP. The present histochemical study demonstrated that the DP, remodeling DP, and remodeled DP have glycogens but not mucins, while immature IBD expressed both glycogens and mucins that contained neutral, carboxylated, and sulfated mucins, suggesting that glycogens are used as energy in human fetal cholangiocytes and that there are no mucins in DP, remodeling DP, and remodeled DP. The data also suggest that the immature IBD in human fetus have neutral, carboxylated, and sulfated mucins. Mucins function as local innate immunity and lubrication. The data suggest that these functions are required in immature IBD but not in DP, remodeling DP, and remodeled DP. The present study showed the expression of CK7 and CK19 in the periportal hepatoblasts. The observation of the expression of biliary-type CK (CK7 and CK19) in periportal hepatoblasts suggests that the periportal hepatoblasts in HFL have cholangiocytic lineage, and that human DP can give rise to periportal hepatocytes.
Immunohistochemistry of NCAM, KIT, MET, and PDGFRA
HSC express HSC-specific several antigens including NCAM, KIT (CD117), CD34, OV6, Thy1 (CD90), CK14, CD133, ALDH, and M2PK.48–53 Among these antigens, NCAM, KIT, CD34, and CK14 are available commercially. In the present study, the author used NCAM and KIT as markers for HSC in humans. The present study showed positive expression of NCAM and KIT in DP and remodeling DP but not in remodeled DP and mature IBD in the HFLs. The data suggest that DP and remodeling DP have lineage to HSC. It is also suggested that KIT/SCF signaling plays an important part in the DD of human IBD.
Recently, Carpentier et al.54 demonstrated that mouse DP cells are among embryonic HSC cells in mouse. They demonstrated that mouse DP cells expressed HSC/biliary cell markers of CK19, SOX9, OPN,23 TROP2,24 and YEP in mouse livers. HSC antigens are much easily analyzed in mouse fetal liver than in HFL. However, this hypothesis has not been verified in HFLs. The findings of the present study strongly suggest that some cells of DP and remodeling DP of humans are already HSC or may give rise to HSC in the human fetal IBD development. Of particular interest was that some hepatoblasts and precursor hematopoietic cells showed immunoreactive NCAM and KIT. These findings suggest that some hepatoblasts of HFLs are HSC or give rise to HSC in the HFLs. These findings also strongly suggest that hematopoietic cells in the human liver contain hematopoietic SCs in human fetal life. Nerve fibers were labeled by NCAM and mast cells labeled by KIT were present in portal mesenchyme of HFL. Nerve fibers and mast cells have not been investigated in HFL. The findings showed that nerve fibers and mast cells are already present in portal mesenchyme of HFL.
HGF/MET signaling and PDGFa/PDGFRA signaling play important roles in fetal organogenesis.48–78 The signaling may cause cellular events including cell proliferation, scatter, cell migration, and cell survival, thus giving contributions to normal organogenesis. HGF/MET and PDGFa/PDGFRA signaling are also associated with carcinogenesis, cancer cell proliferation, invasion, and metastasis.48–78 The present study demonstrated proteins of MET and PDGFRA in HFL. There have been no studies of HGF/MET and PDGFa/PDGFRA signaling in HFLs. There has been only one such a study of mouse embryo, reported by Ishikawa et al.82 They stated that HGF was expressed in hepatocytes and nonparenchymal cells including biliary epithelial cells, periportal connective tissue cells, megakaryocytes, endothelial cells, and sinusoidal cells, throughout mouse fetal liver development.82 They also stated that MET was expressed in hepatocytes throughout the mouse fetal liver development. They did not find MET expression in cholangiocytes and hematopoietic cells.
In the present study, MET was strongly expressed in primitive cholangiocytes including DP, remodeling DP, remodeled DP, and immature IBD in HFLs. These findings strongly suggest that HGF/MET signaling plays an important role in the normal DD of IBD in HFL. The present study did not investigate the expression of HGF because anti-HGF antibody was not available. This is a weak point of the present study. However, the author thinks that receptor expression is more important than growth factor expression because receptor expression may determine the sites of the growth factor/receptor signaling. HGF is known to be produced by mesenchymal cells.46–55 In the liver, portal mesenchyme may be the sites of HGF production. The capillary and sinusoidal endothelial cells are also candidates. The author proved that mesenchymal-DP or mesenchymal-IBD interactions are important to the normal development of DP and IBDs.3,8,12,16 The author demonstrated that mesenchymal elements such as type IV collagen, laminin, tenascin, matrix proteinases, E-cadherin, catenins, and apoptosis-related proteins were present in portal mesenchyme of HFL and that these molecules may induce transformation of neighboring hepatoblasts into the DP in HFL.3,4,8,12,16 The strong expression of MET in the DP and IBDs just adjacent to the portal mesemchyme in HFL also suggest that HGF may be produced by the portal mesenchyma and directly act the neighboring DP and IBDs. The present study demonstrated that primitive hepatocytes and hematopoietic cells also moderately and strongly expressed MET. These findings strongly suggest that the normal DD of hepatocytes and disappearance of hematopoietic cells in the postnatal periods in the human livers may be achieved by HGF/MET signaling.
The author has already proved that transforming growth factor-α and its receptor (TGFα/TGFR signaling) may play an important role during human liver development and maturation,16 prior to the present work. This work of TGFα/TGFR signaling16 was the first report of growth factor/receptor signaling in human liver development. In the present study, MET and PDGFRA were investigated in HFLs. The author recently has studied PDGFRA, their biological behaviors, their mutations. The findings in the present study are that the PDGFRA was strongly expressed in the DP, remodeling DP, remodeled DP, and mature bile ducts. These findings, like HGF/MET signaling, strongly suggest that PDGFa/PDGFRA signaling plays an important role in the normal development of DP and IBDs in HFLs. In this case also, the expression of PDGFa could not be studied because the antibody against PDGFa was not available commercially. Thus, like HGF, this is a weak point of the present study. However, as stressed earlier, the author believes that receptor expression is more important than growth factor expression. PDGFRA is known to be produced by platelets and mesenchymal cells.48–78 As is well recognized, the hematopoietic organ in human fetuses is the liver. In the present study, hepatopoietic cells including megakaryocytes and platelets were present within the liver parenchyma. The author speculates that PDGFa released from platelets and megakaryocytes in HFL binds the PDGFRA present in fetal cholangiocytes. Thus, the PDGFa/PDGFRA signaling plays an important role in DP of human fetal cholangiocytes. The portal mesenchyme is a strong candidate for the cells producing PDGFa.56–59 In the present study, PDGFRA was also strongly expressed in the primitive hepatocytes and primitive hematopoietic cells in HFL. These findings also, like HGF/MET signaling, that the normal development of hepatocytes and disappearance of hemopoietic cells in the postnatal periods in the human livers may be achieved by PDGFa/PDGFRA signaling in addition to the HGF/MET signaling.
MET and PDGFRA were not expressed or very weakly expressed in portal mesenchyme, portal veins, sinusoids, and hepatic veins. These findings suggest that HGF/MET signaling and PDGFa/PDGFRA signaling play no or few roles in the development of the portal mesenchyme, portal veins, sinusoids, and hepatic veins.
Cholangiocellular antigens
The cholangiocellular antigens (mucins, MUC1, MUC2, MUC5AC, MUC6, CEA, CA19-9, EMA, CK7, CK8, CK18, and CK19) examined are not expressed in hepatocytes but expressed in cholangiocytes in HFL. Mucins and MUC are expressed in cholangiocytes but never in hepatocytes. The MUC profile of DP of HFL is of hepatobiliary type.69 It is well known that CEA (carcinoembryonic antigen) and CA19-9 (carbohydrate antigen 19-9) are expressed in cholangiocytes but never in hepatocytes. It is well known that hepatocytes express CK8 and CK18 while cholangiocytes CK7 and CK19, in addition to CK8 and CK18.1,17,21 Thus, the expression of CK7 and CK19 indicates cholangiocellular lineage. The CK profile in HFL is almost similar to previous human studies. Carpentier et al.51 suggested that mouse embryonic DP cells give rise to cholangiocytes, by demonstrating oval cell antigens. Although human DP seems quite different from mouse DP, the findings of the present study is compatible.
Hepatocellular antigens
The hepatocellular antigens examined are HepPar1 (hepatocyte-paraffin 1, a mitochondria-related antigen relatively specific to hepatocytes), AFP, CK8 and CK18. HepPar1 is relatively and AFP is sensitive and specific for hepatocytes.52–54 They are not expressed in cholangiocytes. The expression of CK8 and CK18 are not specific to hepatocytes because it was seen in cholangiocytes. Haruna et al.22 demonstrated that HepPar1 was present in the DP of human fetus. They speculated that these HepPar1-positive and CK19-positive cells of the human DP were bipotential progenitor cells in human liver development. This study is the only one human study examining hepatocellular lineage of the human fetal DP and IBD. Carpentier et al.51 suggested that mouse embryonic DP cells give rise to periportal hepatocytes by demonstrating mouse hepatocyte-specific antigens in the mouse DP. Although human DP seems different from mouse DP, the findings of the present study is compatible. The mild expression of HepPar1 in portal mesenchyme may show that the mesenchyme of HFL has mitochondria-associated antigen such as HepPar1.
HSC and SC antigens
These present study showed that human DP and derivatives are positive for KIT, PDGFRA, ErbB2, bcl2, and MET. They are also positive for chromogranin, synaptophysin, NSE, and CD56. These findings suggest that the DP and derivatives are HSC or SC, or give rise to HSC or SC in HFL.
Neuroendocrine antigens
There have been only two studies of neuroendocrine cells in the liver.83,84 One was performed by Kurumaya et al. in 1989,83 and other one was done by the author84 Neuroendocrine cells positive for endocrine hormones are known to be present in the IBDs and IPG. Kurumaya et al.,83 in 1989, found that argyrophil cells and endocrine cells containing some endocrine hormones such as serotonin, somatostatin, motilin, gastrin, and bombesin were present in the normal adult IBD and IPG. They found many endocrine cells were present in the intramural and extramural glands of the IPG. They also found such endocrine cells in proliferated IBD and IPG in hepatolithiatic livers. They demonstrated that the endocrine cells in hepatolithiatic livers are hyperplastic and contain endocrine cells with many hormones such as somatostatin, motilin, gastrin, and bombesin.83 Interestingly, they also found that neuroendocrine cells were present in the IBD and intrehapatic peribiliary glands in HFL at later gestational weeks.83 They did not use pan-neuroendocrine antigens because such antibodies were not commercially available.
The author demonstrated that chromogranin-positive endocrine cells are present in IBD epithelial cells and epithelial cells of IPG in normal adult livers.84 The author also demonstrated chromogranin-positive endocrine cells in proliferated IBD and IPG in hepatolithiatic livers. The author also showed the presence of chromogranin-positive neuroendocrine cells in hepatobiliary cystadenomas and hepatobiliary cystadenocarcinoma.84 The endocrine cells were numerous in proliferated bile duct elements of hepatolithiatic livers, and it was thought the endocrine cells in hepatolithiasis originated from normally existing endocrine cells in the IBD and peribiliary glands.84 The author also found the association of these neuroendocrine cells and hepatobiliary cystadenoma and cystadenocarcinoma and suggested that hepatobiliary cystadenoma and cystadenocarcinoma may arise from IPG.84
However, there have been no comprehensive studies of neuroendocrine cells in HFL. The present study apparently demonstrated that the cells of DP definitely expressed neuroendocrine features. These findings are new and were confirmed by most reliable neuroendocrine antigens at the present time, i.e. chromogranin, synaptophysin, NSE, and CD56. In particular, as stated early in this report, the positive reactions of chromogranin and synaptophysin always indicate that cells positive for these two antigens are actual neuroendocrine cells.85–87 In the present study, cells of DP were definitely positive for chromogranin, synaptophysin, NSE, and CD56. Therefore, it can be concluded that the cells of human DP have neuroendocrine features. These findings strongly suggest that the cells of human DP are neuroendocrine cells or may give rise to neuroendocrine cells in the fetal and adult IBD epithelial cells including IPG. These findings are the main and most important in the present study.
Recently, it has been suggested in mouse that cells of mouse DP are HSC, and the mouse DP can give rise to hepatocytes, HSC, and cholangiocytes.54 In humans, the author demonstrated that human DP cells have features of HSC, MUC apomucins-positive mucin producing epithelial cells, glandular epithelial cells, periportal hepatocytes, and growth factor/receptor signaling. These studies by demonstrated positive reactions for KIT, NCAM, hepatocyte paraffin 1, AFP, MUC apomucins, mucins histochemistry, CEA, CA19-9, MET, PDGFRA, TGF-α,16 and MIB-1 in human IBD development including the DP. Thus, it is suggested that cells of human DP are HSC and have these phenotypes, and the DP may give rise to hepatocytes, mucin-producing epithelial cells, and glandular epithelial cells in addition to cholangiocytes. In addition, it was suggested that the IPG and pancreatic acinar cells do develop from DP, remodeling DP, remodeled DP, and mature IBD in humans.1,3,5,6 It was demonstrated that mesenchymal–stroma interactions, tenascin, typeIV collagen, laminin, E-cadherin, catenins, and several growth factors/receptors signalings are involved in the normal development of IBD in humans.1–17
In the present study, neuroendocrine cells was not identified in cells of remodeling DP, remodeled DP, and mature IBD in the HFLs. These findings may suggest that the neuroendocrine features of human DP may disappear during the progress of the IBD development in humans. However, Kurumaya et al.83 demonstrated that endocrine cells were present in a few HFLs of late gestational stage. This discrepancy is unclear but may be explained by the speculation that the endocrine features of human DP cells may be superficially undetected by the immunohistochemistry of chromogranin, synaprophysin, NSE, and CD56 but may be persisted in the human fetal life. These undetected neuroendocrine cells in human fetal life exhibit again these four antigens in the postnatal periods when chromogranin-positive and hormone-positive endocrine cells have been detected in normal adult livers, hepatolithiatic livers, and hepatic tumors including cystadenomas.83,84 In the two studies of endocrine cells of the human livers, Kurumaya et al.83 used endocrine hormones, while the author used a pan-neuroendocrine marker chromogranin A.83 The discrepancy may be due to the antigens used for demonstration of endocrine cells. In any way, further meticulous studies on endocrine cells in the stages of the remodeling DP, remodeled DP, and matures IBD are mandatory.
Interestingly, in the current study, the primitive hepatocytes (hepatoblasts) occasionally showed strong expression of chromogranin, synaptophysin, NSE, and CD56. This phenomenon is a new finding. This phenomenon can be explained by the hypothesis that primitive hepatocytes (hepatoblasts) of HFLs, like human DP, have neuroendocrine features and may give rise to endocrine cells in future hepatocytes. The author noticed similar findings with regard to aberrant expressions of CK17, CK19, MUC apomucins, CEA, CA19-9, MET, PDGFRA, KIT, and NCAM (submitted). Taken together, these findings strongly suggest a hypothesis that certain human fetal hepatoblasts can differentiated into cholangiocytes, HSC, mucins-producing epithelial cells, and glandular epithelial cells. More meticulous studies of fetal hepatoblasts remain to be performed.
In the present study, the nerve fibers were positively labeled by chromogranin, synaptoiphysin, NSE, and CD56. These findings imply that these four pan-neuroendocrine markers are very useful for identifying nerve fibers in HFL. Innervation of the human has been studies in adult livers and liver diseases and postnatal animal livers but has not examined the development of nerve fibers in HFLs. This kind of study has never been performed in HFLs. The present study demonstrated that nerve fibers were not present in the portal tracts in the stage of DP. The nerve fibers emerge firstly in the portal tracts of the stages of remodeling DP. They increased in the portal tracts at the stage of remodeling DP and mature IBD in human livers. The author did not find nerve fibers in the HFL parenchyma, indicating that parenchymal innervation is not yet present in the HFLs. These findings indicate the normal development of nerve fibers in HFLs. More meticulous investigations of the development of nerves or innervation during human liver organogenesis are mandatory.
Of interest is that some mesenchymal cells in the portal mesenchyme were positive for choromogranin, synaptophysin, NSE, and CD56 in the HFLs. These mesenchymal cells appear not to be nerve fibers in their morphology. Such cells in the fetal portal mesenchyme have not been discovered. The author speculate that the portal mesenchyme of HFL have such “neoroendocrine” cells of unknown origin and such cells may give rise to neuroendocrine cells present I the fetal and adult livers.84,85
Recently, it is well recognized that many kinds of SCs have been discovered in human bone marrow hepatopoietic cells in adults. The parenchymal hematopoietic cells of the HFLs appeared negative for chromogranin, synaptophysin, NSE, and CD56, suggesting that the hepatopoietic cells in the human fatal liver do not show neuroendocrine cells and do not differentiate into neuroendocrine cells. It was found that no expression of some SCs in the hepatic hematopoietic cells of human fetuses. These findings suggest that the hematopoietic cells of the HFLs do not contain SCs. However, many more meticulous studies are needed to ascertain this hypothesis.
Proliferation antigen Ki67
The present study demonstrated relatively high cell proliferative capacity of human DP. The author previously demonstrated apoptosis and cell proliferation determined by proliferating cell nuclear antigen (PCNA) and argyrophilic nuclear organizer region (AgNOR).7 Thus, it seems that the human DP is relatively actively dividing and apoptosis-prone cells, which may give rise to other cell types in addition to DP cells in humans. Carpentier et al.51 suggested in mouse fetal livers that they never identified apoptosis by TUNEL method in mouse DP, and cell proliferative activity is limited. The findings of mouse are different from the present study of humans. Other studies showed many apoptosis of IBD in humans and animals.70–73 The DP of humans appears different from mouse DP.
Signal transduction
The present study revealed positive expression of KIT, MET, PDGFRA, and ErbB2 suggesting that SCF/KIT, HGF/MET, PDGFa/PDGFRA, and EGF/ErbB2 pathways play an role in the DD of DP and its derivatives.
Summary
It was suggested that human fetal DP can differentiate into periportal hepatocytes, HSC, SC, peribiliary glands, pancreatic acinar cells, neuroendocrine cells, in addition to biliary ductal cells. It was also suggested that CK, NCAM, KIT/SCF signaling, HGF/MET signaling, PDGFa/PDGFRA signaling, NSE, synaptophysin, chromogranin, and CD56 may play roles in the DD in HFL.
Human DP showed Ki67 labeling, suggesting that human fetal DP is not an inert tissue but active tissue.
Acknowledgements
The author has no funds or sponsors. This work was performed only by the author. The informed consent was obtained from each mother or relative. The publication was permitted by the Ethical Committee of the Hospital.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Terada T, Nakanuma Y. Development of human intrahepatic peribiliary glands: histological, keratin immunohistochemical and mucus histochemical analyses. Lab Invest 1993; 68: 261–9. [PubMed] [Google Scholar]
- 2.Terada T, Nakanuma Y. Profiles of expression of carbohydrate chain structures during human intrahepatic bile duct development and maturation: a lectin-histochemical and immunohistochemical study. Hepatology 1994; 20: 388–97. [PubMed] [Google Scholar]
- 3.Terada T, Kitamura Y, Nakanuma Y. Normal and abnormal development of the intrahepatic biliary system: a review. Tohoku J Exp Med 1997; 181: 19–32. [DOI] [PubMed] [Google Scholar]
- 4.Terada T, Ashida K, Kitamura Y, Matsunaga Y, Takashima K, Kato M, Ohta T. Expression of E-cadherin, alpha-catenin and beta-catenin during human intrahepatic bile duct development. J Hepatol 1998; 28: 263–9. [DOI] [PubMed] [Google Scholar]
- 5.Terada T. Differentiation of intrahepatic peribiliary glands and pancreatic acinar cells from the remodeling ductal plate in human fetuses. Hepatology 2012; 56: 2004–5. [DOI] [PubMed] [Google Scholar]
- 6.Terada T, Nakanuma Y. Expression of pancreatic enzymes (α-amylase, trypsinogen and lipase) during human liver development and maturation. Gastroenterology 1995; 108: 1236–45. [DOI] [PubMed] [Google Scholar]
- 7.Terada T, Nakanuma Y. Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am J Pathol 1995; 146: 67–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Terada T, Okada Y, Nakanuma Y. Expression of matrix proteinases during human intrahepatic bile duct development: a possible role in biliary cell migration. Am J Pathol 1995; 147: 1207–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Kato M, Shinozawa T, Kato S, Terada T. Divergent expression of midkine in the human fetal liver and kidney: an immunohistochemical analysis of developmental changes in hilar primitive bile ducts and hepatocytes. Liver 2000; 20: 475–81. [DOI] [PubMed] [Google Scholar]
- 10.Kato M, Shinozawa T, Kato S, Terada T. Immunohistochemical localization of truncated midkine in developing human bile ducts. Histol Histopathol 2003; 18: 129–34. [DOI] [PubMed] [Google Scholar]
- 11.Terada T, Nakanuma Y. Development of human peribiliary capillary plexus: a lectin-histochemical and immunohistochemical study. Hepatology 1993; 18: 529–536. [PubMed] [Google Scholar]
- 12.Terada T, Nakanuma Y. Expression of tenascin, type IV collagen and laminin during human intrahepatic bile duct development and in intrahepatic cholangiocarcinoma. Histopathology 1994; 25: 143–50. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki M, Nakanuma Y, Terada T, Kim YS. Biliary epithelial expression of MUC1, MUC2, MUC3, and MUC5/6 apomucins during human intrahepatic bile duct development and maturation: an immunohistochemical study. Am J Pathol 1995; 147: 574–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Terada T, Kato M, Horie S, Endo K, Kitamura Y. Expression of pancreatic alpha-amylase protein and messenger RNA on hilar primitive bile ducts and hepatocytes during human fetal liver organogenesis: an immunohistochemical and in situ hybridization study. Liver 1998; 18: 313–9. [DOI] [PubMed] [Google Scholar]
- 15.Terada T, Ukita Y, Ueyama J, Ohta T. Protein expression of double-stranded RNA-activated protein kinase (PKR) in intrahepatic bile ducts in normal adult livers, fetal livers, primary biliary cirrhosis, hepatolithiasis and intrahepatic cholangiocarcinoma. Liver 2000; 20: 450–7. [DOI] [PubMed] [Google Scholar]
- 16.Terada T, Ohta T, Nakanuma Y. Expression of transforming growth factor-α and its receptor during human liver development and maturation. Virchows Archiv 1994; 424: 669–75. [DOI] [PubMed] [Google Scholar]
- 17.Terada T. Human fetal ductal plate revisited: II. MUC1, MUC5AC, and MUC6 are expressed in human fetal ductal plate and MUC1 is expressed also in remodeling ductal plate, remodeled ductal plate and mature bile ducts of human fetal livers. Int J Clin Exp Pathol 2013; 6: 571–85. [PMC free article] [PubMed] [Google Scholar]
- 18.Van Eyken P, Sciot R, Callea F, Van der Steen K, Moerman P, Desmet VJ. The development of the intrahepatic bile ducts in man: a keratin immunohistochemical study. Hepatology 1988; 8: 1586–95. [DOI] [PubMed] [Google Scholar]
- 19.Desmet VJ. Congenital disease of intrahepatic bile ducts: variations on the theme “ductal plate malformation.”. Hepatology 1992; 16: 1069–83. [DOI] [PubMed] [Google Scholar]
- 20.Desmet VJ. Intrahepatic bile duct under the lens. J Hepatol 1985; 1: 545–59. [DOI] [PubMed] [Google Scholar]
- 21.Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec 2008; 291: 628–35. [DOI] [PubMed] [Google Scholar]
- 22.Shah KD, Gerber MA. Development of intrahepatic bile ducts in humans: immunohistochemical study using monoclonal cytokeratin antibodies. Arch Pathol Lab Med 1989; 113: 1135–8. [PubMed] [Google Scholar]
- 23.Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology 1996; 23: 476–81. [DOI] [PubMed] [Google Scholar]
- 24.Shar KD, Gerber MA. Development of intrahepatic bile ducts in humans: possible role of laminin. Arch Pathol Lab Med 1990; 114: 597–600. [PubMed] [Google Scholar]
- 25.Terada T, Nakanuma Y, Ohta G. Glandular elements around the intrahepatic bile ducts in man: their morphology and distribution in normal livers. Liver 1987; 7: 1–8. [DOI] [PubMed] [Google Scholar]
- 26.Terada T, Nakanuma Y. Solitary cystic dilation of the intrahepatic bile duct: morphology of two autopsy cases and a review of the literature. Am J Gastroenterol 1987; 82: 1301–5. [PubMed] [Google Scholar]
- 27.Terada T, Takegoshi T, Doishita K, Nakanuma Y. Histological study of intrahepatic cavernous transformation in a patient with primary myelofibrosis and portal venous thrombosis. Virchows Arch A 1988; 412: 339–45. [DOI] [PubMed] [Google Scholar]
- 28.Terada T, Nakanuma Y. Morphological examination of intrahepatic bile ducts in hepatolithiasis. Virchows Arch A 1988; 413: 167–76. [DOI] [PubMed] [Google Scholar]
- 29.Terada T, Ishida F, Nakanuma Y. Vascular plexus around intrahepatic bile ducts in normal livers and portal hypertension. J Hepatol 1989; 8: 139–49. [DOI] [PubMed] [Google Scholar]
- 30.Terada T, Nakanuma Y. Pathologic observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: IV. Hyperplasia of intramural and extramural glands. Hum Pathol 1992; 23: 483–90. [DOI] [PubMed] [Google Scholar]
- 31.Terada T, Nakanuma Y. Innervation of intrahepatic bile ducts and peribiliary glands in normal livers, extrahepatic biliary obsrtruction and hepatolithiasis: an immunohistochemical study. J Hepatol 1989; 9: 141–8. [DOI] [PubMed] [Google Scholar]
- 32.Terada T, Nakanuma Y, Kakita A. Pathologic observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: heterotopic pancreas in the liver. Gastroenterology 1990; 98: 1333–7. [DOI] [PubMed] [Google Scholar]
- 33.Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: II. A possible source of cholangiocarcinoma. Hepatology 1990; 12: 92–7. [DOI] [PubMed] [Google Scholar]
- 34.Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: III. Survey of necroinflammation and cystic dilatation. Hepatology 1990; 12: 1229–33. [DOI] [PubMed] [Google Scholar]
- 35.Terada T, Kono N, Nakanuma Y. Immunohistochemical and immunoelectron microscopical analyses of α-amylase isozymes in the intrahepatic biliary epithelium and hepatocytes. J Histochem Cytochem 1992; 40: 1627–35. [DOI] [PubMed] [Google Scholar]
- 36.Terada T, Nakanuma Y. Intrahepatic cholangiographic appearance simulating primary sclerosing cholangitis in hepatobiliary diseases: a postmortem cholangiographic and histolopathological study in 154 autopsy livers. Hepatology 1995; 22: 75–81. [PubMed] [Google Scholar]
- 37.Terada T, Kida T, Nakanuma Y. Extrahepatic peribiliary glands express α-amylase isozymes, trypsin and pancreatic lipase: an immunohistochemical analysis. Hepatology 1993; 18: 803–8. [DOI] [PubMed] [Google Scholar]
- 38.Terada T, Nakanuma Y. Congenital biliary dilatation in autosomal dominant adult polycystic disease of the liver and kidneys. Arch Pathol Lab Med 1988; 112: 1113–6. [PubMed] [Google Scholar]
- 39.Jorgensen MJ. The ductal plate malformation. Acta Pathol Microbiol Scand Suppl 1977; 257: 1–87. [PubMed] [Google Scholar]
- 40.Summerfield JA, Nagafuchi Y, Sherlock S, Cadafalch J, Scheuer PJ. Hepatobiliary fibropolycystic diseases: a clinical and histological review of 51 patients. J Hepatol 1986; 2: 141–56. [DOI] [PubMed] [Google Scholar]
- 41.Terada T, Moriki T. Monolobar ductal plate malformation disease of the liver. Pathol Int 2010; 60: 407–12. [DOI] [PubMed] [Google Scholar]
- 42.Terada T, Moriki T. Monolobar hepatobiliary fibropolycystic disease. Pathol Oncol Res 2011; 17: 159–65. [DOI] [PubMed] [Google Scholar]
- 43.Nakanuma Y, Terada T, Ohta G, Matsubara F, Kurachi M. Caroli’s disease in congenital hepatic fibrosis and infantile polycystic disease. Liver 1982; 2: 346–54. [DOI] [PubMed] [Google Scholar]
- 44.Desmet VJ. Ductal plates in hepatic ductular reactions: hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch 2011; 458: 251–9. [DOI] [PubMed] [Google Scholar]
- 45.Terada T. Hepatic nodular hamartoma containing liver cysts, ductal plate malformations and peribiliary glands. Hepatol Res 2011; 41: 93–8. [DOI] [PubMed] [Google Scholar]
- 46.Terada T. Projected focal nodular hyperplasia (FNH) of the liver with pronounced atypical ductular reaction resembling ductal plate and expressing KIT. Hepatol Res 2012; 42: 721–6. [DOI] [PubMed] [Google Scholar]
- 47.Nakanuma Y, Sato Y, Ikeda H, Harada K, Kobayashi M, Sano K, Uehara T, Yamamoto M, Arrizumi S, Park YN, Choi JH, Yu E. Intrahepatic cholangiocarcinoma with predominant “ductal plate malformation” pattern: a new subtype. Am J Surg Pathol 2012; 36: 1629–35. [DOI] [PubMed] [Google Scholar]
- 48.Terada T. Mutations and protein expression of KIT and PDGFRA genes in ipsilateral testicular seminomas: an immunohistochemical and molecular genetic study. Appl Immunohistochem Mol Morphol 2011; 19: 450–3. [DOI] [PubMed] [Google Scholar]
- 49.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 2006; 25: 3818–22. [DOI] [PubMed] [Google Scholar]
- 50.Libbrecht L. Hepatic progenitor cells in human tumor development. World J Gastroenterol 2006; 12: 6261–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allison MR. Liver stem cells: implication for hepatocarcinogenesis. Stem Cell Rev 2005; 1: 253–60. [DOI] [PubMed] [Google Scholar]
- 52.Strain AJ, Crosby HA. Hepatic stem cells. Gut 2000; 46: 743–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss TS, Lichtennauer M, Kirchner S, Stock P, Aurich H, Christ B, Brockhoff G, Kunz-Schughart LA, Jauch KW, Schlitt HJ, Thasler WE. Hepatic progenitor cells from adult human livers for cell plantation. Gut 2008; 57: 1129–38. [DOI] [PubMed] [Google Scholar]
- 54.Carpentier R, Suner RS, Van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology 2011; 141: 1432–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terada T. Gastrointestinal stromal tumor of the uterus: a case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Pathol 2009; 28: 29–34. [DOI] [PubMed] [Google Scholar]
- 56.Terada T. Small cell carcinoma of the urinary bladder. Int J Clin Exp Pathol 2012; 5: 596–600. [PMC free article] [PubMed] [Google Scholar]
- 57.Terada T. Small cell neuroendocrine carcinoma of the prostate: incidence and a report of four cases with an examination of KIT and PDGFRA. Prostate 2012; 72: 1150–6. [DOI] [PubMed] [Google Scholar]
- 58.Terada T. Primary small cell carcinoma of the ureter: a case report with immunohistochemical and molecular genetic analysis of KIT and PDGFRA genes. Pathology 2010; 42: 101–2. [DOI] [PubMed] [Google Scholar]
- 59.Terada T. Autopsy case of primary small cell carcinoma of the urinary bladder: KIT and PDGFRA expression and mutations. Pathol Int 2009; 59: 247–50. [DOI] [PubMed] [Google Scholar]
- 60.Terada T. An immunohistochemical and molecular genetic analysis of KIT and PDGFRA in small cell lung carcinoma in Japanese. Int J Clin Exp Pathol 2012; 5: 331–8. [PMC free article] [PubMed] [Google Scholar]
- 61.Terada T. Primary esophageal small cell carcinoma with brain metastasis and with CD56, KIT, and PDGFRA expressons. Pathol Oncol Res 2012; 18: 1091–3. [DOI] [PubMed] [Google Scholar]
- 62.Terada T. KIT and PDGFRA in esophageal pure small cell carcinoma. Int J Clin Exp Pathol 2011; 4: 718–21. [PMC free article] [PubMed] [Google Scholar]
- 63.Terada T. Primary small cell carcinoma of the mediastinum: a case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol 2009; 26: 247–50. [DOI] [PubMed] [Google Scholar]
- 64.Terada T. Primary small cell carcinoma of the pleura: a case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Med Oncol 2010; 27: 1119–22. [DOI] [PubMed] [Google Scholar]
- 65.Terada T. KIT-positive primary small cell carcinoma of the endometrium: a case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Arch Gynecol Obstet 2010; 282: 413–6. [DOI] [PubMed] [Google Scholar]
- 66.Terada T. Myoepithelial carcinoma of pharynx expressing KIT and PDGFRA. Int J Clin Exp Pathol 2013; 6: 313–8. [PMC free article] [PubMed] [Google Scholar]
- 67.Terada T. Expression of NCAM (CD56), chromogranin A, synaptophysin, c-KIT (CD117) and PDGFRA in normal non-neoplastic skin and basal cell carcinoma: an immunohistochemical study of 66 consecutive cases. Med Oncol 2013; 30: 444–444. [DOI] [PubMed] [Google Scholar]
- 68.Terada T. Large cell neuroendocrine carcinoma with sarcomatous changes of the endometrium: a case report with immunohistochemical studies and molecular genetic study of KIT and PDGFRA. Pathol Res Pract 2010; 206: 420–5. [DOI] [PubMed] [Google Scholar]
- 69.Terada T. Pulmonary large cell neuroendocrine carcinoma diagnosed in a brain metastasis. Int J Clin Exp Pathol 2012; 5: 159–62. [PMC free article] [PubMed] [Google Scholar]
- 70.Terada T. Neuroendocrine carcinoma of the esophagus: a case report with immunohistochemical and molecular genetic analysis of KIT and PDGFRA. Med Oncol 2011; 28: 509–12. [DOI] [PubMed] [Google Scholar]
- 71.Terada T. Low incidence of KIT gene mutations and no PDGFRA gene mutations in primary cutaneous melanoma: an immunohistochemical and molecular genetic study of Japanese cases. Int J Clin Oncol 2010; 15: 453–6. [DOI] [PubMed] [Google Scholar]
- 72.Terada T. Gastrointestinal stromal tumor of the digestive organs: a histopathologic study of 31 cases in a single Japanese institute. Int J Clin Exp Pathol 2010; 3: 162–8. [PMC free article] [PubMed] [Google Scholar]
- 73.Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology 2009; 41: 695–7. [DOI] [PubMed] [Google Scholar]
- 74.Terada T. Primary extragastrointestinal stromal tumors of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Med Oncol 2009; 26: 233–7. [DOI] [PubMed] [Google Scholar]
- 75.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol 2008; 14: 7256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int 2002; 52: 740–6. [DOI] [PubMed] [Google Scholar]
- 77.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med 2005; 271: 271–5. [DOI] [PubMed] [Google Scholar]
- 78.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int 2004; 54: 116–23. [DOI] [PubMed] [Google Scholar]
- 79.Funaki N, Sasano H, Shizawa S, Nio M, Iwami D, Ohi R, Nagura H. Apoptosis and cell proliferation in biliary atresia. J Pathol 1998; 186: 429–33. [DOI] [PubMed] [Google Scholar]
- 80.Sasaki H, Nio M, Iwami D, Funaki N, Sano N, Ohi R, Sasano H. E-cadherin, alpha-catenin and beta-catenin in biliary atresia: correlation with apoptosis and cell cycle. Pathol Int 2001; 51: 923–32. [DOI] [PubMed] [Google Scholar]
- 81.Erickson N, Mohanty SK, Shivakumar P, Sabla G, Chakraborty R, Bezerra JA. Temporal-spatial activation of apoptosis and epithelial injury in murine experimental biliary atresia. Hepatology 2008; 47: 1567–77. [DOI] [PubMed] [Google Scholar]
- 82.Ishikawa KS, Masui T, Shikawa K, Shiojiri N. Immunolocalization of hepatocyte growth factor and its receptor (c-MET) during mouse liver development. Histochem Cell Biol 2001; 116: 453–62. [DOI] [PubMed] [Google Scholar]
- 83.Kurumaya H, Ohta G, Nakanuma Y. Endocrine cells in the intrahepatic biliary tree in normal livers and hepatolithisis. Arch Pathol Lab Med 1989; 113: 143–7. [PubMed] [Google Scholar]
- 84.Terada T, Kitamura Y, Ohta T, Nakanuma Y. Endocrine cells in hepatobiliary cystadenoma and cystadenocarcinoma. Virchows Arch 1997; 430: 37–40. [DOI] [PubMed] [Google Scholar]
- 85.Rosai J. Chromogranin. In: Rosai J. (eds). Rosai and Ackerman’s surgical pathology, 9th ed New York: Mosby, 2004, pp. 51–51. [Google Scholar]
- 86.Rosai J. Synaptophysin. In: Rosai J. (eds). Rosai and Ackerman’s surgical pathology, 9th ed New York: Mosby, 2004, pp. 61–61. [Google Scholar]
- 87.Rosai J. Neuron specific enolase. In: Rosai J. (eds). Rosai and Ackerman’s surgical pathology, 9th ed New York: Mosby, 2004, pp. 58–58. [Google Scholar]