Abstract
Stress fractures are common injuries with load-bearing activities. Stress fractures have been reported in the scientific literature for over a century; however, the etiology continues to be investigated with important distinctions made between the contributions of the tissue-level processes of bone remodeling and modeling. In response to novel repetitive loading, increased bone remodeling may serve to replace fatigue-damaged bone while at the same time creating temporary porosity. Much attention has been given to the role of remodeling in the etiology of stress fracture; however, the role of bone modeling has received less attention. Modest increases in modeling, via bone formation on the periosteal surface of long bones in response to mechanical loading, greatly increases the fatigue resistance of bone. Thus, enhancing this adaptive bone formation is a promising target for stress fracture prevention, and a focus on adaptive bone formation may reveal novel risk factors for stress fracture.
Keywords: Stress fracture, bone formation, bone remodeling, bone modeling, military, NSAIDs
Introduction
Stress fractures occur with repetitive loading and are most often seen in physically active populations such as military recruits and endurance athletes.1–3 Although pain in the lower extremities during marching was first described in Soldiers over 120 years ago,4 the etiology of stress fractures continues to be revealed. Recent advancements in the understanding of adaptive bone formation, coupled with reports of structural differences in the bones of individuals with stress fractures, contribute to an improved model of stress fracture etiology and suggest that de novo bone formation following skeletal loading is a critical process for preventing stress fractures. This “adaptive bone formation” can occur via bone deposition on the trabecular, endocortical, and periosteal surfaces. However, as we will review, deposition of new bone on the periosteal surface in particular, especially along the diaphysis of long bones, may have important consequences for improving fatigue resistance. In this review, we outline an etiological model of stress fracture with a focus on this adaptive bone formation. We conclude with a discussion of modifiable factors that may inhibit adaptive bone formation, and therefore provide potential targets for stress fracture prevention. We will focus our discussion of stress fracture etiology on the tibia and femur because these are the two most common sites of stress fracture during military training.5 Metatarsal stress fractures are also common with repetitive loading but tend to occur later in training,6 and the bones of the foot are likely subjected to loading conditions that differ from the long bones of the legs.
Etiology of stress fracture
Stress fracture is the clinical term used to denote bone failure in fatigue. While an overt stress fracture can appear as cortical disruption on a radiograph, the clinical diagnosis is not always straightforward and may vary by imaging modality.7 Over half a century ago, failure of bone was thought to occur solely from the mechanical consequences of repetitive loading, much like other repetitively loaded structures such as bridges and aircraft wings.8 Indeed, stress fractures do not occur from a single traumatic event; rather, they are the product of repetitive submaximal loading.9 Stress fractures often occur after a runner suddenly increases mileage or a military recruit begins basic combat training.10 When a load of sufficient magnitude is applied to a bone, the tissue will deform, or strain.11 If strains are sufficiently high, microscopic cracking and diffuse damage of the tissue will occur.12–14 Continued loading on bone with decreased stiffness will lead to increased strains and further microdamage accumulation that may not be adequately repaired, leading ultimately to a stress fracture.
While this model of failure in fatigue can represent any inert structure subjected to repetitive loads, bones are biologically active. The consequences of this biologic activity are well illustrated when comparing studies that assessed fatigue in isolated tissue and in human subjects. When inert bovine bone tissue was experimentally fatigued in vitro at physiological strains analogous to those measured in humans while running (∼1200–1500 microstrain, 0.12–0.15 percent deformation), the fatigue life (number of loading cycles a material can sustain before failure occurs) was between 12 and 37 million loading cycles.15 The authors of this study estimated that 10 million loading cycles would equate to 11,000 miles of running, or 5 to 10 years of continuous loading, before stress fracture would occur.9 In contrast to this, Soldiers experience stress fractures within only weeks of beginning initial military training. During basic combat training, Soldiers have been reported to average approximately 100 total hours of combined walking, marching, and running over 8 weeks.16 Therefore, Soldiers are exposed to less than 1/400th the duration of loading required to induce a fatigue-related stress fracture under in vitro loading conditions. One explanation for this discrepancy might be that physiological loading within the bones of a Soldier or an athlete may include multi-axial loading (distinct from the uniaxial loading in the in vitro experiment15), which could generate abnormal loading patterns and shear stresses that may reduce the fatigue life of the structure.17 Another potential explanation might be the relatively small volume of bone tested in the in vitro experiment. A human tibia, for example, will have a greater volume than the experimental specimen, and larger specimens are more likely to contain weaker regions than smaller specimens.18 A final explanation for differences in fatigue life between in vitro experimental and clinical observations is that bones undergo remodeling in response to increased mechanical loading. As we review below, bone remodeling is a process that may serve to repair accumulated microdamage, while also temporarily increasing porosity and decreasing the elastic modulus of bone.19,20
The role of bone remodeling in stress fracture
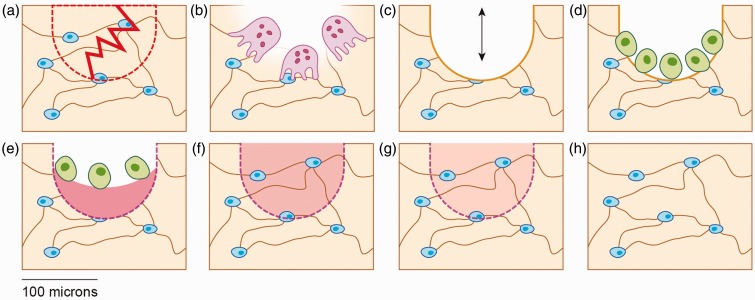
Remodeling is the process whereby a small cavity of bone tissue (∼150–200 µm in diameter)21 is first hollowed out by osteoclastic bone resorption, and followed by filling of the cavity through coupled osteoblastic bone formation (Figure 1).22 The remnants of remodeling cycles are cylindrical osteons in cortical bone and hemiosteons in trabecular bone, produced by concentric or semi-concentric deposition of lamellar bone.22 Osteonal bone has been shown to fatigue sooner than primary bone, and this mechanical disadvantage raises the question as to the purpose of bone remodeling.15 One reason for bone remodeling, as theorized over 50 years ago23 and now experimentally supported,14,24 is to repair fatigue damage. Remodeling aimed at removing microdamage is referred to as “targeted remodeling.” This repair mechanism results in temporary porosity and therefore may contribute to stress fracture risk, although the link between increased porosity and stress fracture risk remains to be demonstrated experimentally.20 In principle, the process of bone remodeling in response to physical training is paradoxical in that it may promote stress fracture development by introducing an acute increase in porosity but may also prevent stress fracture development by replacing fatigue-damaged bone. While experimental data are still needed to fully understand the role of remodeling in stress fracture prevention and promotion, each of these concepts are reviewed below.
Figure 1.
Schematic of bone remodeling following fatigue damage in trabecular bone. (a) Linear microcrack disrupting the osteocyte lacunocanalicular system, leading to osteocyte apoptosis in the affected area (dotted region). (b) Osteoclastic resorption of microdamaged bone. (c) Temporary negative bone space due to osteoclastic resorption. (d) Osteoblast recruitment to the remodeling space. (e) Osteoblastic deposition of unmineralized bone matrix (osteoid). (f) Primary mineralization of newly deposited matrix. (g) Secondary mineralization of bone matrix. (h) Completed remodeling cycle. (A color version of this figure is available in the online journal.)
The role of remodeling in promoting stress fracture
Bone remodeling is a time-dependent process. During the initial period immediately following the introduction of a new repetitive loading regimen, it has been suggested that bone acquires microdamage which decreases the elastic modulus of the bone.15 Diffuse damage and microcracking have been reported to precede osteocyte apoptosis in areas of damaged bone tissue (Figure 1(a)).25,26 Apoptosis of osteocytes at microcrack loci and paracrine signaling by adjacent viable osteocytes have been reported to be necessary for stimulating expression of the pro-osteoclastic proteins, receptor activator of nuclear factor κ-B ligand (RANKL)27 and vascular endothelial growth factor (VEGF).24 With recruitment of osteoclasts to the remodeling site (Figure 1(b)), bone is resorbed,28 and the resultant porous space (Figure 1(c)) represents a temporary negative bone balance that can reduce the stiffness of bone until the cavity is replenished with fully mineralized bone.29
At each remodeling site, bone formation is coupled to resorption (“coupled bone formation”), but deposition of newly formed bone by osteoblasts recruited to the remodeling site (Figure 1(d)) does not immediately restore mechanical stiffness. After deposition of unmineralized bone matrix (Figure 1(e)), a rapid mineralization of the newly deposited matrix occurs.30 This is termed primary mineralization, and during the first few weeks following matrix deposition, approximately 65 to 70% of the final mineralization is completed (Figure 1(f)).19,31 The remainder of mineralization (“secondary mineralization;” Figure 1(g)) occurs gradually over the following year and continues throughout the lifespan of the tissue.31 Therefore, each newly activated remodeling unit will require more than a year before the mechanical properties reflect baseline values (Figure 1(h)). As stress fractures may occur within weeks of onset of physical training,32 newly activated remodeling cycles remain in early stages when a negative bone balance can theoretically contribute to a cycle of increased strain9 and accumulation of microdamage upon continued loading until stress fracture ensues (Figure 2).20
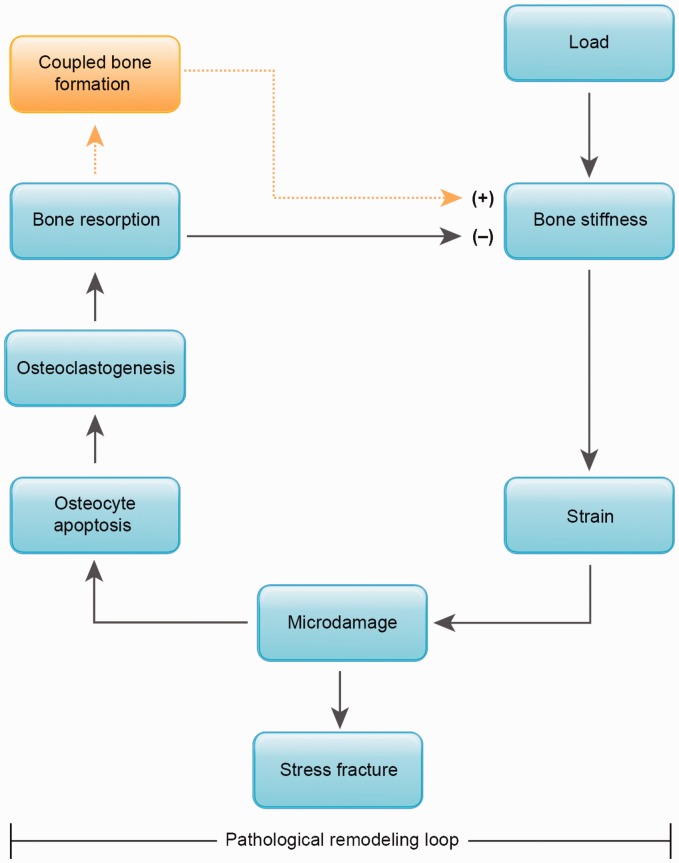
Figure 2.
Schematic diagram of the remodeling loop wherein repetitive loading leads to stress fracture. With loading, a bone of a particular stiffness will experience strains that may result in microdamage generation within the bone tissue. This damaged tissue is targeted for remodeling through focal osteocyte apoptosis, recruitment of osteoclasts, and bone resorption. The dashed lines indicate the process of coupled bone formation within the remodeling cycle. When appropriate time is allowed for bone formation to follow resorption within the remodeling unit (see Figure 1(d) to (g)), microdamage will be replaced and bone stiffness increased. However, when repetitive loading is continued without appropriate rest, the continued loading on a more porous bone may result in a positive feedback cycle of increased strain, microdamage accumulation, and bone resorption until microcracks coalesce and stress fracture occurs. (A color version of this figure is available in the online journal.)
The role of remodeling in preventing stress fracture
Although remodeling temporarily introduces porosity and declines in stiffness, paradoxically, attempting to prevent stress fractures by inhibiting remodeling may not be effective. For example, in an effort to suppress the hypothesized negative effects of remodeling on stress fracture risk, a randomized, placebo-controlled trial of the bisphosphonate, risedronate, was conducted in 324 Israeli infantry recruits. There were no differences in stress fracture incidence reported between those randomized to risedronate (14%) or placebo (13%).33 These results suggest that suppression of bone remodeling through bisphosphonate use does not reduce stress fracture incidence. One possible explanation for these results is that remodeling is needed to repair the microdamage that occurs with repetitive loading.25 Fatigue loading in rat models results in loss of stiffness, microcrack formation (Figure 1(a)), and activation of bone remodeling25 (Figure 1 (b) to (h)). It therefore follows that targeted remodeling may be an important process for replacing fatigue damage during physically active times such as training for an endurance running event or basic military training. Nevertheless, the associations among increased training, microdamage accrual, and stress fracture risk remain under investigation.
Given the theoretical roles of remodeling in both promoting stress fracture (because of temporary porosity) and preventing stress fracture (because of microdamage repair), research is still needed to fully characterize the contributions of remodeling to the etiology of stress fracture. In the interim, we posit a renewed focus on the other biological response to repetitive loading—adaptive bone formation.
The role of adaptive bone formation in preventing stress fracture
Adaptive bone formation occurs when bone is deposited on the periosteal, endocortical, or trabecular surfaces in response to mechanical loading.34 This bone modeling process is distinct from remodeling because it involves the independent action of osteoblasts without prior osteoclastic bone resorption.30 In the process of modeling, osteoblast and osteoclast activity are generally uncoupled. As in targeted remodeling, modeling involves osteocyte activation; but rather than undergoing apoptosis, osteocytes act as mechanotransducers—translating the mechanical deformation of the bone matrix into biochemical signals that lead to de novo bone formation35 (Figure 3).
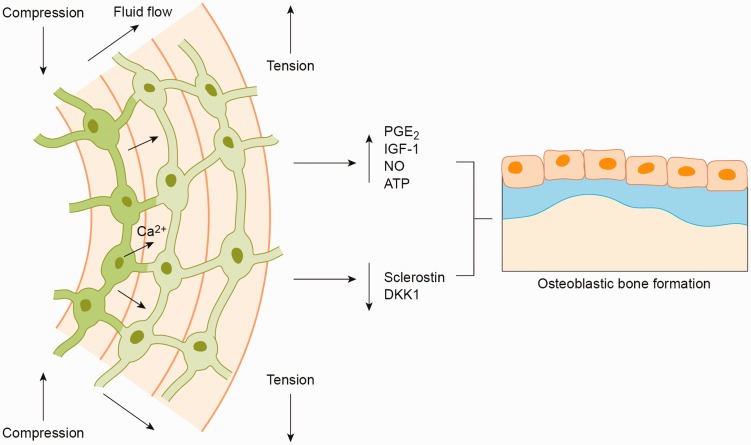
Figure 3.
Schematic diagram demonstrating movement of interstitial fluid through the osteocyte lacunocanalicular network from areas of compression to tension as a result of mechanical loading. This fluid flow stimulates intracellular calcium signaling and osteocytic molecular signaling, osteoblast recruitment, and de novo bone formation. (A color version of this figure is available in the online journal.)
Although significant progress has been made in identifying the pathways that translate mechanical stimuli into bone formation, there is still much to be determined. Several theories have been proposed regarding the mechanisms for stimulation of osteocytes with mechanical loading, including deformation of the bone matrix, electric streaming potentials created by ionic fluid movement through the lacunocanalicular system, and cellular shear stress generated by fluid flow along the osteocyte cell body and dendritic processes.35 Further debate exists as to what components of osteocytes are responsible for mechanosensation, including the cell body, the primary cilia, and the dendritic processes.35 While the particulars of bone mechanotransduction continue to be elucidated, the current theory holds that perturbation of osteocytes and their structural components lead to increased intra-osteocyte calcium signaling and production of pro-osteoblastic molecules such as prostaglandin E2 (PGE2), insulin-like growth factor (IGF-I), nitric oxide (NO), and adenosine triphosphate (ATP).36 These molecular signals have been shown to positively regulate bone formation, as suppression of PGE2,37 IGF-I,38 NO,39 and ATP40 inhibited bone formation in response to mechanical loading. Mechanical loading not only promotes osteocyte signaling but also suppresses osteocyte production of negative regulators of the Wnt/β-catenin pathway, including sclerostin and dickkopf-1 (DKK1).41,42 Osteocyte-mediated biochemical signaling and Wnt/β-catenin pathway activation following mechanical loading are necessary for osteoblast differentiation, proliferation and bone formation.38,39,43–46 Together, these findings reveal a complicated network of signaling pathways regulated by mechanical loading of osteocytes (Figure 3).
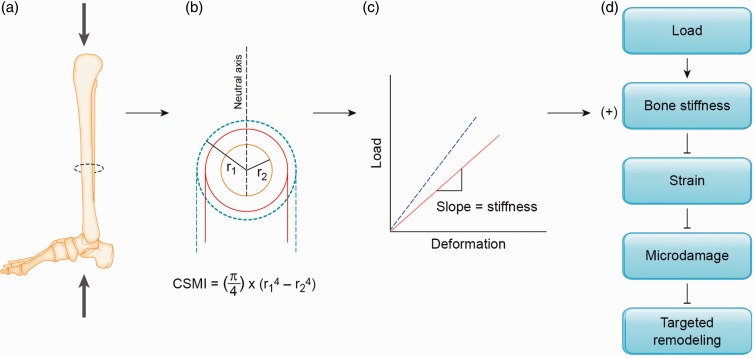
Bone deposition can occur on all surfaces of bone, but along the diaphysis of long bones, deposition on the periosteal surface provides the greatest mechanical advantage.11 This is because most long bones experience bending (Figure 4(a)), and the ability of a structure to resist deformation during bending is largely determined by its cross-sectional moment of inertia (CSMI, Figure 4(b)).11 Therefore, long bones with mass distributed furthest from the neutral axis (e.g. wide bones) are stronger in relation to bones with similar masses that are narrower. In extension of this model, if bone mass is added to the skeleton in response to loading, deposition on the periosteal surface will theoretically provide the greatest mechanical advantage. This mechanical advantage will translate into a decrease in the amount of deformation a bone will experience for a given load (Figure 4(c)), and this increase in stiffness should attenuate generation of microdamage, and therefore, improve the fatigue life of bone. In support of this concept, a modest increase in whole bone mineral content of approximately 8% was reported in the loaded ulnas of rats following 5 weeks of loading (3 days/week, 360 cycles/day at 17N).47 Importantly, the majority of added bone was deposited on the periosteal surface, which increased the cross-sectional moment of inertia by 90%. When the fatigue life of the untrained and trained limb was assessed, the untrained limb fractured after an average of 15,000 cycles compared to the trained limb which failed after an average of 1.5 million cycles. This 100-fold increase in fatigue resistance after a 5-week loading regimen demonstrates the potential importance of adaptive bone formation with physical training—a small increase in bone, deposited on the periosteal surface, may greatly improve the fatigue resistance of a long bone (Figure 4).
Figure 4.
Schematic of the mechanical advantage provided by adaptive periosteal bone formation. (a) Mechanical loading of the lower limb stimulates adaptive bone formation. (b) Adaptive bone formation on the periosteal surface (denoted by dashed lines) adds mass furthest from the neutral axis in bending, and thus, increases the cross-sectional moment of inertia (CSMI, strength in bending). Note that CSMI is proportional to the fourth power of the outer radius (r1), and therefore, bone formation on the periosteum is advantageous in increasing the strength of the bone in bending. (c) An increase in resistance to bending will result in a stiffer bone, or a shift to the left of the linear portion of the load–deformation curve (denoted by the dashed line). In the elastic region where strains are not pathophysiological, the bone will deform less for a given load. (d) An increase in stiffness due to periosteal bone deposition will theoretically result in lower strains for a given load, and therefore, attenuated microdamage generation and suppression of targeted bone remodeling. Suppression of targeted remodeling is expected to inhibit the pathological remodeling loop (depicted in Figure 2). (A color version of this figure is available in the online journal.)
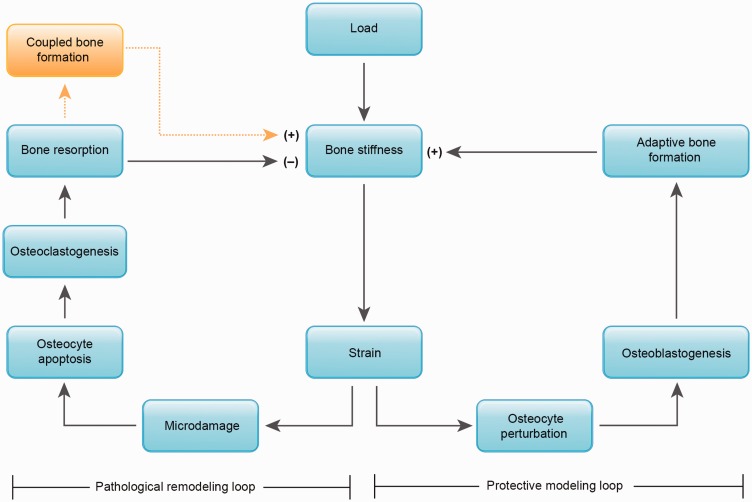
Adaptive bone formation, therefore, provides an ideal target for stress fracture prevention. While repetitive loading and resultant strains may initiate both remodeling and formation modeling, the formation modeling has the potential to offset the transient reduction in bone stiffness caused by targeted remodeling of fatigue-damaged bone. Thus, there exist two different physiological loops to consider in the etiology of stress fracture (Figure 5). First, there is a pathophysiological remodeling loop that is pathophysiological in the sense that generation of new bone resorption cavities temporarily outpaces the filling of the cavities in the process of targeted remodeling (Pathophysiological remodeling loop in Figure 5). The second loop is a protective modeling loop wherein perturbation of osteocytes results in molecular signaling and recruitment of osteoblasts to form new bone (Protective modeling loop in Figure 5). This de novo bone formation has the potential to increase bone stiffness. The protective loop is not commonly targeted for stress fracture prevention, and focus on promoting adaptive bone formation in populations subjected to novel repetitive mechanical loading may provide a new focus for stress fracture prevention.
Figure 5.
Etiological model of stress fracture with competition between a pathological loop on the left and a protective loop on the right. With loading, the strains generated may result in a cycle of microdamage generation and targeted bone remodeling which includes osteoclastic bone resorption. Resorption can lead to temporary porosity, decreased bone stiffness and further microdamage generation. Porosity is only temporary, as coupled bone formation will eventually restore stiffness. However, this process requires up to a year for completion (see Figure 1(d) to (g)). Without appropriate rest, this cycle may lead to stress fracture, and is therefore considered pathophysiological. The same strains that initiate microdamage formation concurrently stimulate adaptive bone formation which increases bone stiffness, thereby preventing increases in strain with continued loading. This protective loop of adaptive bone formation has the potential to offset the pathophysiological loop and prevent stress fracture. (A color version of this figure is available in the online journal.)
Preventing stress fracture by building wider bones
Consistent with the concept that wider long bones provide a mechanical advantage (Figure 4), numerous studies have reported that narrower bones are prone to stress fracture.2,48–51 In a prospective study of bone geometry in male and female Marine recruits, females who suffered a stress fracture during training possessed smaller section moduli and thinner cortices than their non-fracturing female counterparts. Likewise, male Marine recruits who suffered a stress fracture had narrower bones and smaller section moduli compared to male Marines who did not have a stress fracture.2 Similarly, in a study of Israeli Soldiers, increased risk of stress fracture was reported in Soldiers with slender tibias (narrow bones relative to bone length and body size), even with the expected cortical area and tissue-mineral density for body size.50 Case–control studies in athletes further reveal the importance of bone size, with reports of 7.8% lower distal tibia cross-sectional area49 and 9–10% lower strength indices at the midshaft48 in female runners with a history of stress fracture compared to female runners without a history of stress fracture. Collectively, these studies suggest that narrower bones are a risk factor for stress fracture.
The baseline width and other geometrical characteristics of long bones are regulated by a combination of genetic and environmental factors, including sex, age, ethnicity, height, endocrine history, prior nutrition, and prior physical training.52 Of these characteristics, the only modifiable, non-pharmacological mechanism for increasing the width of long bones is through physical training. Therefore, prior to beginning formal training, military recruits and athletes may benefit from pretraining to stimulate adaptive bone formation. In support of the benefit of pretraining, a 50% reduction in stress fracture incidence was reported in Israeli elite infantry recruits who played ball sports immediately prior to basic training.53 Given these findings, recruits would ideally become more physically active prior to training to offset stress fracture risk; and accordingly, once training has begun, any factor suppressing the adaptive bone formation response during military or athletic training may increase the risk of stress fracture.
Risk factors for inhibition of adaptive bone formation
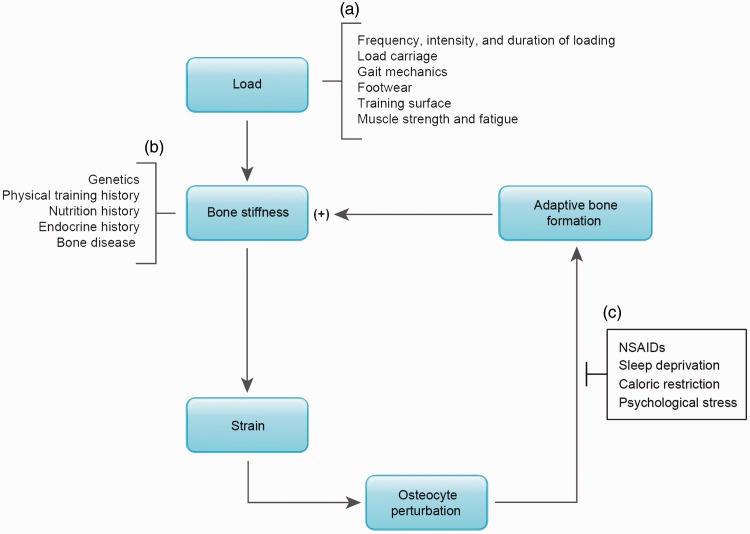
Based on the current understanding of stress fracture etiology, three categories of modifiable risk factors should be considered: factors that affect the load on bone, factors that influence baseline bone stiffness, and factors that inhibit or promote the adaptive response of bone to loading (Figure 6). The first category includes modifiable factors that affect the magnitude or distribution of loading, such that strains can be minimized and microdamage prevented. These factors are the most commonly studied modifiable risk factors for stress fracture and include fitting Soldiers and athletes with proper footwear, minimizing the duration, intensity, and frequency of training, incorporating longer rest periods between training bouts, improving gait mechanics, and training on softer surfaces52 (Figure 6(a)). A second category of risk factors is characterized by their effects on bone stiffness and include genetics, hormone status, and prior nutrition and physical training history (Figure 6(b)). Once a new loading regimen has begun, these risk factors are clearly nonmodifiable. A third category of risk factors, however, provides new modifiable targets for stress fracture. This category includes factors that may inhibit adaptive bone formation (Figure 6(c)).
Figure 6.
(a) Risk factors for stress fracture can be categorized by their relationship to the load placed on bone (i.e. frequency, intensity, and duration of loading, load carriage, gate mechanics, etc.) (b) or by their effect on baseline bone stiffness (genetics, physical training and nutrition history, etc.). (c) A new category of risk factors is presented, characterized by their effect on adaptive bone formation (NSAID use, sleep deprivation, caloric restriction, and psychological stress). (A color version of this figure is available in the online journal.)
A number of potential physical, psychological, and pharmacological risk factors exist that may inhibit adaptive bone formation and promote stress fracture. For example, a 20% reduction in the bone formation markers bone alkaline phosphatase (BAP) and osteocalcin (OCN) following 8 weeks of U.S. Army Ranger School was recently reported.54 Ranger trainees average 19.6 h of training per day and have a daily energy expenditure of 2500–4500 calories.55,56 The reduced concentrations of BAP and OCN immediately following Ranger school suggest that bone formation is inhibited with this intense training regimen. Beyond intensive physical training, Army Ranger trainees experience other physical and psychological stressors that may contribute to suppression of bone formation, and therefore implicate potential risk factors for stress fracture including sleep deprivation, caloric restriction, and psychological stress.57–59 Furthermore, widespread use of non-steroidal anti-inflammatory drugs (NSAIDs) has also been reported throughout military and athletic populations,60,61 and NSAIDs have been shown to inhibit adaptive bone formation.37 Each of these potential inhibitors of adaptive bone formation is further discussed below.
Caloric restriction and bone formation
Studies of caloric restriction in animals provide evidence of deficits in bone strength due to suppressed bone formation.62,63 Six months of 40% caloric restriction in mice and rats resulted in osteopenia and a reduction in bone volume fraction (bone volume/total volume) of 29% in mice and 25% in rats as compared to control animals.62 Calorie-restricted animals also had decreases in trabecular number and thickness. Histomorphometric analysis determined that the decrements in bone mass and structure were due to decreased bone apposition, rather than from bone resorption. In a study of 35% energy restriction in obese rats, similar declines in bone mineral density and trabecular number were reported.63 Further, 30% caloric restriction from weaning until 6 or 12 weeks of age in mice resulted in impaired cortical and trabecular bone acquisition and 62–63% lower ultimate force and 56–62% lower stiffness at both ages.64 Decrements in bone metabolism have also been shown in calorie-restricted humans with a 10% reduction in true fractional calcium absorption and a 13.5% reduction in serum OCN concentrations in overweight postmenopausal women who underwent six weeks of caloric restriction (reduction in daily caloric intake of 500–600 calories/day).65,66
While studies in animals and postmenopausal women suggest reductions in bone formation with caloric restriction, little evidence is available in young athletic and military-aged individuals. However, in a study of 25-year-old male runners, the bone formation marker, procollagen type 1 N-terminal propeptide (P1NP), was suppressed when running with energy restriction but was not suppressed under conditions of energy balance.67 P1NP was also suppressed in 24-year-old, exercising women with energy deficiency compared to energy replete referents, with the most severe P1NP suppression in women who were also estrogen deficient.68 The latter study highlights a potential indirect effect of caloric restriction on adaptive bone formation in physically active women—low energy availability directly contributes to menstrual disturbances,69 which in turn may inhibit bone formation.69 While these studies suggest that caloric restriction inhibits bone formation, it may not be solely a lack of energy intake that suppresses bone formation; in particular, caloric restriction may be accompanied by insufficient dietary intake of calcium and vitamin D. The importance of these two micronutrients for stress fracture prevention was demonstrated in a randomized, placebo-controlled trial of calcium (2000 mg/day) and vitamin D (800 IU/day) supplementation in Navy recruits, in which stress fractures were reduced by 20% in recruits randomized to supplementation.70 Collectively, these studies suggest that adequate energy, calcium, and vitamin D intake are all necessary to promote bone formation in physically active, young adults. Nevertheless, these studies did not test whether adaptive bone formation in particular was suppressed with inadequate energy, calcium, and vitamin D intake. Moreover, bone formation markers reflect global formation throughout the skeleton and therefore do not distinguish between bone deposited at remodeling sites or on the various surfaces of the bone in the process of modeling. Given all of this, skeletal loading intervention studies will be important for determining the contribution of nutrition to adaptive bone formation and ultimately, stress fracture risk.
Sleep deprivation and bone formation
While the effects of sleep deprivation on bone formation have not been intensively studied, some research exists that may link sleep deprivation to suppressed bone formation. For example, 72 days of sleep restriction in rats (cycles of 10 days of 37% reduction in paradoxical sleep followed by 2 days of ad libitum sleep) resulted in a 45-fold reduction in osteoid-lined bone and a reduced osteoid thickness compared to controls,71 suggesting that bone formation is inhibited with sleep restriction. In a cross-sectional analysis of sleep-deprived men and women (<6.5 h per night), sleep-deprived women had lower cortical volumetric bone mineral density, and sleep-deprived men had lower polar strength-strain indices than sleep-replete controls.72 Although these studies suggest that sleep restriction may inhibit bone formation, the extent to which sleep restriction inhibits adaptive bone formation subsequent to exercise requires further study.
Psychological stress and bone formation
Psychological stress is another potential factor that may lead to suppression of bone formation. Psychological stress may promote inflammatory and hormonal insults to bone tissue.73 In particular, the physically and psychologically demanding nature of military and athletic training has been shown to lead to high levels of inflammatory cytokines and stress hormones such as cortisol.59,74–76 High levels of circulating inflammatory cytokines and cortisol have been associated with low bone density, altered bone metabolism, and increased fracture risk.73,77–79 As both physical and psychological stress contribute to a pro-inflammatory state, research is needed to determine the individual contribution of psychological stress to potential reductions in adaptive bone formation.
NSAIDs and bone formation
As discussed above, prostaglandin production by osteocytes in response to fluid flow is an important step in adaptive bone formation.37 NSAIDs inhibit cyclooxygenase (COX), an enzyme responsible for prostaglandin synthesis, and therefore, may block loading-induced bone formation.80 Several animal studies report complete or partial inhibition of adaptive bone formation with indomethacin, an inhibitor of the constitutive isoform (COX-1) and inducible isoform (COX-2), and with NS-398 (COX-2 inhibitor).37,80–82 These studies suggest adaptive bone formation is inhibited with NSAID use. The timing of NSAID use, however, may be a critical factor in suppression of adaptive bone formation.81,83,84 For example, in rats, indomethacin only suppressed bone formation following an acute bout of exercise if it was administered before, but not after, loading.81 Similarly, NS-398 suppressed bone formation of the rat ulna following mechanical loading if it was given 3 hours before loading, but did not suppress bone formation if administered 30 minutes after loading.80 Comparable findings were reported in a 9-month weight-bearing exercise intervention in premenopausal women who were randomized to three groups: placebo before and after each exercise bout, ibuprofen before and placebo after exercise, or placebo before and ibuprofen after exercise.84 In this study, ibuprofen use before exercise ameliorated gains in bone mineral density. However, if ibuprofen was taken after each exercise bout, the adaptive response to exercise was actually enhanced.84 In a study of exercise in older adults, ibuprofen taken before or after exercise had no effect on the response of bone mineral density to exercise.83 This final study may indicate that the effects of NSAIDs on adaptive bone formation are age dependent. Collectively, these studies suggest that use of NSAIDs and the timing of NSAID ingestion may have consequences for adaptive bone formation. Further research is needed to determine if NSAID use increases stress fracture risk.
Conclusions
Stress fractures occur over a relatively short time period following increased repetitive loading in physically active populations. With increased loading, strains in the bone tissue may lead to a pathophysiological loop of microdamage generation, osteocyte apoptosis, osteoclastic bone resorption, increased porosity, and declines in bone stiffness until stress fracture occurs. Paradoxically, the process of bone remodeling may also be important for extending the fatigue life of bone by removing microdamage through targeted remodeling. Nonetheless, the same strains that produce microdamage also cause perturbation of osteocytes, leading to pro-osteogenic molecular signaling, recruitment of osteoblasts, and de novo bone formation. This new, adaptive bone formation can occur on the trabecular, endocortical, and periosteal surfaces. However, when this bone formation occurs on the periosteum in the shaft of long bones, the cross-sectional moment of inertia increases and fatigue resistance is improved. Therefore, establishing novel exercise regimens aimed at promoting periosteal bone formation may reduce stress fracture incidence. Also, several factors including caloric restriction, sleep deprivation, psychological stress, and use of non-steroidal anti-inflammatory drugs may inhibit adaptive bone formation during periods of increased physical activity and therefore provide new targets for stress fracture prevention.
Acknowledgements
Research supported in part by an appointment to the Postgraduate Research Participation Program funded by USARIEM & administered by Oak Ridge Institute for Science and Engineering (JMH).
Authors' contributions
All authors participated in the conceptual development and writing of the paper. RWM and KLP contributed to figure design.
Disclaimer
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army of or the U.S. Department of Defense.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bennell KL, Malcolm SA, Thomas SA, Wark JD, Brukner PD. The incidence and distribution of stress fractures in competitive track and field athletes. A twelve-month prospective study. Am J Sports Med 1996; 24: 211–7. [DOI] [PubMed] [Google Scholar]
- 2.Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone 2000; 27: 437–44. [DOI] [PubMed] [Google Scholar]
- 3.Khan K. Physical activity and bone health, Champaign, IL: Human Kinetics, 2001. [Google Scholar]
- 4.Breithaupt J. Zur pathologic des menschlichen fusses, Berlin: Med Zeitg, 1855. [Google Scholar]
- 5. Cowan D, Jones B, Shaffer R. Musculoskeletal injuries in the military training environment. In: Lounsbury DE, Bellamy RF, Zajtchuk R (eds) Military preventative medicine: mobilization and deployment. Washington DC: Department of the Army, Office of the Surgeon General, 2003, pp.195–210.
- 6.Finestone A, Milgrom C, Wolf O, Petrov K, Evans R, Moran D. Epidemiology of metatarsal stress fractures versus tibial and femoral stress fractures during elite training. Foot Ankle Int 2011; 32: 16–20. [DOI] [PubMed] [Google Scholar]
- 7.Beck BR, Bergman AG, Miner M, Arendt EA, Klevansky AB, Matheson GO, Norling TL, Marcus R. Tibial stress injury: relationship of radiographic, nuclear medicine bone scanning, MR imaging, and CT Severity grades to clinical severity and time to healing. Radiology 2012; 263: 811–8. [DOI] [PubMed] [Google Scholar]
- 8.Donald JG, Fitts WT., Jr March fractures: a study with special reference to etiological factors. J Bone Joint Surg Am 1947; 29: 297–300. [PubMed] [Google Scholar]
- 9.Schaffler M. Bone fatigue and remodeling in the devlopment of stress fractures. In: Burr D, Milgrom C. (eds). Musculoskeletal fatigue and stress fractures, Boca Raton: CRC Press, 2000, pp. 161–82. [Google Scholar]
- 10.Bennell KL, Brukner PD. Epidemiology and site specificity of stress fractures. Clin Sports Med 1997; 16: 179–96. [DOI] [PubMed] [Google Scholar]
- 11.Karim L, Hussein AI, Morgan EF, Bouxsein, ML. The Mechanical Behavior of Bone. In: Marcus R, Feldman D, Nelson D, Rosen CJ (eds) Osteoporosis, 4th ed. Boston: Elsevier Academic Press, 2013, pp. 431–452.
- 12.Schaffler MB, Radin EL, Burr DB. Mechanical and morphological effects of strain rate on fatigue of compact bone. Bone 1989; 10: 207–14. [DOI] [PubMed] [Google Scholar]
- 13.Pattin CA, Caler WE, Carter DR. Cyclic mechanical property degradation during fatigue loading of cortical bone. J Biomech 1996; 29: 69–79. [DOI] [PubMed] [Google Scholar]
- 14.Herman BC, Cardoso L, Majeska RJ, Jepsen KJ, Schaffler MB. Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone 2010; 47: 766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaffler MB, Radin EL, Burr DB. Long-term fatigue behavior of compact bone at low strain magnitude and rate. Bone 1990; 11: 321–6. [DOI] [PubMed] [Google Scholar]
- 16.Simpson K, Redmond JE, Cohen BS, Hendrickson NR, Spiering BA, Steelman R, Knapik JJ, Sharp MA. Quantification of physical activity performed during US Army Basic Combat Training. US Army Med Dep J 2013; Oct-Dec: 55–65. [PubMed] [Google Scholar]
- 17.Milgrom C, Burr DB, Finestone AS, Voloshin A. Understanding the etiology of the posteromedial tibial stress fracture. Bone 2015; 78: 11–14. [DOI] [PubMed] [Google Scholar]
- 18.Taylor D, Kuiper JH. The prediction of stress fractures using a 'stressed volume' concept. J Orthop Res 2001; 19: 919–26. [DOI] [PubMed] [Google Scholar]
- 19.Currey JD. Bones: structure and mechanics, Princeton: Princeton University Press, 2002. [Google Scholar]
- 20.Martin R. The role of bone remodeling in preventing or promoting stress fracture. In: Burr D, Milgrom C. (eds). Musculoskeletal fatigue and stress fracture, Boca Raton: CRC Press, 2001, pp. 184–200. [Google Scholar]
- 21.Currey JD. Some effects of ageing in human Haversian systems. J Anat 1964; 98: 69–75. [PMC free article] [PubMed] [Google Scholar]
- 22.Martin RB, Burr DB, Sharkey NA. Skeletal tissue mechanics, New York, NY: Springer, 1998. [Google Scholar]
- 23.Frost H. Presence of microscopic cracks in vivo in bone. Henry Ford Hosp Med Bulletin 1960; 8: 25–35. [Google Scholar]
- 24.Kennedy OD, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone 2014; 64: 132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone 1998; 23: 275–81. [DOI] [PubMed] [Google Scholar]
- 26.Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J, Skerry TM, Lanyon LE. Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol 2003; 284: C934–43. [DOI] [PubMed] [Google Scholar]
- 27.Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, Bonewald L, Manolagas SC, O'Brien CA. Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One 2015; 10: e0138189–e0138189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res 2009; 24: 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heaney RP. The bone-remodeling transient: implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res 1994; 9: 1515–23. [DOI] [PubMed] [Google Scholar]
- 30.Parfitt AM. Bone remodeling and bone loss: understanding the pathophysiology of osteoporosis. Clin Obstet Gynecol 1987; 30: 789–811. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs RK, Allen MR, Ruppel ME, Diab T, Phipps RJ, Miller LM, Burr DB. In situ examination of the time-course for secondary mineralization of Haversian bone using synchrotron Fourier transform infrared microspectroscopy. Matrix Biol 2008; 27: 34–41. [DOI] [PubMed] [Google Scholar]
- 32.Bennell KL, Malcolm SA, Brukner PD, Green RM, Hopper JL, Wark JD, Ebeling PR. A 12-month prospective study of the relationship between stress fractures and bone turnover in athletes. Calcif Tissue Int 1998; 63: 80–5. [DOI] [PubMed] [Google Scholar]
- 33.Milgrom C, Finestone A, Novack V, Pereg D, Goldich Y, Kreiss Y, Zimlichman E, Kaufman S, Liebergall M, Burr D. The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone 2004; 35: 418–24. [DOI] [PubMed] [Google Scholar]
- 34.Frost HM. Bone's mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol 2003; 275: 1081–101. [DOI] [PubMed] [Google Scholar]
- 35.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell and more. Endocr Rev 2013; 34: 658–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res 2005; 20: 41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res 1996; 11: 1688–93. [DOI] [PubMed] [Google Scholar]
- 38.Lau KH, Baylink DJ, Zhou XD, Rodriguez D, Bonewald LF, Li Z, Ruffoni D, Muller R, Kesavan C, Sheng MH. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab 2013; 305: E271–81. [DOI] [PubMed] [Google Scholar]
- 39.Watanuki M, Sakai A, Sakata T, Tsurukami H, Miwa M, Uchida Y, Watanabe K, Ikeda K, Nakamura T. Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J Bone Miner Res 2002; 17: 1015–25. [DOI] [PubMed] [Google Scholar]
- 40.Wang N, Rumney RM, Yang L, Robaye B, Boeynaems JM, Skerry TM, Gartland A. The P2Y13 receptor regulates extracellular ATP metabolism and the osteogenic response to mechanical loading. J Bone Miner Res 2013; 28: 1446–56. [DOI] [PubMed] [Google Scholar]
- 41.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 2008; 283: 5866–75. [DOI] [PubMed] [Google Scholar]
- 42.Lara-Castillo N, Kim-Weroha NA, Kamel MA, Javaheri B, Ellies DL, Krumlauf RE, Thiagarajan G, Johnson ML. In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone 2015; 76: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genetos DC, Yellowley CE, Loots GG. Prostaglandin E2 signals through PTGER2 to regulate sclerostin expression. PLoS One 2011; 6: e17772–e17772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brady RT, O'Brien FJ, Hoey DA. Mechanically stimulated bone cells secrete paracrine factors that regulate osteoprogenitor recruitment, proliferation, and differentiation. Biochem Biophys Res Commun 2015; 459: 118–23. [DOI] [PubMed] [Google Scholar]
- 45.Yeh LC, Ma X, Matheny RW, Adamo ML, Lee JC. Protein kinase D mediates the synergistic effects of BMP-7 and IGF-I on osteoblastic cell differentiation. Growth Factors 2010; 28: 318–28. [DOI] [PubMed] [Google Scholar]
- 46.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 2006; 281: 23698–711. [DOI] [PubMed] [Google Scholar]
- 47.Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res 2005; 20: 809–16. [DOI] [PubMed] [Google Scholar]
- 48.Popp KL, Hughes JM, Smock AJ, Novotny SA, Stovitz SD, Koehler SM, Petit MA. Bone geometry, strength, and muscle size in runners with a history of stress fracture. Med Sci Sports Exerc 2009; 41: 2145–50. [DOI] [PubMed] [Google Scholar]
- 49.Schnackenburg KE, Macdonald HM, Ferber R, Wiley JP, Boyd SK. Bone quality and muscle strength in female athletes with lower limb stress fractures. Med Sci Sports Exerc 2011; 43: 2110–19. [DOI] [PubMed] [Google Scholar]
- 50.Jepsen KJ, Evans R, Negus CH, Gagnier JJ, Centi A, Erlich T, Hadid A, Yanovich R, Moran DS. Variation in tibial functionality and fracture susceptibility among healthy, young adults arises from the acquisition of biologically distinct sets of traits. J Bone Miner Res 2013; 28: 1290–300. [DOI] [PubMed] [Google Scholar]
- 51.Cosman F, Ruffing J, Zion M, Uhorchak J, Ralston S, Tendy S, McGuigan FE, Lindsay R, Nieves J. Determinants of stress fracture risk in United States Military Academy cadets. Bone 2013; 55: 359–66. [DOI] [PubMed] [Google Scholar]
- 52.Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med 1999; 28: 91–122. [DOI] [PubMed] [Google Scholar]
- 53.Milgrom C, Simkin A, Eldad A, Nyska M, Finestone A. Using bone's adaptation ability to lower the incidence of stress fractures. Am J Sports Med 2000; 28: 245–51. [DOI] [PubMed] [Google Scholar]
- 54.Hughes JM, Smith MA, Henning PC, Scofield DE, Spiering BA, Staab JS, Hydren JR, Nindl BC, Matheny RW., Jr Bone formation is suppressed with multi-stressor military training. Eur J Appl Physiol 2014; 114: 2251–9. [DOI] [PubMed] [Google Scholar]
- 55.Nindl BC, Barnes BR, Alemany JA, Frykman PN, Shippee RL, Friedl KE. Physiological consequences of U.S. Army Ranger training. Med Sci Sports Exerc 2007; 39: 1380–7. [DOI] [PubMed] [Google Scholar]
- 56.Johnson HL, Krzywicki HJ, Canham JE, Skala JH, Daws TA, Nelson RA, Consolazio CF, Waring PP. Evaluation of calorie requirements for ranger training at Fort Benning, Georgia, Presidio of San Francisco, CA: Letterman Army Institute of Research, 1976. [Google Scholar]
- 57.Friedl KE. When does energy deficit affect soldier physical performance? In: Marriott BM (ed) Not eating enough. Washington, DC: National Academy Press, 1995, pp.253–83.
- 58.Lieberman HR, Castellani JW, Young AJ. Cognitive function and mood during acute cold stress after extended military training and recovery. Aviat Space Environ Med 2009; 80: 629–36. [DOI] [PubMed] [Google Scholar]
- 59.Henning PC, Scofield DE, Spiering BA, Staab JS, Matheny RW, Jr., Smith MA, Bhasin S, Nindl BC. Recovery of endocrine and inflammatory mediators following an extended energy deficit. J Clin Endocrinol Metab 2014; 99: 956–64. [DOI] [PubMed] [Google Scholar]
- 60.Zambraski E. Widespread and increasing use of prescription non-steroidal anti-inflammatory drugs (NSAIDs) among active duty soldiers. In: Military health system research symposium, Ft. Lauderdale, FL, 13 August 2012.
- 61.Feucht CL, Patel DR. Analgesics and anti-inflammatory medications in sports: use and abuse. Pediatr Clin North Am 2010; 57: 751–74. [DOI] [PubMed] [Google Scholar]
- 62.Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology 2008; 149: 634–41. [DOI] [PubMed] [Google Scholar]
- 63.Shen CL, Zhu W, Gao W, Wang S, Chen L, Chyu MC. Energy-restricted diet benefits body composition but degrades bone integrity in middle-aged obese female rats. Nutr Res 2013; 33: 668–76. [DOI] [PubMed] [Google Scholar]
- 64.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res 2010; 25: 2078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shapses SA, Sukumar D, Schneider SH, Schlussel Y, Sherrell RM, Field MP, Ambia-Sobhan H. Vitamin D supplementation and calcium absorption during caloric restriction: a randomized double-blind trial. Am J Clin Nutr 2013; 97: 637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sukumar D, Shapses SA, Schneider SH. Vitamin D supplementation during short-term caloric restriction in healthy overweight/obese older women: Effect on glycemic indices and serum osteocalcin levels. Mol Cell Endocrinol 2015; 410: 73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zanker CL, Swaine IL. Responses of bone turnover markers to repeated endurance running in humans under conditions of energy balance or energy restriction. Eur J Appl Physiol 2000; 83: 434–40. [DOI] [PubMed] [Google Scholar]
- 68.De Souza MJ, West SL, Jamal SA, Hawker GA, Gundberg CM, Williams NI. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone 2008; 43: 140–8. [DOI] [PubMed] [Google Scholar]
- 69.Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab 2001; 86: 5184–93. [DOI] [PubMed] [Google Scholar]
- 70.Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res 2008; 23: 741–9. [DOI] [PubMed] [Google Scholar]
- 71.Everson CA, Folley AE, Toth JM. Chronically inadequate sleep results in abnormal bone formation and abnormal bone marrow in rats. Exp Biol Med (Maywood) 2012; 237: 1101–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Specker BL, Binkley T, Vukovich M, Beare T. Volumetric bone mineral density and bone size in sleep-deprived individuals. Osteoporos Int 2007; 18: 93–9. [DOI] [PubMed] [Google Scholar]
- 73.Bedford JL, Barr SI. The relationship between 24-h urinary cortisol and bone in healthy young women. Int J Behav Med 2010; 17: 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diment BC, Fortes MB, Greeves JP, Casey A, Costa RJ, Walters R, Walsh NP. Effect of daily mixed nutritional supplementation on immune indices in soldiers undertaking an 8-week arduous training programme. Eur J Appl Physiol 2012; 112: 1411–8. [DOI] [PubMed] [Google Scholar]
- 75.Kyrolainen H, Karinkanta J, Santtila M, Koski H, Mantysaari M, Pullinen T. Hormonal responses during a prolonged military field exercise with variable exercise intensity. Eur J Appl Physiol 2008; 102: 539–46. [DOI] [PubMed] [Google Scholar]
- 76.Landers-Ramos RQ, Jenkins NT, Spangenburg EE, Hagberg JM, Prior SJ. Circulating angiogenic and inflammatory cytokine responses to acute aerobic exercise in trained and sedentary young men. Eur J Appl Physiol 2014; 114: 1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greendale GA, Unger JB, Rowe JW, Seeman TE. The relation between cortisol excretion and fractures in healthy older people: results from the MacArthur studies-Mac. J Am Geriatr Soc 1999; 47: 799–803. [DOI] [PubMed] [Google Scholar]
- 78.Dennison E, Hindmarsh P, Fall C, Kellingray S, Barker D, Phillips D, Cooper C. Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J Clin Endocrinol Metab 1999; 84: 3058–63. [DOI] [PubMed] [Google Scholar]
- 79.Gertz ER, Silverman NE, Wise KS, Hanson KB, Alekel DL, Stewart JW, Perry CD, Bhupathiraju SN, Kohut ML, Van Loan MD. Contribution of serum inflammatory markers to changes in bone mineral content and density in postmenopausal women: a 1-year investigation. J Clin Densitom 2010; 13: 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J, Burr DB, Turner CH. Suppression of prostaglandin synthesis with NS-398 has different effects on endocortical and periosteal bone formation induced by mechanical loading. Calcif Tissue Int 2002; 70: 320–9. [DOI] [PubMed] [Google Scholar]
- 81.Chow JW, Chambers TJ. Indomethacin has distinct early and late actions on bone formation induced by mechanical stimulation. Am J Physiol 1994; 267: E287–92. [DOI] [PubMed] [Google Scholar]
- 82.Pead MJ, Lanyon LE. Indomethacin modulation of load-related stimulation of new bone formation in vivo. Calcif Tissue Int 1989; 45: 34–40. [DOI] [PubMed] [Google Scholar]
- 83.Jankowski CM, Shea K, Barry DW, Linnebur SA, Wolfe P, Kittelson J, Schwartz RS, Kohrt WM. Timing of ibuprofen use and musculoskeletal adaptations to exercise training in older adults. Bone Rep 2015; 1: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kohrt WM, Barry DW, Van Pelt RE, Jankowski CM, Wolfe P, Schwartz RS. Timing of ibuprofen use and bone mineral density adaptations to exercise training. J Bone Miner Res 2010; 25: 1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]