Abstract
Depression of heart-rate variability (HRV) in conditions of systemic inflammation has been shown in both patients and experimental animal models and HRV has been suggested as an early indicator of sepsis. The sensitivity of HRV-derived parameters to the severity of sepsis, however, remains unclear. In this study we modified the clinically relevant porcine model of peritonitis-induced sepsis in order to avoid the development of organ failure and to test the sensitivity of HRV to such non-severe conditions. In 11 anesthetized, mechanically ventilated and instrumented domestic pigs of both sexes, sepsis was induced by fecal peritonitis. The dose of feces was adjusted and antibiotic therapy was administered to avoid multiorgan failure. Experimental subjects were screened for 40 h from the induction of sepsis. In all septic animals, sepsis with hyperdynamic circulation and increased plasma levels of inflammatory mediators developed within 12 h from the induction of peritonitis. The sepsis did not progress to multiorgan failure and there was no spontaneous death during the experiment despite a modest requirement for vasopressor therapy in most animals (9/11). A pronounced reduction of HRV and elevation of heart rate developed quickly (within 5 h, time constant of 1.97 ± 0.80 h for HRV parameter TINN) upon the induction of sepsis and were maintained throughout the experiment. The frequency domain analysis revealed a decrease in the high-frequency component. The reduction of HRV parameters and elevation of heart rate preceded sepsis-associated hemodynamic changes by several hours (time constant of 11.28 ± 2.07 h for systemic vascular resistance decline). A pronounced and fast reduction of HRV occurred in the setting of a moderate experimental porcine sepsis without organ failure. Inhibition of parasympathetic cardiac signaling probably represents the main mechanism of HRV reduction in sepsis. The sensitivity of HRV to systemic inflammation may allow early detection of a moderate sepsis without organ failure.
Impact statement
A pronounced and fast reduction of heart-rate variability occurred in the setting of a moderate experimental porcine sepsis without organ failure. Dominant reduction of heart-rate variability was found in the high-frequency band indicating inhibition of parasympathetic cardiac signaling as the main mechanism of heart-rate variability reduction. The sensitivity of heart-rate variability to systemic inflammation may contribute to an early detection of moderate sepsis without organ failure.
Keywords: Sepsis, heart-rate variability, experimental model, pig
Introduction
Sepsis remains a major health care problem of the new millennium. Annually, there are up to 20 million diagnosed cases of sepsis worldwide1 and sepsis continues to show progressively increasing incidence and high mortality.2–4
Early and sensitive diagnosis of sepsis that would allow effective early therapeutic interventions is, however, still not available. The classification of sepsis and its progression is currently based mainly on scoring systems that include infection, inflammatory, hemodynamic, and organ failure symptoms5 and inherently cannot precede the overt clinical sepsis to provide a significant therapeutic window for early treatments.
Depression of heart-rate variability (HRV) in conditions of systemic inflammation has been repeatedly shown in both patients6–10 and experimental animal models.11–13 In some studies, a predictive power of HRV was documented, in which depression of HRV-derived parameters preceded the onset of overt clinical sepsis by several hours.11,14,15
The sensitivity of HRV-derived parameters to the severity of sepsis, however, remains unclear. In general, even in experimental models it is difficult to control progression of sepsis and to prevent deterioration to septic shock with multiple organ failure. In our previous study we have shown that in conditions of such a progressive septic shock an early and marked reduction of HRV parameters occurs.11 In this study we extended our observations toward less severe forms of sepsis and hypothesized that HRV is significantly reduced even by moderate sepsis without organ failure. The clinically relevant porcine model of peritonitis-induced septic shock used in the earlier study11 was modified in order to avoid development of organ failure and the sensitivity of HRV to such non-severe conditions was investigated.
Materials and methods
Animal handling was in accordance with the European Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (86/609/EU). The experiments were approved by the Committee for Experiments on Animals of the Charles University Faculty of Medicine in Pilsen (protocol No. MSMT-26770/2012-30). All experiments were performed in the animal research laboratory at the Faculty of Medicine in Pilsen. Sixteen domestic pigs (breed Prestice black-pied pig) of both sexes and of similar weight (41 ± 5 kg) were used for experiments. Pigs were purchased from a commercial farm (ZD Mladotice, Czech Republic) and housed individually in the Central animal facility of the Charles University Faculty of Medicine in Pilsen in pens (size 2 m2) with constant temperature (19℃) and humidity (55%), 12/12 light/dark cycle, unrestrained access to drinking water and normal diet. Enrichment devices and materials were provided and the animals were handled by experienced technicians. Sepsis was induced by fecal peritonitis in 11 pigs (9 boars, 2 sows). Control sham experiments (following an analogous procedure but without sepsis induction) were performed in five pigs (3 boars, 2 sows) and no major nonspecific effects of experimental procedures (surgery, anesthesia, sampling) were found.
Anesthesia and instrumentation
Anesthesia and ventilation protocols were identical to those previously described.11 Instrumentation for hemodynamic monitoring and blood sampling included insertion of femoral artery catheter, triple lumen central venous catheter, pulmonary artery catheter, and silicone drains into Morison and Douglas anatomical spaces for fecal inoculation.
Experimental protocol
Experimental protocols were similar to those previously described11 except for the use of a lower dose of feces for peritonitis induction and application of antibiotic therapy to prevent multiorgan failure. Peritonitis was induced by inoculating 0.5 g/kg of autologous feces (for each animal autologous feces were collected 1 h before induction of anesthesia, suspended in 200 mL isotonic saline solution and incubated at 37℃ until the time of application) into the abdominal cavity. Antibiotic therapy (piperacillin/tazabactam, 2.25 g every 8 h) was started 6 h after induction of sepsis and maintained throughout the entire experiment. In total, the experiment lasted 52–56 h (2 h of surgery, 6 h of recovery and 44–48 h after induction of peritonitis). The animals were maintained throughout the entire experiment in surgical anesthesia, mechanically ventilated and continuously monitored by an experienced researcher with an intensive care medicine background (see above). Eventually the animals were euthanized by potassium chloride injection under deep anesthesia, and necropsy was performed.
Measurements, calculations, and HRV analysis
Hemodynamic parameters (cardiac output (CO, mL/min/kg), systemic vascular resistance (SVR, dyn·s/cm5) and arterial pressure (mmHg), plasma levels of tumor necrosis factor-alpha (TNF-α, ng/L) and interleukin 6 (IL-6, ng/L), and ECG were measured as described previously.11 HRV analysis was described in detail in previous report11 and statistical (SDNN, SDSD, pNN50), geometrical (HRV TI, TINN), Poincaré plot (SD1, SD2, S), and frequency domain parameters of HRV (LF, HF) were obtained.16–18
Statistical analysis
Results are presented as means ± SD. After testing for normality of distribution (Shapiro–Wilk test), statistical comparisons were made with the two-way mixed-design ANOVA (one repeated-measures factor for analysis of the progression of parameter in time and one between-groups factor for comparison between control and septic groups) followed by post hoc Dunn-Sidak test or by one-way ANOVA with repeated measures (analysis of single group) followed by post hoc Dunnett tests. The analysis was performed using software Origin 2017 (OriginLab Corp., Northampton, MA). Values of P < 0.05 were considered significant.
Results
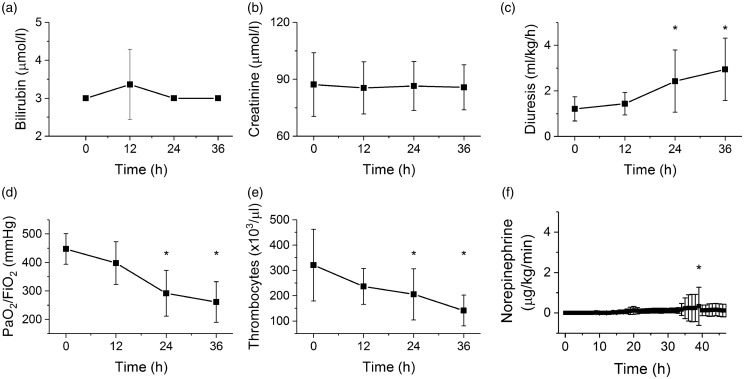
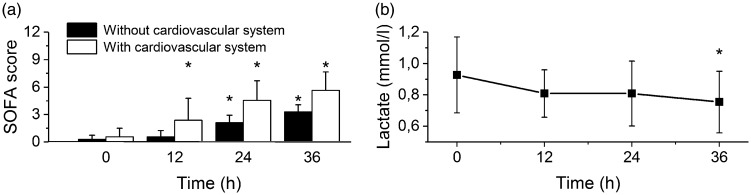
Functions of organ systems were evaluated according to the Sequential Organ Failure Assessment (SOFA) score.5,19 With progression of sepsis, liver (bilirubin plasma levels, Figure 1(a)) and renal parameters (creatinine plasma levels, Figure 1(b)) were not affected and remained stable throughout the experiment. Diuresis was even increased during the experiment probably reflecting volume resuscitation measures (Figure 1(c)). Respiration (PaO2/FiO2, Figure 1(d)) and coagulation (platelet counts, Figure 1(e)) parameters showed a gradual decline, becoming significantly different from baseline 24 h after induction of sepsis. The central nervous system was not evaluated with regard to the general anesthesia applied through the entire experiment. In most septic animals (9 out of 11), norepinephrine had to be administered to maintain MAP above 70 mmHg, although the doses were very low (Figure 1(f)) and administration was usually only transient (in 6 out of 9) indicating a spontaneous recovery. The SOFA score5,19 gradually increased with a dominant contribution (61 ± 43% at 12 h, 42 ± 34% at 24 h and 34 ± 28% at 36 h) of the cardiovascular system and norepinephrine administration (Figure 2(a)). The serum lactate levels were not elevated by the experiment or even decreased (at 36 h from the induction of sepsis, Figure 2(b)).
Figure 1.
Organ function in the porcine model of sepsis. *Significantly different from baseline (P < 0.05), data presented as means ± SD. (a) Plasma levels of bilirubin during progression of sepsis. (b) Plasma levels of creatinine during progression of sepsis. (c) Diuresis (mL/kg/h) during progression of sepsis. (d) Ratio of partial pressure of oxygen in arterial blood and fraction of inspired oxygen during progression of sepsis. (e) Platelet counts during progression of sepsis. (f) Therapeutic doses of norepinephrine during progression of sepsis
Figure 2.
SOFA score and lactate. *Significantly different from baseline (P < 0.05), data presented as means ± SD. (a) SOFA score during progression of sepsis with (empty columns) and without (filled columns) cardiovascular (vasopressor) contribution. (b) Lactate plasma levels during progression of sepsis
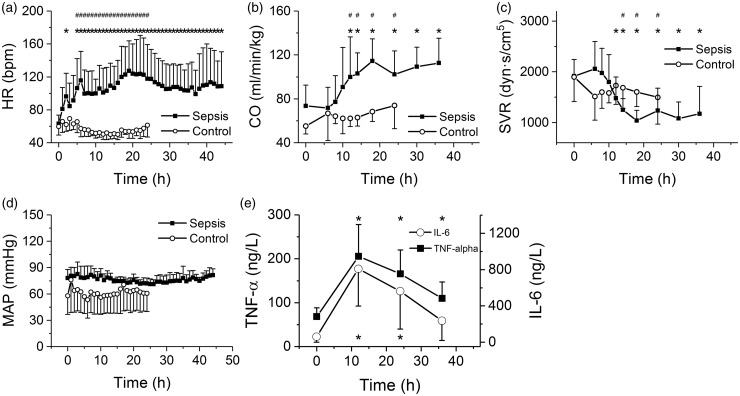
In all septic animals, hyperdynamic circulation with an increased heart rate (Figure 3(a)), increased cardiac output (Figure 3(b)), and reduced systemic vascular resistance (Figure 3(c)) developed. MAP was maintained above 70 mmHg throughout the entire experiment (Figure 3(d)), which required in most septic animals administration of norepinephrine (Figure 1(f)). In control animals, all hemodynamic parameters remained stable throughout the entire experiment (Figure 3(a–d)). Plasma levels of inflammatory mediators (TNF-α, IL-6) increased during the first 12 h of sepsis and then tended to decline (Figure 3(e)).
Figure 3.
Hemodynamic characteristics of porcine sepsis. *Significantly different from baseline (P < 0.05); #significantly different from control, nonseptic group (P < 0.05); data presented as means ± SD. (a) Heart rate (bpm) during progression of sepsis; closed squares, sepsis, open circles, control. (b) Cardiac output (normalized to the body weight) during progression of sepsis; closed squares, sepsis, open circles, control. (c) Systemic vascular resistance during progression of sepsis; closed squares, sepsis, open circles, control. (d) Mean arterial pressure during progression of sepsis; closed squares, sepsis, open circles, control. (f) Plasma levels of IL-6 and TNF-α during progression of sepsis; closed squares, TNF-alpha, open circles, IL-6
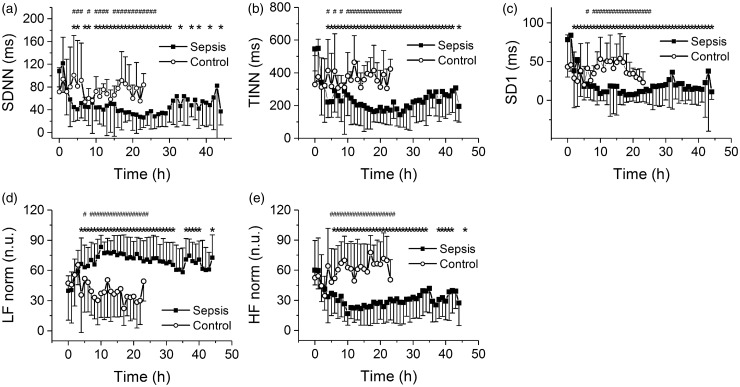
Statistical parameters of HRV (SDNN, SDSD, pNN50) were significantly reduced within several hours from the induction of sepsis and remained low throughout the experiment (SDNN shown in Figure 4(a)), although some tendency to recovery developed at late stages (>40 h) of sepsis. A similar pattern of fast and subsequently maintained reduction was also found for geometrical parameters of HRV (HRV TI and TINN, TINN shown in Figure 4(b)). With non-linear Poincare plot parameters, the pattern was well pronounced for S and SD1 (Figure 4(c)) but not for SD2 (not shown). The frequency domain analysis revealed a reduction of the HF component, whereas the LF component was not altered (not shown). When expressed in normalized units, the reduction of the HFnorm component was accompanied by an increase in LFnorm component (Figure 4(d, e)). Analysis of the time courses of hemodynamic parameters and of HRV revealed that the kinetics of the HRV reduction were faster than those of the hemodynamic parameters (time constant of 1.97 ± 0.80 h for HRV parameter TINN, time constant of 4.82 ± 2.10 h for heart rate increase, time constant of 11.28 ± 2.07 h for systemic vascular resistance decline, P < 0.05), however the onset of significant HRV reduction and heart rate elevation was similar.
Figure 4.
HRV parameters. *Significantly different from baseline (P < 0.05); #significantly different from control, nonseptic group (P < 0.05); data presented as means ± SD; closed squares, sepsis, open circles, control. (a) SDNN, standard deviation of RR intervals, during progression of sepsis. (b) TINN, baseline width of the minimum square difference triangular interpolation, during progression of sepsis. (c) SD1, SD of points perpendicular to the identity line, during progression of sepsis. (d) Normalized low-frequency band component during progression of sepsis. (e) Normalized high-frequency band during progression of sepsis
In control experiments (without the induction of sepsis) the variability parameters (both time and frequency domains) were not altered (no significant difference compared to baseline values) and remained similar throughout the entire 24-h-long experiment (Figure 4(a–e), open symbols).To address the predictive potential of HRV in the conditions of porcine sepsis, the sensitivity, specificity, negative and positive predictive values, and Youden’s index were calculated for several cut-off values of SDNN (Table 1). For the SDNN cut-off value of 50 ms a good combination of sensitivity, specificity, positive, and negative predictive value was obtained beginning 5 h after induction of peritonitis.
Table 1.
Sensitivity, specificity, positive, and negative predictive values for SDNN.a
| SDNN (ms) | Time (h) | Sensitivity | Specificity | PPV | NPV | Youden’s |
|---|---|---|---|---|---|---|
| <100 | 0 | 0.18 | 0.40 | 0.40 | 0.18 | |
| 5 | 1 | 0.4 | 0.79 | 1 | 0.4 | |
| 10 | 1 | 0.2 | 0.73 | 1 | 0.2 | |
| 15 | 1 | 0 | 0.69 | – | 0 | |
| 20 | 1 | 0.2 | 0.73 | 1 | 0.2 | |
| <75 | 0 | 0.09 | 0.8 | 0.5 | 0.29 | |
| 5 | 1 | 0.4 | 0.79 | 1 | 0.4 | |
| 10 | 0.91 | 0.4 | 0.77 | 0.67 | 0.31 | |
| 15 | 0.91 | 0.6 | 0.83 | 0.75 | 0.51 | |
| 20 | 0.91 | 0.4 | 0.77 | 0.67 | 0.31 | |
| <50 | 0 | 0 | 0.8 | 0 | 0.27 | |
| 5 | 0.82 | 0.8 | 0.9 | 0.67 | 0.61 | |
| 10 | 0.82 | 1 | 1 | 0.71 | 0.82 | |
| 15 | 0.82 | 1 | 1 | 0.71 | 0.82 | |
| 20 | 0.82 | 1 | 1 | 0.71 | 0.82 | |
| <25 | 0 | 0 | 1 | – | 0.31 | 0 |
| 5 | 0.09 | 1 | 1 | 0.33 | 0.09 | |
| 10 | 0.27 | 1 | 1 | 0.38 | 0.27 | |
| 15 | 0.55 | 1 | 1 | 0.5 | 0.55 | |
| 20 | 0.55 | 1 | 1 | 0.5 | 0.55 |
PPV: positive predictive value; NPV: negative predictive value; Youden’s: Youden’s index.
Sensitivity, specificity, positive and negative predictive values, and Youden’s index were calculated for several cut-off values of SDNN at times of 0, 5, 10, 15, and 20 h after induction of peritonitis.
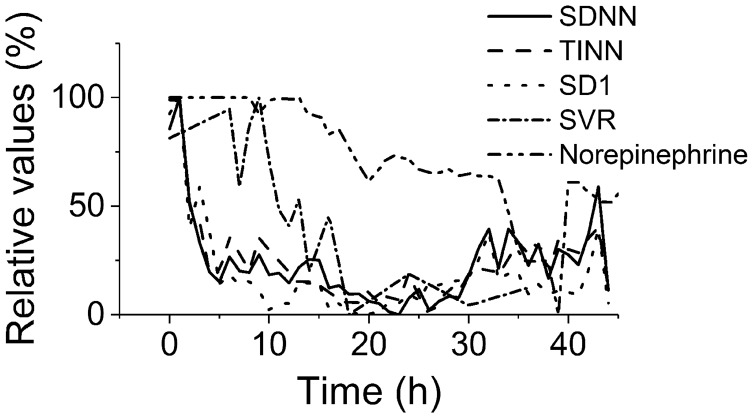
Finally, the HRV and hemodynamic parameters were expressed in relative terms (with the baseline value being 100%) to compare the kinetics of the processes (Figure 5). The kinetics of all HRV parameters (statistical, geometrical, Poincare) were similar and preceded hemodynamic changes by several hours (e.g. reduction of systemic vascular resistance). Therapeutic application of norepinephrine to maintain the MAP above 70 mmHg was further delayed by an additional several hours. There was no premature death due to refractory septic shock; all animals were euthanized according to the original experimental time schedule > 40 h after induction of sepsis. This was very different from our traditional experimental scenario with higher dose of feces, in which refractory septic shock with lethal consequences usually developed within 24 h.11
Figure 5.
Time relationships of HRV and hemodynamics. Expressed in relative values (100% corresponds to baseline). Depression of HRV parameters (SDNN, full line, TINN, dashed line, and SD1, dotted line) preceded the hemodynamic instability (systemic vascular resistance, dash-and-dotted line; reverse norepinephrine dose calculated as a difference of the respective dose and baseline 100%, double-dot-and-dashed line)
Discussion
The principal question of the study was how sensitive the HRV parameters are to less severe forms of sepsis. Previously, we have shown that in the porcine model of peritonitis-induced progressive septic shock, HRV parameters are substantially and quickly reduced.11 In this study the model was modified by decreasing the dose of feces and by administration of antibiotic therapy to prevent multiorgan failure. Analysis of organ dysfunction and tissue perfusion variables revealed that organ failure probably did not develop: diuresis increased during the experiment to values above 2 mL/kg/h; plasma levels of creatinine and bilirubin did not change throughout the experiment; and plasma levels of lactate slightly decreased during the experiment. Platelet counts showed a decreasing trend during the experiment; however, the values remained above 100,000/µL. Although the animals were artificially ventilated, the PaO2/FiO2 ratio gradually decreased during the experiment. However, it only became lower than the borderline value of 300 mmHg after 24 h of progression of sepsis.
Interpretation of the hemodynamic variables, however, is less straightforward. Hyperdynamic circulation with peripheral vasodilation developed in all animals. To maintain the MAP above 65 mmHg, norepinephrine had to be administered in most animals (in 9/11) although usually (in 6/9) only transiently. The requirement for vasopressor is rather incompatible with the other organ failure variables mentioned above, which did not (at least for the first 24 h of progression of sepsis) show any significant organ impairment. It is questionable, however, whether the MAP target of 65 mmHg recommended by clinical guidelines20 is relevant for this porcine model. In a recent porcine study,21 a lower MAP target (50–60 mmHg) did not affect the inflammatory response, mitochondrial respiration, or skeletal muscle ATP content, although it was associated with increased incidence of acute kidney injury. It may be speculated that with a lower MAP target the vasopressor therapy would not be necessary and then, according to the traditional sepsis staging definitions,22 not even severe sepsis developed in the model. Similarly, without the cardiovascular (vasopressor) contribution the SOFA score was below 2 points for the first 24 h. Such a low SOFA score argues against organ dysfunction and in a general hospital population with suspected infection it would reflect a low risk of overall mortality.5 According to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-35), patients with septic shock can be identified by the clinical construct of: sepsis with persisting hypotension requiring vasopressors to maintain MAP 65 mmHg, and a serum lactate level > 2 mmol/L despite adequate volume resuscitation. Although in the porcine model the persisting hypotension requiring vasopressors was often encountered, hyperlactatemia never developed, indicating an adequate tissue oxygenation. Perhaps the discrepancy between mild (if any) organ impairment and requirement of vasopressors reflects species differences, with a higher susceptibility of the porcine model to septic hypotension. All in all, the results indicate that in this porcine model (breed Prestice black-pied pig) multiorgan failure did not develop for (at least) 24 h of progression of sepsis despite a modest requirement of vasopressor therapy.
The time domain parameters of HRV (statistical, geometrical and non-linear Poincare plot) showed a pronounced and fast decrease of HRV. The frequency domain analysis revealed that dominant reduction occurred in the HF band, whereas the LF band was not influenced. Since the HF band represents the parasympathetic modulation of the heart, whereas the LF component represents a mixture of both sympathetic and parasympathetic cardiac modulations, clearly the cardiac sympathovagal balance in porcine sepsis shifts toward sympathetic dominance. A recent series of studies23 introduced the concept of a cholinergic anti-inflammatory pathway, through which the nervous and humoral responses to infection are linked. Vagal stimulation was shown to suppress the production of inflammatory mediators (e.g. TNF-α) as well as systemic inflammation.24 In this line of thinking, inhibition of the parasympathetic system would promote systemic inflammation and progression of sepsis. The reduction of the HF band of HRV, which reflects vagal inhibition, may therefore point to the inhibition and/or insufficient activation of the cholinergic anti-inflammatory pathway as an important pathophysiological mechanism of porcine sepsis.
The HRV reduction and heart rate elevation were very fast (time constants below 5 hours) and preceded the hemodynamic changes (systemic vascular resistance, cardiac output) by several hours. This time relationship of HRV and hemodynamic changes excludes the possibility that HRV reduction occurs secondarily due to hemodynamic changes and consequent autonomic dysregulation. On the other hand, the plasma levels of pro-inflammatory mediators were elevated on a similar time scale as HRV reduction, suggesting a common pathophysiological mechanism for both processes. Suppression of the cholinergic anti-inflammatory pathway would both reduce the HF band of HRV and promote production of pro-inflammatory mediators and therefore it may represent a reasonable candidate of such integrative pathophysiological mechanism. It should be emphasized, however, that this study does not provide any direct evidence of such an integrative mechanism and that the nervous and humoral pathways may be completely separate. In any case, the early response of HRV to the infectious insult, well before the onset of overt clinical sepsis, allows early diagnosis of systemic inflammation and widening the therapeutic windows for early interventions.
The relationship between heart rate, HRV, and sepsis will require further investigation. Although the heart rate changed with slower kinetics (longer time constant) than HRV, it started to rise early and in the early phase of sepsis it paralleled the reduction of HRV. It is possible that, especially in the early phase, the changes in average heart rate contribute in this experimental model to the HRV reduction simply on mathematical basis.25 Nevertheless, although on the one hand it is difficult to dissect the exact contribution of heart rate to HRV changes, on the other hand it is possible by a mathematical modification to strengthen or weaken the dependence of HRV on heart rate (by either multiplying or dividing HRV parameters by the corresponding average RR intervals25) and consequently to optimize the predictive power of HRV parameters. Further investigations with interventions into the autonomic nervous system or pacemaking mechanisms (e.g. inhibition of If channels) will be necessary to clarify these relationships and to define the best set of diagnostic parameters.
In general, the results of this study are in line with a number of previous studies6–15 that have documented the relationship between systemic infection and HRV. Probably the most advanced clinical applications of HRV can be found in neonatal intensive care medicine, in which HRV analysis has already been shown to add independent information to clinical signs scores and to allow for earlier diagnosis of neonatal sepsis.7,26 Randomized clinical trials have revealed that heart rate characteristics monitoring can reduce the mortality rate in very low-birth weight infants27 and is associated with a lower septicemia-associated mortality.28 The main novel finding of this study is that the HRV is affected by even a moderate porcine sepsis without organ failure. However, when the HRV responses to a porcine moderate sepsis without multiorgan failure (this study) and to a porcine progressive lethal septic shock (earlier study by this group11) were compared, no major differences were found. This indicates that the sensitivity of HRV to systemic inflammation is high but, on the other hand, HRV will probably not allow scaling and staging of the systemic inflammation, at least in conditions of similar infectious stimulus as in our experimental setting of fecal peritonitis-induced sepsis. Reliable staging of inflammation processes will probably require an integrative approach with additional parameters like biomarker dynamics29 and/or biochemical variables. In this porcine peritonitis model, the plasma levels of lactate could represent such a discriminating factor. Whereas in the group of moderate sepsis without organ failure (this study) the plasma levels of lactate did not rise, in the group of progressive septic shock11 a significant increase was observed. It remains for future studies to determine whether the pattern of HRV response is largely constant for a variety of infectious stimuli or whether some specific differences can be found.
Study limitations
Only the classical time and frequency domain analyses of HRV together with non-linear Poincare plot analysis were employed in this study. Although a number of more advanced non-linear analyses are now available,30 the signal (HRV reduction) was sufficiently robust to be detected by the classical methods selected.
The causal relationship between the pathophysiological processes of sepsis and the HRV reduction remains unclear. Although some indirect evidence suggests the cholinergic anti-inflammatory pathway as a common denominator of progression of sepsis and HRV, there remain a number of unresolved issues, which will require further attention.31
The septic experiments were performed in nine boars and two sows, which hampers proper analysis of sex-specific differences. Although in none of our previous studies with the porcine peritonitis-induced sepsis model (e.g. Jarkovska et al.11) sex-specific differences in the response to the infection were observed, they cannot be completely excluded.
Although the frequency domain analysis points to autonomic dysbalance as the dominant mechanism of HRV suppression in sepsis, direct effects of inflammatory mediators on myocardium with subsequent septic cardiomyopathy cannot be excluded. Further research will be necessary to elucidate possible interactions of myocardium and inflammatory mediators.
Acknowledgments
The authors thank prof. Sarah Leupen (University of Maryland Baltimore County, USA) for proofreading the manuscript. This study was supported by the Grant Agency of the Czech Republic (project No. 15-15716S), by the Ministry of Health of the Czech Republic (grant No. 15-32801A), by the National Sustainability Program I No. LO1503 provided by the Ministry of Education, Youth and Sports of the Czech Republic and by the Charles University Research Fund (project No. P36).
Authors' contributions
All authors participated in interpretation of the studies, analysis of the data, and review of the manuscript; DJ, LV, JC, JB, and VD conducted the experiments, DJ, JS, and LN participated in HRV analysis; MM and MS conceived of and designed the study and drafted the manuscript.
Declarations of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet 2010; 376: 1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348: 1546–54. [DOI] [PubMed] [Google Scholar]
- 3.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM. ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372: 1301–11. [DOI] [PubMed] [Google Scholar]
- 4.Sartelli M, Catena F, Di Saverio S, Ansaloni L, Malangoni M, Moore EE, Moore FA, Ivatury R, Coimbra R, Leppaniemi A, Biffl W, Kluger Y, Fraga GP, Ordonez CA, Marwah S, Gerych I, Lee JG, Tranà C, Coccolini F, Corradetti F, Kirkby-Bott J. Current concept of abdominal sepsis: WSES position paper. World J Emerg Surg WJES 2014; 9: 22–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnaby D, Ferrick K, Kaplan DT, Shah S, Bijur P, Gallagher EJ. Heart rate variability in emergency department patients with sepsis. Acad Emerg Med 2002; 9: 661–70. [DOI] [PubMed] [Google Scholar]
- 7.Bohanon FJ, Mrazek AA, Shabana MT, Mims S, Radhakrishnan GL, Kramer GC, Radhakrishnan RS. Heart rate variability analysis is more sensitive at identifying neonatal sepsis than conventional vital signs. Am J Surg 2015; 210: 661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godin PJ, Fleisher LA, Eidsath A, Vandivier RW, Preas HL, Banks SM, Buchman TG, Suffredini AF. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit Care Med 1996; 24: 1117–24. [DOI] [PubMed] [Google Scholar]
- 9.Griffin MP, Lake DE, Bissonette EA, Harrell FE, Jr, O'Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 2005; 116: 1070–74. [DOI] [PubMed] [Google Scholar]
- 10.Korach M, Sharshar T, Jarrin I, Fouillot JP, Raphaël JC, Gajdos P, Annane D. Cardiac variability in critically ill adults: influence of sepsis. Crit Care Med 2001; 29: 1380–85. [DOI] [PubMed] [Google Scholar]
- 11.Jarkovska D, Valesova L, Chvojka J, Benes J, Sviglerova J, Florova B, Nalos L, Matejovic M, Stengl M. Heart rate variability in porcine progressive peritonitis-induced sepsis. Front Physiol 2016; 6: 412–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pancoto JA, Corrêa PB, Oliveira-Pelegrin GR, Rocha MJ. Autonomic dysfunction in experimental sepsis induced by cecal ligation and puncture. Auton Neurosci Basic Clin 2008; 138: 57–63. [DOI] [PubMed] [Google Scholar]
- 13.Setoguchi D, Yatsuki H, Sadahiro T, Nakamura M, Hirayama Y, Watanabe E, Tateishi Y, Oda S. Effects of a peripheral cholinesterase inhibitor on cytokine production and autonomic nervous activity in a rat model of sepsis. Cytokine 2012; 57: 238–44. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad S, Ramsay T, Huebsch L, Flanagan S, McDiarmid S, Batkin I, McIntyre L, Sundaresan SR, Maziak DE, Shamji FM, Hebert P, Fergusson D, Tinmouth A, Seely AJ. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One 2009; 4: e6642–e6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W-L, Kuo C-D. Characteristics of heart rate variability can predict impending septic shock in emergency department patients with sepsis. Acad Emerg Med 2007; 14: 392–7. [DOI] [PubMed] [Google Scholar]
- 16.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93: 1043–65. [PubMed] [Google Scholar]
- 17.Lomb NR. Least-squares frequency analysis of unequally spaced data. Astrophys Space Sci 1976; 39: 447–62. [Google Scholar]
- 18.Scargle JD. Studies in astronomical time series analysis. II – Statistical aspects of spectral analysis of unevenly spaced data. Astrophys J 1982; 263: 835–53. [Google Scholar]
- 19.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on ‘sepsis-related problems’ of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41: 580–637. [DOI] [PubMed] [Google Scholar]
- 21.Corrêa TD, Vuda M, Takala J, Djafarzadeh S, Silva E, Jakob SM. Increasing mean arterial blood pressure in sepsis: effects on fluid balance, vasopressor load and renal function. Crit Care Lond Engl 2013; 17: R21–R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy B. Epinephrine in septic shock: Dr. Jekyll or Mr. Hyde? Crit Care Med 2003; 31: 1866–7. [DOI] [PubMed] [Google Scholar]
- 23.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007; 117: 289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–62. [DOI] [PubMed] [Google Scholar]
- 25.Sacha J. Interaction between heart rate and heart rate variability. Ann Noninvasive Electrocardiol 2014; 19: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin MP, Lake DE, O'Shea TM, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr Res 2007; 61: 222–27. [DOI] [PubMed] [Google Scholar]
- 27.Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, Bancalari E, Aschner JL, Whit Walker M, Perez JA, Palmer C, Stukenborg GJ, Lake DE, Michael O'Shea T. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr 2011; 159: 900–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairchild KD, Schelonka RL, Kaufman DA, Carlo WA, Kattwinkel J, Porcelli PJ, Navarrete CT, Bancalari E, Aschner JL, Walker MW, Perez JA, Palmer C, Lake DE, O'Shea TM, Moorman JR. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatr Res 2013; 74: 570–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tambuyzer T, De Waele T, Chiers K, Berckmans D, Goddeeris BM, Aerts JM. Interleukin-6 dynamics as a basis for an early-warning monitor for sepsis and inflammation in individual pigs. Res Vet Sci 2014; 96: 460–63. [DOI] [PubMed] [Google Scholar]
- 30.Gao J, Gurbaxani BM, Hu J, Heilman KJ, Emanuele Ii VA, Lewis GF, Davila M, Unger ER, Lin JM. Multiscale analysis of heart rate variability in non-stationary environments. Front Physiol 2013; 4: 119–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci Basic Clin 2014; 182: 65–9. [DOI] [PubMed] [Google Scholar]