Abstract
The aim of this study was to evaluate the effect of intralesional tamoxifen on keloids, particularly on the concentration of fibroblasts, dermal inflammatory infiltrate, and collagen degeneration. A prospective study was carried out to evaluate keloids in 13 patients of both genders pre- and post-treatment with intralesional tamoxifen. Two samples of keloid lesions were obtained by 4-mm punch biopsies during the study: the first at the time of diagnostic confirmation of keloid and the other eight weeks later at the end of intralesional tamoxifen treatment. The biopsy samples were placed in 10% buffered formalin for HE staining and morphological and morphometric study. The degree of collagen fiber reduction and inflammatory infiltration were analyzed. Student’s t-test was used for statistical analysis of the mean number of fibroblasts before and following tamoxifen treatment (P < 0.05). The degree of collagen fiber reduction and inflammatory infiltration were absent before treatment and present in 100% of cases after treatment, while the mean number of fibroblasts was significantly lower after intralesional tamoxifen treatment (P < 0.0001). We conclude that intralesional administration of tamoxifen promoted an inflammatory stimulus and collagen fiber reduction as well as a significant reduction in the number of fibroblasts that produce collagen.
Impact statement
Effective treatment of keloid that is a commonly recurrent dermatosis is very difficult, even after standard treatment. Standard treatment consists of partial resection of the lesion (shaving excision), in addition to local corticosteroid injection. Therefore, there is interest in alternative forms of topical treatment, e.g., selective estrogen receptor modulators, particularly tamoxifen has demonstrated in vitro studies to be a promising drug. Nevertheless, there is scarcity of publications on the effects of intralesional tamoxifen on keloids have been found, leading us to the conception of the present study. In this study, tamoxifen has proven to be an interesting alternative drug for the topical treatment of keloid, allowing us to conclude that the intralesional application of tamoxifen in keloids promotes a variable but ever-present inflammatory stimulus, associated with intense reduction of collagen fiber, in addition to a significant decrease in the number of fibroblasts that produce collagen and are involved in disease maintenance.
Keywords: Tamoxifen, skin, fibrosis, keloid, collagen fibroblasts, inflammatory infiltration
Introduction
Keloid was first coined by Alibert in 1806,1 referring to a cutaneous lesion with growth similar to the projection of branches, mimicking crab claws. The word is derived from the Greek khele, meaning “crab claw.” It is interesting to differentiate scars of unsightly cosmetic appearance, since a hypertrophic scar is an asymptomatic dermatosis with an appearance similar to keloid, usually arising four weeks after trauma. A hypertrophic scar lasts around six months and regresses spontaneously. In contrast, keloid is an indurated erythematous nodule that extends beyond the boundaries of the original wound. It usually emerges six months after surgery, growing progressively with periods of symptomatic flare-ups interspersed with periods of remission. Keloids are mainly accompanied by symptoms of pruritus, pain, and burning.2
The etiology of keloid has not been fully elucidated. However, the disorder is characterized by the presence of polyclonal fibroblasts that have an exaggerated response to tissue injury, producing a large amount of collagen in response to the stimulation of growth factors, such as transforming growth factor β-1 (TGF-β1), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF), differing from fibroblasts in normal tissue repair.3 Old keloid scars lack the inflammatory response that represents body defense against fibroblast overgrowth. This dermatosis has a genetic predisposition and is more commonly observed in melanodermic individuals and mestizos.4
Keloid is a nosological entity that causes a number of health problems in humans, particularly functional impairment, when located in the joints. It also may cause emotional distress in the affected individual due to an unacceptable cosmetic appearance.5 Effective treatment of this commonly recurrent dermatosis is very difficult, even after standard treatment. Standard treatment consists of partial resection of the lesion (shaving excision), in addition to local corticosteroid injection. Therefore, there is interest in alternative forms of topical treatment, e.g., selective estrogen receptor modulators (SERMs), particularly tamoxifen. In vitro studies have demonstrated that tamoxifen is a promising drug that reduces the action of growth factors involved in fibroblast stimulation.6–10 Nevertheless, to the extent of our investigation, no publications on the effects of intralesional tamoxifen on keloids have been found, leading us to the conception of the present study.
Materials and methods
Patients
Thirteen patients of both genders presenting with keloids, receiving care at the Dermatology Outpatient Facility of the Getúlio Vargas Hospital, Federal University of Piauí, participated in this study. The current investigation was approved by the Internal Review Board of the Federal University of Piaui, and all patients signed an informed consent term prior to study initiation. Patients with keloids for less than one year, aged 18 to 60 years, who had not been receiving hormones in the last six months and had no contraindication to SERM therapy, were included in the study. Patients receiving antibiotic therapy and those with a history of chronic degenerative disease and/or neoplasm were excluded from the study. Before intralesional tamoxifen therapy, patients underwent a biopsy (control test) for confirmation of the keloid. The procedure was performed under local anesthesia administered into the most clinically active site of the lesion, with 4-mm punches. Samples were fixed in 10% buffered formalin for 12–24 h, processed, and stained with hematoxylin-eosin for analysis under light microscopy. Eight days after biopsy for diagnostic confirmation, tamoxifen was administered into the lesion, once a week during eight weeks, at a dose corresponding to 20 mmol/L (0.01 mL/cm2) in the area surrounding the biopsy site, as recommended by Mikulec et al.3
Methods
Morphological study
The morphological study was performed using a Nikon Eclipse E 400 binocular light microscope, at a magnification of × 400, which characterized the conformation and density of collagen fibers, degree of inflammatory infiltration, and fibroblast concentration in the dermis.
Morphometric study
For morphometric study, we used a Nikon Eclipse E 400 binocular light microscope, at a magnification of × 400 and connected to a video camera to quantify the number of fibroblasts. The degree of collagen fiber reduction and inflammatory infiltration were classified as mild, moderate, and marked. Quantification of the number of fibroblasts before and after treatment with tamoxifen was performed using the Image Lab-Softium® software (São Paulo, Brazil). Fibroblasts were counted in five random microscope fields by two observers blinded to patient identity.
Statistical analysis
For analysis of the number of fibroblasts in the keloid lesion before and after tamoxifen treatment, we used the Student’s t-test. Significance level was established at P < 0.05.
Results
Morphological
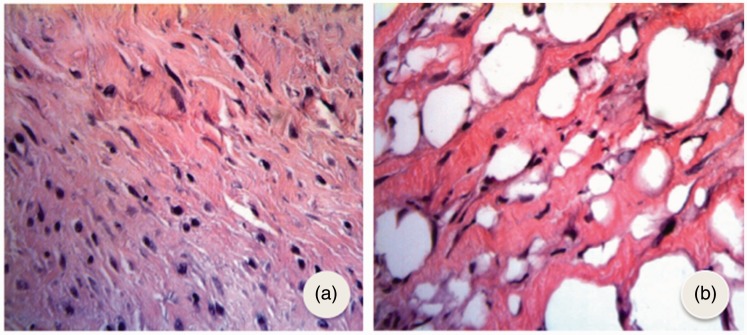
Prior to intralesional tamoxifen injection, keloid lesions exhibited a large fibroblast concentration, thickened dense collagen fibers, and a sparse inflammatory infiltrate. Nevertheless, after tamoxifen treatment, there was reduction of collagen fibers, with loose atrophic collagen fibers. Furthermore, the presence of inflammatory infiltrate and a decrease in fibroblast concentration were observed in all study cases. Following intralesional tamoxifen treatment, keloid scars showed a decreased fibroblast concentration, loose and atrophic collagen fibers, in addition to a marked inflammatory process (Figure 1).
Figure 1.
Photomicrograph of keloid pretreated with tamoxifen (a) showing a large concentration of fibroblasts and thickened collagen fibers and phytomicrograph after treatment with tamoxifen and (b) showing great reduction in the number of fibroblasts, presence of looser collagen fibers with vacuoles and marked inflammatory process (HE × 400). (A color version of this figure is available in the online journal.)
Morphometric
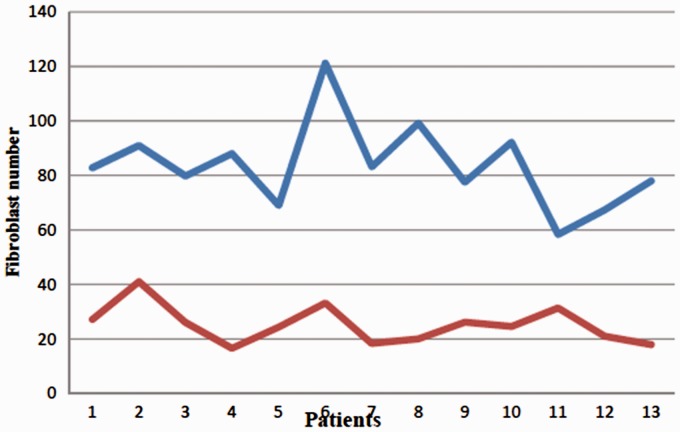
The degree of collagen fiber reduction and the degree of inflammatory infiltration were absent in all keloids pretreated with tamoxifen and were present in 100% of the lesions following tamoxifen treatment. The mean number of fibroblasts was 83.68 ± 4.38 and 25.23 ± 1.92, before and after intralesional tamoxifen treatment, respectively. This difference was statistically significant (P < 0.0001) (Table 1 and Figure 2).
Table 1.
Mean and standard error of fibroblasts in keloid before and after intralesional tamoxifen treatment.
| Pre-treatment | Post-treatment |
|---|---|
| 82.8 | 27.2 |
| 91.0 | 41.0 |
| 78.9 | 26.0 |
| 88.0 | 16.6 |
| 69.0 | 24.4 |
| 121.2 | 33.2 |
| 83.2 | 18.4 |
| 99.2 | 20.0 |
| 77.6 | 26.2 |
| 92.2 | 24.6 |
| 58.4 | 31.4 |
| 67.4 | 21.0 |
| Mean 83.68 ± 4.38 | 25.23 ± 1.92a |
There was a statistically significant reduction in the number of fibroblasts (P < 0.0001).
Figure 2.
Stressing the number of fibroblasts prior to and after intralesional tamoxifen treatment (*Blue line—Pre-treatment; Red line—Post-treatment). (A color version of this figure is available in the online journal.)
Discussion
Keloid is an exophytic dermatitis of unsightly cosmetic appearance that is very common in mestizo or black individuals. It is usually symptomatic and has a progressive growth pattern.11,12 Its standard treatment, i.e., partial removal of the scar plus intralesional corticosteroid, is frequently unsatisfactory, leading to the search for alternative intralesional therapies.13,14 Tamoxifen has shown to be a promising drug, as observed from the results of murine in vitro studies.6 However, further studies in humans need to be conducted. In the present study, we chose a more rapid route of administration that mimicked the intralesional injection used in standard therapy. In this study, the dose used was based on in vitro studies, which showed a considerable inhibition of fibroblast-mediated collage synthesis.9,15,16 The site used for biopsy before intralesional tamoxifen injection was thicker and had greater keloid erythema, probably because fibroblast concentration and activity were higher in the keloid scar.2
The interval between the performance of biopsy and the beginning of drug administration was based on the time for wound healing and suture removal. Tamoxifen was administered during eight weeks at a regimen of one weekly application. The duration of treatment and intralesional drug use in this study were based on those of other studies. According to Ruiz et al.,5 the topical use of tamoxifen citrate in the form of 0.1% cream had a delayed response of around six months. However, the intralesional use of other drugs showed a more rapid response.5
Punch biopsy was performed with the purpose of standardizing the amount of tissue removed and thus avoid error in the removal of the elements analyzed. This method is in agreement with studies of other authors.7 In the present study, intralesional application of tamoxifen once a week during eight weeks showed a considerable decrease in the elements proposed for the study of tamoxifen effects.
A significant reduction in the number and function of fibroblasts was observed, related to the production of new collagen fibers, showing a concordance to studies of mice.8,9 Collagen fiber reduction also occurred, showing that production was inhibited, and therefore, maintenance of the rigid indurated structure, parallel to the keloid collagen bundles. The intense collagen fiber reduction may be partly explained by the presence of inflammatory infiltrate at the site of injection and also by the action of tamoxifen on these fibers. Tamoxifen acts by contracting scars in mice17 and reducing the action of TGF-β1 in vitro, which is a factor responsible for the excessive production of collagen by diseased fibroblasts.9,10
The inflammatory infiltrate predominantly composed of neutrophils and lymphocytes is due to at least two factors: the presence of a foreign body in the dermis and a direct action of tamoxifen in the proinflammatory mechanisms that contribute to dermal vacuolation and also collagen fiber reduction. These facts were also observed by Mikulec et al.3 in experimental murine studies.
Although the literature is scarce, there are already some studies on the action of tamoxifen on the dermis, mainly focused on fibroblasts and cytokines that stimulate the proliferation of these cells and consequently excessive collagen production.3 A beneficial action of tamoxifen on the inhibition of growth factors (mainly TGF-β1, in addition to IGF, VEGF) has been definitively proven.15 However, all studies were performed either in animal samples or in vitro fibroblast cultures. The effects of tamoxifen are already well known in humans, since it is a widely used SERM in the chemoprevention and treatment of breast cancer. However, apart from the use of topical tamoxifen citrate used in the form of cream, its effect on the skin and specifically on keloid scars has still not been fully elucidated.15
Nevertheless, there are no studies on tamoxifen administered into the keloid lesion, which is considered the gold standard of topical drug administration in keloid treatment.2 In the current study, tamoxifen has proven to be an interesting alternative drug for the topical treatment of keloid. However, there is a need for further studies using topical intralesional tamoxifen with a larger sample of patients, since this study allowed us to conclude that the intralesional application of tamoxifen in keloids promotes a variable but ever-present inflammatory stimulus, associated with intense collagen fiber reduction, in addition to a significant decrease in the number of fibroblasts that produce collagen and are involved in disease maintenance.
Authors’ contributions
All authors participated in the design, interpretation of the studies, analysis of the data, and review of the manuscript; BBS and LRSL: Effective scientific, intellectual contribution to the study, creator of the design; BBS, LRSL, IMSL, and LLLF: Technical procedures and acquisition of data; LRSL, BBS, and LLLF: Manuscript writing; BBS, LRSL, and IMSL: Macroscopic and histopathological examinations; APA: Interpretation of data and critical review and approved the version to be published.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Alibert JLM. Description des maladies de la peau observe a’l Hospital Saint Louis e exposition des meilheures methods suives pour leurtraitament. Paris Barroisl’aineetfils 1806; 1: 113–113. [Google Scholar]
- 2.Al-Attar A, Mess S, Thomassen JM, Kauffmann CL, Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg 2006; 117: 286–300. [DOI] [PubMed] [Google Scholar]
- 3.Mikulec AA, Hanosono MM, Lum J, Kadleck JM, Kita M, Koch J. Effect of tamoxifen on transforming growth factor B1 production by keloids and fetal fibroblasts. Arch Facial Plast Surg 2001; 3: 111–4. [DOI] [PubMed] [Google Scholar]
- 4.Rossiello L, D’andrea F, Grella R, Signoriello G, Abbondanza C, De-rosa C, Prudente M, Morlando M, Rossiello R. Differential expression of cyclooxygenases in hypertrophic scar and keloid tissues. Wound Repair Regen 2009; 17: 750–7. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz RO, Warde MJ, Matter CA. Uso do tamoxifeno no tratamento de queloides. Fac Cienc Med Sorocaba 2006; 8: 18–24. [Google Scholar]
- 6.Mancoll JS, Mccaulley RL, Philips LG. The inhibitory effect of tamoxifen on keloid fibroblasts. Surg Forum 1996; 47: 718–20. [Google Scholar]
- 7.Hu D, Hughes MA, Cherry GW. Topical tamoxifen – a potential therapeutic regime in treating excessive dermal scarring? Br J Plast Surg 1998; 51: 462–9. [DOI] [PubMed] [Google Scholar]
- 8.Chau D, Mancoll JS, Zhao J, Phillips LG, Gittes GK, Longaker MT. Tamoxifen down-regulates TGF-beta production in keloid fibroblasts. Ann Plast Surg 1998; 40: 490–3. [DOI] [PubMed] [Google Scholar]
- 9.Hu D, Hughes MA, Cherry GW. The inhibitory effect of tamoxifen on human dermal fibroblast-populated collagen lattices. Zhonghua Zheng Xing Wai Ke Za Zhi 2002; 18: 160–2. [PubMed] [Google Scholar]
- 10.Ruffy MB, Kunnavatana SS, Kock RJ. Effects of tamoxifen on normal human dermal fibroblasts. Arch Facial Plast Surg 2006; 8: 329–32. [DOI] [PubMed] [Google Scholar]
- 11.Chevray PM, Manson PN. Keloid scars are formed by polyclonal fibroblasts. Ann Plast Surg 2004; 52: 605–8. [DOI] [PubMed] [Google Scholar]
- 12.Lu F, Gao J, Ogawa R, Hyakusoku H, Ou C. Biological differences between fibroblasts derived from peripheral and central areas of keloid tissues. Plast Reconstr Surg 2007; 120: 625–30. [DOI] [PubMed] [Google Scholar]
- 13.Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg 2006; 8: 362–8. [DOI] [PubMed] [Google Scholar]
- 14.Akaishi S, Akimoto M, Ogawa R, Hyakusoku H. The relationship between keloid growth pattern and stretching tension. Ann Plast Surg 2008; 60: 445–51. [DOI] [PubMed] [Google Scholar]
- 15.Gragnani A, Warde M, Furtado F, Ferreira LM. Topical tamoxifen therapy in hypertrophic scars or keloids in burns. Arch Dermatol Res 2009; 302: 1–4. [DOI] [PubMed] [Google Scholar]
- 16.Nelson HD, Fu R, Griffin JC, Nygren P, Smith ME, Humphrey L. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med 2009; 151: 703–15. [DOI] [PubMed] [Google Scholar]
- 17.Denton CP, Khan K, Hoyles RK, Shiwen X, Leoni P, Chen Y, Eastwood M, Abraham DJ. Induced lineage-specific deletion of T beta RII in fibroblasts defines a pivotal regulatory role adult skin wound healing. J Invest Dermatol 2008; 129: 194–204. [DOI] [PubMed] [Google Scholar]