Abstract
Objective
The aim of this study was to assess the pattern of cardiovascular diseases (CVDs), their clinical characteristics, and associated factors in the outpatient department of the chronic illness clinic of Gondar University Referral Hospital.
Method
A retrospective cross-sectional study was conducted among patients on follow-up at the outpatient chronic illness clinic of the hospital from October 2010 to October 2015. The source population for the study included patients with a diagnosis of CVD whose medical records have the required socio-demographic information during the study period. The data were collected from August 2015 to December 2015. Chi-square and binary logistic regression tests were performed to test the significance of difference among predictive variables and CVDs.
Results
Of 1105 patient medical records, 862 fulfilled the inclusion criteria. The majority of the patients were females (65%) and living in urban areas (62.7%). Hypertension accounted for the majority (62.3%) of CVDs followed by heart failure (HF) (23.9%). Headache was the leading chief complaint among the patients (37.7%) upon diagnosis and was the prominent clinical feature in more than half of the patients during their course of follow-up. Higher proportions of dyslipidemia (85.7%), hypertension (72.8%), and ischemic heart disease (IHD) (73.2%) were associated with urban residency (P<0.01). Patients from rural areas (crude odds ratio [COR] =1.306 [95% confidence interval 1.026–2.166], adjusted odds ratio [AOR] =1.272 [95% confidence interval 1.017–2.030]) and those with comorbidity illnesses (COR= 1.813 [1.279–2.782], AOR =1.551 [95% confidence interval 1.177–2.705]) were more likely to have poor CVD outcome (P<0.05).
Conclusion
Hypertension was found to be the most frequent CVD followed by HF, and hypertensive heart disease was the leading cause of cardiac diseases. Most of the patients had improved assessment in the last follow-up, but patients from rural regions and those with comorbidty had higher likelihood of poor cardiovascular outcome.
Keywords: cardiovascular disease, clinical characteristics, pattern, Gondar, Ethiopia
Video abstract
Introduction
Cardiovascular disorder comprises various diseases affecting the heart and blood vessels, resulting in different comorbidities and life-threatening complications. Cardiovascular disease (CVD) is causing worldwide concern because of the rising prevalence and consequence of morbidity and mortality rate with huge economic burden.1 The rise in CVDs is being witnessed globally among various cultures and ethnic groups because of epidemiological shifts.2 In low-income countries also, CVDs are among the emerging health problems as major contributors to the disease burden.3 Among sociodemographic variables, age is an important predisposing factor for chronic disorders, CVDs in particular. Factors related to diet and sedentary lifestyle also contribute to increased occurrence of CVDs.4
In the past 20–30 years, the emerging CVD epidemic in developing countries has not been given enough attention, nor adequate public health response, globally and in the countries themselves. The fact that developing countries share a higher proportion of CVD burden than developed nations is not recognized well.5 CVD is the biggest cause of death worldwide and is estimated to remain the leading cause of mortality. An estimated 17.5 million people died from CVD globally, accounting for 30% of all deaths, in 2005. The majority of these deaths occurred in the developing countries.3
According to a systematic review, CVD (24%), cancer (10%), diabetes (5%), and chronic obstructive pulmonary diseases (3%) were found to be important causes of death in different parts of Ethiopia. Predisposing factors such as rheumatic heart disease, khat chewing, obesity, and physical inactivity were responsible for CVD burden especially in the urban Ethiopian population.6–8 In general, despite the increasing threat of noncommunicable diseases (NCDs), communicable diseases are still the main causes of admission to medical wards in developing countries, while NCDs are leading in the developed world.9,10 CVDs account for a significant number of patient visits, though the burden by etiologic type varies in different regions of the globe. This is dependent on epidemiologic transition and the various stages in its development that a country has to undergo.11 The pattern of prevalence of CVDs is distinctly different in sub-Saharan Africa (SSA), compared to the rest of the world, since infectious and inflammatory diseases are relatively more common.12
Although infectious diseases are still the leading causes of morbidity and hospital admission in developing countries like Ethiopia, in recent decades there has been significant epidemiological transition to NCDs.9 In turn, the clinical pattern of the diseases and the shifts need to be characterized. CVDs are responsible for the majority of morbidity and mortality among NCDs in Ethiopia. Despite the rise of NCDs, the country’s full attention was in combating the communicable diseases such as HIV, tuberculosis, and malaria.11,13
Similar to most SSA countries, there is a lack of data on the patterns and clinical characteristics of CVD in Ethiopia. This is due to the lack of study done about CVD patterns in recent years despite the emergence of NCDs in the developing world. Hence, this study was conducted in northwestern Ethiopia, one of the low-income countries. It aimed to explain the pattern and characteristics of CVDs and suggest on the future pattern of CVDs by assessing clinical characteristics and associated factors among patients with CVDs.
Methods
Patients and settings
The study was conducted in Gondar University Referral Hospital (GURH), which is located in Gondar town, 738 km from Addis Ababa, the capital city of Ethiopia. The outpatient department of the hospital has specific clinics where patients with chronic diseases and CVDs are followed-up. The clinics are staffed with internists, residents, and general practitioners with expertise in specific chronic disease patient follow-up. The source population for the study included medical records of all patients diagnosed with a CVD. Medical records that had the required sociodemographic information and medical records during the study period were included in the study. Incomplete medical records were excluded from the study.
Study design and study period
A retrospective cross-sectional study design, based on review of medical records of patients, was followed in this study. The medical records of patients who started their follow-up at the chronic illness clinics between October 2010 and October 2015 were included in the study. Data collection was conducted from August 2015 to December 2015.
Data collection procedure
The data were collected by four trained clinical pharmacists by using a structured pretested checklist. The checklist included basic demographic data including age, sex, residence, and the year in which follow-up started. Records on chief complaint, signs and symptoms, echocardiography results (if available), last follow-up assessment, and summary of important laboratory findings (such as serum electrolytes and organ function tests) were also included. The data collection tool was developed in consultation with senior clinical researchers at GURH, and it was pretested for its validity before the actual data collection commenced.
Diagnosis, prognosis, as well as treatment response information were taken from the evaluation in the last follow-up of patients in the study. Standard laboratory reference range values from clinical chemistry textbooks and Harrison’s Principle of Internal Medicine were used, to classify each laboratory test result as low, normal, or high (Table 3).14,15 Medical records of study participants were selected by their chart number, from the chronic illness clinic CVD patient registration book. Based on this, individual medical records were traced, and pertinent data were filled into the data collection format.
Table 3.
Laboratory characteristics of CVD patients during their first visit at GURH, 2015 (N=862)
| Laboratory parameters with standard values | Test was not done, n (%) | Test was done, n (%) | Normal, n (%) | Low, n (%) | High, n (%) |
|---|---|---|---|---|---|
| Creatinine (0.6–1.1 mg/dL) | 300 (34.8) | 562 (65.2) | 346 (61.6) | 48 (8.5) | 168 (29.9) |
| BUN (7–20 mg/dL) | 352 (40.8) | 509(59.2) | 319 (62.7) | 39 (7.7) | 151 (29.6) |
| K+ (3.5–5 meq/L) | 597 (69.3) | 265(30.7) | 190 (71.7) | 52 (19.6) | 23 (8.7) |
| Na+ (135–145 meq/L) | 594 (68.9) | 268(31.1) | 202 (75.4) | 42 (15.7) | 24 (8.9) |
| Ca+ (2.24–2.46 meq/L) | 620 (71.9) | 242(28.1) | 189 (78.1) | 28 (11.6) | 25 (10.3) |
| SGPT (<35 IU/L) | 376 (43.6) | 486(56.4) | 369 (75.9) | na | 117 (24.1) |
| SGOT (<35 IU/L) | 370 (42.9) | 492(57.1) | 376 (76.4) | na | 116 (23.6) |
| Triglycerides (<150 mg/dL) | 531 (61.5) | 331(38.5) | 239 (72.2) | na | 92 (27.8) |
| Cholesterol (<200 mg/dL) | 535 (62.1) | 327(37.9) | 218 (66.7) | na | 109 (33.3) |
Abbreviations: BUN, blood urea nitrogen; CVD, cardiovascular disease; GURH, Gondar University Referral Hospital; BUN, blood urea nitrogen; na, not applicable.
Data entry and analysis
The data collected were entered into and analyzed by using SPSS for Windows version 20.0. In the analysis, descriptive statistics, chi-square tests, and binary logistic regression were done. In the analysis, 95% confidence interval (CI) and P-value <0.05 were used as cutoff points, to determine the statistical significance of the tests of associations of different variables with CVDs.
Data quality control
The measures taken to ensure data quality included pre-testing of the data collection instrument to check for consistency and validity, training of data collectors, supervision by the principal investigator, and checking completeness and internal consistency of data during and after data collection, as well as entry. Ethical clearance was obtained from the ethical review committee of the University of Gondar. In addition, a letter of permission was obtained from the medical director of GURH. However, patient consent was exempted by the ethical review committee of the University of Gondar, as individual patient data were kept anonymous and confidential by ensuring data taken from patient charts did not include any personal identifiers and was used only for the purpose of this study.
Definitions of terms and operational definitions
For the purpose of this study, CVDs were categorized as diagnosed by the physician and basic diagnostic parameters with standard definitions. On the basis of this, the CVD was categorized as hypertension when blood pressure was ≥140/90 mmHg, congestive heart failure (CHF) if there was presence of two or more of the adapted Framingham criteria or presence of one major criteria and two minor criteria, and rheumatic heart disease if there was presence of two major modified Jones criteria or one major and two minor criteria. Dyslipidemia diagnosis was determined by patient lipid profile of cholesterol and triglycerides exceeding 200 and 150 mg/dL, respectively.16,17
Patients’ clinical condition was classified as good, fair, and poor based on the following definitions.
Good: patients had better prognosis both clinically and in laboratory parameters (signs and symptoms resolved, blood pressure, cholesterol, and other diagnostic parameters far better than initial diagnosis and the previous follow-up).
Fair: there was improvement from state at the previous visit and moderate change in laboratory parameters but not at the level they could be.
Poor: there was no improvement at all or patient conditions might have become worse.
Improved: in this study, it signifies an assessment rated as fair or good on the last follow-up.
The terms cardiovascular outcome and CVD improvement were used interchangeably in this study.
Results
Socio-demographic and related characteristics
Of 1105 patient medical records, 243 were excluded because of incompleteness and the remaining 862 were included in the final analysis. Of these, approximately two thirds (65%) were female patients. Similarly, two thirds (65.2%) of the patients were >50 years of age; the age group 50–59 years constituted nearly a quarter (23.6%) of CVDs that had been documented. The majority (62.7%) of patients were from urban areas. More than half (55%) of the patients did not have a documented comorbidity in the whole follow-up period from first diagnosis to the last follow-up. More than one quarter of the CVD patients started follow-up in 2015. Considering prognosis, nearly two thirds (63.5%) of the patients had good improvement on their last follow-up, whereas 14.4% had poor assessment of their CVD (Table 1). The most frequent comorbidity reported was diabetes mellitus in 9% of the patients, followed by thyroid-related disorders (7%) and peptic ulcer disease/dyspepsia (6%) (Figure 1).
Table 1.
Sociodemographic and related characteristics of the cardiovascular patients, Gondar 2015
| Variables | Frequency (%) |
|---|---|
| Sex | |
| Male | 302 (35) |
| Female | 560 (65) |
| Age (years) | |
| 10–19 | 21 (2.4) |
| 20–29 | 58 (6.7) |
| 30–39 | 89 (10.3) |
| 40–49 | 133 (15.4) |
| 50–59 | 203 (23.6) |
| 60–69 | 198 (23.1) |
| 70–79 | 125 (14.5) |
| ≥80 | 35 (4) |
| Residence | |
| Rural | 322 (37.3) |
| Urban | 540 (62.7) |
| Year of registration | |
| 2010 | 39 (4.5) |
| 2011 | 114 (13.2) |
| 2012 | 129 (15) |
| 2013 | 217 (25.2) |
| 2014 | 135 (15.7) |
| 2015 | 228 (26.5) |
| Time of follow-up | |
| <1 month | 114 (13.2) |
| 1 month | 224 (26) |
| 2 months | 288 (33.4) |
| 3 months | 236 (27.4) |
| Medical status of patients | |
| Good | 547 (63.46) |
| Fair | 191 (22.16) |
| Poor | 124 (14.38) |
Figure 1.
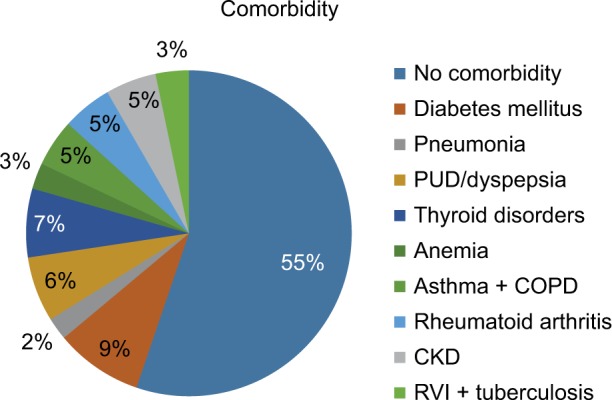
Comorbidities other than cardiovascular disease in the chronic illness clinic of Gondar University Referral Hospital, October 2010 to October 2015.
Abbreviations: PUD, peptic ulcer disease; COPD, Chronic obstructive pulmonary disease; CKD, chronic kidney disease; RVI, retroviral infection.
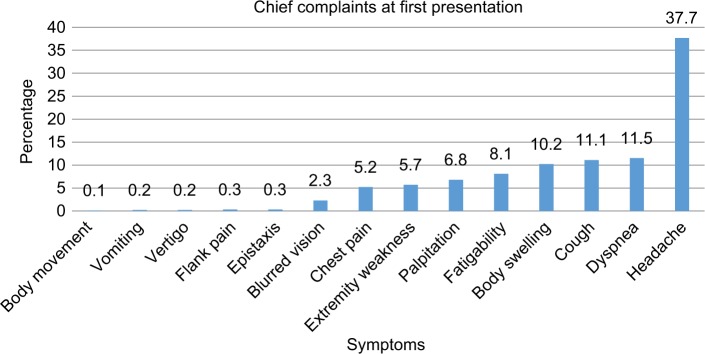
More than a third (37.7%) of patients diagnosed with CVD for the first time came to hospital because of chief complaints of headache, followed by dyspnea (11.5%), cough (11.1%), body swelling (10.2%), easy fatigability (8.1%), and palpitations (6.8%) (Figure 2).
Figure 2.
Cardiovascular patients’ chief complaint at the first visit to the hospital.
Clinical presentation of CVD patients
More than half of the CVD patients complained of headache at least once in their course of follow-up visits, followed by dyspnea in approximately half of the patients. Orthopnea, easy fatigability, palpitations, and cough were also frequently reported signs and symptoms in more than one third of the CVD patients (Table 2).
Table 2.
Clinical presentation of cardiovascular disease patients who had follow-up at Gondar University Referral Hospital during the period from October 2010 to October 2015
| Signs and symptoms | Yes, n (%) | No, n (%) |
|---|---|---|
| Headache | 439 (50.9) | 423 (49.1) |
| Dizziness | 56 (6.5) | 806 (93.5) |
| Vertigo | 70 (8) | 792 (92) |
| Tinnitus | 50 (5.8) | 812 (94.2) |
| Cough | 283 (32.8) | 579 (67.2) |
| Weakness of extremities | 62 (7.2) | 800 (92.8) |
| Blurred vision | 162 (18.8) | 700 (81.2) |
| Chest pain | 237 (27.5) | 625 (72.5) |
| Light headedness | 23 (2.7) | 839 (97.3) |
| Dyspnea | 416 (48.3) | 446 (51.7) |
| Orthopnea | 336 (39) | 526 (61) |
| Easy fatigability | 332 (38.5) | 530 (61.5) |
| Palpitations | 330 (38.3) | 532 (61.7) |
| Facial puffiness | 52 (6) | 810 (94) |
| Body/leg swelling | 248 (28.8) | 614 (71.2) |
| Paroxysmal nocturnal dyspnea | 120 (13.9) | 742 (86.9) |
| Nasal bleeding | 3 (0.3) | 859 (99.7) |
| Loss of consciousness | 14 (1.6) | 848 (98.4) |
Among the patients who had an electrolyte test, approximately three quarters of them had their results within the normal range. One third of the patients, who underwent serum cholesterol test, had hypercholesterolemia. More than 29% of the patients had high serum creatinine and blood urea nitrogen (BUN) level (Table 3). From the total of 505 CVD patients, 31.5% of them had reduced ejection fraction from the echocardiography report (Table 5).
Table 5.
Pattern of valve(s) involvement among RVHD patients registered and who started follow-up at chronic clinic illness of Gondar University Referral Hospital, October 2010 to October 2015
| Echocardiography report on the extent of valvular involvement | Number (%) |
|---|---|
| MS | 57 (55.8) |
| MR | 61 (59.8) |
| AR | 34 (33.3) |
| MS+MR | 18 (17.6) |
| MS+AR | 11 (10.8) |
| MR+TR+AR | 9 (8.8) |
| Others | 15 (13.7) |
| Preserved EF | 346 (69.5) |
| Reduced EF | 159 (31.5) |
Notes: Preserved EF is >40%, reduced EF is <40%.
Abbreviations: AR, aortic regurgitation; EF, ejection fraction; MS, mitral stenosis; MR, mitral regurgitation; RVHD, rheumatic valvular heart disease; TR, tricuspid regurgitation.
CVD distribution
Of the 862 patients in the study, 541 (62.76%) were hypertensive, followed by patients with heart failure (HF) 206 (23.9%), hypertensive heart disease 132 (15.3%), and rheumatic heart disease 102 (11.8%). Regarding sex distribution, females constituted higher proportions in all CVDs except in pulmonary hypertension and congenital heart disease. A chi-square test showed that females (53.1%) had a statistically significant (P=0.042) higher proportion of stroke than males. Statistically significant differences in CVDs were also found in patients from rural areas contributing higher proportions of HF (55.8%), arrhythmia (52.1%), rheumatic valvular heart disease (RVHD) (52%), dilated cardiomyopathy (DCMP) (62.7%), cor pulmonale (84%), degenerative valvular heart disease (DVHD) (60.2%), and pulmonary hypertension (63.6%) disease patterns (P<0.01). Patients from urban areas had higher proportions of dyslipidemia (85.7%), hypertension (72.8%), and ischemic heart disease (IHD) (73.2%) (Table 4). Of 102 RVHD patients with echocardiograph reports, 57 (55.8%), 61 (59.8%), 34 (33.3%), and 18 (17.6%) had mitral stenosis (MS), mitral regurgitation (MR), aortic regurgitations, and combined MS and MR, respectively (Table 5).
Table 4.
Cardiovascular disorder distribution by sex and residency among patients who attended follow-up at the chronic illness clinic of Gondar University Referral Hospital during the period from October 2010 to October 2015
| Cardiovascular disorders | Sex
|
p-value | Residence
|
p-value | Total N=862, n (%) | ||
|---|---|---|---|---|---|---|---|
| Male (%) | Female (%) | Rural (%) | Urban (%) | ||||
| Dyslipidemia | 37 (40.7) | 54 (59.3) | 0.553 | 13 (14.3) | 78 (85.7) | 0.015* | 91 (10.5) |
| Stroke | 30 (46.9) | 34 (53.1) | 0.042* | 20 (31.2) | 44 (68.8) | 0.284 | 64 (7.4) |
| Heart failure | 63 (30.6) | 143 (69.4) | 0.122 | 115 (55.8) | 91 (44.2) | 0.000** | 206 (23.9) |
| Arrhythmia | 33 (34.4) | 63 (65.6) | 0.40 | 50 (52.1) | 46 (47.9) | 0.000** | 96 (11.3) |
| Hypertension | 200 (37) | 341 (63) | 0.14 | 147 (27.2) | 394 (72.8) | 0.000** | 541 (62.76) |
| Rheumatic valvular heart disease (RVHD) | 35 (34.3) | 67 (65.7) | 0.48 | 53 (52) | 49 (48) | 0.003** | 102 (11.8) |
| Ischemic heart disease (IHD) | 16 (28.6) | 40 (71.4) | 0.184 | 15 (26.8) | 41 (73.2) | 0.098 | 56 (6.5) |
| Dilated cardiomyopathy (DCMP) | 32 (40) | 48 (60) | 0.30 | 50 (62.5) | 30 (37.5) | 0.000** | 80 (9.3) |
| Cor pulmonale | 4 (21.1) | 15 (78.9) | 0.196 | 16 (84.2) | 3 (15.8) | 0.000** | 19 (2.2) |
| Pulmonary hypertension | 12 (54.5) | 10 (45.5) | 0.052 | 14 (63.6) | 8 (36.4) | 0.043* | 22 (2.55) |
| Degenerative valvular heart disease (DVHD) | 28 (30.1) | 65 (69.9) | 0.303 | 56 (60.2) | 37 (39.8) | 0.000* | 93 (10.8) |
| Congenital heart disease (CHD) | 2 (66.7) | 1 (33.3) | 0.281 | 2 (66.7) | 1 (33.3) | 0.297 | 3 (0.35) |
| Hypertensive heart disease (HHD) | 53 (40.2) | 79 (59.8) | 0.181 | 52 (39.4) | 80 (61.6) | 0.622 | 132 (15.3) |
| Deep vein thrombosis (DVT) | 5 (45.5) | 6 (54.5) | 0.455 | 4 (36.4) | 7 (63.6) | 0.983 | 11 (1.23) |
| Pulmonary thromboembolism (PTE) | 1 (50) | 1 (50) | 0.566 | 0 (0) | 2 (100) | 0.546 | 2 (0.23) |
Notes:
P<0.05;
P<0.01.
Factors associated with poor CVD improvement
A binary logistic regression analysis revealed that rural residency was more likely to be associated with poor CVD improvement (crude odds ratio (COR)=1.306(1.026–2.166), adjusted odds ratio (AOR) =1.272(1.017–2.030). Less than or equal to 1 month follow-up was associated with less risk of poor CVD improvement (COR =0.561(0.236–0.759), AOR =0.502(0.213–0.741) and COR =0.691(0.451–0.895), AOR =0.623(0.331–0.842), respectively). Patients with comorbid conditions were 1.5 times more likely to have poor cardiovascular outcome/improvement (COR =1.813(1.279–2.782), AOR =1.551(1.177–2.705)) (Table 6).
Table 6.
Factors associated with the outcome of cardiovascular patients
| Factors | Cardiovascular outcome
|
Crude odds ratio (COR) (95% CI) | Adjusted odds ratio (AOR) (95% CI) | p-value | |
|---|---|---|---|---|---|
| Improved (good + fair) | Poor | ||||
| Sex | |||||
| Male | 250 | 52 | 1.410 (0.957–2.078) | 1.433 (0.961–2.129) | 0. 083 |
| Female | 488 | 72 | 1 | 1 | Reference |
| Age in years | |||||
| 10–39 | 142 | 26 | 1 | 1 | |
| 40–69 | 460 | 74 | 0.879 (0.541–1.427) | 1.079 (0.583–1.995) | 0.601 |
| ≥70 | 136 | 24 | 0.971 (0.531–1.774) | 0.981 (0.581–1.638) | 0.924 |
| Residence | |||||
| Rural | 269 | 53 | 1.306 (1.026–2.166) | 1.272 (1.017–2.030) | 0.041* |
| Urban | 469 | 71 | 1 | 1 | Reference |
| Time of follow-up | |||||
| <1 month | 95 | 19 | 0.561 (0.236–0.759) | 0.502 (0.213–0.741) | 0.044* |
| 1 month | 196 | 28 | 0.691 (0.451–0.895) | 0.623 (0.331–0.842) | 0.039* |
| 2 months | 242 | 46 | 0.751 (0.365–1.289) | 0.749 (0.348–1.156) | 0.35 |
| 3 months | 205 | 31 | 1 | 1 | Reference |
| Comorbidty | |||||
| No | 404 | 70 | 1 | 1 | Reference |
| Yes | 334 | 54 | 1.813 (1.279–2.782) | 1.551 (1.177–2.705) | 0.02* |
| Year of registration | |||||
| 2010 | 37 | 2 | 0.298 (0.069–1.293) | 0.315 (0.072–1.378) | 0.106 |
| 2011 | 94 | 20 | 1.173 (0.643–2.142) | 1.376 (0.739–2.561) | 0.603 |
| 2012 | 118 | 11 | 0.514 (0.251–1.051) | 0.565 (0.274–1.162) | 0.068 |
| 2013 | 180 | 37 | 1.133 (0.684–1.878) | 1.189 (0.174–1.980) | 0.627 |
| 2014 | 116 | 19 | 0.903 (0.494–1.653) | 0.966 (0.524–1.781) | 0.741 |
| 2015 | 193 | 35 | 1 | 1 | Reference |
Note:
P<0.05.
Discussion
Nowadays, there is a global epidemiological transition in the developing countries through the changing disease pattern from infectious diseases to NCDs. This study was conducted to evaluate the pattern of CVDs in an Ethiopian university referral hospital. It provided some insight into the current epidemiological pattern of cardiovascular disorders in the country. To the best of the authors’ knowledge, the pattern of CVDs in GURH was assessed two decades ago and it may not give the right current picture of CVDs and of that in the near future. The study reported that rheumatic heart disease was responsible for 42% of CVDs followed by hypertension in 38.1% of CVDs.13 The most prevalent CVD in the present study was hypertension (62.3%), followed by HF (23.9%). This was similar to most of the studies that found that hypertension is the dominant morbidity and is responsible for most of the cardiovascular events among adults with CVDs.18
The leading cardiac diseases among the patients included hypertensive heart disease, rheumatic heart disease, arrhythmia, degenerative heart disease, DCMP, and IHD in decreasing order. This finding was not in agreement with studies conducted in northern Malawi, Addis Ababa, Jimma, and the same institution two decades ago, which concluded that rheumatic heart disease was the leading cause of heart disease followed by hypertensive heart disease.3,11,13,19,20 The leading cardiac disease etiologies identified by the study performed in Bangladesh were IHD, HF, and chronic RVHD.21 In this study, hypertensive heart disease had surpassed rheumatic heart disease as the leading heart disease. This might be due to the increasing prevalence of hypertension, as supported by the high proportion of hypertension of approximately two thirds (62.3%) of CVDs found in this study. It could also partly be justified by the reduction of rheumatic heart disease, due to the early treatment of respiratory tract infection and early detection of rheumatic fever because of better access to health care in Ethiopia.22
Headache was the most common complaint during the first visits. This might be attributed to the high prevalence of hypertension among CVDs in the study, which manifests with the characteristic occipital type of morning time headache, which is a reason for patients to visit health institutions and check their blood pressure. Differential diagnoses such as malignant pheochromocytoma, hypertensive crisis including hypertensive encephalopathy and eclampsia could contribute to this chief presentation.23
Diabetes mellitus was found to be a more prevalent comorbid condition in cardiovascular patients of this study. Similar findings were demonstrated by Shukrala et al in an Eastern Ethiopia hospital on hypertensive patients, where diabetes was a comorbidity and was predominant.24,25 Two thirds of the patients were >50 years of age, and the risk of developing CVD increases with age.12
In this study, 68.5% of CVD patients had preserved ejection fraction based on the documented echocardiography reports. This was much higher than the proportion reported among CHF patients (52.7%) in the same study setup.26 It is reasonable that ejection fraction is reduced more in HF patients than the general cardiovascular patient population.27 The finding in this study was also in agreement, with little variation, with studies done in Spain (72.2%) and Japan (72%).28 The disparity between these studies and the present study might be credited to the difference in geographic location, sample size, age of the patients, race, and cutoff values for ejection fraction within each study study.29
Approximately a third of the patients, among those tested, had high serum creatinine and BUN levels. This suggests that CVD may cause kidney damage and might result in elevation of creatinine and BUN; however, it cannot be generalized based on this study. But a study done at a nephrology clinic in Uganda suggested that CVDs were the primary risk factors for end-stage renal diseases, morbidity, and mortality of renal patients.30
Chi-square test revealed that hypertension (72.8%), dyslipidemia (85.7%), and IHD (73.2%) were found to be highly associated with urban residency. Lifestyle changes and expansion of urbanization in developing countries might have contributed to this association. Sedentary life is more common in urban areas because more of the residents work in offices and other jobs which do not need physical effort. Besides this, dietary habits are different in rural areas.31 As people in rural Ethiopia are daily laborers and farmers who spend their days in physical labor to earn a living, this contributes to lower cardiovascular disorders, dyslipidemia, hypertension, and IHD. In contrast, HF, arrhythmia, stroke, DCMP, RVHD, DVHD, and cor pulmonale were higher in rural areas than urban areas. This could be due to difficulties faced by people in rural areas to make the necessary follow-ups at health institutions; rather they come after the disease becomes more advanced and severe.32
Most of the rheumatic valvular disease patients had mitral involvement, MS (55.8%), MR (59.8%) followed by aortic involvement aortic regurgitation (33.3%). Unlike this finding, a study done in the cardiac clinic of Jimma University Hospital indicated 55% and 25% of cases of MS and MR, respectively. This might be attributed to the increment of other valvular involvement. The impact of rheumatic heart disease on a patient’s loss of productivity is significant, because functionality cannot be reversed by treatment only, and the effective management for valvular heart disease is surgical intervention. Surgical repair and valvular intervention is not routinely practiced in Ethiopian hospitals and other developing countries because of the economic and technological barriers.11,13
The binary logistic regression test indicated that living in rural areas was found to be associated with poor improvement of CVDs. This might be because of poor medication adherence and loss to scheduled follow-up due to various barriers of education (illiteracy) and inaccessibility of the health facility.12,32 Frequent appointments for follow-up was associated with CVD improvement. This might be due to the closer follow-up with physicians and frequent evaluation of adherence, which result in more improvement. Having different morbidity was associated with poor control of CVDs and risks for multiple drug use to manage the comorbidity, which might affect the disease improvement.24
Limitations of the study
The number of visits and the course of the disease at each appointment from initial diagnosis to the last follow-up were not studied explicitly; therefore, it was difficult to consider its contribution for the final cardiovascular outcome assessment and to the disease prognosis. It was studied retrospectively based solely on the secondary data; and it may not give all the required data at prospective follow-up.
In addition to this, this is a single-centered hospital-based study; hence, it may not be representative to the population CVD distribution, unlike population-based studies. Despite this limitation, this is the first study to our knowledge in our country and institution conducted to assess the CVD pattern, clinical characteristics, and associated factors for the disease outcome. We believe that it will provide insights into epidemiological transition of CVDs and a baseline for large population-based prospective research studies.
Conclusion
Hypertension was found to be the most frequent CVD followed by HF and takes the greatest share of CVD burden. Hypertensive heart disease, RVHD, arrhythmia, DVHD, and DCMP were the top five cardiac diseases in decreasing order of frequency. Most of the patients had improved outcome on their last follow-up, but patients from rural areas and those with comorbid conditions had higher likelihood of poor cardiovascular outcome. Clinicians, public health experts, policy makers, and health care administrators should give attention to the changing patterns of CVDs in developing countries and put emphasis on public health strategies of prevention, early detection, and management. Large community-based studies are recommended for better population representativeness and generalization.
Acknowledgments
The authors would like to thank University of Gondar, College of Medicine and Health Sciences, the School of Pharmacy, for the overall support. They would also like to extend their thanks to physicians and nurses who were working in Gondar University Hospital for their indispensable help in the development of the study. Finally, they would like to express their deepest gratitude to Fitsum Sebsibe Teni from Karolinska Institutet, Sweden, for the English language copyediting and valuable comments.
The abstract of this paper was presented at the International Conference on Cardiovascular Medicine at Manchester, UK, as a poster presentation with interim findings. The abstract of the poster was published in poster abstracts of the conference proceeding in Journal of Clinical & Experimental Cardiology.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Raji Y, Mabayoje O, Bello T. Familial clustering of risk factors for cardiovascular disease among first-degree relatives of patients with chronic kidney disease in a sub-Saharan African population. Cardiovasc J Afr. 2015;26:11–14. doi: 10.5830/CVJA-2015-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaziano T. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 8th ed. Philadelphia: Elsevier Saunders; 2007. Global burden of cardiovascular disease; pp. 1–21. [Google Scholar]
- 3.Soliman EZ, Juma H. Cardiac disease patterns in Northern Malawi: epidemiologictransition perspective. J Epidemiol. 2008;18(5):204–208. doi: 10.2188/jea.JE2008006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimemeri AA. Cardiovascular disease in Hajj pilgrims. J Saudi Heart Assoc. 2012;24:123–127. doi: 10.1016/j.jsha.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodu SR. Emergence of cardiovascular diseases in developing countries. Cardiology. 1988;75(1):56–64. doi: 10.1159/000174349. [DOI] [PubMed] [Google Scholar]
- 6.Misganaw A, Mariam DH, Ali A, Araya T. Epidemiology of major non-communicable diseases in Ethiopia: a systematic review. J Health Popul Nutr. 2014;32(1):1. [PMC free article] [PubMed] [Google Scholar]
- 7.Tesfaye F. Epidemiology of cardiovascular disease risk factors in Ethiopia: the rural-ruban gradient. Epidemiology and Public Health Sciences Department of Public Health and Clinical Medicine Umeå University; 2008. [Google Scholar]
- 8.Oli K, Asmera J. Rheumatic heart disease in Ethiopia: could it be more malignant? Ethiopian Med J. 2004;42(1):1–8. [PubMed] [Google Scholar]
- 9.Ali E, Woldie M. Reasons and outcomes of admissions to the medical wards of Jimma University Specialized Hospital, Southwest Ethiopia. Ethiop J Health Sci. 2010;20:113–120. doi: 10.4314/ejhs.v20i2.69437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noor SK, Elmadhoun WM, Bushara SO, Ahmed MH. The changing pattern of hospital admission to medical wards burden of non-communicable diseases at a hospital in a developing country. Sultan Qaboos Univ Med J. 2015;15(4):517–522. doi: 10.18295/squmj.2015.15.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habte B, Alemseged F, Tesfaye D. The pattern of cardiac diseases at the cardiac clinic of Jimma University specialized hospital, south west Ethiopia. Ethiop J Health Sci. 2010;20(2):199–205. doi: 10.4314/ejhs.v20i2.69435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morana A, Forouzanfarb M, Sampson U, Chugh S, Feigin V, Mensah G. The epidemiology of cardiovascular diseases in Sub-Saharan Africa: the global burden of diseases, injuries and risk factors 2010 study. Prog cardiovasc Dis. 2013;56:234–239. doi: 10.1016/j.pcad.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maru M. The changing pattern of cardiovascular diseases in Ethiopia. East Afr Med J. 1993;70(12):772–776. [PubMed] [Google Scholar]
- 14.Burtis C, Ashwood E. Tietz Textbook of Clinical Chemistry. Elsevier Saunders; Philadelphia: 1998. [Google Scholar]
- 15.Kaspe DL, Hauser SL, Jameson JL, Fauci AS, Longo DL, Loscalzo J. Harrison’s Principles of Internal Medicine. McGraw-Hill Companies; New York, NY: 2012. [Google Scholar]
- 16.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed A, Allman RM, Aronow WS, DeLong JF. Diagnosis of heart failure in older adults: predictive value of dyspnea at rest. Arch Gerontol Geriatr. 2004;38(3):297–307. doi: 10.1016/j.archger.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Ker J. Combination treatment for hypertension. SA Fam Pract. 2010;52(5):417–421. [Google Scholar]
- 19.Petros G. Patterns of heart disease Jimma Hospital. Bull JIHS. 1996;6:85–92. [Google Scholar]
- 20.Hodes RM. Pattern of heart disease in Ethiopia as seen in a cardiology referral clinic. Cardiology. 1988;75:458–464. doi: 10.1159/000174417. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury AW, Alam N, Khan HLR, Sabah KMN, Amin MG. The pattern of cardiac disease at coronary care unit of Dhaka Medical College Hospital. Cardiovasc J. 2015;7(2):119–122. [Google Scholar]
- 22.Huihui W, Raman GNV. Ethiopia Universal Health Coverage for Inclusive and Sustainable Development: Country Summary Form. Washington, DC: World Bank Group; 2014. [Google Scholar]
- 23.Assarzadegan F, Asadollahi M, Hesami O, Aryani O, Mansouri B. Secondary headaches attributed to arterial hypertension. Iran J Neurol. 2013;12(3):106. [PMC free article] [PubMed] [Google Scholar]
- 24.Shukrala F, Gabriel T. Assessment of prescribing, dispensing, and patient use pattern of antihypertensive drugs for patients attending outpatient department of Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia. Drug Design Dev Ther. 2015;9:519–523. doi: 10.2147/DDDT.S73670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain Z, Sana A, Mohammed S, Razzaq MA. Patterns of drug therapy among diabetic hypertensive patients with other complications. Int J Pharm Pharm Sci. 2014;6(6):270–277. [Google Scholar]
- 26.Abebe TB, Gebreyohannes EA, Tefera YG, Abegaz TM. Patients with HFpEF and HFrEF have different clinical characteristics but similar prognosis: a retrospective cohort study. BMC Cardiovasc Disord. 2016;16:8. doi: 10.1186/s12872-016-0418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiroyuki T, Miyuki T-M, Shintaro K. Clinical characteristics and outcomes of heart failure with preserved ejection fraction: lessons from epidemiological studies. J Cardiol. 2010;55:13–22. doi: 10.1016/j.jjcc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko H, Suzuki H, Yajima J, et al. Clinical characteristics and long-term clinical outcomes of Japanese heart failure patients with preserved versus reduced left ventricular ejection fraction. J Cardiol. 2013;62(2):102–109. doi: 10.1016/j.jjcc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Magana-Serrano J, Almahmeed W, Gomez E, et al. Prevalence of heart failure with preserved ejection fraction in Latin American, Middle Eastern, and North African Regions in the I PREFER study (Identification of Patients With Heart Failure and PREserved Systolic Function: an epidemiological regional study) Am J Cardiol. 2011;108(9):1289–1296. doi: 10.1016/j.amjcard.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Babua C, Kalyesubula R, Okello E, et al. Pattern and presentation of cardiac diseases among patients with chronic kidney disease attending a national referral hospital in Uganda: a cross sectional study. BMC Nephrol. 2015;16:126. doi: 10.1186/s12882-015-0128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhit MA, Rahman MO, Raihan SZ, et al. Cardiovascular disease prevalence and prescription patterns at a tertiary level hospital in Bangladesh. J Appl Pharm Sci. 2012;02(03):80–84. [Google Scholar]
- 32.Essop MR, Nkomo VT. Rheumatic and nonrheumatic valvular heart disease epidemiology, management, and prevention in Africa. Circulation. 2005;112:3584–3591. doi: 10.1161/CIRCULATIONAHA.105.539775. [DOI] [PubMed] [Google Scholar]