Abstract
A candlelight-style organic light emitting diode (OLED) is a human-friendly type of lighting because it is blue-hazard-free and has a low correlated color temperature (CCT) illumination. The low CCT lighting is deprived of high-energy blue radiation, and it can be used for a longer duration before causing retinal damage. This work presents the comprehensive protocols for the fabrication of blue-hazard-free candlelight OLEDs. The emission spectrum of the OLED was characterized by the maximum exposure time limit of the retina and the melatonin suppression sensitivity. The devices can be fabricated using dry and wet processes. The dry-processed OLED resulted in a CCT of 1,940 K and exhibited a maximum retinal exposure limit of 1,287 s at a brightness of 500 lx. It showed 2.61% melatonin suppression sensitivity relative to 480 nm blue light. The wet-processed OLED, where the spin coating is used to deposit hole injection, hole transport, and emissive layers, making fabrication fast and economical, produced a CCT of 1,922 K and showed a maximum retinal exposure limit of 7,092 at a brightness of 500 lx. The achieved relative melatonin suppression sensitivity of 1.05% is 86% and 96% less than that of the light emitting diode (LED) and compact fluorescent lamp (CFL), respectively. Wet-processed blue-hazard-free candlelight OLED exhibited a power efficiency of 30 lm/W, which is 2 times that of the incandescent bulb and 300 times that of the candle.
Keywords: Engineering, Issue 121, Blue-hazard, candlelight OLED, wet-processed OLED, dry-processed OLED, low color temperature, eye protection, melatonin secretion
Introduction
Nowadays, lighting sources like LED and CFL are abundantly used for indoor and outdoor illumination, partly for energy-saving reasons. However, these lights are rich in blue emission, showing a higher tendency to cause blue-hazards. LED and CFL emit a spectrum enriched with blue light, leading to irreversible damage to retinal cells1,2,3,4. Blue light or intense white light with high CCT suppresses the secretion of melatonin, an oncostatic hormone, which may disrupt the circadian rhythm5,6 and sleeping behavior7,8. Melatonin, an essential hormone for the circadian rhythm, is synthesized in the pineal gland9. A high level of melatonin is observed during the dark period during the 24-h light-dark cycle10. However, intensive light at night suppresses its synthesis and disrupts the circadian rhythm11. Melatonin suppression due to overexposure to bright lights at night can be a risk factor for breast cancer in women12,13,14. Besides these hazards, blue light interrupts the activities of nocturnal amphibians and can be threatening to ecological protection. It has also been reported that LED lighting in museums is discoloring the actual colors of oil paintings painted by Van Gogh and Cézanne15,16.
Thus, a blue-emission free and low CCT candle-like organic LED (OLED) can be a good substitute for LED and CFL. Candles emit a blue-hazard-free and low CCT (1,914 K) illumination, as well as a high-quality (high color rendering index, CRI) emission spectrum. However, most of the electricity-driven lighting devices emit intense blue light with a comparatively high CCT. For example, the lowest CCT is about 2,300 K for incandescent bulbs, while it is 3,000 or 5,000 K for warm or cold white fluorescent tubes and LED luminaires. So far, low CCT OLEDs nearly free of the blue emission have been fabricated for human-friendly lighting. In 2012, Jou's group reported a physiologically friendly, dry-processed, single emissive layer OLED with a CCT of 1,773 K and a power efficiency of 11.9 lm/W17. The device exhibited a much lower CCT as compared to the incandescent bulb (2,300 K), while its power efficiency was not acceptable from an energy-saving point of view. They reported another dry-processed candlelight-style OLED by using double emissive layers along with a carrier modulation layer18. It exhibited a low CCT of 1,970 K and a power efficiency of 24 lm/W. Later on, a dry-processed OLED consisting of three emissive layers along with a carrier modulation layer was reported19. Its power efficiency was from 21 to 3 lm/W and varied with the CCT, which ranged from 2,500 K to 1,900 K. In 2014, Hu et al. reported a dry-processed hybrid OLED with double emissive layers separated by an interlayer, which showed a high power efficiency of 54.6 lm/W and a low CCT of 1,910 K20. Recently, Jou's group has fabricated a high-efficiency candlelight-style OLED by employing double emissive layers21. It exhibited a high power efficiency of 85.4 lm/W with a CCT of 2,279 K. Until now, all efforts have been made to develop high efficiency, low CCT candlelight-style OLED devices by utilizing dry processes and complicated device architectures17,18,19,20,21,22. Devising a candlelight OLED with wet-process feasibility while simultaneously having a low CCT, a high power efficiency, and a high light quality is a challenge. No study has been developed to describe the emission spectrum sensitivity of a given light source with respect to the blue light. The quality of light at night can be decided/improved to minimize the suppression of melatonin secretion.
There are some reported models that calculate the amount of suppression. Firstly, Brainard et al.23 and Thapan et al.24 reported the spectral sensitivity by using monochromatic light. Later on, the effect of polychromatic light on melatonin suppression was described25,26. The latter is adopted in this study, since most of the commercially available luminaires or novel lighting sources are polychromatic and span over the entire visible range (i.e., from deep red to violet).
In this work, we present comprehensive protocols for the fabrication of blue-hazard-free candlelight OLEDs via dry and wet processes. In both processes, the device architecture is simplified by employing a single emissive layer without any carrier modulation layers. The electroluminescent (EL) spectrum of the fabricated OLED is analyzed for the retinal exposure limit and for the level of melatonin secretion suppression. A maximum exposure limit of emitted light to the retina is calculated by using the theoretical aspect that was reported by the International Electrotechnical Commission (IEC) 62471 standard27,28. The maximum exposure limit "t" is calculated by using the emission spectrum of each OLED at the brightness of 100 and 500 lx, sufficient for home and office illumination, respectively. All related calculation steps are sequentially given in the protocol section. Further, the effect of lighting on the melatonin suppression sensitivity is calculated by following the equations of the action spectrum of melatonin suppression29. The calculation is done by following the steps given in the protocol section. The calculated values of the maximum exposure limit "t" and the melatonin suppression sensitivity (%) with respect to CCT are given in Table 3.
Protocol
NOTE: All the materials used are non-carcinogenic, non-flammable, and non-toxic.
1. Fabrication of Blue-hazard-free Candlelight OLED
- Dry process
- Take a glass slide as a substrate to be coated with a 125 nm indium tin oxide (ITO) anode layer. Wash the substrate with 200 mL (50 mL of liquid detergent and 150 mL of deionized water) of soap solution. Rinse the substrate with deionized water. Dry the substrate with a nitrogen jet spray.
- Put the substrate on a glass slide holder and dip the slide holder in acetone solution in a beaker. Put the beaker in an ultrasonic bath. Sonicate the substrate at 50 °C for 10 min.
- Transfer the slide holder with the substrate to isopropanol solution in a beaker and again sonicate at 60 °C for 10 min.
- Take out the substrate from the beaker and put it in the UV/ozone slot for 10 min to dry. Clean the surface completely.
- Break the vacuum of the thermal evaporator chamber by closing the valve of the high vacuum and opening the valve of nitrogen gas to the chamber.
- Load the cleaned substrate in the chamber on the rotating substrate holder. For each layer that will be deposited, load 100 mg of each required organic material, 3 mg of lithium fluoride (LiF), and a 224 mg aluminum (Al) ingot into the crucible inside the chamber.
- Close the door of the chamber and wait for a high vacuum of 5×10-6 Torr. Once the high vacuum has been reached inside the chamber, start the deposition of the organic layers onto the substrate with ITO.
- Deposit a 5 nm hole injection layer at a deposition rate of 0.8-1 Å/s.
- Deposit a 25 nm transport layer at a deposition rate of 1-1.5 Å/s.
- Deposit a 30 nm emissive layer (8 wt.% green dye and 0.85 wt.% deep-red dye doped in 20 mg of a specified host) at a deposition rate of 1-1.5 Å/s.
- Deposit a 30 nm electron transport layer at a deposition rate of 1-1.5 Å/s.
- Deposit a 20 nm layer of electron transport co-evaporate with electron injection material at a deposition rate of 1-1.5 Å/s.
- Deposit a 1-nm electron injection layer of LiF at a deposition rate of 0.3-0.4 Å/s.
- Deposit a 100-nm cathode layer of Al at a deposition rate of 10-15 Å/s.
- Turn off the current controller and wait 10 min under high vacuum. Close the valve for high vacuum and open the valve for nitrogen gas to the chamber to break the high vacuum.
- Move the fabricated OLED device from the chamber to the atmosphere, and then transfer it to a glove box with an encapsulation machine under a nitrogen atmosphere.
- Encapsulate the fabricated OLED device with a top cover made of glass by using glue and then dry the glue by putting the device in UV radiation box for 110 s.
- Eject out the encapsulated OLED device from the glove box and transfer it to the darkroom for measurements.
- Wet process
- Clean the ITO-coated substrate by using the aforementioned cleaning procedures from steps 1.1.2 to 1.1.4.
- Take an aqueous solution of PEDOT: PSS (stored at 4 °C) to deposit the hole injection layer. Filter the solution in a vial by using a 25-mm diameter filter consisting of a nylon fabric with a pore size of 0.45 µm.
- In a vial, prepare the hole transport layer solution of 3,6-bis(4-vinylphenyl)-9-ethylcarbazole (VPEC)30 dissolved in chlorobenzene solvent in the ratio of 3 mg:1,000 µL. Sonicate the solution for 30 min in the ultrasonic bath and filter the sonicated solution in a vial with a 15-mm diameter filter consisting of a nylon fabric with a pore size of 0.45 µm.
- Prepare a solution for the emissive layer.
- Take 5 mg of the specified host material and dissolve it in tetrahydrofuran (THF) in a ratio of 10 mg:1,000 µL. Sonicate the host-solution at 50 °C for 30 min.
- Take 1 mg of each of the required guest materials and dissolve them in THF in a ratio of 1 mg:1,000 µL. Sonicate the guest-solution at 50 °C for 30 min.
- Filter each solution separately in vials with a 15 mm diameter filter consisting of a nylon fabric with a pore size of 0.45 µm.
- Mix the guest-solution into the host-solution according to the given weight percent (3 wt.% of yellow dye, 6 wt.% of orange-dye, and 12.5 wt.% of green dye), doping for the emissive layer.
- Transfer the vials of PEDOT: PSS, VPEC, and emissive layer solutions along with pre-cleaned substrate and pipette them into the glove box.
- Start coating the layers onto the substrate with ITO in the following sequence under a nitrogen atmosphere: the hole injection layer, hole transport layer, and emissive layer.
- Deposit a 35 nm hole injection layer by spin-coating a 750 µL solution of PEDOT: PSS at 4,000 revolutions per min (rpm) for 20 s.
- Dry the PEDOT: PSS layer at 120 °C for 40 min to remove residual solvent.
- Deposit a 10-nm hole transport layer by spin-coating a 400 µL solution of VPEC at 3,000 rpm for 20 s.
- Bake the layer at 120 °C for 20 min to remove residual solvent.
- Heat the layer at 230 °C for 40 min for a crosslinking reaction to occur before depositing the emissive layer30.
- Deposit a 20 nm emissive layer by spin-coating a 400-µL solution at 2,500 rpm for 20 min.
- Eject out the spin-coated substrate from the glove box to the atmosphere and transfer it to the thermal evaporator chamber for the further deposition of layers. Break the vacuum of the thermal evaporator chamber by closing the valve of the high vacuum and open the valve of the nitrogen gas to the chamber.
- Load the substrate in the chamber on the rotating substrate holder. Load the 45 mg of TPBi, 3 mg of LiF, and a 224 mg Al ingot into the crucible inside the chamber for the layers that will be deposited. Deposit the layers onto the substrate with the emissive layer in the following sequence.
- Deposit a 32 nm electron transport layer of TPBi at a deposition rate of 1-1.5 Å/s.
- Deposit a 1 nm electron injection layer of LiF at a deposition rate of 0.3-0.4 Å/s.
- Deposit a 100-nm cathode layer of Al at a deposition rate of 10-15 Å/s.
- Turn off the current controller and wait 10 min under the high vacuum. Follow the aforementioned procedures from steps 1.1.8 to 1.1.11 to complete the encapsulated OLED device.
- Calculation of the retina-permissible exposure limit "t":
- Measure the EL spectrum of the lighting device by using a spectroradiometer. The resulting EL spectrum is shown in Figure 1a.
- Measure the EL spectrum data (intensity versus wavelength) at a CCT.
- Convert the EL spectrum data to spectral radiance Eλ (normalized intensity versus wavelength). Change the spectrum to the format shown in Figure 1b.
- Use the spectral data from the blue light-weighted function for measuring the retinal hazard from lighting source (i.e., draw the blue light hazard function B(λ) with respect to the wavelength)28. The resulting plot is shown in Figure 1c.
- Calculate the value of the radiance (EB) of a given light source by using the spectral radiance Eλ and blue-hazard function B(λ) corresponding to each wavelength.
- Put the values of Eλ and B(λ) from the abovementioned plots into the following formula:
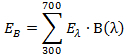 .....(1)
.....(1) - Get the numerical value of EB in W m-2.
- Put the value of EB in the maximum permissible retinal exposure limit "t" formula:
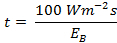 .....(2)
.....(2) - Acquire the exposure limit "t" with respect to the CCT of a given light source.
- Calculation for melatonin suppression sensitivity:
- Measure the EL spectrum of a given lighting device by using spectroradiometer. The resulting spectrum is shown in Figure 2a.
- Get the melatonin suppression power per quantum, SPQ, from the programmed data29. For a given monochromatic light λ, express the SPQ as follows: SPQ (λ) = 10 (λr-λ)/C …………. (3) The values of SPQ (λ) with respect to wavelength are given in Table 1, and the respective graph is shown in Figure 2b.
- Use the photopic luminosity function V (λ) to convert SPQ (λ) into the melatonin suppression power per lux, SLC (λ), in order to give it a practical meaning. The values of V (λ) with respect to the wavelength are given in Table 2, and the respective graph is shown in Figure 2c.
- Express the correlated melatonin suppression power, SLC (λ), for a polychromatic light, as follows:29 SLC (λ) = ∫λSPQ(λ)SI(λ)dλ/∫ V(λ)SI(λ)dλ …………….. (4)
- Put the values of the intensity SI (λ) from the EL spectrum of a given light source along with the values of SPQ (λ) and V(λ) with respect to the wavelength in the above formula and calculate the SLC (λ) as follows: SLC (λ) =
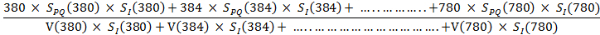
- Retrieve a numerical value of SLC (λ) in lx-1 from the above calculation. For example, by putting the SI (λ) from the EL spectrum of the given candlelight OLED with a CCT of 1,940 K, the melatonin suppression power is: SLC (λ) = 90 lx-1
- Choose a reference light to calculate the relative melatonin suppression sensitivity of a given light source. The reference light can be a wavelength of 460 or 480 nm. Here, we choose a blue light of 480 nm as the reference light.
- Calculate the SLC (λ) for the reference blue light (480 nm) by using the abovementioned formula. SLC (480 nm) = 3,445 lx-1
- Divide the SLC (λ) of a given light source by the SLC (480 nm) and multiply the quotient by 100 to get the melatonin suppression sensitivity percentage (%) of a given light relative to the reference blue light. Relative melatonin suppression sensitivity =
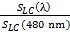 ×100 % ……….... (5) NOTE: For example, relative melatonin suppression sensitivity =
×100 % ……….... (5) NOTE: For example, relative melatonin suppression sensitivity = 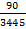 ×100% = 2.61%. Thus, the given candlelight OLED shows a melatonin suppression sensitivity of 2.61% relative to that of the 480-nm blue light.
×100% = 2.61%. Thus, the given candlelight OLED shows a melatonin suppression sensitivity of 2.61% relative to that of the 480-nm blue light.
Representative Results
The current-voltage-luminance characteristics of the resulting candlelight OLEDs are measured by using an electrometer together with a 100 A luminance meter. The emission areas are 9 mm2 for all of the resulting dry-processed devices and are 25 mm2 for wet-processed devices. Here, we used a 125 nm ITO-coated glass substrate with a sheet resistance of 15 Ω/sq as an anode. It has a transparency greater than 84% (Table 4). All the OLED devices consisting of an Al cathode are measured with luminance in the forward direction. The EL spectrum and Commission International de l'Eclairge (CIE) color coordinates are obtained by using a spectroradiometer31. The resulting EL spectrum is used to calculate the retina exposure limit "t" and the melatonin suppression power. All the calculation steps are sequentially given in the protocol section.
The permissible retinal exposure is calculated from the radiance of the given light source that is directed to the human eye. The maximum exposure duration for blue light could be equal to or lower than 100 s if the human eye is directed to a light source of radiation EB = 1 Wm-2. If the radiance is less than 1 Wm-2, the exposure limit will exceed 100 s27. The calculated maximum exposure limit "t" can be used to classify the given light source into one of the four risk groups (i.e., Risk Group 0 (RG0), Risk Group 1 (RG1), Risk Group 2 (RG2), and Risk group 3 (RG 3) if "t" is greater than 10,000 s, between 10,000 and 100 s, between 100 and 0.25 s, or less than 0.25 s, respectively). Figure 3a and 3b shows the effect of CCT at 100 lx and 500 lx on the retinal exposure limit of the blue-hazard-free candlelight OLEDs made via dry and wet processes. Generally, the admissible exposure limit would increase with a decreasing CCT. Most importantly, the applied illuminance has an extremely profound effect on the retinal maximum permissible exposure limit. By reducing the applied brightness from 500 to 100 lx, the entire exposure limit shifts into the RG0 zone, most of which would otherwise be located in the RG1 zone. Those lighting devices that exhibit a CCT lower than 1,922 K especially shift their exposure limits to RG0, as shown in Figure 3a. Taking the radiation at 500 lx, for example, the retina can tolerate 1,020 s at 2,700 K (Device 1-i), 1,226 s at 2,100 K (Device 1-ii), and 6,284 s at 1,864 K (Device 2-i). In other words, light at 1,864 K is 5 and 6.2 times safer than light at 2,100 K and 2,700 K, respectively. As shown in Figure 3b, all the studied OLED devices show exposure limits with a risk group RG1 at 500 lx. By reducing the illumination to 100 lx, the exposure limit will increase by 5 times over the entire CCT studied. In other words, it will be 5 times safer to adopt an illuminance of 100 lx in lieu of 500 lx. As shown in Figure 3a, at 100 lx, the devices (2-i, ii, iii) with a CCT from 1,922 K to 1,864 K show an exposure limit with a RG0 classification. It should be noted that any devices with the RG0 classification are still harmful to the retina, as the exposure time exceeds 100,000 s. Therefore, even the low-CCT OLED shows a permissible exposure time limit beyond which retinal damage can occur.
The melatonin suppression sensitivity is calculated by using the EL spectrum of candlelight OLED, the melatonin suppression power per lux, and the luminosity function. The melatonin suppression power per quantum, SPQ, at varying wavelengths is given in Table 1. The suppression power per photon is then converted into per lux by using the luminosity function V(λ). The average intensities of lights at different wavelengths are given in Table 2. The reference blue light of 480 nm is used to calculate the relative melatonin suppression sensitivity of the candlelight OLED. The whole calculation is done by using the protocol steps 1.4.1 to 1.4.9.
As shown in Figure 4, all the fabricated blue-hazard-free candlelight OLED devices show a melatonin suppression sensitivity below 4%. Device 1-i with a CCT of 2,700 K suppresses the secretion of melatonin to 3.19%, Device 1-ii with a CCT of 2,100 K suppresses it to 2.74%, and Device 1-iii with a CCT of 1,940 K suppresses it to 2.61%. In other words, Device 1-iii suppresses 18% and 14% less melatonin secretion than Devices 1-i and 1-ii, respectively. Moreover, Device 2-iii, with a CCT of 1,922 K, shows the minimum melatonin suppression sensitivity, 1.05%, among all the reported OLED devices. Therefore, Device 2-iii is 67% better than Device 1-i (2,700 K). Moreover, the warm-white LED (CCT: 2,632 K, melatonin suppression sensitivity: 8%) and cold-white CFL (CCT: 5,921 K, melatonin suppression sensitivity: 29%) are 662% and 2,662% more hazardous to melatonin secretion than the OLED Device 2-iii counterpart. Therefore, the blue-hazard-free candlelight OLEDs that exhibit a very low suppression effect on the secretion of melatonin and can be used at night without greatly disturbing the secretion of melatonin.
Moreover, light quality is one critical parameter of any illumination source. The color rendering index (CRI) was once considered the most reliable metric to quantify the light quality of a given lighting source. However, some shortcomings are noticed in CRI values. To improve upon it, a new light quality index, spectrum resemblance index (SRI), is reported. It is defined as the percentage similarity between a given light source and its corresponding blackbody radiation based on the same CCT32,33. In order to create a quality light, a low CCT or blue emission-free lighting device with a high SRI is needed. Nonetheless, the available lighting devices do not demonstrate these qualities. Here, the reported blue-hazard-free candlelight OLED devices exhibit an SRI ranging from 75 to 84, with a low CCT from 1,864 K to 2,700 K. For example, the OLED devices with CCT values of 1,922 K and 1,940 K show SRI values of 76 and 81, respectively (Table 3). The emitted lights of a candle and blue-hazard-free candlelight OLED are shown in Figure 5.
From an energy-saving perspective, candles are considered energy-wasting (0.1-0.3 lm/W). The reported blue-hazard-free candlelight OLED exhibits a power efficiency of 30 lm/W, which is twice that of an incandescent bulb and 300 times that of a candle. The performance of each device is given in Table 3. In addition, this candlelight OLED provides a physically cool but sensationally warm glow. It is energy-saving, non-obtrusive, and free from flickering, glare, and UV radiation. The blue-hazard-free candlelight OLED is safe to use instead of candles or the other current white lights.
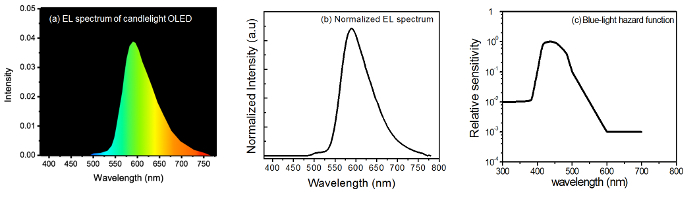 Figure 1: (a) Sample EL spectrum of the given candlelight OLED, (b) normalized EL spectrum of the fabricated candlelight source, and (c) blue-light hazard function with respect to the wavelength and action spectrum of the blue-light hazard with a crystalline lens in the eye28(reproduced from ICNIRP 2013). Please click here to view a larger version of this figure.
Figure 1: (a) Sample EL spectrum of the given candlelight OLED, (b) normalized EL spectrum of the fabricated candlelight source, and (c) blue-light hazard function with respect to the wavelength and action spectrum of the blue-light hazard with a crystalline lens in the eye28(reproduced from ICNIRP 2013). Please click here to view a larger version of this figure.
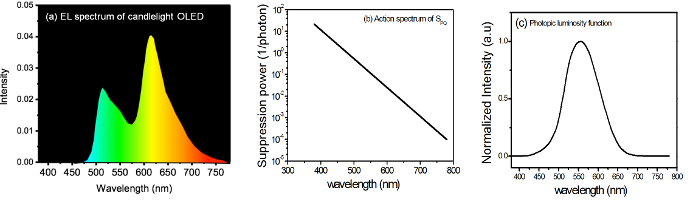 Figure 2: (a) Sample EL spectrum of the fabricated candlelight OLED, (b) melatonin suppression power per quantum, SPQ, versus wavelength29, and (c) luminosity function V(λ) (normalized intensity of different lights versus the wavelength). Please click here to view a larger version of this figure.
Figure 2: (a) Sample EL spectrum of the fabricated candlelight OLED, (b) melatonin suppression power per quantum, SPQ, versus wavelength29, and (c) luminosity function V(λ) (normalized intensity of different lights versus the wavelength). Please click here to view a larger version of this figure.
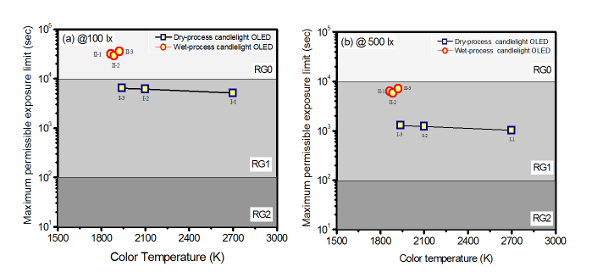 Figure 3: Effect of the CCT of the blue-hazard-free candle light OLEDs on the retinal maximum permissible exposure limit at (a) 100 lx and (b) 500 lx. At a high brightness, even a low CCT OLED may pose a threat to the retina. Please click here to view a larger version of this figure.
Figure 3: Effect of the CCT of the blue-hazard-free candle light OLEDs on the retinal maximum permissible exposure limit at (a) 100 lx and (b) 500 lx. At a high brightness, even a low CCT OLED may pose a threat to the retina. Please click here to view a larger version of this figure.
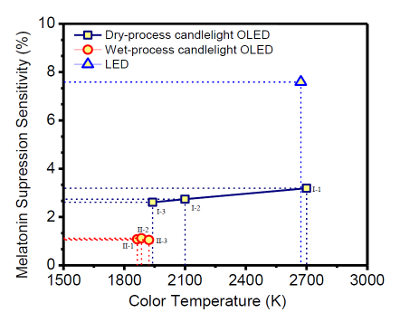 Figure 4: Effect of CCT on the melatonin suppression sensitivity (%) of the blue-hazard-free candlelight OLEDs, made via dry and wet processes, and warm-white LED. The blue-hazard-free candlelight OLED exhibits a very low suppression effect on the secretion of melatonin. Please click here to view a larger version of this figure.
Figure 4: Effect of CCT on the melatonin suppression sensitivity (%) of the blue-hazard-free candlelight OLEDs, made via dry and wet processes, and warm-white LED. The blue-hazard-free candlelight OLED exhibits a very low suppression effect on the secretion of melatonin. Please click here to view a larger version of this figure.
 Figure 5: Photographs of cloud papers with rainbows and white colors illuminated by candles (left) and a blue-hazard-free candlelight OLED (right) at 10 lx34. Please click here to view a larger version of this figure.
Figure 5: Photographs of cloud papers with rainbows and white colors illuminated by candles (left) and a blue-hazard-free candlelight OLED (right) at 10 lx34. Please click here to view a larger version of this figure.
| wavelength(nm) | SPQ | wavelength(nm) | SPQ | wavelength(nm) | SPQ | wavelength(nm) | SPQ |
| 380 | 21.54435 | 484 | 0.88444 | 588 | 0.03631 | 692 | 0.00149 |
| 384 | 19.05461 | 488 | 0.78223 | 592 | 0.03211 | 696 | 0.00132 |
| 388 | 16.85259 | 492 | 0.69183 | 596 | 0.0284 | 700 | 0.00117 |
| 392 | 14.90505 | 496 | 0.61188 | 600 | 0.02512 | 704 | 0.00103 |
| 396 | 13.18257 | 500 | 0.54117 | 604 | 0.02222 | 708 | 9.12E-04 |
| 400 | 11.65914 | 504 | 0.47863 | 608 | 0.01965 | 712 | 8.07E-04 |
| 404 | 10.31177 | 508 | 0.42332 | 612 | 0.01738 | 716 | 7.13E-04 |
| 408 | 9.12011 | 512 | 0.3744 | 616 | 0.01537 | 720 | 6.31E-04 |
| 412 | 8.06616 | 516 | 0.33113 | 620 | 0.01359 | 724 | 5.58E-04 |
| 416 | 7.134 | 520 | 0.29286 | 624 | 0.01202 | 728 | 4.94E-04 |
| 420 | 6.30957 | 524 | 0.25902 | 628 | 0.01063 | 732 | 4.37E-04 |
| 424 | 5.58042 | 528 | 0.22909 | 632 | 0.0094 | 736 | 3.86E-04 |
| 428 | 4.93552 | 532 | 0.20261 | 636 | 0.00832 | 740 | 3.41E-04 |
| 432 | 4.36516 | 536 | 0.1792 | 640 | 0.00736 | 744 | 3.02E-04 |
| 436 | 3.86071 | 540 | 0.15849 | 644 | 0.00651 | 748 | 2.67E-04 |
| 440 | 3.41455 | 544 | 0.14017 | 648 | 0.00575 | 752 | 2.36E-04 |
| 444 | 3.01995 | 548 | 0.12397 | 652 | 0.00509 | 756 | 2.09E-04 |
| 448 | 2.67096 | 552 | 0.10965 | 656 | 0.0045 | 760 | 1.85E-04 |
| 452 | 2.36229 | 556 | 0.09698 | 660 | 0.00398 | 764 | 1.63E-04 |
| 456 | 2.0893 | 560 | 0.08577 | 664 | 0.00352 | 768 | 1.45E-04 |
| 460 | 1.84785 | 564 | 0.07586 | 668 | 0.00311 | 772 | 1.28E-04 |
| 464 | 1.63431 | 568 | 0.06709 | 672 | 0.00275 | 776 | 1.13E-04 |
| 468 | 1.44544 | 572 | 0.05934 | 676 | 0.00244 | 780 | 1.00E-04 |
| 472 | 1.2784 | 576 | 0.05248 | 680 | 0.00215 | ||
| 476 | 1.13066 | 580 | 0.04642 | 684 | 0.00191 | ||
| 480 | 1 | 584 | 0.04105 | 688 | 0.00169 |
Table 1: Intensity versus suppression power per quantum29, SPQ.
| Wavelength (nm) | Intensity | Wavelength (nm) | Intensity | Wavelength (nm) | Intensity | Wavelength (nm) | Intensity |
| 380 | 4.00E-05 | 484 | 0.16366 | 588 | 0.78061 | 692 | 0.00714 |
| 384 | 5.83E-05 | 488 | 0.19197 | 592 | 0.73206 | 696 | 0.00544 |
| 388 | 9.15E-05 | 492 | 0.22777 | 596 | 0.68174 | 700 | 0.00414 |
| 392 | 1.58E-04 | 496 | 0.27123 | 600 | 0.63095 | 704 | 0.00315 |
| 396 | 2.51E-04 | 500 | 0.32467 | 604 | 0.57982 | 708 | 0.00242 |
| 400 | 4.03E-04 | 504 | 0.39087 | 608 | 0.52858 | 712 | 0.00184 |
| 404 | 6.33E-04 | 508 | 0.46488 | 612 | 0.47824 | 716 | 0.0014 |
| 408 | 9.45E-04 | 512 | 0.54392 | 616 | 0.4292 | 720 | 0.00106 |
| 412 | 0.00159 | 516 | 0.6281 | 620 | 0.38107 | 724 | 7.97E-04 |
| 416 | 0.00253 | 520 | 0.70784 | 624 | 0.33365 | 728 | 6.05E-04 |
| 420 | 0.00405 | 524 | 0.77659 | 628 | 0.28762 | 732 | 4.50E-04 |
| 424 | 0.00656 | 528 | 0.83515 | 632 | 0.24551 | 736 | 3.38E-04 |
| 428 | 0.00979 | 532 | 0.88379 | 636 | 0.2086 | 740 | 2.51E-04 |
| 432 | 0.01361 | 536 | 0.92268 | 640 | 0.17539 | 744 | 1.87E-04 |
| 436 | 0.01803 | 540 | 0.95299 | 644 | 0.14556 | 748 | 1.40E-04 |
| 440 | 0.02303 | 544 | 0.97501 | 648 | 0.11924 | 752 | 1.04E-04 |
| 444 | 0.0285 | 548 | 0.98946 | 652 | 0.09655 | 756 | 7.94E-05 |
| 448 | 0.03461 | 552 | 0.99751 | 656 | 0.07745 | 760 | 6.02E-05 |
| 452 | 0.0419 | 556 | 0.99921 | 660 | 0.0613 | 764 | 4.55E-05 |
| 456 | 0.05033 | 560 | 0.99408 | 664 | 0.04778 | 768 | 3.47E-05 |
| 460 | 0.06012 | 564 | 0.9819 | 668 | 0.03686 | 772 | 2.59E-05 |
| 464 | 0.07118 | 568 | 0.96302 | 672 | 0.02833 | 776 | 1.96E-05 |
| 468 | 0.08388 | 572 | 0.9377 | 676 | 0.02212 | 780 | 1.50E-05 |
| 472 | 0.09942 | 576 | 0.9062 | 680 | 0.0171 | ||
| 476 | 0.11778 | 580 | 0.86915 | 684 | 0.0129 | ||
| 480 | 0.13932 | 584 | 0.82678 | 688 | 0.00963 |
Table 2: Intensity of different lights in the visible range.
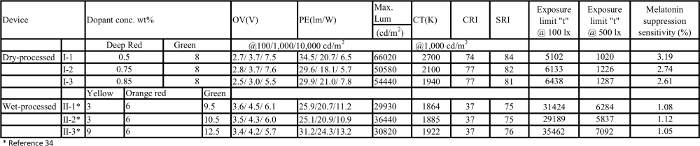 Table 3:. Operation voltage (OV), power efficiency (PE), CCT, light quality spectrum resemblance index (SRI), exposure limit "t", melatonin suppression sensitivity (%), and maximum luminance of the studied blue-hazard-free candlelight OLED devices made via dry and wet processes. Please click here to view a larger version of this table.
Table 3:. Operation voltage (OV), power efficiency (PE), CCT, light quality spectrum resemblance index (SRI), exposure limit "t", melatonin suppression sensitivity (%), and maximum luminance of the studied blue-hazard-free candlelight OLED devices made via dry and wet processes. Please click here to view a larger version of this table.
Discussion
The most critical steps in the fabrication of OLED devices are: 1) cleaning the glass substrate, 2) selecting the appropriate solvent, 3) dissolving the organic materials, 4) uniformly forming the film via spin-coating in the wet process, and 5) controlling the deposition rate and thickness of the organic layer during the thermal evaporation. Initially, cleaning the ITO anode coated substrate is a crucial step to achieve high efficiency. The glass substrate is cleaned with soap solution to remove greasy spots or layers. Then, it is ultra-sonicated in acetone, followed by isopropanol, to eradicate dirt particles from the anode layer. UV/ozone treatment is given to the substrate before depositing any layer on the ITO. UV/ozone treatment not only dries the substrate, but it also increases the surface oxygen and thus enhances the work function of ITO35. It can reduce the hole injection barrier to facilitate more hole transport.
Subsequently, organic layers are deposited on the ITO anode by two separate methods, namely the dry process and the wet process. For the candlelight OLED fabricated with the dry process, all organic molecules are evaporated under high vacuum and deposited successively on the ITO layer. In this process, temperature is increased gradually step by step, and the organic materials are deposited at a certain temperature. It prevents non-uniformity of the thin film and allows for precise layer thickness. Dry-processed candlelight OLED devices are ultra-clean and free from any non-emissive spots. Nevertheless, this process is limited to producing large-area films and is cost-ineffective due to a large consumption of organic materials. On the other hand, the wet process includes spin-coating, inkjet printing, and screen printing of polymer and organic materials, a cost-effective, large-area, and mass-manufacturing procedure for the creation of OLED devices36-38.
For wet-processed candlelight OLEDs, hole injection, hole transporting, and emissive layers are spin-coated at a specified rpm and duration. It is a fast deposition technique, which allows for continuous production. Major challenges in the wet process are the selection of solvent and the prevention of undesirable blending of subsequently coated organic layers. Some organic materials do not dissolve properly in the organic solvent due to a polarity mismatch. Organic solvents also dissolve precast organic layers, resulting in morphological and compositional defects39,40. To avoid such difficulties, we baked the hole injection layer of a conducting polymer, PEDOT:PSS, to make a more hydrophilic surface before coating the hole transport layer. After that, a hole transport layer of VPEC is spin-coated and again baked at 120 °C for 20 min to make it thermally stable and to avoid the presence of residual solvent. Furthermore, the VPEC layer is heated to 230 °C for crosslinking30 the hole transporting layer. Accordingly, the emissive layer is spin-coated onto the hole transporting layer to circumvent any morphological defects. The electron transport layer and cathode layer are deposited via thermal evaporation under high vacuum.
Earlier-reported candlelight-style OLED devices were fabricated by a dry process18,21. These devices were composed of a complex architecture, like double emissive layers and an additional carrier modulation layer18,21,22. In this study, we have modified the OLED device architecture and avoided complexity by using a single emissive layer. The reported blue-hazard-free candlelight OLEDs are also fabricated without using any blue or sky-blue emitters. The EL spectrum of OLED devices can be arbitrarily formed. Dry- and wet-processed OLED devices exhibited differently shaped emission spectra with low CCT values. These spectra showed different effects from the perspective of maximum exposure limit and melatonin suppression sensitivity (Table 3).
The dry process allows the vapor deposition of small molecules and oligomers in the multiple layer architecture. Additionally, the dry process develops various ways to achieve high efficiency. Also, the multiple-layered architecture enables the lower carrier-injection barrier, balanced carrier-injection to emissive layer, and effective recombination zone to facilitate more carriers to recombine40. However, the dry process has some issues, such as the limited thermal stability of the organic molecules, the low throughput due to the need of a high-vacuum fabrication condition, and the material wastage due to the low material utilization rate in deposition, etc.
In contrast, the wet process is more favorable to reduce the production cost and to achieve high efficiency. Low-cost polymeric materials are promising for multiple-layer, wet-processed OLEDs. Their efficiency is comparatively lower than vacuum-deposited, small molecule organic materials. In the wet process, efficiency can be improved by utilizing a combination of successive polymer and small molecule layers. Generally, the employment of a polymeric hole transport layer with high triplet energy is able to stabilize the prior spin-coated hole injection film and also to confine the excitons generated in the emissive layer. Small-molecule organic materials with high glass transition temperatures are not crystalized during spin-coating and uphold the film integrity. Additionally, high triplet energy small molecules can be used as an effective host material to facilitate a host-to-guest energy transfer mechanism. Wet-process fabrication of OLEDs also has some restrictions due to the solubility issue of its materials. Nowadays, to stabilize the multiple layer architecture in the wet process, many approaches have been developed that maintain the solubility from polar to non-polar solvents42,43,44. The wet process enables the devices to be fabricated in large areas and roll-to-roll with high throughput. The wet process offers more design freedom for disruptive characteristics, such as flexibility, transparency, and ultra-thinness. The wet process can be a promising technology for OLED lighting.
Disclosures
We have nothing to disclose.
Acknowledgments
The authors would like to acknowledge the support in part from the Ministry of Economic Affairs and the Ministry of Science and Technology, Taiwan, via Grants MEA 104-EC-17-A-07-S3-012, MOST 104-2119-M-007-012, and MOST 103-2923-E-007-003-MY3.
References
- Melton R. Ultraviolet and blue light. Rev opt. 2014;2:151. [Google Scholar]
- Singerman LJ, Miller DG. Pharmacological Treatments for AMD. Rev Ophthalmol. 2003;10:88–90. [Google Scholar]
- International Energy Agency final report on potential health issues on SSL. 2014. Sep,
- Reilly R. 2016. http://www.dailymail.co.uk/health/article-2324325/Do-environmentally-friendly-LED-lights-cause-BLINDNESS.html.
- Pauley SM. Lighting for the human circadian clock: Recent research indicates that lighting has become a public health issue. Med. Hypotheses. 2004;63:588–596. doi: 10.1016/j.mehy.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Mills PR, Tomkins SC, Schlangen LJM. The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J. Circadian Rhythm. 2007;5:1–9. doi: 10.1186/1740-3391-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sakaguchi T, Morita T. The effects of exposure in the morning to light of different color temperatures on the behavior of core temperature and melatonin secretion in humans. Biol. Rhythm. Res. 2005;36:287–292. [Google Scholar]
- Arendt J. Melatonin, circadian rhythms, and sleep. New Engl. J. Med. 2000;343(15):1114–1116. doi: 10.1056/NEJM200010123431510. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF. Melatonin: parallels in pineal gland and retina. Exp Eye Res. 1986;42(6):507–527. doi: 10.1016/0014-4835(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Brown GM. Light, melatonin, sleep-wake cycle. J. pshychiatry. Neurosci. 1994;19(5):345–356. [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210(4475):1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J. Clin. 2014;64(3):207–218. doi: 10.3322/caac.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Mirick DK, Stevens RG. Night-shift work, light at night, and risk of breast cancer. J. Natl. Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- Kloog I, Haim A, Stevens RG, Barchanade M, Portnov BA. Light at Night Co Distributes with Incident Breast but Not Lung Cancer in the Female Population of Israel. Chronobiology Intl. 2008;25:65–81. doi: 10.1080/07420520801921572. [DOI] [PubMed] [Google Scholar]
- 2016. http://www.vangogh.ua.ac.be.
- Monico L. Degradation Process of Lead Chromate in Paintings by Vincent van Gogh Studied by Means of Spectromicroscopic Methods 3. Synthesis, Characterization, and Detection of Different Crystal Forms of the Chrome Yellow. S. Anal. Chem. 2013;85(2):851–859. doi: 10.1021/ac302158b. [DOI] [PubMed] [Google Scholar]
- Jou JH. Organic light-emitting diode-based plausibly physiologically-friendly low color-temperature night light. Org. Electron. 2012;13(8):1349–1355. [Google Scholar]
- Jou JH. Candlelight-style organic light-emitting diodes. Adv. Funct. Mater. 2013;23(21):2750–2757. [Google Scholar]
- Jou JH. OLEDs with chromaticity tunable between dusk-hue and candle-light. Org. Electron. 2013;14(1):47–54. [Google Scholar]
- Hu Y, Zhang T, Chen J, Ma D, Cheng CH. Hybrid organic light-emitting diodes with low color temperature and high efficiency for physiologically-friendly night illumination. Isr. J. Chem. 2014;54:979–985. [Google Scholar]
- Jou JH. Enabling a blue-hazard free general lighting based on candlelight-style OLED. Optics Express. 2015;23(11):A576–A581. doi: 10.1364/OE.23.00A576. [DOI] [PubMed] [Google Scholar]
- Jou JH. High efficiency low color-temperature organic light emitting diodes with a blend interlayer. J. Mater. Chem. 2011;21:17850–17854. [Google Scholar]
- Brainard GG. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535(Pt 1):261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullough JD, Bierman A, Figueiro MG, Rea MS. Letter On Melatonin Suppression from Polychromatic and Narrowband Light Lighting Research. Chronobiol. Int. 2008;25(4):653–656. doi: 10.1080/07420520802247472. [DOI] [PubMed] [Google Scholar]
- Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Brain Res Rev. 2005;50:213–228. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- International Electrotechnical Commission. IEC 62471: 2006. Geneva: IEC; 2006. Photobiological safety of lamps and lamp systems. [Google Scholar]
- ICNIRP. ICNIRP guidelines on limits of exposure to incoherent visible and infrared radiation. Health Physics. 2013;105(1) doi: 10.1097/HP.0b013e318289a611. [DOI] [PubMed] [Google Scholar]
- Jou JH, inventor. Melatonin suppression extent measuring device. S20120303282 A1. Patent. 2012
- Jou JH. Enabling high-efficiency organic light-emitting diodes with a cross-linkable electron confining hole transporting material. Org. Electron. 2015;24:254–262. [Google Scholar]
- Commission International de l’Éclairage. Method of measuring and specifying colour rendering of light sources. 3rd. Vienna (Austria): 1995. p. 16. CIE. Publication No. 13.3. [Google Scholar]
- Jou JH. A universal, easy-to-apply light-quality index based on natural light spectrum resemblance. Appl. Phys. Lett. 2014;104:203304–203309. [Google Scholar]
- Jou JH. Pseudo-natural light for displays and lighting. Adv. Optical mater. 2015;3:95–102. [Google Scholar]
- Jou JH. Wetprocess feasible candlelight OLED. J. Mater. Cem. C. 2016.
- Kim BS. UV-ozone surface treatment of indium-tin-oxide in organic light emitting diodes. J. Korean Phys. Soc. 2007;50:1858–1861. [Google Scholar]
- Lee TW. Characteristics of solution-processed small-molecule organic films and light-emitting diodes compared with their vacuum-deposited counterparts. Adv. Mater. 2009;19(10):1625–1630. [Google Scholar]
- Duan L. Solution processable small molecules for organic light-emitting diodes. J. Mater. Chem. 2010;20:6392–6407. [Google Scholar]
- Kim SK. Low-power flexible organic light-emitting diode display device. Adv. Mater. 2011;23:3511–3516. doi: 10.1002/adma.201101066. [DOI] [PubMed] [Google Scholar]
- Kaake LG, Barbara PF, Zhu XY. Intrinsic charge trapping in organic and polymeric semiconductors: a physical chemistry perspective. J. Phys. Chem. Lett. 2010;1(3):628–635. [Google Scholar]
- Yersin H, Rausch AF, Czerwieniec R, Hofbeck T, Fischer T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 2011;255:2622–2652. [Google Scholar]
- Jou JH, Kumar S, Agarwal A, Lia TH, Sahoo S. Approaches for fabricating high efficiency organic light emitting diodes. J. Mater. Chem. C. 2015;3:2974–3002. [Google Scholar]
- Volz D. Auto-catalysed crosslinking for next-generation OLED-design. J. Mater. Chem. 2012;22:20786–20790. [Google Scholar]
- Furuta PT, Deng L, Garon S, Thompson ME, Frechet JMJ. Platinum functionalized random copolymers for use in solution-processible, efficient, near-white organic light-emitting diodes. J. Am. Chem. Soc. 2004;126(47):15388–15389. doi: 10.1021/ja0446247. [DOI] [PubMed] [Google Scholar]
- Biwu M. New thermally cross-linkable polymer and its application as a hole-transporting layer for solution processed multilayer organic light emitting diodes. Chem. Mater. 2007;19:4827–4832. [Google Scholar]


